Abstract
Background
Multimorbidity (multiple co-occurring chronic conditions) may be an important contributor to disability and poor health-related quality of life. The functional consequences of specific combinations of somatic and mental health conditions are unclear.
Methods
Nationally representative prospective cohort study using the National Health and Aging Trends Study data of Medicare beneficiaries. We included 4,017 participants aged 65 years or older interviewed in 2013 and 2014. The primary outcome was prospective activities of daily living (ADL)–instrumental ADL (IADL) index (range = 0–11) assessed in 2014. All other measures were assessed in 2013. Chronic conditions included heart disease, hypertension, stroke, diabetes, arthritis, lung disease, osteoporosis, cancer, depression, and cognitive impairment. Analyses were adjusted for age, sex, education, race/ethnicity, body mass index, and baseline ADL–IADL.
Results
Thirty-four percent of multimorbidity combinations included depression, cognitive impairment, or both. Relative to multimorbidity combinations of exclusively somatic conditions, combinations that included both depression and cognitive impairment were associated with 1.34 times greater ADL–IADL in adjusted models (95% confidence interval [CI]: 1.09, 1.64). Relative to combinations of both depression and cognitive impairment, combinations of cognitive impairment and somatic conditions were associated with 0.84 times lower ADL–IADL in adjusted models (95% CI: 0.74, 0.96); combinations of depression and somatic conditions were associated with 0.72 times lower ADL–IADL in adjusted models (95% CI: 0.62, 0.85).
Conclusions
Depression and/or cognitive impairment was identified in one-third of older adults with multimorbidity, and these combinations were associated with substantially greater prospective disability than combinations comprised exclusively of somatic conditions. This argues for identifying and managing mental health conditions that co-occur with somatic conditions.
Keywords: Multimorbidity, Comorbidity, Multiple chronic conditions, Chronic disease combinations, Disability
Multimorbidity, defined as the co-occurrence of two or more chronic conditions, has been identified in more than 50% of U.S. adults aged 50 years and older, and in 70% of Medicare beneficiaries (1–3). Multimorbidity is not only highly prevalent, but has also been associated with greater disability (4–6), poorer health-related quality of life (7,8), and higher health care costs (8). A growing body of work indicates that the risks of multimorbidity exceed the risks attributable to the individual conditions (9–11). Multiple methods for assessing multimorbidity have been suggested, such as counting the number of chronic conditions, applying weighted frequency and severity-based algorithms, identifying common clusters, and examining pathophysiologically related combinations.
The U.S. Department of Health and Human Services (HHS) has endorsed the practice of considering both somatic and mental health conditions in concert when conceptualizing and measuring multimorbidity (12). In particular, HHS has earmarked dementia and depression—two conditions of particular importance in older adult populations—as critical components in the operationalization of multimorbidity (12). However, few studies have explored the functional consequences of co-occurring somatic and mental chronic health conditions (11,12). Two such studies examined co-occurring somatic-mental multimorbidity among older adults in a geographically confined area (13), and assessed the associations between prevalent multimorbidity combinations in a nationally representative sample of older adults (4), respectively. Both highlight the prevalence of multimorbidity, but do not clarify the role of mental health on the associations between multimorbidity and disability.
This study aimed to examine the relationship between combinations of somatic-mental multimorbidity and prospective disability in a nationally representative sample of Medicare beneficiaries. We hypothesized that multimorbidity combinations including depression, cognitive impairment, or both are associated with significantly greater future disability burden compared with similar combinations that involve only somatic conditions.
Methods
Data Sources
The National Health and Aging Trends Study (NHATS) is a publicly available, population-based longitudinal study. Beginning in 2011, NHATS interviews a nationally representative sample of Medicare beneficiaries living in the contiguous United States. In-person self or proxy interviews are conducted annually. Full details of NHATS design have been published elsewhere (14).
Study Population
Our study included NHATS respondents who completed the sample person questionnaire in 2013 and 2014. Of the 5,799 respondents interviewed in 2013, 82% were also interviewed in 2014 (4,737/5,799) and 91% of these respondents completed the sample person questionnaire (4,321/4,737). Because proxy interviews for persons deceased between the two data collection rounds were not asked comparable activities of daily living (ADL)–instrumental ADL (IADL) questions, we omitted these individuals from the analyses (n = 304).The final analytic sample was 4,017 respondents, including 229 proxy interviews (6%).
