Abstract
Text-based interventions are effective for smoking cessation, but have not been tested in rural older adults. The purpose of this study was to compare the feasibility, acceptability and preliminary efficacy of a text-based Scheduled Gradual Reduction (SGR) program to a non-SGR text messaging support condition among rural older adults. Adults over 60 years were randomized to either: (i) the SGR program (n = 20), a text-based program to reduce smoking over 4-weeks plus text-based support messages; or (ii) control (n = 20), receipt of text-based support messages only. Participants completed surveys at baseline and end of program to assess feasibility and acceptability of the intervention, and biochemically validated 7-day point prevalence cessation was assessed at end of treatment. Most participants (81%) reported reading all the messages they received. Participants found both interventions useful in quitting smoking (SGR = 57%, Control = 63%) and would recommend it to a friend (SGR = 72%, Control = 79%). Although not statically significant, the SGR group had a higher rate of biochemically validated cessation (SGR = 15%, Control = 5%, Cohen d = 0.67). Among those still smoking, the median percent reduction in cigarettes was 33.3% for both groups. Text-based cessation interventions are feasible, acceptable and can be easily disseminated to rural older adult tobacco users.
Introduction
Approximately 3.8 million older adults (65 years and older) currently smoke, which puts them at risk for smoking-related morbidities and mortality [1, 2]. Older adult smokers are interested in quitting, and cessation is highly beneficial to them. Cardiac risk drops substantially within 5 years of quitting, and there is an overall decrease in all-cause mortality [1, 3, 4]. Even those who quit smoking over the age of 65 can gain one to four additional years of longevity [5]. Despite the need, cessation initiatives have not focused on older adults [2]. An even more high-risk group involves rural older adult smokers. Rural older adults smoke at higher rates than their urban counterparts, yet have access to few cessation services [6]. They also receive less cessation advice from providers than their younger and urban counterparts [7, 8].
Text messaging may be an effective way to increase the reach of smoking cessation interventions among rural older adults. Mobile phones are in use across socioeconomic and demographic groups [9]. As many as 78% of older adults and 9 of 10 rural residents have cell phones with texting ability [10, 11], and text-based interventions targeting other health issues, including physical activity and chronic disease management in older adults, have been feasible and efficacious [12–14]. This suggests that cell phones have the potential to be an effective delivery mode in this population, who traditionally lack access to cessation interventions [6]. A recent Cochrane review supports the efficacy of text-based smoking interventions in promoting cessation [15], but to our knowledge, there are currently no text-based cessation interventions that have specifically targeted rural older adult smokers.
Rural older adult smokers are a unique group given that their smoking habits have been engrained in their social environment for decades compared to those habits of younger smokers. Therefore, text-based interventions such as Smokefree TXT, may not be enough to help them quit. However, combining a text-based support intervention with a Scheduled Gradual Reduction (SGR) program may be more efficacious. SGR of smoking first assesses smokers’ patterns, then over the course of 3 weeks, gradually reduces the number of cigarettes used per day by a third, thus lengthening the time interval between use [16]. SGR disentangles tobacco use from situational cues and helps to mitigate withdrawal symptoms that might otherwise decrease motivation and confidence in quitting [17]. SGR has also been shown to promote smoking cessation with quit rates as high as 50%, and using reduction methods with older adults have resulted in greater long-term cessation rates compared to non-reduction cessation methods [16, 18]. Our study uses methods similar to previous SGR studies, although unlike prior work that used small hand-held computers to deliver the intervention [16], we delivered the SGR program via text message on a mobile phone platform.
Methods
Design
This study was a randomized control trial comparing a text-based SGR intervention plus support messages to an intervention that did not use reduction and included only text-based support messages. Face-to-face interviews were conducted with participants at baseline and at the end of the intervention. This study was approved by the Duke University Institutional Review Board.
