Abstract
Background: The efficacy of low- and middle-income countries’ (LMIC) national drug policies in managing antiretroviral (ARV) pharmaceutical prices is not well understood. Though ARV drug prices have been declining in LMIC over the past decade, little research has been done on the role of their national drug policies. This study aims to (i) analyse global ARV prices from 2004 to 2013 and (ii) examine the relationship of national drug policies to ARV prices.
Methods: Analysis of ARV drug prices utilized data from the Global Price Reporting Mechanism from the World Health Organization (WHO). Ten of the most common ARV drugs (first-line and second-line) were selected. National drug policies were also assessed for 12 countries in the South African Development Community (SADC), which self-reported their policies through WHO surveys.
Results: The best predictor of ARV drug price was generic status—the generic versions of 8 out of 10 ARV drugs were priced lower than branded versions. However, other factors such as transaction volume, HIV prevalence, national drug policies and PEPFAR/CHAI involvement were either not associated with ARV drug price or were not consistent predictors of price across different ARV drugs.
Conclusion: In the context of emerging international trade agreements, which aim to strengthen patent protections internationally and potentially delay the sale of generic drugs in LMIC, this study shines a spotlight on the importance of generic drugs in controlling ARV prices. Further research is needed to understand the impact of national drug policies on ARV prices.
Keywords: Drug policy, developing countries, economic evaluation, effectiveness, essential drugs, evidence-based policy, health systems research, HIV, multivariate analysis
Key Messages
The impact of national drug policies in the Southern African Development Community (SADC) on antiretroviral (ARV) medicine prices is not well understood.
The most consistent predictor of lower ARV prices is generic status.
Other factors such as transaction volume, HIV prevalence, national drug policies and PEPFAR/CHAI involvement were either not associated with ARV drug price or were not consistent predictors of price across different ARV drugs.
As new international trade agreements emerge, nations seeking to expand access to life-saving ARV drugs should pay special attention to protecting the availability of generic drugs in low- and middle-income countries.
Introduction
Growing global action to tackle the AIDS epidemic has made life-saving antiretroviral (ARV) treatment a possibility for many who were previously unable to afford and access medicines. Over the past 15 years, ARV prices have dropped 99%—from a cost of USD $10 000 per person per year in 2000 to around USD $100 per person per year in 2014 for first-line ARVs (UNAIDS 2015). In July 2015, the Joint United Nations Program on HIV/AIDS (UNAIDS) reported that 15 million people worldwide were using ARV therapy—beating the ‘15 by 15’ AIDS target of Millennium Development Goal 6 by 9 months (UNAIDS 2015).
A number of international organizations and programs have contributed to the fall in ARV drug prices and their increased availability including: the ‘3 by 5’ and ‘15 by 15’ initiative by the WHO and UNAIDS, the President’s Emergency Plan for AIDS Relief (PEPFAR) and the Clinton Health Access Initiative (CHAI) among many others. These organizations have provided free HIV/AIDS medicines or engaged in price negotiations with pharmaceutical manufacturers to make drugs more affordable and available for LMICs in need of assistance.
Beyond international aid, various economic and political factors have impacted ARV prices. As prices have dropped, the ARV market has grown increasingly competitive. Nakakeeto and Elliott (2013) estimate that recent price declines have corresponded to gross profit margin declines for generic manufacturers.
However, less attention has been focused on the efforts of national governments receiving AIDS assistance and policies they have implemented to increase access to ARV drugs. Recent global economic concerns have challenged LMICs to meet ARV needs with declining international support. Some have recognized a need to begin scaling local ARV production for autonomy in guaranteeing ARV access for their citizens. For LMICs, establishing local ARV production requires a mix of effective government policy, adequate infrastructure, qualified human resources and a distribution logistics system—yet these policies have yet to be implemented or studied in a widespread manner (Pinheiero et al. 2014).
Price negotiations have been one area in which national governments have demonstrated mixed efficacy at controlling ARV prices. Adesina et al. (2013) study the impact of a national commission created in Mexico in 2008 to negotiate ARV price reductions. Though ARV prices dropped significantly after the first round of price negotiations, Mexico continued to pay a sizable multiple of what other upper-middle-income countries paid (Adesina et al. 2013). Another case study involving Andean countries (Bolivia, Chile, Colombia, Ecuador, Peru and Venezuela) demonstrated that negotiated prices were not adhered to due to voluntary agreements, a lack of pooled procurement processes and multi-national coordination of drug regulations (Seoane-Vazquez and Rodriguez-Monquio 2007). These cases suggest that national governments can have modest success negotiating ARV prices with the right policies.
Significant variation in ARV prices exists at the country level, where annual first-line procurement prices varied as much as ten-fold across Latin American countries in 2008 (Wirtz et al. 2012). Price variation has not been as apparent across regional country groups (e.g. comparing prices in Latin America to those in Sub-Saharan Africa) (Wafula et al. 2014). This suggests studying ARV prices may be more reflective of country characteristics and that analysis at this level will yield greater price heterogeneity.