Measures
Main outcome variable
All respondents were asked about their difficulty performing a variety of everyday tasks to evaluate function within the month prior to the interview. To ensure time-sequencing, multimorbidity combinations were measured in 2013 and function was assessed in 2014.
ADL index
Respondents were asked whether they experienced difficulty or needed help performing the following ADLs: dressing, eating, bathing, toileting, transferring from bed, and getting around inside. Each of the items was scored 0 for no difficulty/need for help and 1 for difficulty/need for help. A summary ADL impairment variable was generated for each respondent who had a least one nonmissing ADL response (range = 0–6). One additional item—going outside—was also assessed though not included in many prior ADL studies. We added this item to the ADL sum (range = 0–7) in sensitivity analyses presented in the Supplementary Material (Tables A2–A5).
IADL index
IADL included cleaning laundry, preparing hot meals, grocery shopping, taking medications, and managing money. Respondents were asked about difficulty with each IADL task, if they received assistance, and why. IADL impairment was defined as reported difficulty performing the IADL or needing assistance with the IADL due to a health or functioning reason. A summary IADL disability variable was generated for each respondent who had least one nonmissing IADL response (range = 0–5).
ADL–IADL index
The primary outcome for this study is the combined ADL–IADL index (15) representing the sum of ADL and IADL impairments (range = 0–11).
Somatic and Mental Chronic Conditions
Somatic conditions/diseases
Self- and proxy-reported, physician-diagnosed conditions included: heart disease (myocardial infarction or heart disease including angina or congestive heart failure), hypertension, stroke, diabetes, arthritis, lung disease, osteoporosis, and cancer. Each condition was coded 1-if answered yes in 2013 or prior, 0-otherwise.
Depression
The Patient Health Questionnaire 2 (PHQ-2), a valid (16) and reliable screening instrument for depression in older adults (17), was administered to self- and proxy-respondents. Respondents were asked two questions: “Over the last month, how often have you: 1) had little interest or pleasure in doing things; 2) felt down, depressed, or hopeless?” PHQ-2 scores were calculated by summing the responses to each question: 0 = not at all, 1 = several days, 2 = more than half the days, 3 = nearly every day. As suggested by validation studies, a PHQ-2 score of ≥ 3 indicated a positive screen for depression (here forward, depression) (18).
Cognitive impairment
NHATS uses three types of information to classify respondent cognitive function: (i) self- or proxy-report of physician-diagnosed dementia or Alzheimer’s disease; (ii) the AD8 Dementia Screening Interview for proxy reports (memory, temporal orientation, judgment, and function); and (iii) cognitive tests including immediate and delayed 10-word recall (memory); naming the date, month, year, day of the week, the President, and Vice President (orientation); and, clock drawing (executive function). Respondents were classified as having probable dementia (self- or proxy-reported dementia/Alzheimer’s; AD8 ≥ 2; or 2–3 impairments on cognitive tests), possible dementia (1 impairment on cognitive tests), or no dementia (AD8 < 2; or 0 impairments on cognitive tests). Cognitive impairment was defined as having either probable or possible dementia according to NHATS study algorithms (19).
Covariates
Sociodemographic characteristics included age, sex, education (less than high-school diploma vs high-school diploma or more), and race/ethnicity (mutually exclusive categories: Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic other). Body mass index (BMI) was calculated employing the established formula (BMI = weight [pounds] × 703/height2 [inches]) using self-reported height and weight in 2013. Baseline ADL–IADL reflects impairments reported in 2013.
Statistical Analysis
We provided descriptive characteristics of our study population. To assess the relationship between combinations of chronic conditions (in 2013) and ADL–IADL index (in 2014), we first identified respondents who had the same combinations of two or more chronic conditions, and calculated the prevalence and mean ADL–IADL index of each multimorbidity combination. Because many of the combinations contained only a small number of individuals, we analyzed four groups of clinically relevant somatic-mental multimorbidity combinations that included: (i) both depression and cognitive impairment (at minimum and may include additional somatic conditions); (ii) cognitive impairment (and somatic conditions) without depression; (iii) depression (and somatic conditions) without cognitive impairment; and (iv) somatic conditions only (i.e., excluded depression and cognitive impairment). Combinations were sorted into these four groups to test whether multimorbidity combinations that comprise clinically important mental health conditions, individually and/or in combination, were associated with significantly greater prospective ADL–IADL burden compared with combinations of exclusively somatic conditions.