Participants
We enrolled 40 rural smokers from two Duke Primary Care Clinics. Rural status was determined by Rural-Urban Commuting Area (RUCA) codes designated as non-metropolitan (RUCA code of 4 or above) [19]. Inclusion criteria were: (i) over 60 years of age; (ii) smoked at least 100 cigarettes in their lives and currently smoke an average of five cigarettes per day; (iii) residential address in a rural census tract defined by a RUCA code of 4–10; (iv) general knowledge of text messaging and (v) interest in quitting. A permuted block randomization was used to assign participants to the SGR (n = 20) or control (n = 20) group. We opted for a block size of 2 to ensure balance in the sample size of the arms in the event that we were unable to recruit 40 participants or the attrition was higher than expected for this pilot in which the primary outcome was an event rate.
Study procedure
We pulled from the Electronic Medical Record all smokers 60 and older seen at two Duke Primary Care Clinics in the past 12 months. We sent these potential participants a letter describing the study and gave them a number to call to opt out. We contacted those patients who did not opt out and screened them for eligibility. Staff met with those who passed the telephone screen and obtained informed consent, conducted a face-to-face baseline assessment, and completed the randomization. If subjects did not have a cell phone with unlimited texting, they were provided with one. Participants completed the intervention and completed an end-of-program survey. Those participants who reported complete abstinence from tobacco use were asked to provide a saliva sample to biochemically confirm their self-reports. All participants received $35 for completing both surveys.
Treatment groups
Control group: text-based support messages alone
Participants received supportive text messages over the 4-week study period and were asked to set a quit date within 2 weeks of the start of the intervention. The support messages were developed based on constructs of the Health Belief Model (perceived benefits, susceptibility, barriers, motivation and self-efficacy) and on The National Cancer Institute’s Clear Horizons Cessation Intervention designed for adults ages 50 and older. These messages were pilot tested with eight rural older adults for content and readability. Participants were asked to read each message and comment on the helpfulness and clarity of the message. Those messages that the majority of participants found unhelpful or unclear were removed. Participants received two messages a day during Weeks 1 and 4 and up to three a day during Weeks 2 and 3 when they were attempting to quit smoking altogether. All messages were sent at 10 a.m. each day. Text messages were delivered by a computer program, Mobile Health Platform [20] at Duke.
SGR group: text-based SGR intervention plus support messages
Participants received the same 4 weeks of support text messages as the control group as well as the SGR intervention. During Week 1, participants were asked to smoke according to their usual habit and text ‘s’ every time they smoked. At the completion of Week 1, the mean number of cigarettes was calculated. We used an algorithm based on the work of Cinciripini et al. [16, 21], which involved using the calculated number of cigarettes smoked per day and reducing this number by a third over the next 3 weeks to reach 0. The last day of program, when reduction to zero occurred was considered their quit date, and participants received a reminder text on both Days 2 and 1 prior to the quit date. Participants over the reduction period (Weeks 2–4) were instructed not to smoke unless they received a text message telling them to do so. The number of SGR texts received per day varied based on baseline smoking levels (range 2–18). Participants were instructed to respond to the aforementioned text with ‘s’ if they smoked and ‘ns’ if they did not smoke. Participants were given a booklet outlining the SGR process (reduction by a third each week) after randomization with the main goal of quitting the last day of the reduction.
Study measures
At the end of the study, we asked participants the following questions: ‘What did you typically do when you received a support text message?’ (1 = Ignored it completely, 5 = Read it right away) and ‘On a typical day, did you read messages you received?’ (1 = Not at all, 5 = read the whole message) [22]. A priori, the intervention was deemed feasible if the majority of participants (more than 50%) read all the messages received, and if participants in the SGR group responded to 50% or greater of the texts to smoke.
To determine the program’s success, we asked the following questions tailored to each group: ‘How useful was the intervention in helping you to quit smoking?’ (1 = Not at all useful, 5 = Extremely useful) and ‘Would you recommend the program to a friend?’ (1 = Not at all recommend, 5 = Extremely likely to recommend). A priori, the intervention was deemed acceptable if the majority of participants (more than 50%) found the intervention very or extremely useful and would be very or extremely likely to recommend it to a friend.
