Abstract
Aims
Inflammation plays an important role in the neointima formation of grafted veins. However, the initiation of inflammation in grafted veins is still unclear. Here, we investigated the role and underlying mechanism of an innate immunity signalling protein, caspase-associated recruitment domain 9 (CARD9) in vein grafts in mice.
Methods and results
In early murine vein grafts, we observed robust death of smooth muscle cells (SMCs), which was accompanied by infiltration of macrophages and expression of pro-inflammatory cytokines. Meanwhile, SMC necrosis was associated with the expression of pro-inflammatory cytokines in macrophages in vitro. To explore the mediators of necrotic SMC-induced inflammation in grafted veins from mice, we examined the expression of CARD family proteins and found CARD9 highly expressed in infiltrated macrophages of grafted veins. CARD9-knockout (KO) inhibited necrotic SMC-induced pro-inflammatory cytokine expression and NF-κB activation. Furthermore, CARD9-KO suppressed necrotic SMC-induced expression of VEGF in macrophages. Finally, CARD9-KO decreased neointima formation of grafted veins in mice.
Conclusion
The innate immune protein CARD9 in macrophages may mediate necrotic SMC-induced inflammation by activating NF-κB and contributed to neointima formation in the vein grafts.
Keywords: Vein graft, CARD9, Macrophage, Cardiovascular inflammation, Neointima
1. Introduction
Autologous vein grafts are widely used for many types of vascular reconstruction such as coronary artery bypass grafting. However, the long-term vein graft patency is limited by progressive intimal hyperplasia and atherosclerosis.1 When grafted veins are exposed to arterial blood flow, increased mechanical stretch stimulates excess loss and proliferation of smooth muscle cells (SMCs) at early and late stages, eventually leading to neointima formation of grafted veins.2–4 Recent studies showed that therapeutic strategies in clinical trials targeting the proliferation of SMCs did not protect against vein graft failure,5,6 so novel insights are needed into the mechanism of remodelling in grafted veins.
Inflammation contributes to the pathogenesis of neointima formation of grafted veins. In grafted veins, infiltration of inflammatory cells (e.g. macrophages, granulocytes, and lymphocytes) and expression of pro-inflammatory cytokines (e.g. TNF-α and IL-1β) precede the onset of neointima development and remain elevated throughout its formation in grafted veins.7,8 Depletion of macrophages attenuated the neointima formation and decreased pro-inflammatory cytokine expression in rat vein grafts,9 which indicates the critical role of macrophages in initiating inflammation in grafted veins. Several studies showed that inhibition of pro-inflammatory cytokine expression could protect against neointima formation of grafted veins. Blocking C–C chemokine receptor type 2, the monocyte chemotactic protein-1 (MCP-1) receptor, inhibited the proliferation of SMCs and attenuated neointima formation in grafted veins.10 Knockout (KO) of TNF receptor 2 reduced neointima formation in grafted veins.11 However, the initiator of inflammation in the grafted veins remains to be understood.
Necrosis provokes pathological inflammation in many other types of diseases such as acute pancreatitis.12 The necrotic cells activate innate immune responses via multiple danger signals, which serve as damage-associated molecular patterns (DAMPs) or alarmins.13 Necrosis of SMCs is induced in early grafted veins.14 All these studies suggested that necrotic SMCs could be a strong candidate to initiate the inflammation in grafted veins.
The initial sensing of stimuli from necrotic cells is mediated by innate immunity. Necrotic cells release multiple internal constituents, which are recognized by pattern recognition receptors (PRRs) expressed mainly on antigen-presenting cells, including macrophages and dendritic cells, to induce inflammation.13 Recent studies showed that the intracellular caspase-associated recruitment domain (CARD) family of proteins mediates downstream signalling of PRRs on microbial infection.15 CARD4 and CARD15 are cytosolic sensors of peptidoglycans of bacterial cell walls, and CARD4-deficient mice infected with invasive Listeria monocytogenes show increased bacterial numbers in the spleen.16 CARD12 and CARD5 form a complex to activate caspase-1 and subsequent release of mature IL-1β and IL-18 on stimulation with invasive pathogens, and CARD12-deficient macrophages failed to release IL-1β stimulated with Salmonella typhimurium.17 CARD9 is a downstream adaptor of fungal- and bacteria-induced activation of Toll-like receptors (TLRs), immunoreceptor tyrosine-based activation motif-containing non-TLRs and dectin-1, mediating the expression of pro-inflammatory cytokines.18,19 However, the role of the CARD family in necrotic cell-induced inflammation and neointima formation in vein grafts is still unclear.
In this study, we examined necrosis in grafted veins in mice, then investigated the mechanism of necrotic SMC-induced pro-inflammatory cytokines in grafted veins. We found that necrosis of SMCs was accompanied by robust infiltration of macrophages in grafted veins. Among the CARD family, CARD9 was most highly expressed in macrophages of grafted veins. Furthermore, necrotic SMCs stimulated the expression of pro-inflammatory cytokines in macrophages via CARD9-mediated NF-κB activation. Finally, KO of CARD9 inhibited neointima formation of mouse vein grafts. Thus, CARD9 mediated necrotic SMC-induced inflammation and contributed to neointima formation in vein grafts.
2. Methods
Refer Supplementary material online for more detailed experimental methods.
2.1. Animals and vein graft procedure
CARD9-KO mice with a C57BL/6 genetic background were generated as described.20 Three-month-old CARD9-KO and littermate wild-type (WT) male mice were used, which were given standard rodent chow and water ad libitum. The experiments were approved by the Institutional Animal Care and Use Committee of the Capital Medical University and conformed to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Vein grafting was performed as we described previously.21,22 Mice were anaesthetized with ketamine (200 mg/kg) and xylazine (10 mg/kg) by an intraperitoneal injection (i.p.). Anaesthesia was monitored by pinching the toe. The right common carotid artery of mice was mobilized and divided. Inferior venae cavae from donor mice were grafted between the two ends of the carotid artery by sleeving the ends of the vein over the artery cuff and secured with 8.0 silk sutures (n = 6 for each group). Post-operative analgesia (buprenorphine, 0.05 mg/kg/12 h, i.p.) was administered for 48 h.
After 12 h, 3 days, and 1, 2, 3, and 4 weeks, mice were sacrificed with carbon dioxide (CO2) and vein grafts were harvested. The intima plus media was measured as the region between the lumen and adventitia. Vessel wall thickness was measured as the area of the vessel minus that of the lumen by using NIH Image Program Image J and AxioVision software.
2.2. Histopathology
Mouse vein grafts were fixed with 4% paraformaldehyde in PBS and snap-frozen in OCT embedding compound. Then, vessel sections were stained with haematoxylin and eosin (H&E) as described.23 Immunohistochemical and IF staining was performed as described in Supplementary material online.24
2.3. Electron microscopy
Normal veins and 3-day vein grafts were harvested and fixed in 2% glutaraldehyde solution buffered with 0.1 M sodium cacodylate (pH 7.4). Samples were rinsed, fixed, dehydrated, and embedded in Durcupan ACM. Ultrathin sections were stained with uranyl acetate and lead citrate, and viewed on transmission EM (JEOL JEM-1230).