Negative binomial regression models fit for complex survey design estimate the count of ADL–IADL disability and account for observed overdispersion. We estimated models that compared each of the four groups of multimorbidity combinations to: (i) healthy respondents, defined as reporting zero chronic conditions; (ii) respondents reporting one somatic condition only; and (iii) the other three multimorbidity groups. For each comparison, unadjusted and adjusted (for age, sex, education, race/ethnicity, BMI, and ADL–IADL in 2013) models were tested. We report the exponentiated regression coefficients estimating the difference in the number of ADL–IADL impairments between each multimorbidity combination group and its comparator, as well as the associated 95% confidence intervals.
To explore whether there is a “dose response” to greater numbers of conditions within multimorbidity combination groups, we estimated unadjusted and adjusted negative binomial regression models of ADL–IADL index on the number of chronic conditions for each of the four groups. Finally, two sensitivity analyses were conducted, repeating the above analyses: (i) using an expanded ADL–IADL index (0–12 range) and (ii) excluding proxy interviews. All analyses were performed in STATA/SE 13.1.
Results
Sample Characteristics
For the 4,017 older adults in this study, the mean age was 79 years, 59% were female, and the majority self-identified as non-Hispanic whites (72%). Thirty-four percent of multimorbidity combinations included depression, cognitive impairment, or both. Demographic characteristics are reported in Table 1 (full descriptive characteristics in Supplementary Table A1). The mean number of chronic conditions was 3.1 (SD = 1.7), with hypertension reported as the most prevalent single condition (72%), followed by arthritis (64%). Stroke and depression were the least frequently reported conditions (13%, respectively). Cognitive impairment was found in one-fifth of the study sample (21%).
Table 1.
Sample Characteristics, National Health and Aging Trends Study (NHATS) 2013–2014
| N | 4,017 (100) |
| Age, in years, mean (standard deviation) | 78.9 (7.5) |
| Female, n (%) | 2,375 (59) |
| High-school diploma or higher, n (%) | 3,052 (77) |
| Non-Hispanic white, n (%) | 2,863 (72) |
| Non-Hispanic black, n (%) | 823 (21) |
| Hispanic, n (%) | 208 (5) |
| Non-Hispanic other, n (%) | 96 (2) |
| Proxy interview, n (%) | 229 (6) |
| Body mass index, mean (standard deviation) | 27.5 (5.7) |
| Heart disease, n (%) | 1,218 (30) |
| Hypertension, n (%) | 2,898 (72) |
| Arthritis, n (%) | 2,550 (64) |
| Osteoporosis, n (%) | 1,079 (27) |
| Diabetes, n (%) | 1,097 (27) |
| Lung disease, n (%) | 732 (18) |
| Stroke, n (%) | 523 (13) |
| Cancer, n (%) | 1,187 (30) |
| Depression, n (%) | 531 (13) |