To determine preliminary efficacy, we used a 7-day abstinence from smoking as cessation. We asked participants: ‘In the past 7 days, have you smoked any cigarettes in the past 7 days, even a puff?’ Tobacco use status was biochemically confirmed using NicAlert Semiquantitative test strips with a cut-off of 10 ng ml−1 indicating exposure. Mean number of cigarettes per day was assessed as a secondary outcome for those who did not report quitting. Participants were asked ‘How many cigarettes do you smoke a day’ if they had smoked cigarettes in the past 7 days.
Data analysis
Non-directional statistical tests were performed with the level of significance set at 0.05. The significance level was not adjusted for multiple outcomes due to the exploratory nature of this small pilot study. Non-parametric Wilcoxon Two-Sample Tests for continuous measures and Fisher’s Exact Tests for categorical measures were used to test for between-group differences. For the end of program biochemically validated quit rate, intent-to-treat and completers analyses were performed. Failure to quit was assumed for missing data in the intent-to-treat analysis. Multiple open-ended questions to obtain feedback about the intervention were asked of a subsample of participants (n = 14; Control = 7, SGR = 7). Content analysis was done and comments were abstracted.
Results
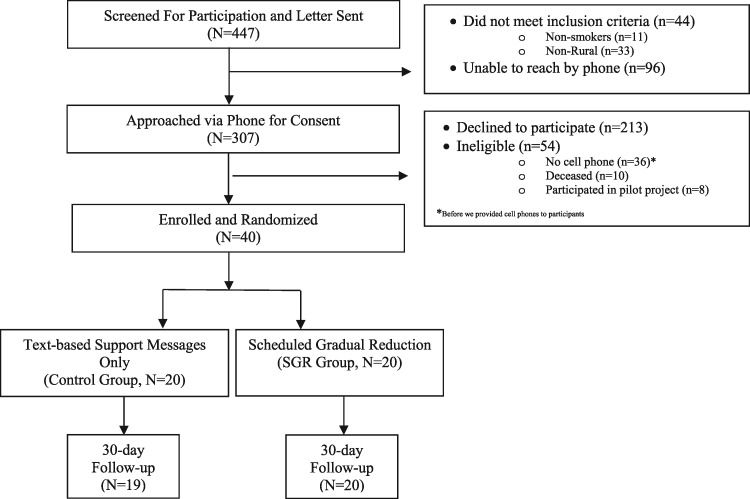
Flow of recruitment and follow-up is shown in Fig. 1. We recruited 40 participants over 6 months. Our refusal rate was 69%, the main reason for refusal was not interested in quitting at this time. We obtained follow-up data on 39 of 40 participants. Table I details the baseline sociodemographic and smoking characteristics, as well as the acceptability, feasibility and smoking outcomes. Table II provides a detailed description of the participants’ responses to the feasibility and acceptability questions for each group along with indicators of fidelity for the SGR group.
Fig. 1.
Flow of recruitment, enrollment and follow-up of older adults in the North Carolina (2015–16).
Table I.