2.4. Cell isolation and culture
WT or CARD9-KO mice (8–10 weeks old) were sacrificed with CO2 narcosis, and macrophages were obtained from bone marrow as described in Supplementary material online.24 The isolation of SMCs from vena cava of C57BL/6 WT male mice (8–10 weeks old) and from human saphenous vein involved enzymatic digestion as described in Supplementary material online.25 The human saphenous vein specimens were trimmings that are usually discarded during coronary artery bypass surgery, and informed consent was obtained for use of these specimens. Use of the discarded tissue was approved by the Medical Ethics Committee of Capital Medical University, Beijing, and complied with the principles outlined in the Declaration of Helsinki. Death of SMCs used for stimulating macrophages in vitro was induced by treating with 500 µM H2O2 for 8 h.26
2.5. Three-dimensional culture of SMCs
For three-dimensional (3D) peptide culture of SMCs, polypeptides K2 (QL) 6K2 were synthesized, HPLC-purified, and dissolved in acidic solution, which could self-assembly into nanofibers in neutralized pH media.27 The polypeptide was dissolved in 0.1 M acetic acid, then mixed with 1 × 106 SMCs, adding NaH2PO4, NaOH, and media to initiate the self-assembly into 3D peptide gel. For 3D collagen culture, 1 × 106 vein SMCs were mixed with type I collagen (1.5 mg/mL) and incubated at 37°C with 5% CO2 for gelation.
2.6. Cyclic stretch
SMCs in 3D peptide or collagen gel or macrophages were cultured on silicone, elastomer-bottomed, collagen-coated plates (Flexcell, Hillsborough, NC, USA), then underwent cyclic mechanical stretch with use of the computer-controlled Mechanical Strain Unit (Flexcell 4000) at uniform deformation of 15% elongation. For treatment with necrostatin-1 (Nec-1; Sigma-Aldrich, St. Louis, MO, USA), SMCs cultured in 3D peptide gel were administered with 30 µM Nec-1 or the same volume of vehicle 1 h before mechanical stretch performed.
2.7. Assessment of death
Death of SMCs in vein grafts was analysed with the DeadEnd™ Fluorometric TUNEL System (Promega, Madison, WI, USA) according to the manufacturer's instructions. The death of SMCs in response to mechanical stretch was measured by annexin V and PI staining as described in Supplementary material online.28
2.8. CARD9 knockdown
Small interfering RNA (siRNA) was used for knockdown of CARD9 in THP1 cells as described in Supplementary material online.
2.9. Western blot analysis
Western blot analysis was performed as described in Supplementary material online.26 The images were quantified by the use of the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
2.10. Quantitative real-time PCR
Total RNA was isolated from macrophages with Trizol Reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized with AMV reverse transcriptase and random primers. Quantitative real-time PCR (qPCR) was performed with iQ™ SYBR Green PCR Supermix in the DNA Engine Opticon real-time system (Bio-Rad), with α-tubulin as an internal control. The primer sequences are listed in Supplementary material online.
2.11. NF-κB luciferase assay
NF-κB transcriptional activity in macrophages was evaluated by the use of an adenovirus NF-κB-Luc luciferase reporter (Ad.NF-κB-Luc) as described in Supplementary material online.29
2.12. Growth factor measurement
WT and CARD9-KO macrophages were stimulated with necrotic SMCs for 4 h, then the supernatant was discarded and fresh medium was added to the macrophages and cultured for an additional 16 h. The levels of nine growth factors in the medium were measured by the use of the Bio-Plex™ 200 System (Bio-Rad, USA) according to the manufacturer's protocol.27
2.13. SMC migration assay
SMC migration was determined in Boyden chambers (Cell Biolabs, USA) as described in Supplementary material online.
2.14. SMC proliferation assay
Proliferation of SMCs was detected by BrdU incorporation as described in Supplementary material online.
2.15. Statistics
Data are presented as mean ± SEM. Differences between groups were determined by non-parametric tests. The Mann–Whitney test was used to compare two groups, and Dunn's test was used after Kruskal–Wallis testing to compare multiple groups; a value of P < 0.05 was considered statistically significant.
3. Results
3.1. Necrosis of SMCs was induced in vein grafts
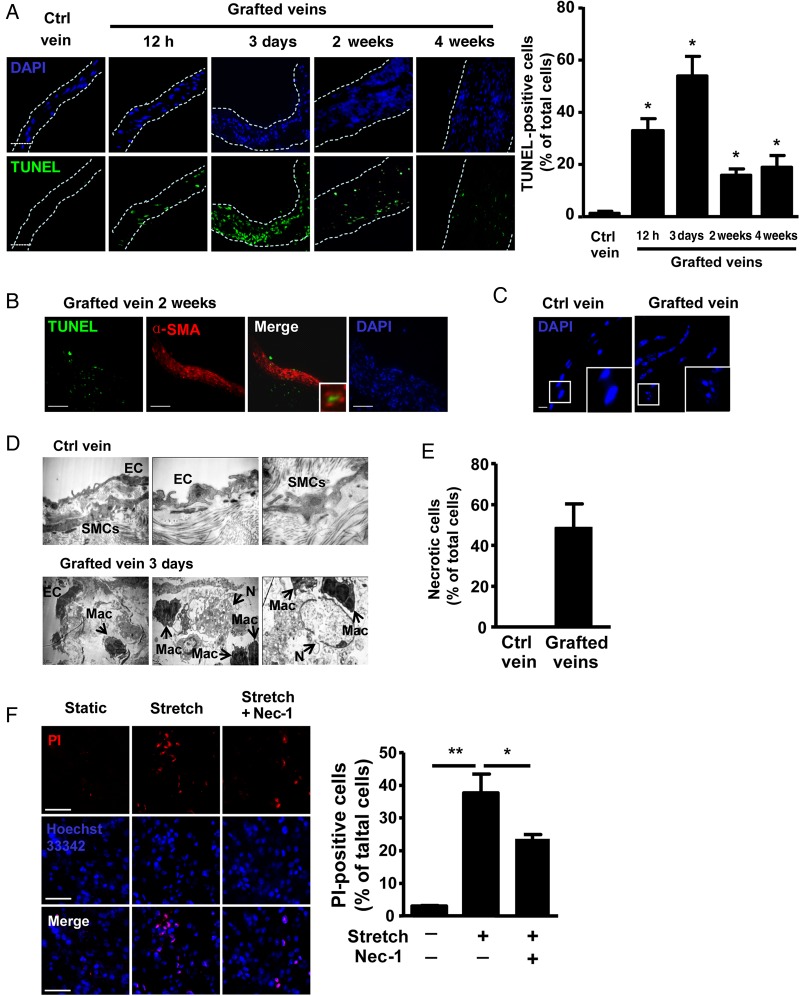
Vein grafts were performed by grafting the inferior vena cava from donor mice to the right common carotid artery of recipient mice.21,22 We first examined SMC death in vein grafts by TUNEL staining, which was reported to detect both apoptosis and necrosis.30 As early as 12 h after grafting, robust death was induced (Figure 1A). Cell death peaked at Day 3 and decreased at Week 2 in grafted veins. Figure 1B shows that the TUNEL-positive cells were α-SMA positive, suggesting that the death of SMCs was induced in grafted veins.