| Cognitive impairment, n (%) | 829 (21) |
| Multimorbidity combinations, n (%) | |
| Combinations that include both depression and cognitive impairment | 204 (6) |
| Combinations that include cognitive impairment (without depression) | 596 (18) |
| Combinations that include depression (without cognitive impairment) | 311 (9) |
| Combinations of somatic conditions only | 2,206 (66) |
| No. of chronic conditions, mean (standard deviation) | 3.1 (1.7) |
| Baseline ADL–IADL index, median (25th percentile, 75th percentile) [range] | 1 (0, 3) [0, 11] |
| ADL–IADL index in 2014, median (25th percentile, 75th percentile) [range] | 1 (0, 3) [0, 11] |
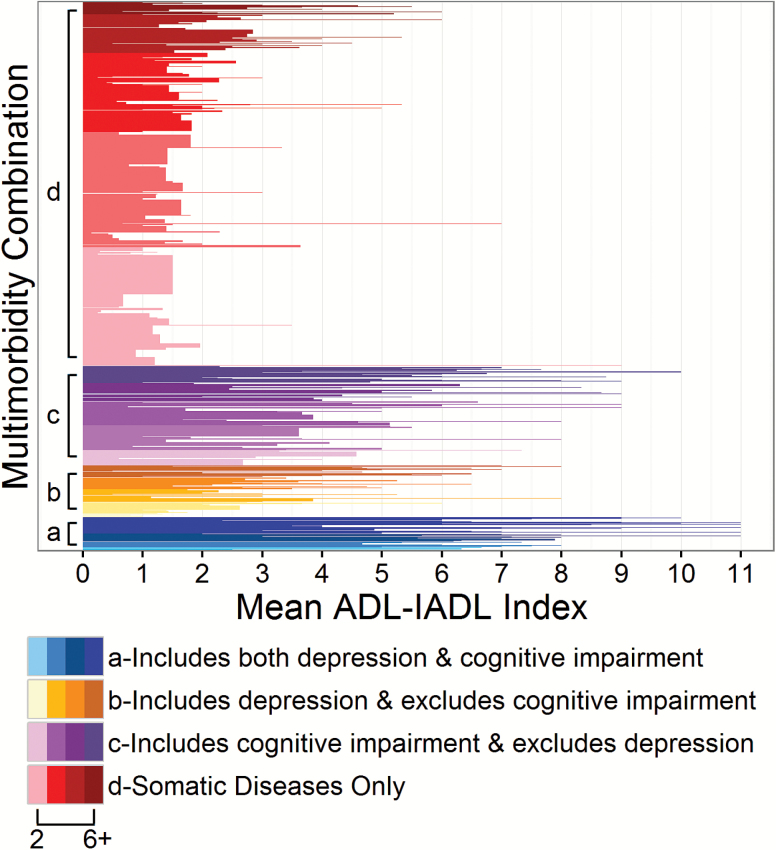
We identified 573 unique multimorbidity combinations with widely varying membership size. The largest multimorbidity combination (hypertension and arthritis) included 236 individuals. A substantial proportion of all cases (44% or 252 multimorbidity combinations) involved a single individual. Twelve multimorbidity combinations comprised 50 or more individuals and only the most prevalent combination (hypertension and arthritis) comprised more than 100 individuals. Multimorbidity combinations aggregated into the four mental-somatic comorbidity groupings as follows (i) both depression and cognitive impairment (and any other somatic conditions), 6% of the sample (n = 204); (ii) cognitive impairment (and somatic conditions) without depression, 18% (n = 596); (iii) depression (and somatic conditions) without cognitive impairment, 9% (n = 311); and (iv) somatic conditions only, 66% (n = 2,206). Figure 1 shows the associations of these four groups of multimorbidity combinations with prospective ADL–IADL disability and indicates greater mean ADL–IADL disability reported for the group of multimorbidity combinations that include depression, cognitive impairment, or both.
Figure 1.
Multimorbidity combination groups and associated mean prospective ADL–IADL, National Health and Aging Trends Study 2013–2014. Each horizontally-oriented bar represents a unique condition combination where taller bar height denotes greater numbers of individuals populating that unique combination. Each group of multimorbidity combinations is ordered on the y-axis from fewest numbers of morbidities (2) to greatest (6+) and graded from lightest to darkest. ADL = activities of daily living; IADL = instrumental activities of daily living.
The results from unadjusted and adjusted negative binomial regression models are shown in Table 2. Overall, all four groups of multimorbidity combinations were associated with significantly greater numbers of ADL–IADL impairments compared to healthy respondents with no reported chronic conditions, as well as compared to respondents with only one somatic condition, in both unadjusted and adjusted models.
Table 2.
