Abstract
OBJECTIVES
The latest World Health Organization (WHO) histological classification divides thymic epithelial tumours in thymomas and thymic carcinomas (TCs), the latter also including the neuroendocrine thymic tumours (NETTs). NETTs and other TC histotypes have been described to have a significantly lower survival than thymomas, but these two groups of tumours have rarely been compared directly. Using the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group datasets, we wanted to study this issue.
METHODS
This is a retrospective multicentre cohort study of patients operated for TC. Outcome measures were overall survival (OS) and recurrence-free survival (RFS). OS was analysed using the Kaplan–Meier method and RFS was assessed using competing risk analysis. The association with clinical and prognostic factors for OS and RFS was evaluated with log-rank test and Gray's test, respectively.
RESULTS
A total of 1247 tumours (1042 TCs) were collected between 1984 and 2012. A R0 resection was performed in 363 TCs and in 52 NETTs. The median follow-up was 4.4 years for TCs and 4.1 years for NETTs. Owing to the missing values for survival information, a total of 728 TC patients and 132 NETTs were included in the OS analysis. Among them, 262 TC and 39 NETT patients died. The median OS was 6.6 years for TC and 7.5 years for NETTs. The overall 5-year survival rates were 60% for TC and 68% for NETTs; 10-year survival rates were 40% for TCs and 39% for NETTs (P = 0.19). Five-year RFS was 0.35 and 0.34 for TCs and NETTs (P = 0.36). On multivariate analysis, histology did not influence either OS (P = 0.79) or RFS (P = 0.59).
CONCLUSIONS
This represents the largest clinical series of TCs and NETTs collected. Despite the biological aggressiveness of these rare neoplasms, the 5-year survival rate after resection is over 60% and TCs and NETT showed a similar rate of survival and recurrences after surgery.
Keywords: Thymus, Thymic epithelial tumours, Thymic carcinoma, Thymic neuroendocrine tumour, Surgery, Survival, Histology
INTRODUCTION
Thymic epithelial neoplasms (TENs) are rare tumours although they represent the most common anterior mediastinal lesions in adults. The latest World Health Organization (WHO) histological classification of thymic tumours divides TENs into thymomas and thymic carcinomas (TCs). The latter also include neuroendocrine thymic tumours (NETTs) [1]. Our knowledge of TCs and NETTs behaviour, prognostic factors and outcome is still limited to small retrospective series. It has been reported that TCs and NETTs portend a significantly lower survival compared with thymomas [2, 3], with higher incidence of local recurrence and/or distant metastases [4, 5].
Clinical differences and outcome comparison between these two groups of thymic neoplasms have been rarely investigated, due to their rarity and the lack of multicentre studies. The European Society of Thoracic Surgeons (ESTS) and the International Thymic Malignancy Interest Group (ITMIG) joined their retrospective datasets on thymic malignancies with the aim to evaluate factors influencing the outcome of TCs and NETTs [6, 7].
Using these retrospective datasets, the aim of this paper was to compare the clinical characteristics and outcome of TCs and NETTs.
MATERIALS AND METHODS
ESTS and ITMIG retrospective databases
The ESTS retrospective database was started in 2011 and data of surgically treated primary thymic tumours were collected between 1990 and 2011. The ITMIG database, with similar purpose, was started in 2012 with 67 participating institutions. A central data handling team and database committee overlooked the process for each dataset. Both datasets have similar data fields and variables, including gender, previous malignancy, tumour histology, lymph node involvement, tumour local recurrence and distant metastases, clinical and pathological Masaoka or Masaoka-Koga staging system, resection status, chemotherapy (CT) and/or radiotherapy (RT) treatment.
Clinicopathological variables
For the purpose of this study, data regarding demographic and clinical characteristics, tumour histology, size and invasion, staging, type of surgical resection, completeness of resection, CT and RT administration were collected, along with the information concerning survival and tumour recurrence.
Moreover, duplicate cases from ESTS centres that were already participating in ITMIG dataset have been removed for the analyses in this study.
Tumour staging system
A dedicated staging system for TCs and NETTs does not exist [8] and the different institutions worldwide used either the Masaoka [9] or the Masaoka-Koga [10] staging system. These systems differ only in how Stages I and II are defined, and it has been reported that there is no statistically significant difference in clinical outcome between those two stages. Therefore, for the purpose of this study, patients with Stages I and II were joined and analysed together, and cases staged with Masaoka and Masaoka-Koga staging systems were combined together.
