Abstract
Background. The gold standard for diagnosis of enteric fever caused by Salmonella Typhi or Salmonella Paratyphi A or B is bone marrow culture. However, because bone marrow aspiration is highly invasive, many hospitals and large health centers perform blood culture instead. As blood culture has several limitations, there is a need for novel typhoid diagnostics with improved sensitivity and more rapid time to detection.
Methods. We developed a clyA-based real-time polymerase chain reaction (qPCR) method to detect Salmonella Typhi and Salmonella Paratyphi A simultaneously in blood. The sensitivity and specificity of this probeset was first evaluated in vitro in the laboratory and then in a typhoid-endemic population, in Karachi, Pakistan, and in healthy US volunteers.
Results. We optimized a DNA extraction and real-time PCR-based method that could reliably detect 1 colony-forming unit/mL of Salmonella Typhi. The probe set was able to detect clinical Salmonella Typhi and Salmonella Paratyphi A strains and also diarrheagenic Escherichia coli, but not invasive E. coli or other invasive bacteria. In the field, the clyA qPCR diagnostic was 40% as sensitive as blood culture. However, when qPCR-positive specimens were considered to be true positives, blood culture only exhibited 28.57% sensitivity. Specificity was ≥90% for all comparisons and in the healthy US volunteers. qPCR was significantly faster than blood culture in terms of detection of typhoid and paratyphoid.
Conclusions. Based on lessons learned, we recommend that future field trials of this and other novel diagnostics that detect typhoidal and nontyphoidal Salmonella employ multiple methodologies to define a “positive” sample.
Keywords: diagnostic, blood, Salmonella, typhoid, PCR
Collectively, invasive infections by Salmonella species are estimated to cause >30 million new illnesses annually [1–3]. The majority of enteric fever cases (caused by Salmonella enterica serovars Typhi and Paratyphi A) occur in South and Southeast Asia, whereas invasive infections with nontyphoidal Salmonella (iNTS) are primarily observed in sub-Saharan Africa [4–6]. Clinical diagnosis of invasive Salmonella infection is complicated due to similarity of symptoms to other febrile illnesses such as malaria, dengue, and rickettsioses. Ambulatory healthcare facilities in endemic settings frequently lack laboratory-based diagnostics, resulting in the majority of diagnoses being made clinically and antimicrobials given empirically [4, 6–8].
Early and reliable detection is an essential step in delivering successful patient care, controlling disease spread, and determining treatment outcome. Additionally, the ability to reliably implicate Salmonella as a cause of disease would permit improved estimates of burden at a national and global level, thereby creating a case for future investment in treatment and preventive methods and ultimately enabling accurate evaluation of such interventions [9]. Diagnostic tests that can detect invasive Salmonella require different attributes depending on the goal. A diagnostic for patient care should be rapid, sensitive, and specific; economical; simple to operate; and ideally able to detect antibiotic resistance. A diagnostic that would be used to measure disease burden should have the following attributes: sensitive and specific; economical; and able to differentiate between Salmonella Typhi, Salmonella Paratyphi A (in Asia), Salmonella Typhimurium, Salmonella Enteritidis (in Africa) and other Salmonella serovars.
The most widely available methods of laboratory diagnosis of Salmonella bloodstream infection rely on direct culture [10]. However, culture-based methods require that viable bacteria be present in detectible quantities at the time of specimen sampling; this has proven to be a major hurdle in diagnosis of Salmonella as reports of bacterial burden at systemic sites during invasive Salmonella infection are universally low and many patients have received antibiotics prior to their arrival at the hospital [11, 12].
Bacterial burden varies throughout the course of infection but is reported to be similar for both typhoidal and nontyphoidal Salmonella, which establish an intracellular niche at a density of <1 colony-forming unit (CFU)/mL of peripheral blood or 10 CFU/mL of bone marrow [13–15]. Bacterial burden is an order of magnitude higher in the bone marrow than in peripheral blood and is increased relative to that of peripheral blood during antibiotic therapy and relapse of infection [13–15]. Blood culture exhibits approximately 60%–80% sensitivity during the first 7 days of infection. The sensitivity drops to 20%–30% at subsequent time-points and although positively correlated with blood draw volume, the recommended sample volume of 10 mL can be difficult to obtain from acutely ill children [10, 16, 17].
