The fungal pathogen P brasiliensis activates caspase-8 downstream of dectin-1 recognition leading to ASC oligomerization, caspase-1 activation, and IL-1β processing and release. Activated caspase-1, in turn, negatively regulates caspase-8 activity controlling procaspase-8 cleavage into their processed p18 form.
Keywords: caspase-8, IL-1β production, noncanonical inflamassome pathway, P brasiliensis, paracoccidioidomycosis
Abstract
Background
Paracoccidioides brasiliensis is equipped with an arsenal of virulence factors that are crucial for causing infection. Our group previously defined the NLRP3 inflammasome as a mediator of P brasiliensis-induced cell damage recognition and induction of effective Th1 immune responses. However, deficiency of caspase-1 only partially reduced interleukin (IL)-1β levels.
Methods
In this study, using chemical inhibitors as well as genetically modified mice, we identify an additional pathway for IL-1β production in response to P brasiliensis infection.
Results
Paracoccidioides brasiliensis initiated caspase-8-mediated IL-1β production, an event that was necessary for transcriptional priming and posttranslational processing of pro-IL-1β. Caspase-8 synergizes with the canonical NLRP3 inflammasome pathway to control caspase-1 processing and IL-1β maturation, providing a regulatory role for caspase-8 in host resistance to in vivo P brasiliensis infection.
Conclusions
Taken together, these findings revealed an important role for caspase-8 in the innate immune response of host cells to P brasiliensis infection, demonstrating a connected network between noncanonical and canonical inflammasomes to coordinate IL-1β production during fungal challenge.
The systemic granulomatous disease, paracoccidioidomycosis (PCM), is initiated when airborne particles produced by the mycelial stage of Paracoccidioides brasiliensis are inhaled and converted into pathogenic yeast in the lungs due to an increase in temperature [1, 2]. This morphological transition, crucial for the establishment of infection, is accompanied by extensive structural changes in cell wall architecture and composition. The polysaccharide shift has been proposed as a virulence factor during P brasiliensis infection [3–5], because it presumably enhances fungal survival in the mammalian host by modulating β-glucan-triggered inflammatory response [6, 7].
β-1,3-glucans are recognized by dectin-1 (Clec7A), a C-type lectin receptor (CLR) that drives the CARD9/Bcl-10/MALT1 scaffold assembly via Src/Syk-dependent signaling to activate the transcription factor nuclear factor (NF)-κB [8, 9]. Besides dectin-1-induced interleukin (IL)-1β transcription, recent studies have suggested that this CLR also functions as an extracellular sensor that directly activates caspase-8 to cleave pro-IL-1β [10, 11]. Once the dectin-1 pathway is activated by P brasiliensis [12], we expanded our views to clarify the interplay between the noncanonical caspase-8 and the canonical caspase-1 inflammasome pathway after P brasiliensis infection, because our earlier work had revealed that caspase-1 activation alone could not fully account for the regulation of IL-1β levels in infected macrophages [13]. In this study, we show that caspase-8, activated after dectin-1 engagement by P brasiliensis, orchestrates the caspase-1/11-independent IL-1β release and impairs fungal growth. The involvement of the noncanonical caspase-8 inflammasome as a partner in controlling IL-1β secretion during P brasiliensis infection demonstrates the versatility of this platform in recruiting distinct effector proteins to tailor immune responses, and it sheds new light on the complexity of this host-pathogen interaction.
MATERIAL AND METHODS
Mice
All mouse strains (on C57BL/6 background) were maintained in specific pathogen-free conditions and bred at the University of Massachusetts Medical School (UMMS). The experiments involving animals were approved by UMMS Institutional Animal Care and Use Committee (protocol no. 1633) and carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Fungal Infection
The yeast cells of P brasiliensis (Pb) 60855 strain (American Type Culture Collection 60855), obtained from Dr. B. S. Klein (University of Wisconsin, Madison, WI), were cultured for 7 days at 37°C in brain heart infusion agar medium (BHI) supplemented with 5% fetal bovine serum (FBS). To prepare the inocula, the yeast cells were harvested and grown overnight under agitation in F12 Coon’s Modification medium at 37ºC before the analysis of viability by the fluorescein diacetate (5 mg/mL)-ethidium bromide (1 mg/mL) method. The cells were infected with viable fungi at a multiplicity of infection (MOI) of 1 or 5, whereas each animal was inoculated intravenously with 1 × 106 yeast cells.
Recovery of Colony-Forming Units
The numbers of viable yeast cells in the organs of the Pb60855-infected mice were determined at 30 days postinfection (dpi) by counting the CFUs. The resulting macerate of lung, liver, and spleen was diluted 10-fold in sterile phosphate-buffered saline and plated onto Petri dishes containing BHI agar enriched with 5% FBS. The plates were incubated at 37°C for 7 days, and the number of CFUs per gram of tissue was calculated.
Histopathological Analysis
Animals selected at random from each group were sacrificed at 30 dpi. The lungs were excised, fixed with 10% formalin for 48 hours, and embedded in paraffin. Tissue sections (5 mm) were stained with hematoxylin and eosin stain (H&E) for analysis of the lesions in the lung tissue using standard protocols.
Bone Marrow-Derived Macrophage and Bone Marrow-Derived Dendritic Cell Differentiation and Stimulation
Bone marrow progenitor cells were obtained from femurs and cultivated for 7–10 days either in (1) L929-conditioned Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin-streptomycin to differentiate into macrophages or in (2) Roswell Park Memorial Institute (RPMI) 1640 medium with 10% heat-inactivated serum, 1% penicillin-streptomycin, 1% sodium pyruvate, 1% nonessential amino acids, 20 ng/mL recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), and 50 mM 2-mercaptoethanol to differentiate into dendritic cells (DCs). On day 7, bone marrow-derived macrophages (BMDMs) or bone marrow-derived dendritic cells (BMDCs) were seeded in cell culture plates, and the next day they were primed or not with lipopolysaccharide ([LPS] 200 ng/mL) or Pam3CSK4 (200 ng/mL) for 3 hours, and then they were infected with P brasiliensis at an MOI of 1 or 5 for 24 hours. Where indicated, Pam3CSK4-primed BMDCs were treated either with caspase-1 (z-YVAD-fmk) or caspase-8 inhibitors (z-IETD-fmk) (both from Santa Cruz Biotechnology; 50 µM) for 2 hours before adding the fungus. As a control, cells were incubated overnight with a canonical inflammasome activator such as pdAdT (poly(deoxyadenylic-deoxythymidylic) acid).