Standard outcome measures
According to the ITMIG standards [11], we evaluated the following outcome measure:
Overall survival
Overall survival (OS) was calculated as the time interval between the date of surgery (or the last day of non-surgical treatment for patients with unresectable disease) and the date of death or the date of the last follow-up.
Recurrence-free survival
Recurrence-free survival (RFS) was defined as the time interval between the date of surgery and the last day of therapy if no surgery and the date of first recurrence, or the date of the last follow-up without recurrence.
Moreover, surgery was intended radical if a complete tumour resection (R0) was achieved and, contrariwise, in case of micro–macroscopic residuals (R1 and R2), incomplete.
Statistical analysis
All analyses were performed by the ITMIG statistical team (Xiaopan Yao), using SAS version 9.3 (SAS: http://www.sas.com/en_us/legal/editorial-guidelines.html) and R version 3.1.2 (R: http://cran.r-project.org/doc/FAQ/R-FAQ.html#Citing-R). Concerning patients’ clinical characteristics, continuous data are presented as median (range) and categorical ones as frequency with relative percentage. Group comparisons were performed with the use of Fisher's exact test or χ2 tests for categorical variables and Mann–Whitney U-tests for continuous variables, as appropriate.
OS was analysed by the Kaplan–Meier method; the association of OS with clinical variables was tested using the log-rank test.
RFS was assessed using competing risk analysis, with death included as the competing event (curves of death were not shown in cumulative incident plot). The difference on freedom from recurrence between two histology groups (TC versus NETT) was assessed using Gray's test [12]. Multivariate analyses were performed using Cox proportional hazard model for both OS and RFS, with age, sex, pathological stage, resection status, CT, RT, tumour size and histology (TC versus NETT).
RESULTS
This is a retrospective multicentric cohort study of patients treated for TCs and NETTs between 1984 and 2012. Overall, 1247 patients (1042 TCs) were collected using the ESTS and ITMIG retrospective databases.
Patients characteristics
Thymic carcinoma
A total of 1042 TC cases (624 males, 61%) were identified; the median age was 56 years (range: 12–88 years). Para-neoplastic syndromes were observed in 76 [56 myasthenia gravis (MG)] and second tumours in 78 cases, respectively. The median tumour size was 6.2 cm (range: 0–20 cm). Squamous cell carcinoma was the most common histological subtype (79%), followed by lymphoepithelioma-like and basaloid one. Details of surgical resection status were available in 607 cases; a R0 resection was achieved in 363 cases (60%), an R1 in 87 and an R2 in 157 patients, respectively. Data on pathological tumour stage were available in 803 patients and were as follows: Stage I/II 180, Stage III 370, Stage IV 253 cases, respectively.
CT was not administered to most patients with Stage I and II TC; 65% Stage III TCs received CT (neoadjuvant in 19% and adjuvant in 37% of cases, respectively). In Stage IV TCs, 30% of patients received CT, both in the neoadjuvant and adjuvant setting.
RT was offered to more than 70% of TC cases, mostly in the adjuvant setting. Overall, 15% of patients received neither CT nor RT.
On univariate analysis, Masaoka stage was found to be significantly associated with both OS (P < 0.001) and RFS (P < 0.001); no association between tumour histology and OS/RFS was observed.
Thymic neuroendocrine tumours
A total of 205 NETTs (155 males, 77%) were collected; the median age was 55 years (range: 19–83 years). Previous malignancies/second tumours were observed in 17 cases (1 colorectal, 1 lymphoma/leukaemia, 1 breast cancer, 5 prostate cancer, 2 skin cancer and 7 not otherwise specified neoplasms); no data concerning endocrine/para-neoplastic disorders were available in both datasets. The median tumour size was 7.9 cm (range: 2.1–30 cm). Data concerning tumour histology were available in 178 cases: typical carcinoid was observed in 49 (28%), atypical carcinoid in 71 (40%) and poorly differentiated carcinoma (both large-cell neuroendocrine carcinoma or small cell carcinoma) in 49 cases. The histological subtype of carcinoid was not otherwise specified in 9 patients.
Data concerning the resection status were available in 96 cases: a R0 resection was achieved in 52 and an R1/R2 in 44 cases.
Data on pathological tumour stage were available in 146 cases: Stage I/II NETTs were 45, Stage III 56 and Stage IVa 45 cases.
CT and RT were offered to 66 and 85 NETTs, respectively.