Culture of bone marrow aspirates is considered the most accurate method of identifying acute Salmonella Typhi infection [18], but requires technical skill and appropriate equipment, is invasive, and does not eliminate the need for laboratory culture and subsequent serological identification. Bone marrow culture has not been fully evaluated as a diagnostic for invasive NTS infections. For both typhoidal and nontyphoidal Salmonella, most diagnostic laboratories use blood culture methodology for detection. Laboratory methods most commonly used for blood culture employ an automated blood culture instrument followed by traditional bacteriology identification and susceptibility testing [19].
Diagnostic targets for new typhoid detection tests include bacterial proteins, metabolites, and nucleic acid using technologies such as mass spectroscopy, polymerase chain reaction (PCR), and DNA hybridization [20–23]. Of particular note, Zhou and Pollard [24] have shown that preincubation of blood in bacteriological medium can improve sensitivity of molecular techniques. Several groups have taken advantage of this and are currently evaluating novel typhoid molecular diagnostic assays following preincubation. Other methods used to diagnose acute Salmonella Typhi infection involve detection of host response markers. Some of the most popular tests are the Widal, Tubex-TF, Typhidot IgG, and Typhidot IgM tests, which have low to moderate sensitivity and specificity [25]. One assay that shows promise is the TPTest, which detects Salmonella Typhi and Salmonella Paratyphi A antibody secretions by isolated lymphocytes [26, 27]. Most of the diagnostic assays developed to date target typhoidal Salmonella, but with additional modifications, many of these tests could be adapted for detection of iNTS.
Our goal here was to develop a quantitative real-time PCR (qPCR)–based methodology to detect Salmonella Typhi and Salmonella Paratyphi A directly from blood without the need for a preincubation step. We aimed to design an assay that would be more sensitive than blood culture but just as specific, and significantly faster in terms of time to detection.
MATERIALS AND METHODS
Bacterial Strains and Blood
Salmonella Typhi Ty2 and Salmonella Paratyphi A ATCC9150 were used for assay optimization. Additional clinical invasive Salmonella enterica strains (serovars Typhi, Paratyphi A, Paratyphi B, Paratyphi C, Typhimurium, Enteritidis, and Dublin) were previously obtained from blood or other sterile sites in Mali (Typhi and NTS) or Chile (Typhi, Paratyphi A, and Paratyphi B). Other bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella enterica serovars Choleraesuis and Newport) were from the Centers for Disease Control and Prevention or the Center for Vaccine Development culture collection. Salmonella species, E. coli, P. aeruginosa, and K. pneumoniae were grown in HS bacteriological medium (5 g sodium chloride, 10 g soytone [Teknova, Hollister, California], 5 g Hy Yest 412 [Sigma Aldrich, St Louis, Missouri] in 1 L distilled water) at 37°C. Streptococcus pneumoniae and H. influenzae were subcultured on commercially available plates (BD BBL Trypticase Soy Agar with 5% Sheep Blood/Chocolate II, I Plate Prepared Media [Fisher Scientific, Pittsburgh, Pennsylvania]) at 37°C. Whole human blood with sodium heparin anticoagulant was purchased from Biological Specialty Corporation (Colmar, Pennsylvania) or from healthy donors from the Baltimore area under the approval of the University of Maryland, Baltimore Institutional Review Board. All blood donors provided informed written consent.
DNA Extraction
The white blood cell fraction (WBC) was isolated from ≤3 mL of whole blood using erythrocyte lysis buffer as previously described [28]. The supernatant was decanted and DNA extractions were performed using the QIAamp Blood DNA Mini kit (Qiagen) on the WBC pellets with modifications to the manufacturer's protocol as described in the Supplementary Data. DNA was eluted in 32 µL of nuclease-free water.