Unadjusted and Adjusted Negative Binomial Regression of ADL–IADL Index, NHATS 2013–2014
| Healthy comparator (zero chronic conditions) (N = 193) | Any one somatic condition (N = 458) | ||||
|---|---|---|---|---|---|
| Multimorbidity combination groups | N | Unadjusted eβ (95% CI) | Adjusted eβ (95% CI) | Unadjusted eβ (95% CI) | Adjusted eβ (95% CI) |
| Combinations that include both depression and cognitive impairment | 204 | 12.63 (7.60, 20.99) | 5.24 (2.61, 10.52) | 11.23 (8.96, 14.08) | 2.65 (1.78, 3.93) |
| Combinations that include cognitive impairment (without depression) | 596 | 7.96 (4.67, 13.56) | 3.26 (1.86, 5.69) | 7.08 (5.54, 9.05) | 2.39 (1.72, 3.31) |
| Combinations that include depression (without cognitive impairment) | 311 | 6.03 (3.53, 10.29) | 2.44 (1.47, 4.05) | 5.36 (4.13, 6.96) | 1.89 (1.38, 2.59) |
| Combinations that include somatic conditions only | 2,206 | 2.88 (1.75, 4.73) | 1.84 (1.19, 2.87) | 2.56 (2.01, 3.27) | 1.66 (1.29, 2.12) |
Note: Models adjusted for complex survey design; significant differences are bolded; adjusted for age, sex, education, race/ethnicity, body mass index, and baseline ADL–IADL. ADL = activities of daily living; IADL = instrumental activities of daily living; CI = confidence interval; NHATS = National Health and Aging Trends Study.
Table 3 reports head-to-head comparisons between each of the groups of multimorbidity combinations:
Table 3.
Head-to-Head Unadjusted and Adjusted Negative Binomial Regression of ADL–IADL Index, NHATS 2013–2014
| Comparisons between multimorbidity combination groups | Unadjusted eβ (95% CI) | Adjusted eβ (95% CI) | |
|---|---|---|---|
| Combinations that include Both depression and cognitive impairment (n = 204) | vs. combinations of somatic conditions only (n = 2,206) | 4.38 (3.91, 4.92) | 1.34 (1.09, 1.64) |
| Combinations that include cognitive impairment (without depression) (n = 596) | vs. combinations of somatic conditions only (n = 2,206) | 2.76 (2.43, 3.15) | 1.23 (1.01, 1.49) |
| Combinations that include depression (without cognitive impairment) (n = 311) | vs. combinations of somatic conditions only (n = 2,206) | 2.06 (1.72, 2.47) | 1.07 (0.90, 1.28) |
| Combinations that include cognitive impairment (without depression) (n = 596) | vs. combinations that include both depression and cognitive impairment (n = 204) | 0.63 (0.54, 0.73) | 0.84 (0.74, 0.96) |
| Combinations that include depression (without cognitive impairment) (n = 311) | vs. combinations that include both depression and cognitive impairment (n = 204) | 0.47 (0.39, 0.56) | 0.72 (0.62, 0.85) |
| Combinations that include cognitive impairment (without depression) (n = 596) | vs. combinations that include depression (without cognitive impairment) (n = 311) | 1.34 (1.11, 1.61) | 1.10 (0.92, 1.31) |
Note: Models adjusted for complex survey design; significant differences are bolded; adjusted for age, sex, education, race/ethnicity, body mass index, and baseline ADL–IADL. ADL = activities of daily living; IADL = instrumental activities of daily living; CI = confidence interval; NHATS = National Health and Aging Trends Study.
Comparator—somatic conditions
Combinations that included both depression and cognitive impairment were associated with 4.38 times greater ADL–IADL in unadjusted models (95% confidence interval [CI]: 3.91, 4.92) and 1.34 times greater in adjusted models (95% CI: 1.09, 1.64); cognitive impairment (without depression) combinations were associated with 2.76 times greater ADL–IADL in unadjusted models (95% CI: 2.43, 3.15) and 1.23 times greater ADL–IADL in adjusted models (95% CI: 1.01, 1.49); and combinations including depression (without cognitive impairment) were associated with 2.06 times greater ADL–IADL in unadjusted models (95% CI: 1.72, 2.47) but were not significantly different in adjusted models.