At univariate analysis, Masaoka stage (P = 0.025) and R0 resection (P = 0.027) were significant prognostic factors for OS, whereas tumour histology did not significantly influence both OS and RFS.
Comparison between the two groups
Table 1 summarizes the comparison between TCs and NETTs according to the identified clinical variables. On univariate analysis, a male predominance was observed in NETTs (P < 0.001). TCs presented in advanced stage more frequently than NETTs (P < 0.001); resection status did not statistically differ between the two groups of patients. CT and RT were more frequently administered in TCs (P < 0.001 and P = 0.026, respectively).
Table 1:
Comparison between the clinical variables of TCs and NETTs
|
All |
Histology |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Neuroendocrine tumour |
Thymic carcinoma |
||||||
| n | % | n | % | n | % | ||
| Gender | |||||||
| Female | 448 | 37 | 47 | 23 | 401 | 39 | <0.001 |
| Male | 779 | 63 | 155 | 77 | 624 | 61 | |
| Age, median (range) (years) | 56 (12–88) | 55 (19–83) | 56 (12–88) | 0.16 | |||
| Tumour size, median (range) (cm) | 6.5 (0–30) | 8 (2–30) | 6.2 (0–20) | <0.001 | |||
| Previous malignancies | |||||||
| No | 656 | 87 | 105 | 86 | 551 | 88 | 0.64 |
| Yes | 95 | 13 | 17 | 14 | 78 | 12 | |
| Pathological stage | |||||||
| I/II/IIa/IIb | 225 | 24 | 45 | 31 | 180 | 23 | 0.008 |
| III | 426 | 45 | 56 | 38 | 370 | 46 | |
| IVa | 125 | 13 | 11 | 8 | 114 | 14 | |
| IVb | 173 | 18 | 34 | 23 | 139 | 17 | |
| Resection status | |||||||
| R0 | 415 | 59 | 52 | 54 | 363 | 60 | 0.30 |
| R1/2 | 288 | 41 | 44 | 46 | 244 | 40 | |
| Chemotherapy | |||||||
| No | 338 | 38 | 68 | 51 | 270 | 35 | <0.001 |
| Yes | 561 | 62 | 66 | 49 | 495 | 65 | |
| Radiotherapy | |||||||
| No | 259 | 29 | 50 | 37 | 209 | 28 | 0.026 |
| Yes | 633 | 71 | 85 | 63 | 548 | 72 | |
NETT: neuroendocrine thymic tumour; TC: thymic carcinoma.
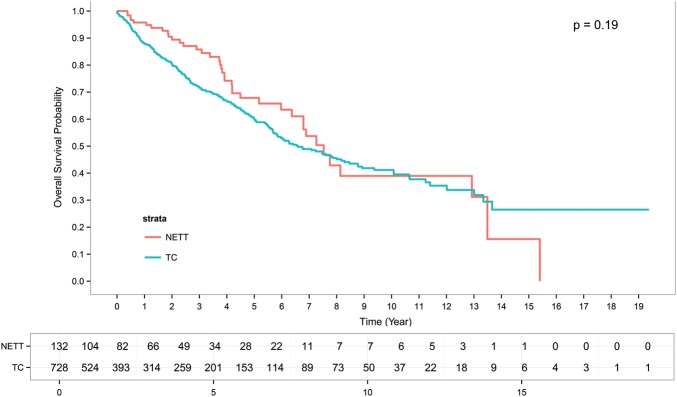
The median follow-up in TC cases was 4.4 years, and the median OS was 6.6 years [95% confidence interval (CI): 5.8–8.3]; overall 5- and 10-year survival rates were 60 and 40%, respectively. The overall CIR was 35% at 5 years and 40% at 10 years.
The median follow-up in NETTs was 4.1 years, and the median OS was 7.5 years (95% CI: 6.79–not reached); 5- and 10-year survival rates were 68 and 39%, respectively. CIR was 34% at 5 years and 54% at 10 years.
On univariate analysis, a total of 728 TC and 132 NETTs patients were included in the OS analysis. Among them, 262 TCs and 39 NETTs died. Tumour histology did not influence either OS (P = 0.19) or RFS (P = 0.35) (Figs 1 and 2). At the multivariate analysis, pathological stage, resection status and RT showed statistically significant impact on both OS and RFS (Tables 2 and 3).
Figure 1:
Overall survival curves of thymic carcinomas and neuroendocrine tumours.