Sample characteristics of rural older adults in North Carolina (2015–16) and study outcomes
| Variables | Total | Control group | SGR group | P-value |
|---|---|---|---|---|
| (n = 40) | (n = 20) | (n = 20) | ||
| Baseline characteristics | ||||
| Age, in years | 68.0 (65.0, 72.0) | 66.5 (64.5, 71.0) | 69.0 (66.0, 73.0) | 0.13 |
| Females | 25 (62.5%) | 10 (50.0%) | 15 (75.05) | 0.10 |
| Post-secondary education | 20 (50.0%) | 9 (45.0%) | 11 (55.0%) | 0.53 |
| Black/African American | 17 (42.5%) | 9 (45.0%) | 8 (40.0%) | 0.75 |
| Hispanic/Latino | 2 (5.0%) | 1 (5.0%) | 1 (5.0%) | 1.0 |
| Household Income: $15 000 or lessa | 20 (55.6%) | 10 (55.6%) | 10 (55.6%) | 1.0 |
| Married/living with partner | 18 (45.0%) | 9 (45.0%) | 9 (45.0%) | 1.0 |
| Unemployed | 37 (92.5%) | 17 (85.0%) | 20 (100.0%) | 0.23 |
| Days smoked in past 30 days | 30 (30.0, 30.0) | 30 (30.0, 30.0) | 30 (30.0, 30.0) | 0.49 |
| Number of cigarettes/day smoked | 12.0 (6.5, 20.0) | 10.5 (5.5, 20.0) | 15.0 (9.0, 20.0) | 0.32 |
| Age started smoking, in years | 15.0 (14.0, 17.5) | 15.5 (14.5, 18.5) | 15.0 (14.0, 16.0) | 0.31 |
| Feasibility outcomes | ||||
| Read the whole message | 30 (81.1%) | 16 (88.9%) | 14 (73.7%) | 0.40 |
| Read all of the support texts | 31 (88.6%) | 15 (93.8%) | 16 (84.2%) | 0.60 |
| Read support text right awayb | 31 (83.8%) | 14 (77.8%) | 17 (89.5%) | 0.40 |
| Acceptability: very much/extremely | n = 38 | n = 19 | n = 19 | |
| Usefulness of the intervention | 22 (57.9%) | 12 (63.2%) | 10 (57.6%) | 0.74 |
| Recommend intervention to a friendc | 28 (75.7%) | 15 (79.0%) | 13 (72.2%) | 0.71 |
| 30-day self-reported smoking outcomes | ||||
| Self-reported smoking cessationd | 8 (20.5%) | 2 (10.5%) | 6 (30.0%) | 0.24 |
| Percent reduction in cigarettes smoked/daye | 33.3% (0.0, 66.7%) | 40.0% (0.0, 66.7%) | 30.0% (0.0, 50.0%) | 0.66 |
| 30-day biochemically validated quit rate | ||||
| Smoking cessation (intent-to-treat analysis)f | 4 (10.0%) | 1 (5.0%) | 3 (15.0%) | 0.60 |
| Smoking cessation (completers analysis)g | 4 (10.5%) | 1 (5.3%) | 3 (15.8%) | 0.60 |
Continuous measures: median (25th, 75th percentile) reported; Categorical measures: n (%) of non-missing reported; P-values for the Wilcoxon Two-Sample Tests for continuous or the Chi-square/Fisher’s Exact Test for categorical measures.
n = 36.
n = 35 (16 control, 19 SGR) due to missing data.
n = 37 (19 control, 18 SGR) due to missing data.
n = 39 for self-reported smoking cessation due to one participant who dropped out before 30 days.
n = 31 for baseline minus day 30% reduction in cigarettes smoked among those who self-reported as non-quitters; 30-day biochemically validated quit rate.
Intent-to-treat analysis: n = 40, 20/group, no cessation (non-quitter) imputed for missing due to dropout or insufficient saliva sample.
Completers analysis: n = 38, 19 control, 19 SGR due to one participant who dropped out and one participant with insufficient saliva sample.
Table II.