Additionally, previous research has identified HIV prevalence, ARV drug volume, third party negotiators such as CHAI and PEPFAR, access to generics and national socioeconomic status as factors potentially responsible for heterogenous ARV drug prices. Lower drug prices have been associated with generic status, CHAI involvement and low national income but not drug procurement volume (Vasan et al. 2006; Chien 2007; Wirtz et al. 2009; Waning et al. 2009). Past researchers have used the Global Price Reporting Mechanism (GPRM) database to analyse ARV prices and reach these conclusions.
Meanwhile, evaluation of national pharmaceutical policies in LMICs has been scarce. Kaplan et al. (2012) conducted a literature review of policies designed to increase uptake of generic medicines in LMICs and find there has been little policy evaluation of which pro-generic policies have increased generic utilization. Within LMICs, do national drug policies like price controls, national health insurance schemes, essential medicine lists, procurement strategies or capacity for research and development predict ARV drug prices? Understanding this inquiry could help to quantify the impact of LMIC regulations on drug prices and their influence in the procurement process.
This study estimates the relationship between ARV drug prices and national drug policies to expand on past research into factors influencing ARV prices. We focus on countries in the Southern African Development Community (SADC)—an inter-governmental organization of African states—to examine how factors like national drug policies, generic drugs and HIV prevalence relate to ARV drug price at the country level. We hypothesize that policies such as pricing regulation, national health insurance and participation in PEPFAR and CHAI programs to correlate with lower ARV drug prices.
Methods
Data sources
For this study, HIV drug transaction data was obtained from the WHO Global Price Reporting Mechanism (GPRM) database for 12 SADC countries from 2004 to 2013 (World Health Organization 2015a). Using the GPRM database offers two advantages: it covers around 75% of all ARV transactions in LMICs and provides information on prices countries paid in ARV transactions (instead of manufacturers’ quotes) (World Health Organization 2015c). Data on national HIV prevalence (% of population 18–49 living with HIV) was obtained from World Bank World Development Indicators (WDI) for the years 2004–2013 (World Bank 2015).
The GPRM is a publicly available database recording international transactions of HIV, tuberculosis and malaria commodities purchased by national programs in LMIC. The GPRM database sources data from both funders and procurement organizations: including the Global Fund, PEPFAR, CHAI and the United Nations Development Program (UNDP), among others. Duplicate transactions have been identified and removed from the GPRM. SADC countries were selected because the WHO assisted them in completing comprehensive evaluations of each country’s pharmaceutical situation, permitting side-by-side policy comparisons among countries.
Data on national pharmaceutical policies for SADC countries was gathered from WHO Country Data Profiles, collected in 2009 (World Health Organization 2015c). The 12 SADC countries analysed in this study were: Angola, Botswana, Democratic Republic of the Congo, Lesotho, Malawi, Mozambique, Namibia, South Africa, Swaziland, Tanzania, Zambia and Zimbabwe. The three remaining SADC countries—Madagascar, Mauritius and Seychelles—were omitted from the study due to missing data on drug price, HIV prevalence or pharmaceutical situations.
WHO Country Data Profiles for SADC countries collected binary (yes/no) survey responses from country representatives on whether a country had certain pharmaceutical policy elements. For example, one question in the WHO Country Data profile solicited yes/no response on whether a country had price controls. GPRM and SADC country profile data are freely accessible via the WHO websites (World Health Organization 2016).1,2
Using WHO Country Data Profiles, each SADC country was rated for its performance on ten key components of national drug policies, as defined by WHO guidelines (World Health Organization 2003). Ten key components of a national drug policy are: selection of essential medicines, affordability, financing options (split into national health insurance and free HIV/AIDS medicine programs), supply systems, regulation and quality assurance, rational use, research, human resources and monitoring and evaluation. [See Appendix 1 for performance ratings]
Ten of the most commonly used first-line and second-line adult ARV drugs (according to volume of transactions in the GPRM) were selected to analyse price trends. Six first-line ARV drugs included: Efavirenz 600 mg, Lamivudine 150 mg, Lamivudine 150 mg/Zidovudine 300 mg, Nevirapine 200 mg, Stavudine 30 mg and Zidovudine 300 mg. Four second-line ARV drugs included: Abacavir 300 mg, Didanosine 400 mg, Ritonavir 100 mg and Tenofovir 300 mg. We study first-line ARVs separate from second-line ARVs because first-line drugs are most commonly used whereas second-line drugs may reflect higher medical need due to potential resistance or adverse effects with first-line drugs.
This study looked at two overlapping time periods: (1) descriptive drug price trends from 2004 to 2013 and (2) regression model including country pharmaceutical policies from 2009 to 2013 (country pharmaceutical policy profiles were only collected in 2009/10). Regression analysis was limited to 2009–2013 because 2009 was the most recent year in which SADC Country Data profiles on pharmaceutical policies were available and 2013 was the most recent year for which a full year’s data of ARV transactions was accessible. From 2009 to 2013, a total of 8096 transactions were analysed for the 10 ARV drugs in the 12 selected SADC countries (minimum of 270 transactions for Didanosine 400 mg and maximum of 1340 for Efavirenz 600 mg).
Regression model
We used a cross-sectional regression equation because country pharmaceutical policy profiles were collected only once in 2009–2010. The empirical equation estimated was:
ARV = α1 + Xβ 1 + β2POLICY + β3COUNTRY + β4YEAR + β5COUNTRY*YEAR + ε
where the unit of analysis is at the individual transaction level of ARV purchases, according to specific drugs and doses, by national LMIC programs. We did not aggregate transactions for different drugs or doses because the ARV market is highly heterogeneous and doing so would hide significant price variations.