Real-time PCR
Primers and probes used in this study are shown in Table 1. Probes were designed in Primer express version 2.0 (Applied Biosystems). Primers were designed using Primer3 version 4.0.0 [29, 30]. Real-time PCR was performed using a 20-µL reaction containing 10 µL TaqMan Fast Universal PCR Master Mix (2×) (Life Technologies, Grand Island, New York), 1 µL probeset mix (900 nM probe, 250 nM forward primer, and 250 nM reverse primer), and 9 µL template. The instrumentation used was the 7500 FAST Dx Real-time PCR machine (Life Technologies). Initial denaturation was at 95°C for 20 seconds followed by 50 cycles of 95°C denaturation for 3 seconds, and 60°C annealing and extension for 30 seconds. Data was collected during the annealing and extension step. A positive was determined if amplification intersected with the threshold within 50 cycles. Results reported as an undetermined cycle threshold (Ct) were examined for indications of late amplification as described in the Supplementary Data. Controls were run with every reaction as described in the Supplementary Data.
Table 1.
Primers and Probes Used in This Study
| Oligo | Sequence (5′ to 3′) and Fluorophore | Target | Reference |
|---|---|---|---|
| Cloning primers | |||
| ST-Fc | GGAGTCGCCGTTTTTAGACA | Salmonella Typhi STY0201 | This work |
| ST-Rc | TCCTTCAGCCAGCAGAGAAT | Salmonella Typhi STY0201 | This work |
| PA-Fc | AATTGGCGGCGTAGTGATAG | Salmonella Paratyphi A SSPA2308 | This work |
| PA-Rc | GTGAGGGGACAGATGTGGAG | Salmonella Paratyphi A SSPA2308 | This work |
| clyA559-F | ATAGTCGCCGGTCCGTTTG | Salmonella Typhi clyA | This work |
| clyA722-R | GCCGCATCGATATCTTTATTCG | Salmonella Typhi clyA | This work |
| Diagnostic primers and probes | |||
| ST-Probe | FAM-CATTTGTTCTGGAGCAGGCTGACGG-TAMRA | Salmonella Typhi STY0201 | [22] |
| ST-Frt | CGCGAAGTCAGAGTCGACATAG | Salmonella Typhi STY0201 | [22] |
| ST-Rrt | AAGACCTCAACGCCGATCAC | Salmonella Typhi STY0201 | [22] |
| Pa-Probe | CY5-CCCATACAATTTCATTCTTATTGAGAATGCGC-BHQ2 | Salmonella Paratyphi A SSPA2308 | [22] |
| Pa-Frt | ACGATGATGACTGATTTATCGAAC | Salmonella Paratyphi A SSPA2308 | [22] |
| Pa-Rrt | TGAAAAGATATCTCTCAGAGCTGG | Salmonella Paratyphi A SSPA2308 | [22] |
| phHV-Probe | CY5-TTTTTATGTGTCCGCCACCATCTGGATC-BHQ2 | Recombinant pCR TOPO 2.1 gB | [22] |
| PhHV-Frt | GGGCGAATCACAGATTGAATC | Recombinant pCR TOPO 2.1 gB | [22] |
| PhHV-Rrt | GCGGTTCCAAACGTACCAA | Recombinant pCR TOPO 2.1 gB | [22] |
| clyA542-Probe | FAM-CCGGTGCTGCAGCAGGCATA-TAMRA | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA498-F | TTATTTCCAGTCACAGGTGGATAG | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA677-R | CTAGTAAAGAAATTTTGCACTGCTTTTA | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA383-Probe | FAM-AACTGAATGAAGCGCAAAAATCTCTCCTGG-TAMRA | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA335-F | CAGCGCAGAAAGACATTCTCATC | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA440-R | GAAGCGTTGTTGAAACTTTGTGAA | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
| clyA598-Probe | FAM-ATTGCTGCGGGCGTGATTGAAGGGA-TAMRA | Salmonella Typhi and Salmonella Paratyphi A clyA | This work |
In Vitro Evaluation of Specificity
Specificity of primers was tested on clarified boiled lysate of Salmonella enterica (serovars Typhi, Paratyphi A, Paratyphi B, Paratyphi C, Typhimurium, Enteritidis, Dublin, Choleraesuis, and Newport) and other bacteria including S. pneumoniae, K. pneumoniae, H. influenzae, P. aeruginosa, and E. coli (Table 2). Three to 5 colonies were suspended in molecular-grade water and boiled at 95°C for 10 minutes in a thermal cycler. The lysate was centrifuged at maximum speed in a microcentrifuge for 1 minute. The supernatant was transferred into a fresh tube and 2 µL of a 1:10 dilution of the clarified lysate was tested in a 20-µL reaction for amplification of the clyA amplicon.