Figure 1.
Necrosis of SMCs in vein grafts of mice. (A) TUNEL staining and quantification of control (Ctrl) veins and vein grafts at the indicated times. Nuclei were stained with DAPI. Dotted lines indicate the cross-section of vein grafts; n = 6 per group. Scale bar: 50 µm. (B) IF staining of α-SMA (TRITC) in vein grafts at 2 weeks; n = 6 per group. Scale bar: 50 µm. (C) Frozen sections from control veins and vein grafts at 12 h were stained with DAPI. Scale bar: 5 µm; n = 6 per group. (D) EM of control vein and vein grafts at 3 days; n = 6 per group. (E) Qualification of necrotic SMCs in grafted veins at 3 days. Data represent the mean ± SEM; n = 6 per group. (F) PI staining of necrosis in SMCs cultured on 3D peptide gel and subjected to mechanical stretch (15% elongation, 12 h), with or without 30 µM Nec-1 pre-treatment and quantification. Data represent the mean ± SEM; five independent experiments were performed. *P < 0.05, **P < 0.01.
To further verify the necrosis of SMCs in vein grafts, we first stained the sections of grafted veins and control veins with DAPI, and observed nuclear fragmentation in 12-h vein grafts (Figure 1C), indicating cell necrosis.31 Then, the sections of grafted veins were viewed by EM. Control veins showed well-lined extracellular matrix, endothelial cells, and SMCs (Figure 1D). Three-day grafted veins showed macrophage recruitment beneath the endothelial cells. Differentiating SMCs from macrophages on electronic microscopy was based on the different cellular structures: the cytoplasm of SMCs is rich in myofilaments, dense plaques, and dense bodies, which form the cytoskeletal system,32 whereas that of macrophages contains a large number of lysosomes, phagosomes, and endocytic vesicles.33 Meanwhile, 3-day vein grafts showed typical necrotic cells (Figure 1D and E), which featured swelling, organelle disruption, and preserved nucleus. Of note, macrophages accumulated around necrotic cells (Figure 1D).
When grafting veins into arteries, high wall pressure induced by arterial flow causes mechanical stretch to SMCs in grafted veins. To mimic the in vivo condition, vein SMCs were cultured on 3D peptide gel27 and subjected to mechanical stretch. Stretch significantly increased the TUNEL staining of SMCs (Figure 1F), which suggests stretch-induced death of SMCs. To determine whether stretch-induced death of SMCs was caspase-dependent or -independent, a pan-caspase inhibitor, Z-VAD-fmk, was used. Z-VAD-fmk did not significantly affect stretch-induced TUNEL staining of SMCs (see Supplementary material online, Figure S2A), indicating that stretch-induced death of SMCs was caspase-independent. The caspase-independent cell death could result from either inefficient clearance of apoptotic SMCs or primary necrotic SMCs. To characterize the type of stretch-induced cell death, annexin V and propidium iodide (PI) staining were used. Phosphatidyl serine (PS) is translocated from the cytoplasmic surface to the outer surface of cell membrane in apoptotic cells. Annexin V, a Ca2+-dependent phospholipid-binding protein, has a high affinity for PS, so annexin V is commonly used for identifying apoptotic cells. In addition, PI, the red fluorescent nucleic acid-binding dye, is impermeable to live cells and apoptotic cells, but stains dead cells.28 We found that PI-positive cells were increased in number, but annexin V staining was not changed (see Supplementary material online, Figure S2B), so necrosis rather than apoptosis of SMCs was involved in stretch-induced death of SMCs. Necroptosis is a process of regulated caspase-independent cell death that results in morphological features resembling necrosis and activation of autophagy.34,35 To address the extent of necroptosis in the necrotic cell population, we used a specific small molecule inhibitor, necroptosis inhibitor Nec-1,36 which decreased stretch-induced cell death by 35% in SMCs (Figure 1F). Our results suggested that robust death of SMCs was induced in early vein grafts.
3.2. Pro-inflammatory cytokine expression in macrophages was induced in vein grafts and in vitro
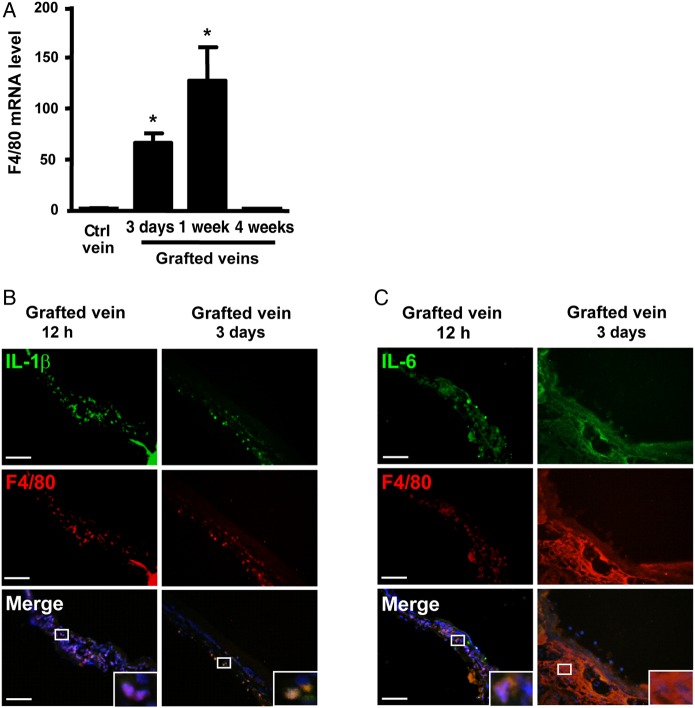
To demonstrate inflammation in the grafted veins, we examined gene expression of the pro-inflammatory cytokines IL-1β, IL-6, and chemokine MCP-1 in grafted veins at different times. The expression of IL-1β, IL-6, and MCP-1 was strongly induced at Day 3, continued at Day 7, and decreased at Week 4 (see Supplementary material online, Figure S3A); this induction was accompanied by the increased presence of the macrophage marker, F4/80 (Figure 2A).
Figure 2.
Inflammation was induced in macrophages of vein grafts. (A) qPCR of the expression of F4/80 in control (Ctrl) veins and vein grafts at 3 days, 1 week and 4 weeks. Data represent the mean ± SEM fold induction compared with the control after normalization to α-tubulin; n = 6 per group. The localization of F4/80 and (B) IL-1β or (C) IL-6 in vein grafts was examined with specific antibodies and TRITC-labelled or FITC-labelled secondary antibodies. Nuclei were stained with DAPI. Data represent the mean ± SEM; n = 6 per group. Scale bar: 50 µm. *P < 0.05.