Figure 2:
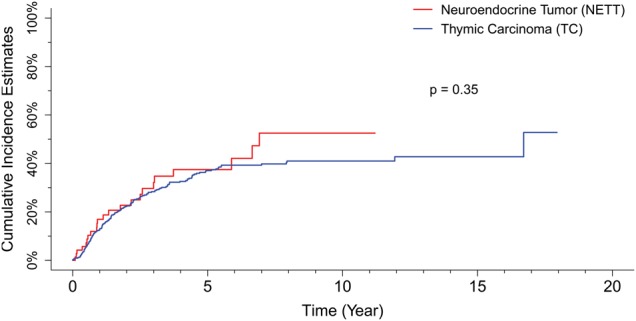
Recurrence-free survival curves of thymic carcinomas and neuroendocrine tumours.
Table 2:
Multivariate analysis of prognostic and treatment variables associated with overall survival
| Parameter | Hazard ratio (95% confidence interval) | P-valuea |
|---|---|---|
| Age | 1.010 (0.995, 1.026) | 0.17 |
| Histology | ||
| NETT (vs TC) | 1.077 (0.620, 1.872) | 0.79 |
| pStage | 0.019 | |
| III (vs I/II/IIa/IIb) | 1.593 (0.900, 2.819) | 0.11b |
| IVa (vs I/II/IIa/IIb) | 2.073 (0.991, 4.399) | 0.053b |
| IVb (vs I/II/IIa/IIb) | 2.861 (1.457, 5.616) | 0.002b |
| Resection status | ||
| R1/2 (vs R0) | 1.623 (1.083, 2.431) | 0.019 |
| Chemotherapy | ||
| Yes (vs no) | 1.171 (0.762, 1.800) | 0.47 |
| Radiotherapy | ||
| Yes (vs no) | 0.560 (0.370, 0.848) | 0.006 |
| Tumour size | 0.983 (0.928, 1.042) | 0.57 |
| Gender | ||
| Female (vs male) | 1.132 (0.759, 1.689) | 0.54 |
NETT: neuroendocrine thymic tumour; TC: thymic carcinoma.
aLikelihood ratio P-value from a Cox model.
bWald P-values.
Table 3:
Multivariate analysis of prognostic and treatment variables associated with recurrence-free survival
| Parameters | Hazard ratio (95% confidence interval) | P-valuea |
|---|---|---|
| Age | 1.000 (0.988, 1.012) | 1.00 |
| Histology | ||
| NETT (vs TC) | 1.156 (0.680, 1.967) | 0.59 |
| pStage | 0.005 | |
| III (vs I/II/IIa/IIb) | 1.912 (1.108, 3.301) | 0.020b |
| IVa (vs I/II/IIa/IIb) | 2.957 (1.562, 5.596) | <0.001b |
| IVb (vs I/II/IIa/IIb) | 2.706 (1.429, 5.124) | 0.002b |
| Resection status | ||
| R1/2 (vs R0) | 1.622 (1.136, 2.317) | 0.008 |
| Chemotherapy | ||
| Yes (vs no) | 1.293 (0.867, 1.927) | 0.21 |
| Radiotherapy | ||
| Yes (vs no) | 0.506 (0.351, 0.731) | <0.001 |
| Tumour size | 1.004 (0.953, 1.058) | 0.89 |
| Gender | ||
| Female (vs male) | 1.378 (0.968, 1.961) | 0.075 |
NETT: neuroendocrine thymic tumour; TC: thymic carcinoma.
aLikelihood ratio P-value from a Cox model.
bWald P-values.
DISCUSSION
The present study, based on the joint analysis of ESTS and ITMIG retrospective databases, represents to our knowledge, the largest series of TCs and NETTs ever reported.
Several papers in the literature have combined TCs and thymomas in the same analysis, sometimes grouping the first with advanced stage thymomas, due to significant confusions in regard to TC's histological classification. The latest WHO histological classification, however, clearly stated that TCs are a separate entity from thymomas. Our study confirms the biological aggressiveness of such tumours, with great incidence of advanced stages at presentation, along with high incidence of local recurrences/distant metastases.
The results of our study demonstrate that (i) tumour histology (TC versus NETT) did not demonstrate an influence on survival; (ii) RFS did not seem to be statistically affected by the histological tumour subtype; (iii) pathological stage, resection status and RT showed statistically significant impact on both OS and RFS.