Cytokine Analysis
Cytokines in culture supernatants were measured by enzyme-linked immunosorbent assay (eBioscience or R&D Systems), according to the manufacturers’ instructions.
Western Blotting
Cell lysates and culture supernatants, either combined or separated, were denatured in loading buffer containing sodium dodecyl sulfate (SDS) and dithiothreitol, boiled, and subjected to 13% SDS-polyacrylamide gel electrophoresis (PAGE) gel. The SDS-PAGE-separated proteins, transferred to nitrocellulose membranes, were immunoblotted with primary antibodies against caspase-1 (Adipogen), pro-caspase-8 (Enzo Life Sciences or Cell Signaling Technology), cleaved caspase-8 (Cell Signaling Technology), IL-1β (R&D Systems), ASC (Millipore or Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich). In some cases, proteins from the cell culture supernatants were precipitated by the methanol-chloroform extraction method.
Caspase-8 Activity and Cell Death Assay
Caspase-8 activity in cell lysates was assayed using the Caspase-Glo 8 Assay kit (Promega). Cell death was assessed detecting the release of lactate dehydrogenase (LDH) by CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega), according to the manufacturer’s instructions. The percentage of cytotoxicity was calculated as (LDH infected − LDH uninfected)/(LDH total lysis − LDH uninfected) × 100. The LDH total lysis was determined by lysing the cultures with Triton X-100.
ASC Oligomerization Assay
The BMDCs were primed either with LPS or Pam3CSK4 (50 ng/mL) overnight and incubated with pan-caspase inhibitor z-VAD (25 µM) 30 minutes before the 24-hour stimulation with P brasiliensis or pdAdT. Cytosolic lysates from the cells were enriched for inflammasome fractions by low-speed centrifugation and subjected to cross-linking with disuccinimidyl suberate (2 mM). The cross-linked samples were analyzed for ASC oligomerization by immunoblotting.
Confocal Immunofluorescence Microscopy
After infection, BMDCs were washed and fixed in 4% paraformaldehyde for 30 minutes, followed by blocking with 5% goat serum (Dako) in Perm/Wash buffer (BD Biosciences) for 1 hour. Cells were incubated with a mouse anti-ASC antibody (Millipore) overnight followed by incubation with a rabbit anti-caspase-8 (CST) for an additional 1 hour. The secondary antibodies used were Alexa Fluor 488 anti-rabbit immunoglobulin (Ig)G and Alexa Fluor 633 anti-mouse IgG. Cells were counterstained in diamidino-2-phenylindole mounting medium (Vector Laboratories), and inflammasome assembly was visualized and imaged using a Leica TCS SP8 confocal microscope at the Research Core Facility at UMMS.
Statistical Analysis
GraphPad Prism 5.0 software was used for statistical analysis. The data were represented as mean ± standard errors of the means, and the differences observed among the experimental groups after infection were examined by applying one-way analysis of variance followed by the parametric Tukey’s test for comparing multiple groups. P < .05 was considered statistically significant.
RESULTS
Paracoccidioides brasiliensis 60855 Strain Also Induces Interleukin-1β Secretion In Vitro
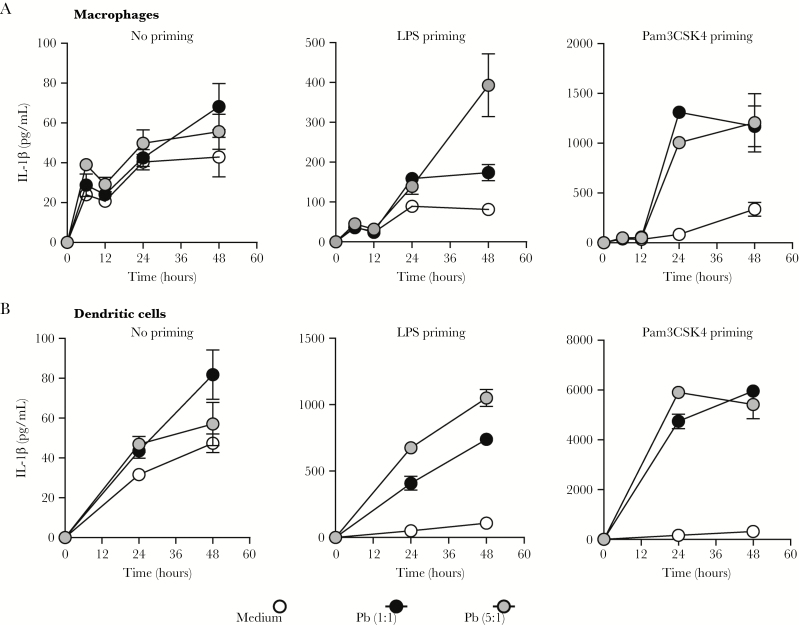
We and others have previously demonstrated that P brasiliensis- induced IL-1β is controlled by the NLRP3-ASC-caspase-1 inflammasome [13–15]. However, it is important to highlight that the activation of inflammasome in response to infection with highly virulent fungi (Pb18) may not be relevant with other strains of P brasiliensis. Because the experimental model of paracoccidioidomycosis in this study used strain 60855 of live P brasiliensis, we first examined whether this strain also triggers IL-1β production. We observed that P brasiliensis 60855 induces IL-1β release in both BMDCs and BMDMs. It is notable that, even though BMDMs (Figure 1A) did not respond to Pb60855 challenge as robustly as BMDCs (Figure 1B), we observed a similar increase in IL-1β levels in both. Moreover, the higher amounts of IL-1β in culture supernatants only occurred with either LPS (Toll-like receptor [TLR]4) or Pam3CSK4 (TLR2) priming. Furthermore, we tested different MOI of P brasiliensis at different time points and determined that 24-hour postinfection using MOI-5 was optimal for characterizing the mechanism of inflammasome activation in P brasiliensis-infected BMDCs primed with Pam3CSK4. Taken together, these results indicate that IL-1β production is not limited to the isolate 18 of P brasiliensis, and that inflammasome activation also occurs required in response to a less virulent strain of P brasiliensis.