Comparator—both depression and cognitive impairment
Cognitive impairment (without depression) combinations were associated with 0.63 times lower ADL–IADL in unadjusted models (95% CI: 0.54, 0.73) and 0.84 times lower ADL–IADL in adjusted models (95% CI: 0.74, 0.96); depression (without cognitive impairment) combinations were associated with 0.47 times lower ADL–IADL in unadjusted models (95% CI: 0.39, 0.56) and 0.72 times lower ADL–IADL in adjusted models (95% CI: 0.62, 0.85).
Depression combinations versus cognitive impairment combinations
Cognitive impairment (without depression) combinations were associated with 1.34 times greater ADL–IADL in unadjusted models (95% CI: 1.11, 1.61); however, the ADL–IADL indices were not significantly different in adjusted models.
Table 4 reports the results from negative binomial regression models, both unadjusted and adjusted for the number of chronic conditions for each of the four groups of multimorbidity combinations. The number of conditions was not significantly associated with the ADL–IADL index for multimorbidity combinations that included both depression and cognitive impairment, cognitive impairment (without depression), and depression (without cognitive impairment); for somatic multimorbidity combinations, an increasing number of conditions were associated with higher ADL–IADL.
Table 4.
Unadjusted and Adjusted Negative Binomial Regression of ADL–IADL Index Regressed on Number of Chronic Conditions, NHATS 2013–2014
| Multimorbidity combination groups | N | Unadjusted eβ (95% CI) | Adjusted eβ (95% CI) |
|---|---|---|---|
| Combinations that include both depression and cognitive impairment | 204 | 1.03 (0.98, 1.08) | 1.03 (0.99, 1.07) |
| Combinations that include cognitive impairment (without depression) | 596 | 1.10 (1.03, 1.17) | 1.02 (0.97, 1.08) |
| Combinations that include depression (without cognitive impairment) | 311 | 1.25 (1.13, 1.39) | 1.07 (0.95, 1.21) |
| Combinations that include somatic conditions only | 2,206 | 1.23 (1.17, 1.30) | 1.09 (1.02, 1.15) |
Note: Models adjusted for complex survey design; significant differences are bolded; adjusted for age, sex, education, race/ethnicity, body mass index, and baseline ADL–IADL. ADL = activities of daily living; IADL = instrumental activities of daily living; CI = confidence interval; NHATS = National Health and Aging Trends Study.
Sensitivity analyses
Supplementary tables provide the results of sensitivity analyses using the expanded ADL–IADL index (Supplementary Tables A2–A5) and excluding proxy responses (Supplementary Tables A6–A8). The use of the expanded ADL–IADL index showed only minor differences in the magnitude of associations and no differences in the substantive findings. Analyses excluding proxy interviews not only showed attenuated magnitude of associations but also rendered head-to-head comparisons between multimorbidity combinations to be not significantly different in adjusted models.
Discussion
This study established the association between somatic-mental multimorbidity combinations and prospective ADL–IADL disability in a nationally representative sample of Medicare beneficiaries. Overall, depression and/or cognitive impairment was identified in one-third of older adults with multimorbidity. This prevalence is particularly important considering our main finding that older adults with somatic-mental multimorbidity combinations had a substantially greater burden of prospective disability compared to those with exclusively somatic multimorbidity, as well as compared to those with no reported chronic condition or with only one somatic condition. Our results show that multimorbidity combinations that included both depression and cognitive impairment indicated the highest burden of prospective ADL–IADL disability among all the combinations tested here. One nuance to our findings is that multimorbidity combinations of depression (excluding cognitive impairment) were no longer associated with significantly different prospective disability relative to somatic multimorbidity combinations in adjusted models. Finally, there was no statistically discernible difference in prospective disability between combinations that included depression (without cognitive impairment) or cognitive impairment (without depression) in adjusted models, suggesting that depression and cognitive impairment may have roughly the same association with disability once age, sex, education, race/ethnicity, BMI, and baseline disability were considered.
We observed a consistent “dose response” to greater numbers of chronic conditions only for somatic multimorbidity combinations. We hypothesize that co-occurrence of both depression and cognitive impairment, or presence of either may be sufficient to render the marginal disability from additional somatic conditions irrelevant. This is noteworthy because it implies that simply counting morbidities—as is often done in multimorbidity research—obscures the importance of mental health conditions in the configuration of multimorbidity combinations.