Thymic malignancies are rare and extremely heterogeneous, with a large spectrum of morphological forms and histological subtypes. From a histological point of view, thymic epithelial tumours comprise thymomas and TCs (the latter also including NETTs). While thymomas present with organotypic characteristics, TCs usually are not immunologically active, lacking also the organotypic features. Patients with TCs generally are more frequently associated with direct tumour invasion or the presence of distant metastases.
Here, we present to our knowledge the larges series of TCs and NETTs ever reported, based on the joint analysis of the ESTS and ITMIG retrospective databases.
We did not find a significant difference in survival nor RFS between TC and NETT. Similar results were recently observed by de Montpreville et al. [13] who analysed a single-institution series of 37 cases. Our data are consistent with the previously published literature: Kondo and Monden [14] reported a 5-year survival of 67% after complete resection and 30% in the case of a subtotal resection in TCs. Other studies with fewer patients found 5-year survival ranging from 27 to 61% [15, 16] for TCs. As regards NETTs, 5-year OS may vary between 26 and 84%, according to the different clinical series reported [7, 17]. However, since our patient cohort was significantly larger in size, our data are much more robust than the relative small series published thus far and provide an important benchmark to which future study outcomes can be compared.
In this study, we compared the clinical characteristics of TCs and NETTs, showing that both tumours frequently present at an advanced stage at the time of diagnosis, along with a male predominance. Moreover, our results also showed that NETTs lesions were larger than TC ones, but this finding apparently did not significantly affect tumour resectability: resection status, in fact, did not differ between the two groups. Finally, TC patients were older than NETT ones.
Para-neoplastic syndromes were observed in 76 TC cases, and MG was the commonest one. Unfortunately, data concerning endocrine syndromes were not available for NETTs, in both databases. TC-associated MG has been previously described: Filosso et al. [4] reported an incidence of 32.5% (9 squamous cell, 1 sarcomatoid and 3 not otherwise specified carcinomas); Ruffini et al. [18] showed MG associated in 31 of 229 TCs (14%) in a large cohort of patients derived from the ESTS retrospective database.
The overall TC R0 resection rate may vary between 21 and 90%, based on the published data [4, 6, 7, 14, 15]; our results are in line with the literature, with 63 and 54% R0 resections in TCs and NETTs, respectively. This rate decreased with increasing tumour stage. Furthermore, R0 resection was associated with significantly increased OS for both TCs and NETTs. We, therefore, advocate that complete surgical resection should be attempted whenever possible, even in patients with advanced TC or NETT; incomplete resections mostly result from highly locally invasive tumours.
Consistent with the recent literature [6, 7, 17, 19, 20], we also found that Masaoka-Koga tumour stage along with resection status was the only other prognostic factor found.
Moreover, there are limited data about the role of RT and/or CT in the treatment of TCs and NETTs. We found that most patients at any stage received RT, usually in the adjuvant setting. In some of the most recent clinical experiences, a neoadjuvant treatment (usually CT) was advocated to increase the chance of achieving tumour shrinkage and, consequently, a R0 resection [13, 17]. Only Tiffet et al. [3] demonstrated the adjuvant RT beneficial effect (no recurrences) in those NETT patients in which RT was administered after a complete tumour resection. However, Cardillo et al. [17] reported a detrimental RT effect for those patients who received it.
We did not find a statistically significant advantage in OS for adjuvant CT/RT, and RFS was not modified by the administration of such treatment.
Instead, our study demonstrates a tendency to offer RT as an induction treatment in advanced stages, and CT ± RT as an adjuvant setting on the basis of tumour invasiveness, resection status and possible presence of lymphatic involvement. However, the statistical power to detect small differences in outcomes based on the administration of CT or RT was limited despite our large dataset.
The limitations of our study are inherent to its design: this is a retrospective study and it suffers from its potential biases. Moreover, a centralized histological review was not available, but all the histological specimens were evaluated by local pathologists who were experts in TENs. The TC/NETT definitive diagnosis was made according to the international histological guidelines. Nevertheless, the chance to use both ESTS and ITMIG datasets allowed us to collect a very large cohort of patients treated for such rare neoplasms and to strengthen our results.
In conclusion, TCs and NETTs are rare mediastinal tumours, with an aggressive behaviour. Masaoka-Koga stage and completeness of resection, and not tumour histology were found to be independent prognostic factors for both OS and RFS.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr J. Salo (Helsinki, Finland): I am interested in knowing in how many patients was the operation was performed with the help of cardiopulmonary bypass? Do you have the numbers?
Dr Filosso: If I remember correctly, we had only two patients with thymic carcinoma who received cardiopulmonary bypass.