Figure 1.
Pb60855 also triggers interleukin (IL)-1β production. (A) IL-1β production in resting or primed C57BL/6 bone marrow-derived macrophages and (B) bone marrow-derived dendritic cells incubated with viable yeast of Paracoccidioides brasiliensis at a multiplicity of infection of 1 or 5 after 12, 24, or 48 hours of infection. Each point represents the mean ± the standard deviation of the parameter in question. The results are representative of 3 experiments performed in triplicate. Abbreviation: LPS, lipopolysaccharide.
Paracoccidioides brasiliensis-Induced Interleukin-1β Secretion Is Dependent on Both Caspase-1 and Caspase-8
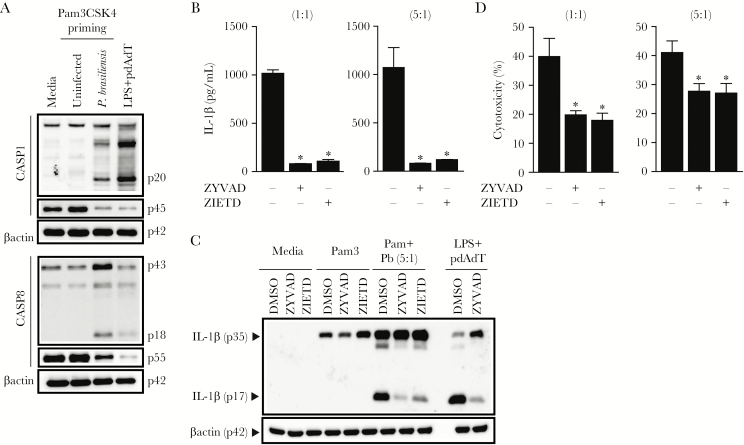
Regulation of IL-1β production in macrophages and DCs has been focused on caspase-1, the best characterized enzyme involved in the proteolytic maturation of IL-1β [16]. However, some studies have also implicated caspase-8 either as a direct executioner caspase for generating the mature 17-kDa IL-1β or as an initiator caspase acting upstream of the activation of caspase-1 [17, 18]. Because the contribution of caspase-8 activation during P brasiliensis infection is still unknown, we monitored the activation status of caspase-8 by Western blotting and analyzed the presence of the caspase-8 p18 subunit, which is generated upon procaspase-8 proteolysis [19]. The procaspase-8 processing into the small catalytic (p18) subunit as well as the intermediate 43kD form, which avoids apoptosis [20], was markedly augmented in Pam3CSK4-primed DCs infected with P brasiliensis, which explains the reduced procaspase-8 (p55) levels in wild-type (WT) cells after incubation with the yeast form (Figure 2A).
Figure 2.
Caspase-8 signaling is important for Paracoccidioides brasiliensis-triggered interleukin (IL)-1β processing and release. (A) wild-type (WT) bone marrow-derived dendritic cells (BMDCs) were primed with 200 ng/mL Pam3CSK4 for 3 hours and infected with P brasiliensis (multiplicity of infection 5) for 24 hours before lysates, and supernatants were collected and immunoblotted for caspase-8 and caspase-1, respectively. Control cells were stimulated with canonical inflammasome activator pdAdT after lipopolysaccharide priming. (B) Culture supernatants harvested from WT BMDCs activated for 3 hours with Pam3CSK4, treated the last 2 hours with caspase-1 (z-YVAD-fmk) or caspase-8 (z-IETD-fmk) inhibitors, and subsequently challenged for 24 hours with P brasiliensis were analyzed either by enzyme-linked immunosorbent assay to measure the levels of secreted IL-1β, or (C) a combination of supernatants and cell lysate were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, blotted, and probed with a monoclonal IL-1β antibody. (D) Pam3CSK4-primed BMDCs untreated or treated with z-YVAD-fmk or z-IETD-fmk were cultured with P brasiliensis before the assessment of extracellular lactate dehydrogenase. Data show the averages ± standard deviation from triplicate wells of at least 3 independent experiments. The symbol (*) indicates a significant difference between dimethyl sulfoxide (DMSO)-treated samples (B and D) (P < .05).
Because caspase-8 has been linked to pro-IL-1β maturation, we next investigated whether caspase-8 was involved in P brasiliensis-induced IL-1β production. Blocking caspase-8 activity with a chemical inhibitor (z-IETD-fmk) significantly attenuated the release of active IL-1β (Figure 2B). Consistently, we also found suppressed cleavage of pro-IL-1β in z-IETD-treated cells compared with the untreated cells (Figure 2C), demonstrating the importance of caspase-8 for pro-IL-1β processing. Accordingly, we noted that caspase-8 inhibition prevented cell cytotoxicity, resulting in lower LDH release, which is a marker of cell death (Figure 2D). Altogether, these data suggest that infection with P brasiliensis leads to processing of pro-IL-1β after fungal recognition not only through caspase-1-dependent inflammasome responses but through caspase-8 as well.