Linear regression models were used to identify significant predictors of ARV drug prices, with the main effects for policies (POLICY) and country dummies (COUNTRY), year dummies (YEAR) and an interaction variable for country and year. Transaction prices were transformed into the logarithm form because they were not normally distributed. Additional independent variables (vector X) normalized ARV drug volume (normalized by dividing total ARV drug units by the number of HIV-positive people in a country), national income level, HIV prevalence, PEPFAR focus/CHAI country status, a drug’s generic status and national pharmaceutical policies (Kaplan et al. 2012; World Health Organization 2015a).
The following five national drug policies were chosen based on their theoretical importance for determining ARV drug price: whether a country had an essential medicines list, sufficient pricing regulations, national/social health insurance, a national procurement strategy and capacity for pharmaceutical research and development. Essential medicines, procurement strategies and domestic production capacity have been shown to influence ARV drug prices (Ripin et al. 2014; Teixeira et al. 2004). Other national drug policy variables, including regulation and quality assurance, rational use, human resources and monitoring and evaluation, were not included in the regression model but may also influence ARV drug price. One national drug policy was excluded from the regression model due to lack of variation among SADC countries: having a free HIV/AIDS medicines program (all 12 SADC countries in this study satisfied this criteria and had free HIV/AIDS medicines programs).
All statistical analyses for this study were performed using STATA software version 13.0.
Results
Six out of 12 SADC countries analysed were PEPFAR focus countries (as of 2008) while nine out of 12 countries were CHAI program countries (as of 2014). These 12 countries demonstrated policy variations in five of six essential components of national drug policies, as defined by the WHO guidelines How to Develop and Implement a National Drug Policy: Essential Medicines List, Price Regulation, National/Social Health Insurance, Procurement Strategy and R&D capacity. Out of the 12 countries, 11 had an essential medicines list, four had price regulation, four had national or social health insurance programs, nine had procurement strategies and three had R&D capacity. No country indicated having all five policy components, though three countries had four of the five policies: Democratic Republic of the Congo, Mozambique and South Africa. All 12 countries indicated they had a free HIV/AIDS medicines program, as a result, this policy component was not included our regression models. See Appendix 1 for pharmaceutical policy evaluation criteria and Appendix 2 for national scores.
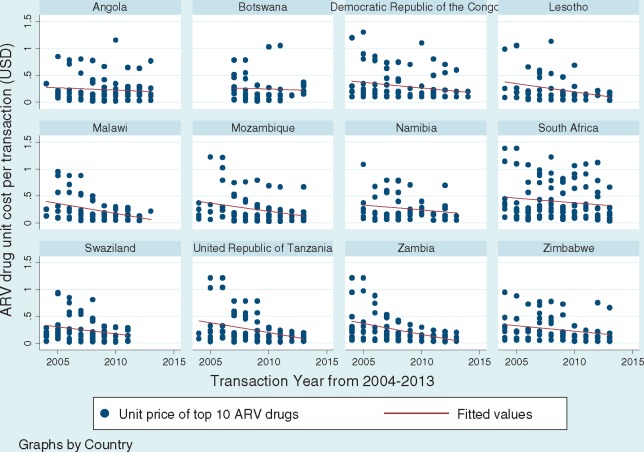
Figure 1 shows that in all SADC countries, the average unit price of the top 10 ARV drugs experienced a decline from 2004 to 2013 (see Figure 1). All six first-line ARV drug unit prices decreased over time, from a 46% price decrease for Lamivudine to 90% price decrease for Efavirenz.
Figure 1.
Price trends of top 10 ARV drugs by Southern African Development Community (SADC) country from 2004-2013. Data source: World Health Organization Global Price Reporting Mechanism 2009–2013. Tweleve Southern African Development Community (SADC) countries included in analysis are Angola, Botswana, Democratic Republic of the Congo, Lesotho, Malawi, Mozambique, Namibia, South Africa, Swaziland, Tanzania, Zambia and Zimbabwe
Tables 1 and 2 show the linear regression outputs of factors associated with first-line and second-line ARV drug prices. The regression results show the strongest predictor of ARV drug price is whether an ARV is generic or brand-name. For 9 out of 11 ARV drugs, the generic ARV was purchased for a lower price than the brand-name ARV. The two exceptions where the generic ARV was more expensive were second-line ARV drugs: Ritonavir and Tenofovir. This may be explained by lower generic competition for select second-line ARVs, which the WHO has cited as a trade policy issue that may keep ARV prices high (World Health Organization 2015c). Médecins sans Frontières reports that Ritonavir prices should soon drop as remaining patent barriers fall and production of generics increases (Médecins sans Frontières 2015).
Table 1.