To further verify the cellular type for expression of inflammatory cytokines, we performed immunofluorescent staining. Both IL-1β and IL-6 were abundantly expressed in grafted veins at 12 h and Day 3, and co-localized with infiltrated F4/80+ macrophages (Figure 2B and C). Our results implied that macrophages were the major source of pro-inflammatory cytokines in the grafted veins.
We next determined whether necrotic debris induces inflammation in macrophages. We used H2O2-induced necrotic SMCs to stimulate mouse bone marrow-derived macrophages (BMDMs). Necrotic SMCs induced the mRNA expression of IL-1β, IL-6, and MCP-1 (see Supplementary material online, Figure S3D). Furthermore, SMCs cultured in 3D collagen gel underwent mechanical stretch with 15% elongation, then were released by incubating with type I collagenase to stimulate macrophages. The expression of pro-inflammatory cytokines was also increased under stretch (see Supplementary material online, Figure S3D).
3.3. CARD9 was most highly expressed in macrophages of vein grafts among CARD family genes
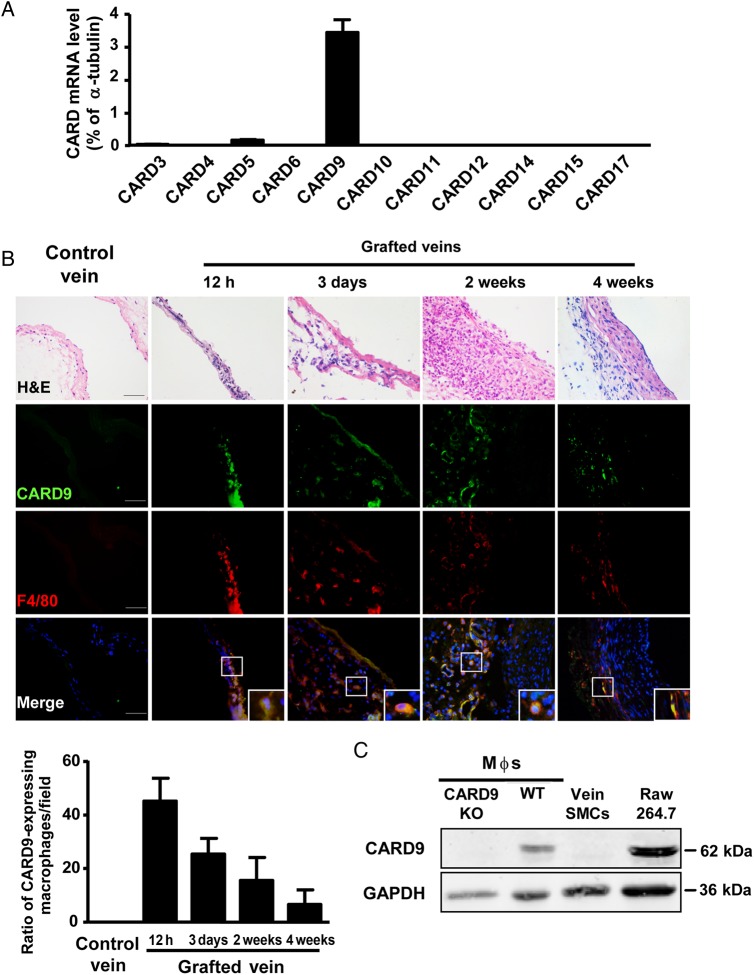
Necrotic cells can provoke an immune response, and CARD family members are crucial for initiation of inflammation in innate immunity. To explore the mediator of pro-inflammatory cytokines induced by necrotic SMCs in grafted veins, we first examined the gene expression of CARD family members in grafted veins. The mRNA level of CARD9 was predominantly expressed in vein grafts at Day 3 (Figure 3A), suggesting that CARD9 may participate in vein graft remodelling.
Figure 3.
Expression of CARD9 in macrophages from vein grafts. (A) qPCR analysis of the expression of CARD family members in 3-day vein grafts expressed as the percentage of α-tubulin; n = 6 per group. (B) Co-localization of CARD9 and F4/80 in H&E-stained vein grafts at 12 h, 3 days, 2 weeks, and 4 weeks and quantification. Nuclei were stained with DAPI. The bar graph was expressed as CARD9-expressing macrophages per field. Data represent the mean ± SEM; n = 6 per group. (C) Western blot analysis of CARD9 and GAPDH as a loading control in BMDMs from WT mice, CARD9-KO mice, vein SMCs, and macrophage cell line RAW 264.7 cells. Three independent experiments were performed. Scale bar: 50 µm.
To examine the identity of CARD9+ cells in grafted veins, we performed immunofluorescent staining in vein grafts. CARD9 was expressed in macrophages in vein grafts at 12 h, 3 days, and 2 and 4 weeks (Figure 3B). CARD9 is also expressed in dendritic cells,19 so we detected dendritic cell infiltration in vein grafts by staining of CD11c, which was commonly used as a dendritic cells marker. Few CD11c+ cells were observed in vein grafts, but robust staining of F4/80 was present in vein grafts (see Supplementary material online, Figure S4).
To confirm the specific expression of CARD9 in macrophages in vein grafts, we examined the protein expression of CARD9 in cultured macrophages and SMCs. CARD9 was highly expressed in macrophages, including BMDMs and the RAW264.7 cell line, and was difficult to detect in SMCs (Figure 3C). Thus, our results indicated that CARD9 expression in vein grafts was mainly in macrophages.