Caspase-1/11 Deficiency Enhances Paracoccidioides brasiliensis- Induced Caspase-8 Maturation
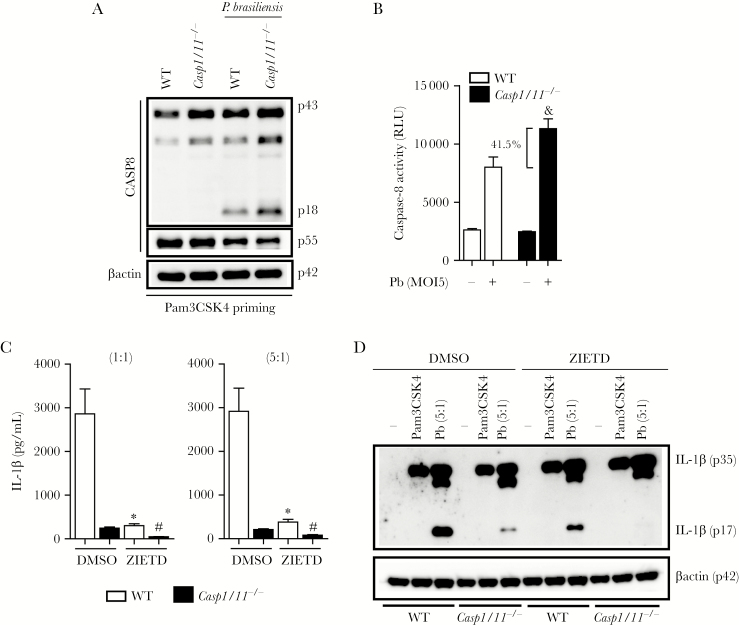
Although IL-1β secretion is primarily dependent on caspase-1 activity [13, 21], P brasiliensis-infected BMDMs lacking caspase-1 still produce moderate amounts of this cytokine. To explore the relative contribution of caspase-8 in the caspase-1/11-independent IL-1β secretion, we analyzed caspase-8 activation and its enzymatic activity in DCs lacking caspase-1 and 11. We were surprised to find that P brasiliensis-induced caspase-8 processing and activity were greatly enhanced (41.5%) in caspase-1/11-deficient BMDCs (Figure 3A and B). These data are consistent with the key role of caspase-8 in mediating IL-1β secretion we observed in Casp1/11−/− cells, because the administration of a caspase-8 inhibitor completely abolished the residual IL-1β production from the untreated DCs that have no caspase-1 or 11 (Figure 3C). In agreement with the hypothesis that caspase-8 is essential for IL-1β release when caspase-1 and 11 are not present, the expression of the processed mature form of IL-1β, p17, that was severely impaired in BMDCs from Casp1/11−/− mice, completely disappeared upon caspase-8 blockade (Figure 3D). Therefore, we conclude that the canonical caspase-1 inflammasome pathway normally restricts the noncanonical caspase-8 inflammasome activation. In the context of caspase-1/11 deficiency, this brake is released and IL-1β processing is then dependent on the increased caspase-8 activity.
Figure 3.
Caspase-8 is responsible for the noncanonical caspase-1-independent interleukin (IL)-1β secretion triggered by Paracoccidioides brasiliensis. (A) Bone marrow-derived dendritic cells (BMDCs) from indicated mice were primed with Pam3CSK4, infected with P brasiliensis at 5:1 for 24 hours. The cell lysates were subjected to caspase-8 staining by Western blotting. (B) Caspase-8 activity in Pam3CSK4-primed wild-type (WT) or Casp1/11−/− BMDCs 24 hours after infection with Pb60855 was presented as relative light units (RLU). (C) The BMDCs obtained from WT or Casp1/11−/− mice were primed with Pam3CSK4 and under caspase-8 activity blockade were incubated for 24 hours with P brasiliensis at a multiplicity of infection (MOI) of 1:1 or 5:1. The amounts of IL-1β were quantified by enzyme-linked immunosorbent assay. (D) Interleukin-1β processing on whole cell extracts was detected after incubation of Pam3CSK4-primed WT and Casp1/11−/− BMDCs with dimethyl sulfoxide (DMSO) or z-IETD-fmk and infection with 5 yeast cells per BMDC for 24 hours. Data are mean ± standard deviation from 1 of 3 independent experiments. (&), (*), and (#) denote P < .05 compared with P brasiliensis-infected WT cells (B), DMSO-treated WT cells (C), and DMSO-treated Casp1/11−/− cells (C), respectively.
Caspase-8 Is Recruited to ASC Oligomeric Complexes (Specks) Upon Paracoccidioides brasiliensis Infection Promoting Interleukin-1β Release Through Caspase-1 Activation
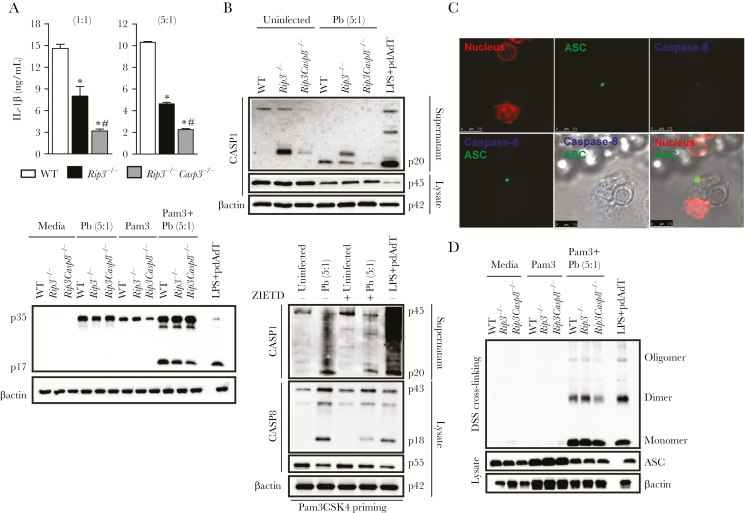
Because caspase-8 plays a role in the regulation of inflammasome-generated IL-1β, we sought genetic evidence to link caspase-8 to the canonical inflammasome. We differentiated BMDCs from caspase-8-deficient mice (generated on a RIP3-deficient background, because caspase-8-deficient mice are embryonic lethal) and examined IL-1β release and caspase-1 activation after P brasiliensis infection. It is interesting to note that P brasiliensis-induced IL-1β production was diminished in Rip3−/−Casp8−/− BMDCs (Figure 4A, upper panel), and, similarly, the amount of mature IL-1β in the supernatants of stimulated BMDCs was also decreased in Rip3−/−Casp8−/− compared with WT and Rip3−/− cells (Figure 4A, bottom panel). Nevertheless, the reduction in the quantity of mature IL-1β from the P brasiliensis-infected Rip3−/−Casp8−/− cells could reflect both attenuation of inflammasome assembly as well as less pro-IL-1β substrate available for activation. As shown in Figure 4A, bottom panel, the levels of pro-IL-1β after Pam3CSK4 priming were intact in WT and Rip3−/−, whereas DCs lacking both caspase-8 and RIP3 had less of the 35kD precursor form. However, this decrease did not occur when the Pam3CSK4-primed cells were infected with P brasiliensis, which confirms the effect of caspase-8 on signal 2 (inflammasome-mediated pro-IL-1β processing) instead of signal 1 (NF-κB-mediated pro-IL-1β induction). Consistently, Rip3−/−Casp8−/− BMDCs infected with P brasiliensis expressed normal levels of procaspase-1 but had a significant defect in inducing caspase-1 activation compared with WT and Rip3−/− cells (Figure 4B, top panel). This correlated with the decreased caspase-1 maturation observed on TLR2 agonist-primed WT cells treated with z-IETD-fmk, before P brasiliensis stimulation (Figure 4B, bottom panel). To further extend our findings, we evaluated whether even with the decreased caspase-1 activation in Rip3−/−Casp8−/− BMDCs, caspase-1 could still contribute to IL-1β maturation. Caspase-1 inhibition clearly limited the ability of Pam3CSK4-primed Rip3−/−Casp8−/− BMDCs to produce mature IL-1β in response to P brasiliensis (Supplemental Figures 1A and B). Thus, these data indicate that caspase-8 licenses caspase-1 activation in the P brasiliensis-triggered inflammasome, mediating efficient canonical inflammasome activation.