Factors associated with first-line ARV drug prices from 2009 to 2013
| Antiretroviral drug | EFV 600mg | 3TC 150mg | 3TC 150mg + ZDV 300mg | NVP 200mg | d4T 30mg | ZDV 300mg |
|---|---|---|---|---|---|---|
| Transaction volume | −0.0700 | −0.0798 | −0.000776 | −0.00196 | −0.471 | −0.0346 |
| (−1.03) | (−1.37) | (−0.64) | (−0.70) | (−0.86) | (−1.06) | |
| Lower middle income | 1.760*** | 6.038 | −1.783*** | −5.266*** | 0.648 | 9.398*** |
| (7.76) | (1.78) | (−272.78) | (−12.83) | (1.29) | (49.05) | |
| Upper middle income | 1.601** | 6.553 | −1.734*** | −5.445*** | −1.051* | 10.23*** |
| (3.52) | (1.72) | (−300.18) | (−12.09) | (−2.95) | (43.92) | |
| HIV prevalence | −0.0292* | −0.179 | 0.0653*** | 0.169*** | −0.0761* | −0.230*** |
| (−2.33) | (−1.66) | (116.10) | (14.21) | (−2.46) | (−100.25) | |
| Generica | −0.509*** | −0.519*** | −0.463*** | −1.022*** | −0.688*** | −0.409*** |
| (−139.87) | (−29.85) | (−3551.14) | (−3502.37) | (−18.64) | (−104.78) | |
| Essential medicines lista | 2.289*** | 7.143 | −2.029*** | −5.096*** | 0.160 | 9.455*** |
| (47.50) | (1.86) | (−351.01) | (−12.07) | (0.33) | (987.65) | |
| Price regulationa | 0.811*** | 1.549* | 0.0180*** | −0.256** | −1.674 | 1.647*** |
| (72.62) | (2.55) | (185.87) | (−4.24) | (−1.42) | (271.38) | |
| NHI or SHIa | 1.406* | 4.775 | −1.351*** | −4.041*** | −1.228** | 7.990*** |
| (2.99) | (1.88) | (−1665.37) | (−12.15) | (−4.55) | (36.07) | |
| Procurement strategya | −0.256 | 0.000 | 0.243*** | 0.590*** | 0.000 | −0.183 |
| (−1.99) | (0.00) | (218.65) | (121.88) | (0.00) | (−1.47) | |
| R&D capacitya | −0.324 | −0.0707 | −0.0629*** | −0.0612*** | 0.000 | 0.283** |
| (−1.11) | (−0.41) | (−15.28) | (−5.68) | (0.00) | (3.25) | |
| PEPFAR focus country | 0.0776 | −1.720 | 0.670*** | 1.634*** | −0.0483 | −2.413*** |
| (0.92) | (−2.00) | (22756.95) | (13.59) | (−0.46) | (−50.47) | |
| CHAI program country | −0.756* | −1.417* | 0.534*** | 0.670*** | 0.000 | −2.605*** |
| (−2.62) | (−2.30) | (449.29) | (7.84) | (0.00) | (−16.15) | |
| Year dummies | Yes | Yes | Yes | Yes | Yes | Yes |
| Country dummies | Yes | Yes | Yes | Yes | Yes | Yes |
| Year and Country interaction | Yes | Yes | Yes | Yes | Yes | Yes |
| # of Transactions | 1340 | 1056 | 1235 | 1125 | 548 | 615 |
| R-squared | 0.731 | 0.536 | 0.541 | 0.629 | 0.555 | 0.509 |
Linear regression model with dependent variable = log of ARV drug price in US dollars. Reference category = low income country. Data source: World Health Organization Global Price Reporting Mechanism 2009 to 2013. Tweleve countries included in analysis are Angola, Botswana, Democratic republic of the congo, lesotho, malawi, Mozambique, Namibia, South Africa, Swaziland, Tanzania, Zambia and Zimbabwe.
EFV, Efavirenz; 3TC, Lamivudine, 3TC + ZDV , Lamivudine + Zidovudine; NVP, nevirapine; d4T, Stavudine; ZDV, zidovudine; CHAI, clinton foundation HIV/AIDS initiative; PEPFAR, president’s emergency plan for AIDS relief; NHI or SHI, national health insurance or social health insurance; R&D, research & development.
For definitions of ‘generic’, ‘essential medicines list’, ‘price regulation’, ‘NHI or SHI’, ‘procurement strategy’ or ‘R&D capacity’, see Appendix 1.
= Significant at 5%,
= Significant at 1%,
= Significant at 0.1%.
Table 2.