Feasibility and acceptability: descriptive statistics
| Domain | Control group | SGR group |
|---|---|---|
| Feasibility | ||
| Read messages your received | n = 18 | n = 19 |
| Not at all | 0 (0.0%) | 2 (10.5%) |
| Read about a quarter of a message | 1 (5.6%) | 0 (0.0%) |
| Read about a half of a message | 0 (0.0%) | 2 (10.5%) |
| Read about a three quarters of a message | 1 (5.6%) | 1 (5.3%) |
| Read the whole message | 16 (88.9%) | 14 (73.7%) |
| How many support texts read | n = 16 | n= 19 |
| Did not read any of the support messages | 0 (0.0%) | 1 (5.3%) |
| Read some of the support messages | 1 (6.3%) | 1 (5.3%) |
| Read most of the support messages | 0 (0.0%) | 1 (5.3%) |
| Read all of the support texts | 15 (93.8%) | 16 (84.2%) |
| When read the support texts | n= 18 | n = 19 |
| Ignored support messages completely | 0 (0.0%) | 1 (5.3%) |
| Read support messages when I had time | 4 (22.2%) | 1 (5.3%) |
| Read support messages right away | 14 (77.8%) | 17 (89.5%) |
| Acceptability | ||
| Usefulness of the intervention in helping you to quit smoking | n = 19 | n = 19 |
| Not at all useful | 2 (10.5%) | 1 (5.3%) |
| Somewhat useful | 4 (21.1%) | 2 (10.5%) |
| Undecided | 1 (5.3%) | 6 (31.6%) |
| Very useful | 10 (52.6%) | 7 (41.2%) |
| Extremely useful | 2 (10.5%) | 2 (15.8%) |
| Would you recommend the intervention to a friend | n = 19 | n = 18 |
| Not at all | 2 (10.5%) | 2 (11.1%) |
| Somewhat | 2 (10.5%) | 2 (11.1%) |
| Undecided | 0 (0.0%) | 1 (5.6%) |
| Very much | 9 (47.4%) | 7 (38.9%) |
| Extremely | 6 (31.6%) | 6 (33.3%) |
| Fidelity: response to ‘alert’ text messages sent | ||
| Number alerts sent per participant | n = 17 | |
| Median (25, 75th percentile) | 84.0 (56.0, 91.0) | |
| Minimum, maximum | 18, 147 | |
| Number responses to alerts per participant | n = 17 | |
| Median (25, 75th percentile) | 58.0 (18.0, 77.0) | |
| Minimum, Maximum | 0, 100 | |
| Percent alerts with response per participant | n = 17 | |
| Median (25, 75th percentile) | 65.4 (42.9, 83.5) | |
| Minimum, maximum | 0, 95.6 |
Among the 40 participants, 56% had an annual household income of $15 000 or less, 78% had their own cell phones and used their phones for the study and 100% of those had unlimited texting. Most participants reported that they read all messages (81%), read all support texts (89%), read support texts immediately (84%), found the assigned intervention very or extremely useful in helping them quit smoking (58%) and would be very or extremely likely to recommend the intervention to a friend (76%). There were no significant group differences on the baseline, feasibility and acceptability measures.
The self-reported quit rates were 30% (n = 6/20) and 10.5% (n = 2/19) for those in the SGR and control groups, respectively (P = 0.24, Cohen d = 0.28). Among 31 participants who reported continued smoking, the median percent reduction in cigarettes smoked per day was 33.3% for both groups (SGR: 30.0%, n = 14, Control = 40.0%, n = 17, P = 0.66).
Although not statistically significant, when the SGR group was compared to the control group, it had a higher rate of biochemically validated cessation (intent-to-treat: SGR = 15%, n = 3/20, Control = 5%, n = 1/20), completers: SGR = 15.8%, n = 3/19, Control = 5.3%, n = 1/19), representing a medium effect (both Cohen d = 0.67, see Table I). Within the SGR group, the median number of alerts per participant was 84, and median percent of alerts with a response per participant was 65%.
Participants had constructive things to say about our intervention. A control participant commented that our intervention was, ‘unobtrusive and a reliable, positive daily reminder of doing something good by quitting smoking.’ Three participants in the SGR group stated how the reduction helped them gain confidence in their ability to quit. One SGR participant said, ‘I kept on coming down. That made me more confident.’ Another SGR participant said, ‘I learned I could wait for a cigarette.’ Multiple SGR participants thought the intervention was not long enough, ‘For me it would need to be at least 60 days, to help me cut down even more.’ Multiple SGR participants said waiting for the texts was stress inducing and difficult, but that over time it was less stressful.