Figure 4.
Caspase-8 is required for efficient activation of the canonical caspase-1 inflammasome. (A) Resting or Pam3CSK4-primed bone marrow-derived dendritic cells (BMDCs) were left uninfected or infected with Paracoccidioides brasiliensis for 24 hours. The supernatant with or without the cell lysate was used to quantify interleukin (IL)-1β (top panel) or was resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis to detect IL-1β cleavage (bottom panel). (B) p20 and p45 caspase-1 subunits were determined in supernatants and lysate, respectively, collected from P brasiliensis-infected wild-type (WT), Rip3−/−, and Rip3−/−Casp8−/− BMDCs, previously primed with PamCSK4 (top panel). Wild-type BMDCs were stimulated with 200 ng/mL Pam3CSK4 for 3 hours, with the last 2 hours preceding the addition of P brasiliensis in the presence of 50 µM z-IETD. Precipitated supernatants and lysates were immunoblotted for the indicated proteins (bottom panel). (C) Caspase-8 (blue) and ASC (green) confocal micrographs taken from Pam3CSK4-primed BMDCs seeded on coverslips and stimulated with P brasiliensis during 24 hours. 4’,6-Diamidino-2-phenylindole (red) was used for nuclear staining. Scale bars, 7.5 µm. (D) ASC oligomerization in inflammasome-enriched and cross-linked lysates of Pam3CSK4-primed WT, Rip3−/−, and Rip3−/−Casp8−/− BMDCs that were infected with P brasiliensis for 24 hours. All cultures contained the pan-caspase inhibitor z-VAD-fmk (25 µM). Where indicated, WT BMDCs stimulated for 3 hours with lipopolysaccharide ([LPS] 200 ng/mL) and treated with pdAdT overnight were used as positive control. Statistical analysis considers the mean ± standard deviation from 3 independent experiments conducted in triplicate. The results of 1 representative experiment are presented. (*) and (#) P < .05 compared with WT and Rip3−/− DCs, respectively.
A key question that arises from these results is how caspase-8 impacts caspase-1 inflammasome activation. Hypothesizing that caspase-8 may be recruited to the P brasiliensis-induced ASC inflammasome platform to facilitate ASC oligomerization, we first stained for caspase-8 and ASC to determine whether caspase-8 forms a distinct speck-like protein and/or colocalizes to the same ASC puncta. Comparing the distribution of the fluorescently labeled proteins, our immunofluorescence analysis showed a significant colocalization of caspase-8 and ASC in DCs stimulated with Pam3CSK4 and P brasiliensis (Figure 4C). As caspase-8 colocalized with ASC inflammasome complex during P brasiliensis infection, we precipitated ASC aggregates with relatively low-speed centrifugation and subjected it to chemical cross-linking to find out the oligomeric state of ASC in P brasiliensis-infected WT, Rip3−/−, and Rip3−/−Casp8−/− cells. ASC was predominantly found as monomers and dimers in the infected WT, Rip3−/ , and Rip3−/−Casp8−/− BMDCs, and it was isolated in equal amounts between WT and Rip3−/− cells. In contrast, the oligomerization of ASC in Rip3−/−Casp8−/−-infected DCs was strikingly reduced (Figure 4D). Thus, these results show that caspase-8 colocalizes with ASC and influences its oligomerization, demonstrating a connection between canonical and noncanonical inflammasome pathways during P brasiliensis infection.
Paracoccidioides brasiliensis Recognition by Dectin-1 Engages Interleukin-1β Processing Through a Noncanonical Caspase-8 Inflammasome Pathway
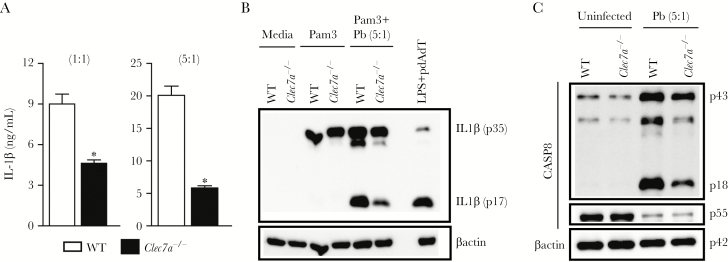
Hereafter, we wanted to understand how P brasiliensis activates the noncanonical caspase-8 inflammasome, by checking whether dectin-1 was involved in caspase-8-induced pro-IL-1β processing. To exclude the effects of dectin-1 on pro-IL-1β accumulation, we primed Clec7a−/− cells with Pam3CSK4 to guarantee dectin-1 independent pro-IL-1β production. Even though dectin-1 may be involved in priming, acting upstream of inflammasome signaling, this C-type lectin receptor was also important for pro-IL-1β/IL-1β conversion after P brasiliensis infection, as validated by a prominent reduction in the levels of processed IL-1β (Figure 5A and B). As stated, this defect was not caused by less pro-IL-1β production, but because Pam3CSK4-primed Clec7a−/− DCs infected with P brasiliensis did not stimulate adequate caspase-8 activation (Figure 5C).
Figure 5.
Paracoccidioides brasiliensis sensing by dectin-1 induces noncanonical caspase-8 inflammasome-mediated interleukin (IL)-1β maturation. (A) Interleukin-1β concentration in bone marrow-derived dendritic cells (BMDCs) from wild-type (WT) or Clec7a−/− mice stimulated with Pam3CSK4 and P brasiliensis at a multiplicity of infection of 1 or 5 for 24 hours. (B) Western blotting of the processed IL-1β p17 subunit in whole cell extracts from Pam3CSK4-primed WT and Clec7a−/− DCs infected with P brasiliensis. Lipopolysaccharide (LPS)-primed WT BMDCs transfected with pdAdT overnight were used as the control for IL-1β production. (C) The lysates from Pam3CSK4-treated WT and Clec7a−/− BMDCs, which were infected or not with P brasiliensis for 24 hours, were fractionated by sulfate polyacrylamide gel electrophoresis and blotted with caspase-8 antibody. Data are representative of at least 3 experiments. Error bars indicate standard deviation of technical triplicates. The symbol (*) represents P < .05 in relation to WT mice.