Factors associated with second-line ARV drug prices from 2009 to 2013
| Antiretroviral drug | ABC 300mg | ddI 400mg | RTV 100mg | TDF 300mg |
|---|---|---|---|---|
| Transaction volume | −0.102 | −0.123 | −0.613*** | −0.521 |
| (−1.79) | (−1.69) | (−31.20) | (−1.03) | |
| Lower middle income | −7.428*** | −3.605*** | −35.57*** | −16.79*** |
| (−19.94) | (−14.70) | (−377.48) | (−6.36) | |
| Upper middle income | −7.501*** | −3.132*** | −47.21*** | −16.08*** |
| (−18.28) | (−10.46) | (−376.37) | (−8.81) | |
| HIV prevalence | 0.221*** | 0.135*** | 5.092*** | 0.710*** |
| (21.89) | (16.49) | (383.66) | (6.59) | |
| Generica | −0.292*** | −0.366** | 0.186*** | 0.286*** |
| (−17.72) | (−3.88) | (1651.69) | (56.96) | |
| Essential Medicines Lista | −8.192*** | −4.284*** | −192.2*** | −21.62*** |
| (−20.53) | (−11.93) | (−380.32) | (−6.65) | |
| Price regulationa | −0.894*** | −0.635*** | −31.72*** | −4.133*** |
| (−13.60) | (−5.69) | (−381.23) | (−6.74) | |
| NHI or SHIa | −5.715*** | −2.611*** | 0.000 | −9.818*** |
| (−18.33) | (−12.09) | (0.00) | (−10.95) | |
| Procurement strategya | 0.781*** | 1.249*** | 43.76*** | 4.825*** |
| (27.80) | (8.32) | (387.71) | (6.88) | |
| R&D capacitya | −0.0620** | −0.182 | 0.000 | 2.163 |
| (−3.16) | (−1.09) | (0.00) | (2.30) | |
| PEPFAR focus country | 2.336*** | 1.200*** | 51.15*** | 4.451*** |
| (19.44) | (9.10) | (376.34) | (6.09) | |
| CHAI program country | 1.605*** | 0.000 | 0.000 | 0.000 |
| (21.40) | (0.00) | (0.00) | (0.00) | |
| Year dummies | Yes | Yes | Yes | Yes |
| Country dummies | Yes | Yes | Yes | Yes |
| Year and Country interaction | Yes | Yes | Yes | Yes |
| # of Transactions | 743 | 270 | 303 | 861 |
| R-squared | 0.656 | 0.433 | 0.611 | 0.824 |
Linear regression model with dependent variable = log of ARV drug price. Reference category = low income country.
ABC, abacavir; ddl, didanosine; ; CHAI , clinton foundation HIV/AIDS initiative; LPV/r, lopinavir/ritonavir; PEPFAR , president’s emergency plan for AIDS relief; R&D , research & development; RTV , ritonavir; NHI or SHI , national health insurance or social health insurance; TDF, tenofovir.
Data source: World Health Organization Global Price Reporting Mechanism 2009 to 2013. 12 countries included in analysis are Angola, Botswana, Democratic republic of the congo, Lesotho, Malawi, Mozambique, Namibia, South Africa, Swaziland, Tanzania, Zambia and Zimbabwe.
For definitions of ‘generic’, ‘essential medicines list’, ‘price regulation’, ‘NHI or SHI’, ‘procurement strategy’ or ‘R&D capacity’, see Appendix 1.
= Significant at 5%,
= Significant at 1%,
= Significant at 0.1%.
While conventional knowledge may suggest larger transaction volumes lower ARV prices, the data did not find a statistically significant relationship between drug volume and price. Only for Ritonavir was higher volume associated with lower price.
Higher national income level was significantly associated with lower prices for all second-line ARV drugs, Lamivudine/Zidovudine and Nevirapine among first-line ARV drugs. However, lower middle income and upper middle income countries paid more than low income countries for Efavirenz and Zidovudine.
Countries with higher HIV prevalence paid more for all second-line ARV drugs, Lamivudine/Zidovudine and Nevirapine among first-line ARV drugs. For Efavirenz, Stavudine and Zidovudine, countries with higher HIV prevalence paid less. (Note: In 2010, the WHO recommended countries to phase out Stavudine from ARV therapy regiments due to well-documented toxicity) (World Health Organization 2013).
With regard to national drug policies, both having an essential medicines list and price regulations was associated with lower prices for all second-line ARV drugs but also higher prices for the first-line drugs Efavirenz and Zidovudine. National health insurance (NHI) schemes were associated with lower prices for Abacavir, Didanosine and Tenofovir among second-line ARV drugs. Among first-line ARV drugs, NHI was not a consistent predictor of drug prices—countries with NHI paid higher prices for Efavirenz and Zidovudine but lower prices for Lamivudine/Zidovudine, Nevirapine and Stavudine. National procurement strategies were linked to higher prices for second-line ARV drugs but were not significant for first-line ARV drugs. Research and development capacity was not a consistent predictor of prices for either first-line or second-line ARV drugs.
National status as a PEPFAR focus country was only associated with lower prices for one ARV drug—Zidovudine—but higher prices for Lamivudine/Zidovudine, Nevirapine and all second-line ARV drugs studied. A country’s involvement with CHAI was linked to lower prices for Efavirenz, Lamivudine and Zidovudine but higher prices for Lamivudine/Zidovudine, Nevirapine and Abacavir.
Overall, country characteristics such as national income, HIV prevalence, PEPFAR/CHAI assistance and national pharmaceutical policies were not consistent predictors of first-line ARV drug price. Across first-line and second-line ARV drugs in 12 SADC countries, a drug’s generic status was found to be the most consistent predictor of lower prices.
Discussion
This study analysed publicly available data on national drug policies and ARV procurement from the WHO and Global Fund to study factors that influence ARV drug price. Our results show that the most consistent predictor of ARV drug price is a drug’s generic status, reinforcing conventional wisdom that generics are more affordable than brand-name drugs. Surprisingly, there was no consistent association between ARV prices and transaction volume, national drug policies or PEPFAR/CHAI involvement. Our results largely agree with previous research, especially on the price influence of generic ARVs, with a few exceptions.