If mental health conditions are assumed to be antecedents (rather than consequences) of disability, our results have several important implications for the care of older adults. First, they emphasize the importance of identifying depression and cognitive impairment as part of the functional assessment of older patients. This would argue, in turn, for assessing functioning more directly to address any unmet ADL or IADL needs. Second, it seems essential to manage the combination of these mental health conditions actively and persistently with deliberate, comprehensive, and collaborative treatment plans (20).
In sensitivity analyses excluding proxy interviews, the strength of associations was attenuated and associations were no longer significant in adjusted models. These changes are perhaps unsurprising because two of the multimorbidity combination groups had large numbers of individuals excluded due to proxy response: both depression and cognitive impairment (a 38% reduction in group size) and cognitive impairment excluding depression (a 18% reduction in group size). These divergent findings argue against the exclusion of proxy interviews from the analyses, at the risk of introducing selection bias and underestimating the high disability potential of multimorbidity combinations with depression and cognitive impairment.
This study adds to our understanding of somatic-mental multimorbidity combinations in important ways, and draws from a number of strengths. First, NHATS yields a relatively recent and strong set of robust longitudinal data that largely generalize to the Medicare population, including older adults with possible and probable dementia, who are frequently excluded from panel surveys. Second, the prospective design assesses multimorbidity prior to the reports of ADL–IADL deficits to provide an indication of time-sequencing in the relationship between the two. Third, this study identifies and compares somatic-mental multimorbidity combinations as they occur in a nationally representative sample, an improvement over earlier studies that examine either single conditions in isolation or prespecified dyads/triads of co-occurring conditions. Our study contributes to the evolving literature by providing direct comparisons of somatic-mental multimorbidity groups with healthy peers, single-condition peers, and head-to-head comparisons between multimorbidity combinations. These findings highlight the consequences of compounding combinations which include both depression and cognitive impairment, namely that these combinations involve the highest ADL–IADL disability needs, which in turn may lead to loss of independence and institutionalization.
Several limitations should also be noted. First, somatic conditions and disability were self-reported. This may be of particular concern, given the sizable proportion of cognitively impaired participants in the study. However, several studies have shown concordance between participant reports of physician-diagnosed conditions and other sources of condition ascertainment (21,22). In addition, it is increasingly important to account for self-reports because they reflect conditions patients (and their proxies) believe they do or do not have and resulting self-management behaviors. Second, our findings are susceptible to underrepresentation of membership in multimorbidity groups for which respondents were lost to follow-up or died prior to observation. Because NHATS is a relatively new longitudinal cohort and we leverage the two most recent rounds available, losses to follow-up and death are minimized. In addition, NHATS boasts a high response and re-interview rate (14). Finally, we are limited by the number of chronic conditions assessed in NHATS and cannot ascertain the severity of conditions with these data. Severity may influence the reporting of conditions and disability; further examination of condition presentation and severity using data linked to clinical or administrative records is warranted. In addition, future work should explore potential mediation and moderation effects socioeconomic characteristics may exert on the relationship between mental-somatic multimorbidity combinations and disability.
These results corroborate and expand on recently published findings using nationally representative cohorts (4,13). Associations between functional decline and both cognitive impairment and depression (separately) have been previously reported (23,24). There are indications in the literature highlighting loss of functional capacity onward to disability and a range of health and social risk factors, including but not exclusive to somatic and/or mental multimorbidity, high BMI, and low frequency of social contacts (23,25). Together, these findings argue for continued integration of behavioral health service delivery into primary care. It is increasingly evident that the prevalence of co-occurring mental and physical health concerns is substantial among older adults, and thus it is important for both clinical practice and research to address somatic and mental health in combination and not as separate conditions with unrelated functional consequences in this population (24,26). Organizing health care delivery to better address these simultaneous concerns should continue to be a priority to address the functional needs of a burgeoning older demographic contingent.
Conclusions
In a large group of Medicare-representative participants, multimorbidity combinations that included depression and/or cognitive impairment were associated with substantially greater prospective disability than any combination comprised exclusively of somatic conditions. These findings make a compelling argument to include mental health conditions as a central part of the multimorbidity framework. Our results suggest that clinicians might improve functioning by better addressing mental health issues for older adults with multimorbidity.