Caspase-8 Augments Host Resistance to Paracoccidioides brasiliensis Infection
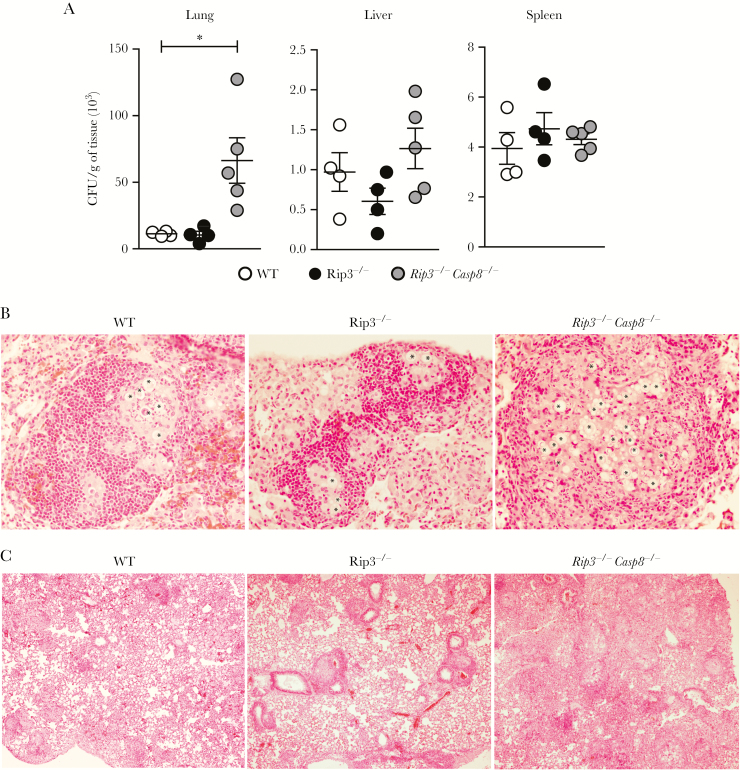
Subsequently, to ascertain whether noncanonical caspase-8 inflammasome response protects against challenge with P brasiliensis, we infected WT, Rip3−/−, and Rip3−/−Casp8−/− mice with 1 × 106 viable yeasts of P brasiliensis and analyzed fungal colonization in tissues. The infected Rip3−/−Casp8−/− mice had much higher fungal loads in their lungs compared with the Rip3−/− mice, which behaved similarly as the WT control mice, and no significant difference was observed in the liver and spleen (Figure 6A). We studied the pulmonary fungal distribution using H&E staining and found that P brasiliensis was localized within granulomatous foci in WT and Rip3−/− mice, but was more widespread in the Rip3−/−Casp8−/− mice, that failed to confine the pathogen inside granulomas (Figure 6B). In addition, at 30 dpi, immune cells surrounded the granulomatous areas in the lung tissues from WT and Rip3−/− mice, but the intense inflammatory reaction of Rip3−/−Casp8−/− mice was diffuse and had infiltrated the alveolar spaces (Figure 6C). Together, these findings show that caspase-8 plays a critical role in mediating immune defense in vivo, and that deficiency of caspase-8 leads to a dysregulated inflammatory response, which is unable to control P brasiliensis replication.
Figure 6.
Caspase-8, but not RIP3, deficiency confers susceptibility to Paracoccidioides brasiliensis infection. (A) The fungal burden in the lungs, livers, and spleens from wild-type (WT), Rip3−/−, and Rip3−/−Casp8−/− mice after 30 days postinfection (dpi) with 1 × 106 yeast cells of P brasiliensis 60855 strain. (B) Photomicrographs of granuloma lesions from the WT, Rip3−/−, and Rip3−/−Casp8−/− mice at 30 dpi taken using magnification of ×40 and (C) ×4. The lung sections were fixed in formalin, paraffin-embedded, stained with hematoxylin and eosin stain, and analyzed by light microscopy. The asterisks indicate the fungi in the tissue. Each point represents the mean ± standard error of the mean of the organs from 4 mice. (*) P < .05 for the knockout versus the C57BL/6 (WT) mice. Abbreviation: CFU, colony-forming units.
DISCUSSION
Substantial evidence has emerged regarding the nonapoptotic functions of caspase-8, including activation of the transcription factor NF-κB [22–24] and production of mature IL-1β [17]. In this study, we show that the moderate level of caspase-8 activation observed in Pam3CSK4-primed WT DCs incubated with P brasiliensis was restrained by caspase-1/11, because in caspase-1 and -11-sufficient cells, the expression and activity of these 2 proteases bypass vigorous caspase-8 proteolysis and activity. However, caspase-8 serves an alternative route to induce IL-1β production in P brasiliensis-infected BMDCs under conditions in which canonical caspase-1 inflammasome activation was impaired (eg, Casp1/11−/− cells). An explanation for the improved caspase-8 activation in Casp1/11−/− cells is that caspase-8 and caspase-1/11 compete with each other. Consequently, caspase-1 and -11 ablation assures that more ASC would be available to bind to 55-kDa procaspase-8. We found that caspase-8 interacts and colocalizes with the adapter protein ASC in a single cytoplasmic speck during P brasiliensis infection, similar to earlier published observations [25–29]. It will be interesting to investigate the spatial orientation of caspase-1 and Nod-like receptor proteins in the ASC-caspase-8 inflammasome in future studies to understand whether the components of the canonical inflammasome pathway also reside in the same ASC complex.