3.4. CARD9 mediated necrotic SMC-induced inflammation in macrophages
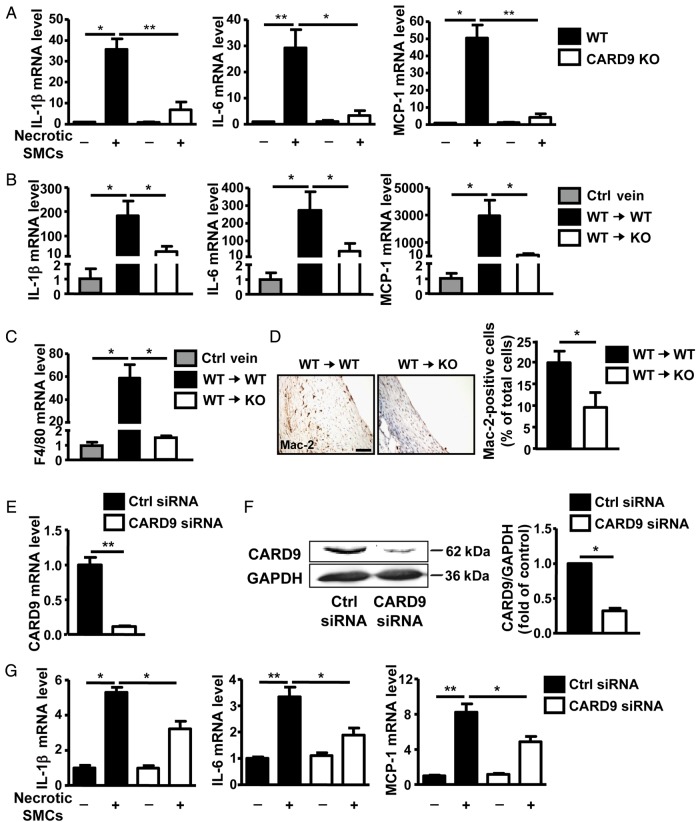
To explore the role of CARD9 in mediating pro-inflammatory cytokine expression in macrophages, WT or CARD9-KO macrophages were stimulated with H2O2-induced necrotic SMCs. Necrotic SMCs showed significantly increased expression of IL-1β, IL-6, and MCP-1, which was inhibited in CARD9-KO macrophages (Figure 4A). Similar results were present in grafted veins. At 3 days, the mRNA expression of IL-1β, IL-6, and MCP-1 was higher in WT vein grafts than in control veins, whereas the levels of the cytokines were attenuated markedly in WT veins grafted into CARD9-KO mouse arteries (Figure 4B). Furthermore, CARD9-KO vein grafts also showed reduced mRNA expression of the macrophage marker F4/80 (Figure 4C), which was further confirmed by immunohistochemistry staining of another macrophage marker, Mac-2, in grafted veins (Figure 4D).
Figure 4.
CARD9-KO attenuated the level of the necrotic SMC-induced pro-inflammatory cytokines in vitro and in vivo. (A) BMDMs derived from WT or CARD9-KO mice were stimulated with necrotic SMCs (H2O2 treated for 8 h) for 20 h. qPCR analysis of the expression of IL-1β, IL-6, and MCP-1 expressed as fold induction compared with the control (Ctrl) after normalization to α-tubulin. Data represent the mean ± SEM; five independent experiments were performed. The mRNA expression of (B) IL-1β, IL-6, MCP-1, and (C) F4/80 in control veins and vein grafts at 3 days presented as fold induction compared with the control after normalization to α-tubulin; n = 6 per group. (D) Expression of Mac2 in sections of vein grafts at 4 weeks from WT and CARD9-KO mice and quantification. Data represent the mean ± SEM; n = 6 per group. THP1 cells were transfected with CARD9 or control siRNA for 36 h; (E) mRNA and (F) protein expression of CARD9 show as fold change compared with the control siRNA group after normalization to α-tubulin and GAPDH, respectively. (G) THP1 cells with or without CARD9 knockdown were stimulated with necrotic HSVSMCs (H2O2 treated for 8 h). qPCR analysis of the expression of IL-1β, IL-6, and MCP-1 expressed as fold induction compared with the control after normalization to α-tubulin. Data represent the mean ± SEM; five independent experiments were performed for (E) and (G), and three independent experiments were performed for (F). Scale bar: 50 µm. *P < 0.05, **P < 0.01.
To investigate whether a similar mechanism operated in human cells, we isolated human saphenous vein SMCs (HSVSMCs) and knocked down CARD9 expression in THP1 cells, the human monocyte lineage, by siRNA. Then, H2O2-induced necrotic HSVSMCs were used to treat THP1 cells, and the mRNA expression of pro-inflammatory cytokines was determined. Consistent with the observation in mouse cells, siRNA knockdown of CARD9 inhibited necrotic SMC-stimulated cytokine expression (Figure 4E–G). The results suggested that CARD9 mediated the necrotic SMC-induced pro-inflammatory cytokine expression and macrophage recruitment in vivo and in vitro.
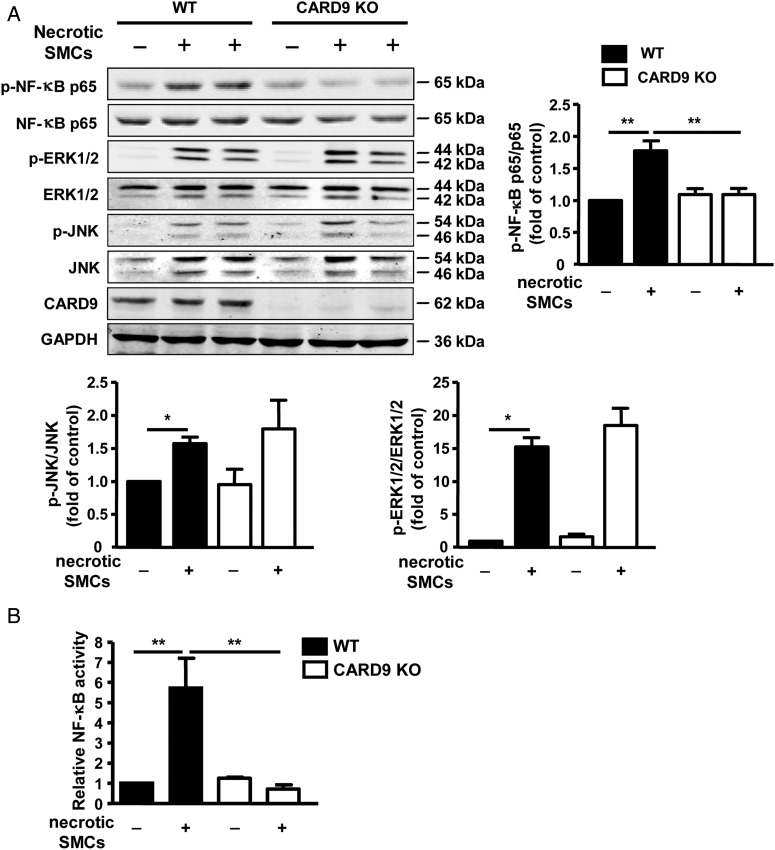
3.5. CARD9 mediated necrotic SMC-stimulated activation of NF-κB in macrophages
Immune cells, including macrophages, can recognize DAMPs released from necrotic cells with the PRRs on the membrane and activate downstream mitogen-activated protein kinases (MAPKs) and NF-κB.37 Either NF-κB38 or MAPK20 signalling pathways would be downstream of CARD9 depending on the conditions. To further determine the mechanism of CARD9 mediating the pro-inflammatory cytokine expression induced by necrotic SMCs, we examined the activation of MAPKs and NF-κB. WT and CARD9-KO macrophages were stimulated with H2O2-induced necrotic SMCs for 15 min. CARD9-KO macrophages showed inhibited necrotic SMC-induced phosphorylation of NF-κB p65 subunit in macrophages under mechanical stretch (Figure 5A). Meanwhile, the phosphorylation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) was not changed in CARD9-KO macrophages. Luciferase assay was further used to examine the transcriptional activity of NF-κB in CARD9-KO macrophages. WT and CARD9-KO macrophages were infected with ad-NF-κB reporter luciferase for 24 h, then co-cultured with H2O2 -induced necrotic SMCs in collagen gel for further 48 h. Luciferase activity of NF-κB in CARD9-deficient macrophages was significantly decreased (Figure 5B).