Wirtz et al. (2009) find lower drug prices are associated with whether an ARV drug is generic versus brand-name, lower national income level and whether a country is a CHAI member. They also find that procurement volume, HIV prevalence and association as a PEPFAR focus country are not significantly associated with drug price. Waning et al. (2009) and Chien (2007) similarly conclude generic ARV drugs and CHAI involvement predict lower prices while larger purchase volume does not. Vasan et al. (2006) demonstrate tiered pricing by national income level in ARV procurement, where prices in low income countries are consistent with the lowest differential prices charged by pharmaceutical companies but prices in lower middle-income countries were more varied.
We originally expected lower ARV prices to be linked to higher volume, ARV-related national drug policies and PEPFAR/CHAI involvement. While these relationships held for select ARV drugs, they did not hold true for all. Our study did not find a statistically significant relationship between transaction volume and ARV price, a finding supported by Wirtz et al. (2009), Waning et al. (2009) and Chien (2007). Taken together, these results suggest that individual countries do not receive volume discounts on ARVs at the transaction level. Conventional knowledge that countries receive volume discounts may depend on assumptions of ARV substitutability and buyer power. As this study demonstrated, a relatively few number of ARVs (six first-line and four second-line) comprise the bulk of ARV transactions which may suggest countries have little flexibility in the ARVs they purchase, giving more leverage to sellers. Patent status and limited generic competition for ARVs suggest price inelasticity of demand and may help explain the non-responsiveness of price to volume.
We also did not find CHAI involvement to be significantly associated with lower drug prices, contrary to the conclusions reached by Wirtz et al. (2009), Waning et al. (2009) and Chien (2007). It is not immediately clear why programs like CHAI and PEPFAR do not consistently predict lower ARV prices for the countries studied. There may be ARV-specific effects—Efavirenz, Lamivudine and Zidovudine are all associated with lower prices in CHAI countries—or country-specific effects that require further investigation. One consideration that deserves further study relates to the structure of CHAI and PEPFAR’s purchasing agreements. Both programs establish long-term ARV purchasing contracts with specific manufacturers, which help to negotiate low prices through bulk purchasing at the outset, but may cause price competition to decrease over time (Sharma 2003; U.S. President's Emergency Plan for AIDS Relief (PEPFAR) 2016).
Finally, unlike Vasan et al. (2006), who found low-income countries tended to pay lower ARV prices, our study discovered mixed associations with national income level and price (Vasan et al. 2006). For most ARVs, prices tended to drop as national income level rose. The mixed association between national income level and price may suggest competition between government policy actions, international aid and market forces. At the lowest of income levels, countries may have less leverage to negotiate price with sellers because they may be more reliant on international aid. However, these discrepancies suggest there is more work to do in studying the relationship between country characteristics, national drug policies and ARV price.
As for national drug policies, there is insufficient evidence to evaluate their efficacy in the 12 SADC countries studied. National drug policies such as price regulation, R&D capacity and whether a country had national health insurance did not demonstrate consistent associations with ARV drug price. We recommend additional research be conducted on the efficacy of national drug policies by gathering more detailed knowledge on national drug policy implementation. Analyzing ARV transactions both before and after national policy changes while controlling for exogenous factors will lead to better understanding of national policy impacts. Given the influential role generic status appears to play in ARV drug prices, subsequent studies should shed light on how changes to intellectual property laws in developing countries may affect the availability of ARV drugs.
When interpreting our results, it is important to remember that they represent correlation, not causation. One limitation to this study is limited knowledge on the implementation and quality of national drug policies. This study relied upon WHO surveys in which SADC countries self-reported the presence or absence of pharmaceutical policies and structures (such as price regulations) through yes/no questions. The yes/no structure of these WHO survey questions did not capture important details on when policies were implemented, how strongly they were enforced in country and how far-reaching policies were. Consequently, even ‘yes’ survey responses are likely disguising significant variation in national drug policies among countries. More detailed information on what each ‘yes’ or ‘no’ survey response to national drug policies should be goal of future survey research. Nevertheless, the current WHO surveys used in this study are invaluable in quantitatively comparing otherwise qualitative national drug policies. Other data sources do not allow for aggregate study of myriad national drug policies.
Furthermore, the timeframe of regression analysis relies on incomplete information. National drug policy information was only available as a cross-sectional data point in 2009 or 2010 (depending on the country). Though our regression analysis is limited to ARV price data after this point (from 2009–2013) to study the potential impact of these national drug policies, we do not have knowledge of new policies that may have arisen after 2009–2010.
In our analysis, we restrict study of national drug policy variables and do not consider policies including regulation and quality assurance, rational use of medicines, human resources and monitoring and evaluation. However, these policies may have potentially significant impacts on ARV price and are an opportunity for subsequent analysis. We also do not consider price per defined daily dose (DDD), the assumed average maintenance dose per day for a drug used for its main indication in adults. Integrating price per DDD would be appropriate for future analyses on the relationship between ARV price and transaction volume.
Another limitation is the restricted sample size of countries studied, including only 12 countries from the South African Development Community (due to their completed WHO surveys on their respective national drug policies). Developing alternate methods to quantify national drug policies could help both to improve knowledge of national drug policy implementation and expand sample size to include countries beyond the SADC. Some of the negotiations for better access to generic drugs could be implemented at the regional rather than at the country level. Further research is needed to understand the impact of national drug policies on ARV prices.