We demonstrate that even in the presence of caspase-1 and 11, caspase-8 also contributes to IL-1β maturation upon P brasiliensis infection. The reduced IL-1β processing due to the chemical inhibition of caspase-8 was corroborated using the Rip3−/−Casp8−/− DCs. Caspase-8 deficiency, more than RIP3 deficiency, reduced P brasiliensis-induced IL-1β secretion in Pam3CSK4-primed BMDCs, disrupting caspase-1 activation and ASC oligomerization. The requirement of caspase-8 for caspase-1 activation suggests that caspase-8 might be acting upstream of caspase-1. It is possible that caspase-8 could be regulating caspase-1 by activating cellular inhibitors of apoptosis proteins (cIAPs) to support either the assembly of inflammasome or coordinating its activity [30]. Several groups have demonstrated the involvement of caspase-8 in the cleavage of procaspase-1 over the last few years [29, 31, 32]. Nevertheless, how caspase-1 and caspase-8 operate as well as the nature of the activators that determine this relationship remain to be fully elucidated.
In addition, we demonstrate that dectin-1-mediated IL-1β production after P brasiliensis challenge is mediated by the noncanonical caspase-8 inflammasome, permitting us to uncover a previously unknown pathway for dectin-1 in P brasiliensis-infected DCs. Indeed, in our fungal model, dectin-1 is connected to the posttranslational cleavage of pro-IL-1β, but it can also regulate IL-1β transcription on priming, similar to the dectin-1-ligand, β-glucan, that participates in both NLRP3 inflammasome activation and NF-κB-mediated priming [33, 34]. Moreover, we observed that IL-1β secretion and inflammasome activation upon infection with strain 60855 of live P brasiliensis was amplified in DCs and were more robust relative to that observed in macrophages. Interleukin-1β secretion in myeloid cell population is enhanced by GM-CSF treatment that maximizes the NF-κB-dependent pro-IL-1β synthesis [35], and the increased amounts of the precursor IL-1β in DCs [36, 37] allow the cells to achieve inflammasome activation. Also different from the isolate 18 of P brasiliensis, the in vitro infection with 60855 strain was only able to induce higher IL-1β levels in primed cells, indicating that the distinct phenotypic characteristics of the 2 strains, such as virulence, geographical source, genetic diversity, and variable concentration of cell wall contents, are important factors in pathogenicity [38].
CONCLUSIONS
Finally, we demonstrate a higher fungal load in P brasiliensis- infected Rip3−/−Casp8−/− mice, denoting a critical role for caspase-8 in host protection. Likewise, a similar phenotype was described in response to Citrobacter rodentium [32], Aspergillus fumigatus [29], and Yersinia pestis [31, 39]. Because mice lacking caspase-8 on a Rip3-deficient background (Rip3−/−Casp8−/−) or DCs with deleted caspase-8 (Casp8fl/flItgaxcre) also manifest defects in cell death causing cellular accumulation [40, 41], we speculate that caspase-8-mediated cell death could prevent the excessive immune response that causes local tissue damage and favors P brasiliensis growth. However, the potential role of caspase-8 in controlling IL-1β production could also be pivotal in regulating host immunity against P brasiliensis. Adequate IL-1β production is critical for proper granuloma formation and determining disease outcome. For instance, IL-1β activates leukocytes to effectively kill the ingested P brasiliensis yeasts [14, 42], potentiates the fungicidal activity of interferon-γ [43], and their signaling is involved in fungal restriction in vivo [13]. Furthermore, the IL-1β levels found in the serum of disseminated PCM patients positively correlates with the production of TNF-α and IL-6 [44], key cytokines important in granuloma development during experimental P brasiliensis infection [45, 46]. These observations help to explain the enhanced susceptibility of P brasiliensis- infected Rip3−/−Casp8−/− mice that is associated with a disorganized inflammatory reaction. However, it remains to be studied whether the reduced IL-1β secretion is directly responsible for the inability of caspase-8-deficient animals to form granulomas structures and clear P brasiliensis. These new insights, which were gained from deciphering how pathogen recognition shapes the development of appropriate innate immune responses during P brasiliensis infection, allow a better understanding of the underlying causes of inflammation after host-pathogen interactions.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We sincerely thank that following contributors: Jennifer Bernier, Gail Germain, and Kelly Army for animal care and handling; Jason McGowan, Zhaozhao Jiang, Mona Motwani, and Shruti Sharma for insightful comments and technical help; Bruce S. Klein for the fungal strain; Chrono K. Lee and Charles A. Specht for reagents and suggestions; and all Fitzgerald and Levitz laboratory members for helpful technical advice and discussions.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. The research leading to these results has received funding from the São Paulo Research Foundation (FAPESP) under grant agreement nos. 2012/14524-9 (Thematic Project; to J. S. d. S.) and 2013/08216-2 (Center for Research in Inflammatory Disease), as well as from the University of São Paulo NAP-DIN under grant agreement no. 11.1.21625.01.0. J. S. d. S. is a research fellow from Conselho Nacional de Desenvolvimento Científico e Tecnológico. N. K.-C. is supported by a doctoral fellowship from FAPESP grant no. 2013/21295–9 and by a scholarship linked to the research internship abroad (no. 2014/24503-4) also funded by FAPESP. S. G. and K. A. F. were supported by grants from the Kenneth Rainin Foundation, Lupus Research Foundation, and National Institutes of Health (NIH) (AI067497). S. M. L. was supported by grants from the NIH (AI025780, HL112671).
Potential conflicts of interest. All Authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: XLI Congress of the Brazilian Society of Immunology, Campos do Jordão, São Paulo, Brazil, November 2016.