Figure 5.
Necrotic SMC-induced activation of NF-κB in macrophages depended on CARD9. (A) BMDMs from WT and CARD9-KO mice were stimulated with necrotic SMCs (H2O2 treated for 8 h) for 15 min. Western blot analysis of protein levels of phosphorylated NF-κB p65, ERK 1/2, and JNK, and quantification presented as fold induction compared with the control of each independent experiment after normalization to their total forms of protein. Data represent the mean ± SEM; three independent experiments were performed. (B) BMDMs from WT and CARD9-KO mice were infected with ad-NF-κB-reporter luciferase for 24 h, and co-cultured with necrotic SMCs on collagen gel for 48 h. Then, the collagen gel was digested, lysed, and subjected to the measurement of luciferase activity, which was expressed as fold induction of the basal level. Data represent the mean ± SEM; three independent experiments were performed. **P < 0.01.
3.6. CARD9-KO inhibited the neointima formation of vein grafts by suppressing the proliferation and migration of SMCs
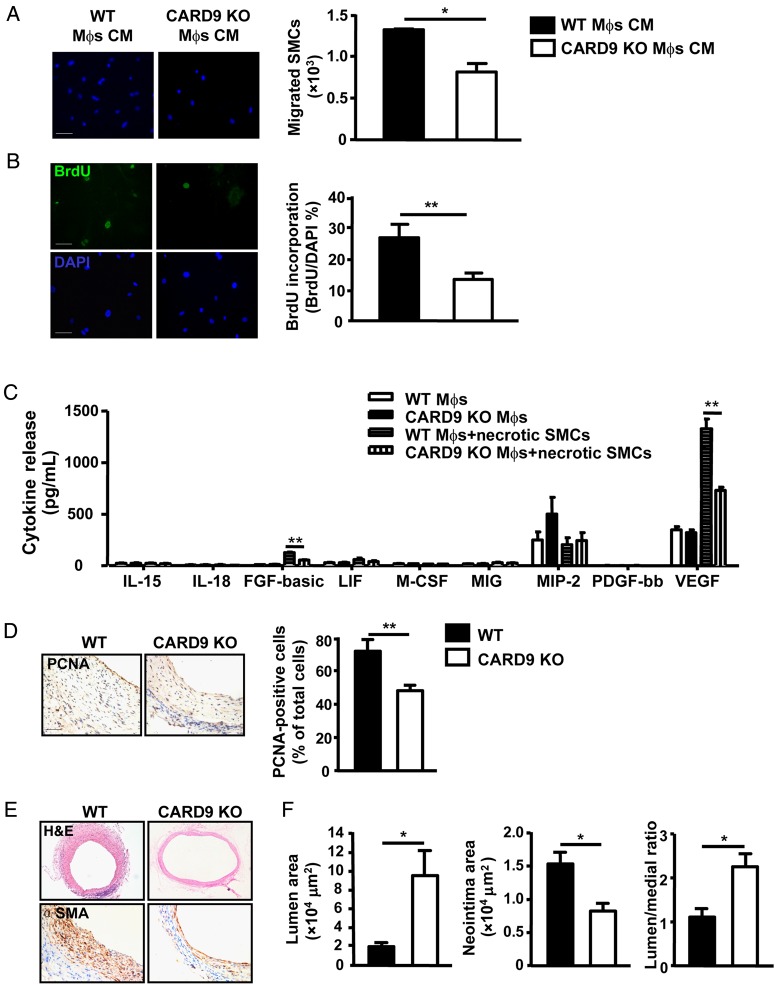
To examine the pathological effect of pro-inflammatory cytokines derived from macrophages in the grafted veins, the conditioned media from WT and CARD9-KO macrophages stimulated with necrotic SMCs were used to stimulate the migration and proliferation of SMCs. Transwell migration was lower with conditioned medium from CARD9-KO macrophages than WT macrophages (Figure 6A), as was proliferation of SMCs which was detected by BrdU incorporation (Figure 6B).
Figure 6.
CARD9-KO inhibited the neointima formation of vein grafts. (A) SMC migration was assessed with 8 µm pore size transwell filters. Representative images are SMC migration response to medium derived from WT and CARD9-KO BMDMs stimulated with necrotic SMCs for 20 h and quantification. Data represent the mean ± SEM; three independent experiments were performed. (B) SMC proliferation response to the conditional medium was assessed by staining of BrdU. Data represent the mean ± SEM; three independent experiments were performed. (C) Growth factors released into culture supernatants from necrotic SMC-treated WT and CARD9-KO BMDMs were determined. Data represent the mean ± SEM; five independent experiments were performed. (D) Staining of PCNA in vein grafts from WT mice and CARD9-KO mice at 4 weeks; n = 6 per group. (E) Staining of H&E and α-SMA in vein grafts from WT mice and CARD9-KO mice at 4 weeks; n = 6 per group. (F) Quantification of the area of lumen and neointima and the ratio of lumen to neointima area. Data represent the mean ± SEM; n = 6 per group. Scale bar: 50 µm. *P < 0.05, **P < 0.01.
To identify the effect of CARD9 on levels of growth factors secreted by macrophages, we analysed the levels of growth factors in culture supernatant from macrophages stimulated with necrotic SMCs. Basic fibroblast growth factor (bFGF) and VEGF were significantly decreased in supernatant of CARD9-KO macrophages (Figure 6C). Notably, the level of VEGF in supernatant of macrophages was much higher than bFGF and PDGF levels, suggesting that VEGF may predominantly mediate the proliferation and migration of SMCs.
To determine the function of CARD9 in remodelling of vein grafts, inferior venae cavae from WT or CARD9-KO mice were placed into carotid arteries of WT or CARD9-KO mice, respectively. The vein grafts were collected at 4 weeks and examined for morphology. Immunohistochemistry showed that the level of proliferating cell nuclear antigen (PCNA), the proliferation marker of SMCs, was significantly decreased in vein grafts from CARD9-KO mice (Figure 6D). H&E and α-SMA staining showed a thinner vascular wall in vein grafts from CARD9-KO mice than WT mice (Figure 6E). Vein grafts from CARD9-KO mice had a significant larger lumen area, higher lumen/neointima ratio, and smaller neointima than those from WT mice (Figure 6F), indicating that KO of CARD9 inhibited neointima formation in vein grafts.