Discussion
This is one of the first studies to establish feasibility and acceptability of a text-based, SGR intervention. Our study demonstrated that using cell phones is a feasible and acceptable way to deliver smoking cessation interventions, both using reduction methods and non-reduction methods [12–14]. Participants in both groups found the intervention feasible and acceptable. Delivering these interventions via text has the potential to reach many vulnerable smokers.
We also found that the SGR intervention resulted in higher quit rates compared to the text-based support messages alone, although this result was in the expected direction it was not significant. SGR has been shown to be an effective cessation method [15], and it may be an efficacious method of cessation in rural older smokers. Stimulus control, the principle that underlies SGR is an important determinate of quitting in all smokers [23], and a signal is provided in this study that this is a method worthy of further testing in this group of smokers. SGR was well received among this group of older smokers. Participants commented on how the gradual reduction helped them build confidence in the ability to quit. Given that many older adults have failed to quit in multiple attempts, this increase in self-confidence from SGR may be especially beneficial for this population. Of note, we did not provide pharmacotherapy in this study, and pharmacotherapy paired with reduction has been shown to be efficacious [24]. We would expect quit rates to increase in both groups with the addition of pharmacotherapy, and this is fertile ground for future studies.
From this feasibility study, we learned valuable information that will inform future research. First, our refusal rate was high at 69%. The majority refused because they were not willing to quit. Second, our recruitment strategy of mailing letters from the study team may not be the best method to recruit rural older adult smokers. In-person recruitment may be more effective, such as approaching smokers immediately after a visit with their providers. This strategy has the potential to capitalize on a teachable moment following a health care appointment and may be more effective in recruitment.
We also learned that the length of the SGR program was not long enough for some participants. For heavier smokers (e.g. those who smoke more than a pack a day), the 3-week reduction period was very short. Extra time to reduce cigarettes may help increase quit rates among these individuals.
Limitations of this analysis include the high refusal rate and small sample. We also were not able to objectively determine if participants smoked when they texted they did or if participants read the support messages received. We did ask participants in the SGR group if they smoked off schedule, and 75% said they either never of very few times smoked off schedule.
Another limitation is that we changed our inclusion criteria half way through the study to provide cell phones to individuals without them; this may have affected the demographics of our study. Finally, we did not examine sustainability of the intervention on long-term cessation. However, rural older adults found this text-based SGR intervention feasible and acceptable and reported higher quit rates compared to those in the text-based support messages only group. Further testing of this intervention in a larger clinical trial with at least 80% power is warranted with the goal of bringing accessible and efficacious cessation interventions to older adults in underserved communities.
Acknowledgements
The authors wish to thank the providers at Duke Primary Care Oxford and Duke Primary Care Henderson for support of this study. We also thank Michael Kahl, MBA for data extraction. We also wish to thank the participants of this study.
Funding
Alliance National Cancer Institute Community Oncology Research Program (NCORP) Research Base grant (1UG1CA189823 to D.N.). This trial is registered at Clinicaltrials.gov: NCT02510716.
Conflict of interest statement
None declared.
References
- 1. LaCroix AZ, Omenn GS.. Older adults and smoking. Clin Geriatr Med 1992; 8: 69–87. [PubMed] [Google Scholar]
- 2. Zbikowski SM, Magnusson B, Pockey JR. et al. A review of smoking cessation interventions for smokers aged 50 and older. Maturitas 2012; 71: 131–41. [DOI] [PubMed] [Google Scholar]
- 3. Gellert C, Schöttker B, Brenner H.. Smoking and all-cause mortality in older people: Systematic review and meta-analysis. Arch Intern Med 2012; 172: 837–44. [DOI] [PubMed] [Google Scholar]
- 4. Gellert C, Schöttker B, Müller H. et al. Impact of smoking and quitting on cardiovascular outcomes and risk advancement periods among older adults. Eur J Epidemiol 2013; 28: 649–58. [DOI] [PubMed] [Google Scholar]
- 5. Taylor DH Jr, Hasselblad V, Henley SJ. et al. Benefits of smoking cessation for longevity. Am J Public Health 2002; 92: 990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Lung Association. Cutting Tobacco’s Rural Roots: Tobacco Use in Rural Communities 2012. Available at: http://www.lung.org/assets/documents/publications/lung-disease-data/cutting-tobaccos-rural-roots.pdf. Accessed: 9 January 2016.