Conclusion
In the context of emerging international trade agreements, which aim to strengthen patent protections and potentially delay the release of generic drugs in low- and middle-income countries, this study shines a spotlight on the importance of generic drugs in controlling antiretroviral prices. We find that an ARV drug’s generic status is the best predictor of lower price, a crucial measure when expanding ARV accessibility worldwide. Accordingly, Médecins sans Frontières writes of ‘severe consequences’ following ‘harsh new precedents for intellectual property provisions’ (Médecins sans Frontières 2015). As nations seek to expand access to life-saving ARV drugs worldwide, special attention should go towards protecting the availability of generic drugs in low- and middle-income countries.
Conflict of interest statement. None declared.
Appendix 1. Methodology for assessment of national drug policies
The World Health Organization (WHO) assisted Southern African Development Community (SADC) countries in completing country profile questionnaires as part of conducting a comprehensive evaluation of their country’s pharmaceutical situation. SADC member states provided self-reports on their national drug policies in the document Country Data Profile on the Pharmaceutical Situation in the Southern African Development Community (SADC).10
Questionnaires were developed by the WHO, filled in with data from WHO libraries and global sources (e.g. WHO Statistical System), then sent to all 15 SADC countries for senior public officials to review and—if necessary—fill in missing data. The data collected in this process have been used to develop a pharmaceutical profile for each of the SADC member states and to develop a report on the pharmaceutical situation in the Southern African Community.
The 12 SADC countries analyzed in this study were: Angola, Botswana, Democratic Republic of the Congo, Lesotho, Malawi, Mozambique, Namibia, South Africa, Swaziland, Tanzania, Zambia and Zimbabwe. The three remaining SADC countries—Madagascar, Mauritius and Seychelles—were omitted from the study due to missing data on drug price, HIV prevalence or pharmaceutical situations.
For our paper, each country was assessed on the following six components of national drug policies, as highlighted by the WHO guidelines How to Develop and Implement a National Drug Policy, based on survey responses in their Country Data Profile(World Health Organization 2003). All tables and data listed below refer to a country’s Country Data Profile document:
1. Essential Medicines list: A country was determined to have this component if that country had a national Essential Medicines List (EML), indicated in Table 7.1 ‘National Structure’ of the Country Data Profile document.
2. Price regulation: A country was determined to have this component if that country had at least 3 out of 7 measures of ‘Pricing Regulation’, indicated by Table 4.4 ‘Pricing Regulation’ of the Country Data Profile document.
3. NHI or SHI: A country was determined to have this component if that country had National Health Insurance (NHI) or Social Health Insurance (SHI) providing at least partial medicines coverage indicated by Table 4.2 ‘Health Insurance and Free Care’ of the Country Data Profile document.
4. Free HIV/AIDS medicines program: A country was determined to have this component if that country had public programs providing free HIV/AIDS medicines to three of five of its citizen groups (patients who cannot afford medicine, children under 5, older children, pregnant women and elderly persons) indicated by Table 4.2 ‘Health Insurance and Free Care’ of the Country Data Profile document.
5. Procurement strategy: A country was determined to have this component if that country had a written public sector procurement strategy, indicated by Table 6.1 ‘Procurement’ of the Country Data Profile document.
6. R&D capacity: A country was determined to have this component if that country had the capacity for research and development (R&D) to discover new active substances indicated by Table 3.5 ‘Manufacturing’ of the Country Data Profile document.
Five of these policy components—essential medicines list, price regulation, NHI or SHI, procurement strategy and R&D capacity—were included in our paper’s regression models. However, whether a country had free HIV/AIDS medicines program was not included in the regression model because there was no variation in this policy among SADC countries (e.g. all 12 SADC countries in the regression model had free programs for HIV/AIDS medicine.
Appendix 2. Assessment of National Drug Policies for SADC countries
| Components of National Drug Policya | Angola | Botswana | Dem. Rep. of the Congo | Lesotho | Malawi | Mozam- bique | Namibia | South Africa | Swaziland | Tanzania | Zambia | Zimbabwe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential medicines list | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Price regulation | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| NHI or SHI | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Free HIV/AIDS medicines programb | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Procurement Strategy | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| R&D capacity | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| Year of survey | 2009 | 2009 | 2009 | 2009 | 2009 | 2010 | 2009 | 2009 | 2009 | 2009 | 2009 | 2009 |
| PEPFAR focus country? (as of 2008) | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| CHAI Program Country? (as of 2014) | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
Appendix 2 shows our assessment of countries’ national drug policies based on responses given in their Country Data Profile and our methodology detailed in Appendix 1. For our paper, each country was assessed on six components of national drug policies, as highlighted by the WHO guidelines How to Develop and Implement a National Drug Policy, based on survey responses in their Country Data Profile.11 Five of these policy components—essential medicines list, price regulation, NHI or SHI, procurement strategy and R&D capacity—were included in our paper’s regression models (regression results shown in Tables 1 and 2) based on their theoretical importance to ARV price. However, whether a country had a free HIV/AIDS medicines program was not included in our regression model because there was no variation in this policy among SADC countries (e.g. all 12 SADC countries in the regression model had free programs for HIV/AIDS medicines).