References
- 1. Franco M, Peracoli MT, Soares A, Montenegro R, Mendes RP, Meira DA. Host-parasite relationship in paracoccidioidomycosis. Current Topics in Medical Mycology 1993; 5:115–49. [PubMed] [Google Scholar]
- 2. Terçarioli GR, Bagagli E, Reis GM et al. Ecological study of Paracoccidioides brasiliensis in soil: growth ability, conidia production and molecular detection. BMC Microbiol 2007; 7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanetsuna F, Carbonell LM. Cell wall glucans of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol 1970; 101:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. San-Blas G, San-Blas F. Paracoccidioides brasiliensis: cell wall structure and virulence. A review. Mycopathologia 1977; 62:77–86. [DOI] [PubMed] [Google Scholar]
- 5. San-Blas G, Niño-Vega G, Iturriaga T. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med Mycol 2002; 40:225–42. [DOI] [PubMed] [Google Scholar]
- 6. Anjos AR, Calvi SA, Ferracini R, Peraçoli MT, Silva CL, Soares AM. Role of Paracoccidioides brasiliensis cell wall fraction containing beta-glucan in tumor necrosis factor-alpha production by human monocytes: correlation with fungicidal activity. Med Mycol 2002; 40:377–82. [DOI] [PubMed] [Google Scholar]
- 7. Silva CL, Alves LM, Figueiredo F. Involvement of cell wall glucans in the genesis and persistence of the inflammatory reaction caused by the fungus Paracoccidioides brasiliensis. Microbiology 1994; 140:1189–94. [DOI] [PubMed] [Google Scholar]
- 8. Gross O, Gewies A, Finger K et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651–6. [DOI] [PubMed] [Google Scholar]
- 9. Rogers NC, Slack EC, Edwards AD et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005; 22:507–17. [DOI] [PubMed] [Google Scholar]
- 10. Gringhuis SI, Kaptein TM, Wevers BA et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 2012; 13:246–54. [DOI] [PubMed] [Google Scholar]
- 11. Ganesan S, Rathinam VAK, Bossaller L et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol 2014; 193:2519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loures FV, Araújo EF, Feriotti C et al. Dectin-1 induces M1 macrophages and prominent expansion of CD8+IL-17+ cells in pulmonary paracoccidioidomycosis. J Infect Dis 2014; 210:762–73. [DOI] [PubMed] [Google Scholar]
- 13. Ketelut-Carneiro N, Silva GK, Rocha FA et al. IL-18 triggered by the Nlrp3 inflammasome induces host innate resistance in a pulmonary model of fungal infection. J Immunol 2015; 194:4507–17. [DOI] [PubMed] [Google Scholar]
- 14. Tavares AH, Magalhães KG, Almeida RD, Correa R, Burgel PH, Bocca AL. NLRP3 inflammasome activation by Paracoccidioides brasiliensis. PLoS Negl Trop Dis 2013; 7:e2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feriotti C, Bazan SB, Loures FV, Araújo EF, Costa TA, Calich VL. Expression of dectin-1 and enhanced activation of NALP3 inflammasome are associated with resistance to paracoccidioidomycosis. Front Microbiol 2015; 6:913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol 2012; 13:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maelfait J, Vercammen E, Janssens S et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med 2008; 205:1967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bossaller L, Chiang PI, Schmidt-Lauber C et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J Immunol 2012; 189:5508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van de Craen M, Van Loo G, Declercq W et al. Molecular cloning and identification of murine caspase-8. J Mol Biol 1998; 284:1017–26. [DOI] [PubMed] [Google Scholar]
- 20. Kawadler H, Gantz MA, Riley JL, Yang X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol Cell 2008; 31:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27:519–50. [DOI] [PubMed] [Google Scholar]
- 22. Kang TB, Oh GS, Scandella E et al. Mutation of a self-processing site in caspase-8 compromises its apoptotic but not its nonapoptotic functions in bacterial artificial chromosome-transgenic mice. J Immunol 2008; 181:2522–32. [DOI] [PubMed] [Google Scholar]
- 23. Hu WH, Johnson H, Shu HB. Activation of NF-kappaB by FADD, Casper, and caspase-8. J Biol Chem 2000; 275:10838–44. [DOI] [PubMed] [Google Scholar]
- 24. Su H, Bidère N, Zheng L et al. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 2005; 307:1465–8. [DOI] [PubMed] [Google Scholar]
- 25. Pierini R, Juruj C, Perret M et al. AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 2012; 19:1709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masumoto J, Dowds TA, Schaner P et al. ASC is an activating adaptor for NF-kappa B and caspase-8-dependent apoptosis. Biochem Biophys Res Commun 2003; 303:69–73. [DOI] [PubMed] [Google Scholar]
- 27. Chen M, Xing Y, Lu A et al. Internalized Cryptococcus neoformans activates the canonical caspase-1 and the noncanonical caspase-8 inflammasomes. J Immunol 2015; 195:4962–72. [DOI] [PubMed] [Google Scholar]
- 28. Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J Immunol 2013; 191:5239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karki R, Man SM, Malireddi RK et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015; 17:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Labbé K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity 2011; 35:897–907. [DOI] [PubMed] [Google Scholar]
- 31. Weng D, Marty-Roix R, Ganesan S et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci USA 2014; 111:7391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gurung P, Anand PK, Malireddi RK et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 2014; 192:1835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar H, Kumagai Y, Tsuchida T et al. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol 2009; 183:8061–7. [DOI] [PubMed] [Google Scholar]
- 34. Kankkunen P, Teirilä L, Rintahaka J, Alenius H, Wolff H, Matikainen S. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol 2010; 184:6335–42. [DOI] [PubMed] [Google Scholar]
- 35. Khameneh HJ, Isa SA, Min L, Nih FW, Ruedl C. GM-CSF signalling boosts dramatically IL-1 production. PLoS One 2011; 6:e23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol 2013; 190:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 2012; 189:4175–81. [DOI] [PubMed] [Google Scholar]
- 38. Munoz JF, Farrer RA, Desjardins CA et al. Genome diversity, recombination, and virulence across the major lineages of Paracoccidioides. mSphere 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Philip NH, Dillon CP, Snyder AG et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci USA 2014; 111:7385–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oberst A, Dillon CP, Weinlich R et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471:363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 2013; 38:27–40. [DOI] [PubMed] [Google Scholar]
- 42. Kurita N, Oarada M, Brummer E. Fungicidal activity of human peripheral blood leukocytes against Paracoccidioides brasiliensis yeast cells. Med Mycol 2005; 43:417–22. [DOI] [PubMed] [Google Scholar]
- 43. Kurita N, Oarada M, Miyaji M, Ito E. Effect of cytokines on antifungal activity of human polymorphonuclear leucocytes against yeast cells of Paracoccidioides brasiliensis. Med Mycol 2000; 38:177–82. [DOI] [PubMed] [Google Scholar]
- 44. Silva CL, Silva MF, Faccioli LH, Pietro RC, Cortez SA, Foss NT. Differential correlation between interleukin patterns in disseminated and chronic human paracoccidioidomycosis. Clin Exp Immunol 1995; 101:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS. Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol 2000; 156:1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tristão FSM, Rocha FA, Carlos D et al. Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front Immunol 2017; 8:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.