- 7. Hutcheson TD, Greiner KA, Ellerbeck EF. et al. Understanding smoking cessation in rural communities. J Rural Health 2008; 24: 116–24. [DOI] [PubMed] [Google Scholar]
- 8. Shadel WG, Elliott MN, Haas AC. et al. Clinician advice to quit smoking among seniors. Prev Med 2015; 70: 83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riley W, Rivera D, Atienza A. et al. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med 2011; 1: 53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pew Center. Older Adults and Technology 2014. Available at: http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/. Accessed: 24 October 2016.
- 11. Pew Center. The Demographics of Device Ownership 2015. Available at: http://www.pewinternet.org/2015/10/29/the-demographics-of-device-ownership/. Accessed: 9 September 2016.
- 12. Durso SC, Wendel I, Letzt AM. et al. Older adults using cellular telephones for diabetes management: a pilot study. Medsurg Nurs 2003; 12: 313–7. [PubMed] [Google Scholar]
- 13. Kim BH, Glanz K.. Text messaging to motivate walking in older African Americans: a randomized controlled trial. Am J Prev Med 2013; 44: 71–5. [DOI] [PubMed] [Google Scholar]
- 14. Müller AM, Khoo S, Morris T.. Text messaging for exercise promotion in older adults from an upper-middle-income country: randomized controlled trial. J Med Internet Res 2016; 18: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittaker R, McRobbie H, Bullen C. et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016; 4: CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cinciripini PM, Wetter DW, McClure JB.. Scheduled reduced smoking: Effects on smoking abstinence and potential mechanisms of action. Addict Behav 1997; 22: 759–67. [DOI] [PubMed] [Google Scholar]
- 17. Beard E, Aveyard P, McNeill A. et al. Mediation analysis of the association between use of NRT for smoking reduction and attempts to stop smoking. Psychol Health 2012; 27: 1118–33. [DOI] [PubMed] [Google Scholar]
- 18. Falba T, Jofre-Bonet M, Busch S. et al. Reduction of quantity smoked predicts future cessation among older smokers. Addiction 2004; 99: 93–102. [DOI] [PubMed] [Google Scholar]
- 19. U.S. Department of Agriculture, Economic Research Service. Urban Influence Codes 2013. Available at: http://www.ers.usda.gov/data-products/urban-influence-codes.aspx. Accessed: 1 September 2015.
- 20. Shaw RJ, Steinberg DM, Bonnet J. et al. Mobile health devices: will patients actually use them? J Am Med Inform Assoc 2016; 23: 462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cinciripini PM, Lapitsky L, Seay S. et al. The effects of smoking schedules on cessation outcome: can we improve on common methods of gradual and abrupt nicotine withdrawal? J Consult Clin Psychol 1995; 63: 388–99. [DOI] [PubMed] [Google Scholar]
- 22. Shaw RJ, Steinberg DM, Zullig LL. et al. mHealth interventions for weight loss: a guide for achieving treatment fidelity. J Am Med Inform Assoc 2014; 21: 959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiftman S, Dunbar MS, Ferugson SG.. Stimulus control in intermittent and daily smokers. Psychol Addict Behav 2015; 29: 847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stead LF, Perera R, Bullen C. et al. Nicotine replacement therapy for smoking cessation (review). Cochrane Database Syst Rev 2008; CD000146. [DOI] [PubMed] [Google Scholar]