Dr C. Kang (Seoul, Republic of Korea): Did you carry out a subgroup analysis of the neuroendocrine types of tumour, such as carcinoid versus other neuroendocrine types, in small cell lung cancer? The second question is, could you find any difference in the recurrence pattern, because neuroendocrine tumours are usually associated with more frequent distant metastases compared to squamous cell carcinoma and thymic carcinoma?
Dr Filosso: We observed that there was no influence on survival or on cumulative incidence of recurrence by tumour subtype in both thymic carcinoma and in neuroendocrine tumours. I did not report the numbers of local recurrences or distant metastases for both tumours since there are not so many cases. However, we found that neuroendocrine tumours had a less aggressive biological behaviour.
REFERENCES
- 1. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004. [Google Scholar]
- 2. Greene MA, Malias MA. Aggressive multimodality treatment of invasive thymic carcinoma. J Thorac Cardiovasc Surg 2003;125:434–6. [DOI] [PubMed] [Google Scholar]
- 3. Tiffet O, Nicholson AG, Ladas G, Sheppard MN, Goldstraw P. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest 2003;124:141–6. [DOI] [PubMed] [Google Scholar]
- 4. Filosso PL, Guerrera F, Rendina AE, Bora G, Ruffini E, Novero D et al. . Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 2014;83:205–10. [DOI] [PubMed] [Google Scholar]
- 5. Lausi PO, Refai M, Filosso PL, Ruffini E, Oliaro A, Guerrera F et al. . Thymic neuroendocrine tumors. Thorac Surg Clin 2014;24:327–32. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad U, Yao X, Detterbeck F, Huang J, Antonicelli A, Filosso PL et al. . Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95–100. [DOI] [PubMed] [Google Scholar]
- 7. Filosso PL, Yao X, Ahmad U, Zhan Y, Huang J, Ruffini E et al. . Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103–9. [DOI] [PubMed] [Google Scholar]
- 8. Filosso PL, Ruffini E, Lausi PO, Lucchi M, Oliaro A, Detterbeck F. Historical perspectives: the evolution of the thymic epithelial tumors staging system. Lung Cancer 2014;83:126–32. [DOI] [PubMed] [Google Scholar]
- 9. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485–92. [DOI] [PubMed] [Google Scholar]
- 10. Koga K, Matsuno Y, Noguchi M, Mukai K, Asamura H, Goya T et al. . A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359–67. [DOI] [PubMed] [Google Scholar]
- 11. Huang J, Detterbeck FC, Wang Z, Loehrer PJ Sr. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017–23. [DOI] [PubMed] [Google Scholar]
- 12. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 2008;16:1141–54 . [Google Scholar]
- 13. de Montpréville V, Ghigna MR, Lacroix L, Besse B, Broet P, Dartevelle P et al. . Thymic carcinomas: clinicopathologic study of 37 cases from a single institution. Virchows Arch 2013;462:307–13. [DOI] [PubMed] [Google Scholar]
- 14. Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878–84. [DOI] [PubMed] [Google Scholar]
- 15. Liu HC, Hsu WH, Chen YJ, Chan YJ, Wu YC, Huang BS et al. . Primary thymic carcinoma. Ann Thorac Surg 2002;73:1076–81. [DOI] [PubMed] [Google Scholar]
- 16. Chung DA. Thymic carcinoma-analysis of nineteen clinicopathological studies. Thorac Cardiovasc Surg 2002;50:189–94. [DOI] [PubMed] [Google Scholar]
- 17. Cardillo G, Carleo F, Giunti R, Lopergolo MG, Salvadori L, De Massimi AR et al. . Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819–23. [DOI] [PubMed] [Google Scholar]
- 18. Ruffini E, Detterbeck F, Van Raemdonck D, Rocco G, Thomas P, Weder W et al. . Thymic carcinoma: a cohort study of patients from the European Society of Thoracic Surgeons database. J Thorac Oncol 2014;9:541–8. [DOI] [PubMed] [Google Scholar]
- 19. Cardillo G, Rea F, Lucchi M, Paul MA, Margaritora S, Carleo F et al. . Primary neuroendocrine tumors of the thymus: a multicenter experience of 35 patients. Ann Thorac Surg 2012;94:241–5. [DOI] [PubMed] [Google Scholar]
- 20. Weksler B, Dhupar R, Parikh V, Nason KS, Pennathur A, Ferson PF. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299–303. [DOI] [PubMed] [Google Scholar]