Key: 1 = policy component met, 0 = policy component not met.
Not included in regression models.
Notes
References
- Adesina A, Wirtz VJ, Dratler S. 2013. Reforming antiretroviral price negotiations and public procurement: the Mexican experience. Health Policy and Planning 28: 1–10. [DOI] [PubMed] [Google Scholar]
- Chien C. 2007. HIV/AIDS Drugs for Sub-Saharan Africa: How do Brand and Generic Supply Compare? PLoS ONE 2: e278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan WA, Ritz LS, Vitello M. et al. 2012. Policies to promote use of generic medicines in low and middle income countries: A review of published literature, 2000-2010. Health Policy 106: 211–24. [DOI] [PubMed] [Google Scholar]
- Médecins sans Frontières: Campaign for Access to Essential Medicines. Untangling the web of antiretroviral price reductions. http://www.msfaccess.org/content/untangling-web-antiretroviral-price-reductions-17th-edition-%E2%80%93-july-2014, accessed 30 July 2015.
- Nakakeeto ON, Elliott BV. 2013. Antiretrovirals for low income countries: an analysis of the commercial viability of a highly competitive market. Globalization and Health 9: 6–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiero ES, Bruning K, Macedo MF, Siani AC. 2014. Production of antiretroviral drugs in middle- and low-income countries. Antiviral Therapy 19: 49–55. [DOI] [PubMed] [Google Scholar]
- Ripin DJ, Jamieson D, Meyers A. et al. 2014. Antiretroviral procurement and supply chain management. Antiviral Therapy 19: 79–89. [DOI] [PubMed] [Google Scholar]
- Seoane-Vazquez E, Rodriguez-Monquio R. 2007. Negotiating antiretroviral drug prices: the experience of Andean countries. Health Policy and Planning 22: 63–72. [DOI] [PubMed] [Google Scholar]
- Sharma DC. 2003. ARV prices nosedive after Clinton brokering. The Lancet 362: 1467.. [DOI] [PubMed] [Google Scholar]
- Teixeira PR, Vitoria MA, Barcarolo J. 2004. Antiretroviral treatment in resource-poor settings: the Brazilian experience. AIDS 18: S5–S7. [DOI] [PubMed] [Google Scholar]
- U.S. President's Emergency Plan for AIDS Relief (PEPFAR). PEPFAR Secures Cost Savings and Low Prices on Lifesaving Medications for Developing Countries. https://2006-2009.pepfar.gov/press/83466.htm, accessed 15 July 2016.
- UNAIDS. Press Release – Millennium Development Goal (MDG) 6 Report. http://www.unaids.org/sites/default/files/20150714_PR_MDG_en.pdf. accessed 30 July 2015.
- Vasan A, Hoos D, Mukherjee JS. et al. 2006. The pricing and procurement of antiretroviral drugs: an observational study of data from the Global Fund. Bulletin of the World Health Organization 84: 393–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafula F, Agweyu A, Macintyre K. 2014. Trends in Procurement Costs for HIV Commodities. A 7-Year Retrospective Analysis of Global Fund Data across 125 Countries. Journal of Acquired Immune Deficiency Syndrome 65: 134–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waning B, Kaplan W, King AC. et al. 2009. Global strategies to reduce the price of antiretroviral medicines: evidence from transactional databases. Bulletin of the World Health Organization 87: 520–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz VJ, Santa-Ana-Tellez Y, Trout CH, Kaplan WA. 2012. Allocating scarce financial resources for HIV treatment: benchmarking prices of antiretroviral medicines in Latin America. Health Policy and Planning 27: 638–48. [DOI] [PubMed] [Google Scholar]
- Wirtz V, Forsythe S, Valencia-Mendoza A, Bautista-Arredondo S. 2009. Factors influencing global antiretroviral procurement prices. BMC Public Health 9: S6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. AIDS medicines and diagnostics service (AMDS) - Global Price Reporting Mechanism, 2004-2015 http://apps.who.int/hiv/amds/price/hdd/Export.aspx, accessed 20 January 2015.
- World Health Organization. Technical Report: Access to Antiretroviral Drugs in Low- and Middle-Income Countries http://www.who.int/hiv/pub/amds/access-arv-2014/en/, accessed 30 July 2015.
- World Bank. World DataBank World Development Indicators: Prevalence of HIV, total http://data.worldbank.org/indicator/SH.DYN.AIDS.ZS?page=1, accessed 20 January 2015.
- World Health Organization. Development of Country Profiles and monitoring of the pharmaceutical situation in countries – Assessment of the Pharmaceutical Situation in SADC Member State. (2016) http://www.who.int/medicines/areas/coordination/coordination_assessment/en/index3.html, accessed 19 August 2016.
- World Health Organization. 2003. How to Develop and Implement a National Drug Policy. WHO Policy Perspectives on Medicines 6: 1–6. [Google Scholar]
- World Health Organization. Supplement to Chapter 9 - Phasing Out Stavudine: Progress and Challenges. In: World Health Organization HIV/AIDS Department. 2013 WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization, 2013, 69–74. [Google Scholar]