4. Discussion
In this study, necrosis of SMCs was induced in grafted veins and accompanied by the induced expression of pro-inflammatory cytokines. CARD9 in macrophages mediated the expression of IL-1β, IL-6, and MCP-1 induced by necrotic SMCs, which further increased the recruitment of macrophages. KO of CARD9 significantly attenuated the neointima formation of vein grafts. Our results revealed that CARD9 played an essential role in the remodelling of vein grafts. Our results highlight the role of SMC necrosis in the vein grafts. Previous studies have showed apoptosis of SMCs induced in pig vein grafts;39 however, human vein grafts showed both apoptosis and necrosis.14 We found necrosis of SMCs in vein grafts on EM (Figure 1). The necrotic SMCs in grafted veins could be necrosis post-apoptosis caused by inefficient clearance of apoptotic SMCs or primary necrosis. We found that stretch-stimulated SMCs cultured on 3D gel were annexin V-negative and PI-positive, and the death could not be inhibited by a caspase inhibitor, suggesting that the death of SMCs was not apoptosis. Necroptosis is a regulated caspase-independent cell death mechanism that results in morphological features resembling necrosis and activation of autophagy.35 We found that the necroptosis inhibitor Nec-1 decreased the stretch-induced death of SMCs by nearly 35% (Figure 1), so necroptosis contributed in part to the necrosis of SMCs. Moreover, in contrast to vein SMC culture under 2D conditions, SMCs cultured on 3D peptide gel were more sensitive to mechanical stretch-induced death (see Supplementary material online, Figures S1 and S2), which provides new insights into research of mechanical stretch in SMCs in vitro.
Our data show that necrotic SMCs induced the expression of pro-inflammatory cytokines IL-1β, IL-6, and MCP-1, indicating that necrotic SMCs were strong stimuli to induce an inflammatory response in grafted veins. CARD9 was required for the innate immune responses to pathogen-associated molecular patterns (PAMPs) on microbial infection.19,20 DAMPs, which could be released by necrotic cells, are endogenous equivalents of PAMPs.40 Previous studies showed that IL-1α was a key danger signal released on primary necrosis of vascular SMCs and induced IL-6 and MCP-1 production by binding to IL-1 receptor 1.41,42 DAMPs, such as the heat shock protein and high-mobility group box 1, could induce inflammation by activating PRR TLR4,40 and TLR4 was recently reported to be upstream of CARD9.43 Along with these studies, our working hypothesis for the role of CARD9 in necrotic cell-induced inflammation is that DAMPs released from necrotic SMCs could be recognized by some PRRs, which further activate the adaptor CARD9, thereby mediating production of pro-inflammatory cytokines in macrophages.
Previous studies demonstrated that with microbial infection, CARD9 interacting with Bcl10 and MALT1 mediated IKK ubiqutination or activation of MAPKs in macrophages.20,38 We found that CARD9-KO significantly decreased the phosphorylation of NF-κB but not other MAPKs induced by necrotic SMCs in macrophages (Figure 5). Therefore, necrotic SMCs activated CARD9, then mediated pro-inflammatory cytokine production selectively by activating NF-κB signalling.
Our results suggest the importance of CARD9 from macrophages in the remodelling process of vein grafts. Previous studies showed that depletion of macrophages was accompanied with decreased expression of MCP-1 and TGF-β, and repressed the neointima formation of rat vein grafts.9,44 We found that CARD9-KO decreased macrophage infiltration and SMC proliferation, and attenuated the neointima formation of vein grafts (Figures 4 and 6).
Neointima formation of vein grafts is a complex process with de-differentiation and consequent proliferation and migration of SMCs. Multiple studies showed that exposure to pro-inflammatory cytokines stimulated the proliferation and de-differentiation of SMCs;45 specifically, IL-1β could decrease the expressions of SMC differentiation marker genes, accompanied by phenotype change in SMCs from differentiated SMCs to proliferating SMCs by inducing the expression of PDGF.46 Both IL-6 and MCP-1 were shown to stimulate proliferation of SMCs via distinct mechanisms.47,48 Furthermore, we and others showed that targeting IL-1β, IL-18, IL-6, or MCP-1 attenuated neointima formation in animal models.10,26 Thus, elevated levels of pro-inflammatory cytokines, including IL-1β, IL-6, and MCP-1, from CARD9-expressing macrophages could simulate proliferation of SMCs. Furthermore, studies have suggested that multiple growth factors cooperate to regulate the phenotypic switch and growth of SMCs,46 and VEGF was reported to mediate the mitogenic effect of MCP-1 in SMCs.49 Growth factors, such as PDGF and VEGF, are induced by inflammatory cytokine stimulation, which further promote the proliferation or de-differentiation of SMCs.47,49 In the current study, VEGF secretion was lower in necrotic SMC-stimulated CARD9-KO macrophages than WT cells, which could mediate the effect of CARD9 on SMC proliferation and migration.
In conclusion, our study demonstrated that the innate immune protein CARD9 was required for the pro-inflammatory cytokine expression induced by necrotic SMCs by activating NF-κB and further contributed to neointima formation of vein grafts. It is suggested that CARD9 was a potential molecular target for preventing vein graft failure.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the Chinese Ministry of Science and Technology (grant no. 2012CB945104), National Natural Science Foundation of China (grant nos 81300120 and 81230006), Beijing Nova Program (grant no. Z151100000315067), Beijing Natural Science Foundation (grant no. 7142030), and Beijing Collaborative Innovative Research Center for cardiovascular diseases (PXM2014_014226_000002).
Supplementary Material
Acknowledgements
J.C. is a visiting professor from Baylor College of Medicine.
Conflict of interest: none declared.