Supplementary Material
Supplementary data is available at Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This study was supported by the American Diabetes Association career development award to Dr. Quiñones (ADA 7-13-CD-08). Dr. Botoseneanu was supported by grants AG-024824 (University of Michigan Claude D. Pepper - Older Americans Independence Center) and UL1TR000433 (Michigan Institute for Clinical and Health Research). A prior version of this work was accepted for presentation at the Gerontological Society of America’s 69th Annual Scientific Meeting in New Orleans, Louisiana.
Conflict of Interest
None declared.
Supplementary Material
References
- 1. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases–a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi:10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 2. Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:120137. doi:10.5888/pcd10.120137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494. doi:10.1001/jama.2012.5265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:823–830. doi:10.1093/gerona/glw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L. The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med. 2009;265:288–295. doi:10.1111/ j.1365-2796.2008.02017.x [DOI] [PubMed] [Google Scholar]
- 6. Barile JP, Thompson WW, Zack MM, Krahn GL, Horner-Johnson W, Bowen SE. Multiple chronic medical conditions and health-related quality of life in older adults, 2004-2006. Prev Chronic Dis. 2013;10:E162. doi:10.5888/pcd10.120282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leroy L, Bayliss E, Domino M et al. The agency for healthcare research and quality multiple chronic conditions research network: overview of research contributions and future priorities. Med Care. 2014;52(Suppl 3):S15–S22. doi:10.1097/MLR.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 8. Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi:10.1093/epirev/mxs009 [DOI] [PubMed] [Google Scholar]
- 9. Vogeli C, Shields AE, Lee TA et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(Suppl 3):391–395. doi:10.1007/s11606-007-0322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–185. doi:10.1016/j.amjmed.2003.09.031 [DOI] [PubMed] [Google Scholar]
- 11. Parekh AK, Goodman RA, Gordon C, Koh HK. Managing multiple chronic conditions: a strategic framework for improving health outcomes and quality of life. Public Health Rep Wash DC 1974. 2011;126:460–471. doi:10.1177/003335491112600403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi:10.5888/pcd10.120239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bobo WV, Yawn BP, Sauver JLS, Grossardt BR, Boyd CM, Rocca WA. Prevalence of combined somatic and mental health multimorbidity: patterns by age, sex, and race/ethnicity. J Gerontol A Biol Sci Med Sci. 2016:glw032. doi:10.1093/gerona/glw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Montaquila J, Freedman VA, Edwards B, Kasper JD. National Health and Aging Trends Study Round 1 Sample Design and Selection. NHATS Technical Paper# 1. Baltim MD Johns Hopkins Univ Sch Public Health. 2012. [Google Scholar]
- 15. Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. J Gerontol B Psychol Sci Soc Sci. 1998;53:S46–S57. [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi:10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- 17. Li C, Friedman B, Conwell Y, Fiscella K. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007;55:596–602. doi:10.1111/j.1532-5415.2007.01103.x [DOI] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi:10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the National Health and Aging Trends Study. Technical Paper #5. Baltimore MD Johns Hopkins Univ Sch Public Health. 2013. [Google Scholar]
- 20. Thielke S, Vannoy S, Unützer J. Integrating mental health and primary care. Prim Care. 2007;34:571–592, vii. doi:10.1016/j.pop.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 21. Weir D. Elastic powers: the integration of biomarkers into the Health and Retirement Study. In: Biosocial Surveys National Academy of Sciences;2008:78–95. [Google Scholar]
- 22. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi:10.1016/j.jclinepi.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 23. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. doi:10.1016/S0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 24. Steffens DC, Otey E, Alexopoulos GS et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63:130–138. doi:10.1001/archpsyc.63.2.130 [DOI] [PubMed] [Google Scholar]
- 25. Ickowicz E. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. J Am Geriatr Soc. 2012;60:E1–E25. doi:10.1111/j.1532-5415.2012.04188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scott KM, Lim C, Al-Hamzawi A et al. Association of mental disorders with subsequent chronic physical conditions: World Mental Health Surveys From 17 Countries. JAMA Psychiatry. 2016;73:150–158. doi:10.1001/jamapsychiatry.2015.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.