References
- 1. Bourassa MG. Fate of venous grafts: the past, the present and the future. J Am Coll Cardiol 1991;17:1081–1083. [DOI] [PubMed] [Google Scholar]
- 2. Bassiouny HS, White S, Glagov S, Choi E, Giddens DP, Zarins CK. Anastomotic intimal hyperplasia: mechanical injury or flow induced. J Vasc Surg 1992;15:708–716; discussion 16–17. [DOI] [PubMed] [Google Scholar]
- 3. Muto A, Model L, Ziegler K, Eghbalieh SD, Dardik A. Mechanisms of vein graft adaptation to the arterial circulation: insights into the neointimal algorithm and management strategies. Circ J 2010;74:1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Predel HG, Yang Z, von Segesser L, Turina M, Buhler FR, Luscher TF. Implications of pulsatile stretch on growth of saphenous vein and mammary artery smooth muscle. Lancet 1992;340:878–879. [DOI] [PubMed] [Google Scholar]
- 5. Kent KC, Liu B. Intimal hyperplasia—still here after all these years! Ann Vasc Surg 2004;18:135–137. [DOI] [PubMed] [Google Scholar]
- 6. Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43:742–751; discussion 751. [DOI] [PubMed] [Google Scholar]
- 7. Sterpetti AV, Cucina A, Lepidi S, Randone B, Corvino V, D'Angelo LS, Cavallaro A. Formation of myointimal hyperplasia and cytokine production in experimental vein grafts. Surgery 1998;123:461–469. [PubMed] [Google Scholar]
- 8. Jiang Z, Berceli SA, Pfahnl CL, Wu L, Goldman D, Tao M, Kagayama M, Matsukawa A, Ozaki CK. Wall shear modulation of cytokines in early vein grafts. J Vasc Surg 2004;40:345–350. [DOI] [PubMed] [Google Scholar]
- 9. Wolff RA, Tomas JJ, Hullett DA, Stark VE, van Rooijen N, Hoch JR. Macrophage depletion reduces monocyte chemotactic protein-1 and transforming growth factor-beta1 in healing rat vein grafts. J Vasc Surg 2004;39:878–888. [DOI] [PubMed] [Google Scholar]
- 10. Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol 2006;26:2063–2069. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol 2008;28:284–289. [DOI] [PubMed] [Google Scholar]
- 12. Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell 2010;140:798–804. [DOI] [PubMed] [Google Scholar]
- 13. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 14. Kanellaki-Kyparissi M, Kouzi-Koliakou K, Marinov G, Knyazev V. Histological study of arterial and venous grafts before their use in aortocoronary bypass surgery. Hellenic J Cardiol 2005;46:21–30. [PubMed] [Google Scholar]
- 15. Hruz P, Eckmann L. Caspase recruitment domain-containing sensors and adaptors in intestinal innate immunity. Curr Opin Gastroenterol 2008;24:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005;307:731–734. [DOI] [PubMed] [Google Scholar]
- 17. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 2007;7:31–40. [DOI] [PubMed] [Google Scholar]
- 18. Hara H, Ishihara C, Takeuchi A, Xue L, Morris SW, Penninger JM, Yoshida H, Saito T. Cell type-specific regulation of ITAM-mediated NF-kappaB activation by the adaptors, CARMA1 and CARD9. J Immunol 2008;181:918–930. [DOI] [PubMed] [Google Scholar]
- 19. Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol 2007;8:619–629. [DOI] [PubMed] [Google Scholar]
- 20. Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol 2007;8:198–205. [DOI] [PubMed] [Google Scholar]
- 21. Wu X, Cheng J, Li P, Yang M, Qiu S, Liu P, Du J. Mechano-sensitive transcriptional factor Egr-1 regulates insulin-like growth factor-1 receptor expression and contributes to neointima formation in vein grafts. Arterioscler Thromb Vasc Biol 2010;30:471–476. [DOI] [PubMed] [Google Scholar]
- 22. Shi HT, Wang Y, Jia LX, Qin YW, Liu Y, Li HH, Qi YF, Du J. Cathepsin S contributes to macrophage migration via degradation of elastic fibre integrity to facilitate vein graft neointimal hyperplasia. Cardiovasc Res 2014;101:454–463. [DOI] [PubMed] [Google Scholar]
- 23. Cheng J, Wang Y, Liang A, Jia L, Du J. FSP-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ Res 2012;110:230–240. [DOI] [PubMed] [Google Scholar]
- 24. Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008;2008:pdb prot5080. [DOI] [PubMed] [Google Scholar]
- 25. Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol 2007;27:1744–1751. [DOI] [PubMed] [Google Scholar]
- 26. Li P, Li YL, Li ZY, Wu YN, Zhang CC, X A, Wang CX, Shi HT, Hui MZ, Xie B, Ahmed M, Du J. Cross talk between vascular smooth muscle cells and monocytes through interleukin-1beta/interleukin-18 signaling promotes vein graft thickening. Arterioscler Thromb Vasc Biol 2014;34:2001–2011. [DOI] [PubMed] [Google Scholar]
- 27. Yang M, Zheng J, Miao Y, Wang Y, Cui W, Guo J, Qiu S, Han Y, Jia L, Li H, Cheng J, Du J. Serum-glucocorticoid regulated kinase 1 regulates alternatively activated macrophage polarization contributing to angiotensin II-induced inflammation and cardiac fibrosis. Arterioscler Thromb Vasc Biol 2012;32:1675–1686. [DOI] [PubMed] [Google Scholar]
- 28. Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 2013;3:200–210. [DOI] [PubMed] [Google Scholar]
- 29. Zhang L, Ma Y, Zhang J, Cheng J, Du J. A new cellular signaling mechanism for angiotensin II activation of NF-kappaB: an IkappaB-independent, RSK-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol 2005;25:1148–1153. [DOI] [PubMed] [Google Scholar]
- 30. Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 1995;21:1465–1468. [DOI] [PubMed] [Google Scholar]
- 31. Cummings BS, Schnellmann RG. Measurement of cell death in mammalian cells. Curr Protoc Pharmacol 2004; Chapter 12:Unit 12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Herrera AM, Pare PD, Seow CY. Dense-body aggregates as plastic structures supporting tension in smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2010;299:L631–L638. [DOI] [PubMed] [Google Scholar]
- 33. Ecker J, Liebisch G, Englmaier M, Grandl M, Robenek H, Schmitz G. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc Natl Acad Sci USA 2010;107:7817–7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J Cell Mol Med 2011;15:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol 2008;9:378–390. [DOI] [PubMed] [Google Scholar]
- 36. Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1:112–119. [DOI] [PubMed] [Google Scholar]
- 37. Wu X, Mi Y, Yang H, Hu A, Zhang Q, Shang C. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol Cell Biochem 2013;380:249–257. [DOI] [PubMed] [Google Scholar]
- 38. Bertin J, Guo Y, Wang L, Srinivasula SM, Jacobson MD, Poyet JL, Merriam S, Du MQ, Dyer MJ, Robison KE, DiStefano PS, Alnemri ES. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem 2000;275:41082–41086. [DOI] [PubMed] [Google Scholar]
- 39. Rodriguez E, Lambert EH, Magno MG, Mannion JD. Contractile smooth muscle cell apoptosis early after saphenous vein grafting. Ann Thorac Surg 2000;70:1145–1153. [DOI] [PubMed] [Google Scholar]
- 40. de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm 2013;2013:206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res 2010;106:363–372. [DOI] [PubMed] [Google Scholar]
- 42. Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity 2013;38:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phongsisay V, Iizasa E, Hara H, Yoshida H. Evidence for TLR4 and FcRgamma-CARD9 activation by cholera toxin B subunit and its direct bindings to TREM2 and LMIR5 receptors. Mol Immunol 2015;66:463–471. [DOI] [PubMed] [Google Scholar]
- 44. Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery 1999;126:428–437. [PubMed] [Google Scholar]
- 45. Nilsson J. Cytokines and smooth muscle cells in atherosclerosis. Cardiovasc Res 1993;27:1184–1190. [DOI] [PubMed] [Google Scholar]
- 46. Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics 2012;44:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stamatiou R, Paraskeva E, Gourgoulianis K, Molyvdas PA, Hatziefthimiou A. Cytokines and growth factors promote airway smooth muscle cell proliferation. ISRN Inflamm 2012;2012:731472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kubler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol 2002;22:914–920. [DOI] [PubMed] [Google Scholar]
- 49. Parenti A, Bellik L, Brogelli L, Filippi S, Ledda F. Endogenous VEGF-A is responsible for mitogenic effects of MCP-1 on vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2004;286:H1978–H1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.