Table 2.
Specificity of the clyA Probeset
| Species | Serovar, Pathotype, or Strain Name (Source) | No. of Strains Tested | Positive or Negative by qPCR |
|---|---|---|---|
| Escherichia coli | BORT (CVD) | 1 | − |
| E. coli | EPEC E2348/69 (CVD) | 1 | + |
| E. coli | EAEC O42 (CVD) | 1 | + |
| Streptococcus pneumoniae | Serotypes 6b, 14, 19f, 23 (CVD) | 4 | − |
| Haemophilus influenzae | Strains 0183 and 0255 (CVD-Mali) | 2 | − |
| Klebsiella pneumoniae | B5055 (CVD) | 1 | − |
| Pseudomonas aeruginosa | PAO1 (CVD) | 1 | − |
| Salmonella enterica | Paratyphi B MNZ6203 (CVD-Chile) | 1 | − |
| S. enterica | Paratyphi C P53 (CVD-Mali) | 1 | − |
| S. enterica | Typhimurium SL13444 (CVD), I77 (CVD-Mali) | 2 | − |
| S. enterica | Enteritidis R11 (CVD-Mali) | 1 | − |
| S. enterica | Dublin P10, R17 (CVD-Mali) | 2 | − |
| S. enterica | Choleraesuis 06–0868 (CDC) | 1 | − |
| S. enterica | Newport 07-0044 (CDC) | 1 | − |
| S. enterica | Multiple Salmonella Typhi clinical strains (CVD-Mali and CVD-Chile) | 24 | + |
| S. enterica | Multiple Salmonella Paratyphi A clinical strains (CVD-Mali and CVD-Chile) | 12 | + |
Abbreviations: CDC, Centers for Disease Control and Prevention; CVD, CVD culture collection; CVD-Chile, isolated in Chile; CVD-Mali, isolated in Mali; EAEC, enteroaggregative Escherichia coli; EPEC, enteropathogenic Escherichia coli; qPCR, real-time polymerase chain reaction.
In Vitro Evaluation of Sensitivity
Salmonella Typhi reference strain Ty2 was grown overnight in HS media to approximately 1 × 109 CFU/mL. Serial 1:10 dilutions of bacteria were made in phosphate-buffered saline. A total of 100–200 µL of bacterial suspension was spiked onto the WBC pellet that was generated following erythrocyte lysis. Total genomic DNA was extracted as described above. Actual CFU used to spike WBC pellets was determined by performing viable counts using the dilutions expected to contain 100–1000 CFU/mL.
Study Sites
Evaluation of the Salmonella qPCR-based diagnostic was performed in Karachi, Pakistan. The study sites for the enrollment of typhoid cases included (1) the emergency department of the Aga Khan University (AKU) Hospital; (2) the main laboratory of the AKU Hospital; and (3) specimen collection point at Garden (AKU satellite laboratory). Controls were enrolled at AKU's Department of Paediatrics–run primary healthcare centers, situated in low-income areas of Karachi (Rehri Goth, Bhains Colony).
Participants
Participants with the following inclusion criteria participated in the study: 5–18 years old, clinically suspected typhoid or paratyphoid fever (enteric fever), with documented fever ≥38°C and no other identified focus of infection at the time of presentation, who provided consent and assent to obtain blood for culture and qPCR. Participants meeting any of the following criteria were excluded from study participation: fever with clinical signs indicating a clear focus of infection making a diagnosis of enteric fever unlikely; diagnosed cases of hematological malignancies presenting with febrile neutropenia; or refusal for consent or assent. Control participants who met the following inclusion criteria participated in the study: children aged 5–18 years with no signs of active infection, who provided consent and assent to obtain blood for culture and qPCR.
Clinical Study Laboratory Methods
Equal volumes of blood were tested by blood culture and real-time PCR in a blinded fashion. For children aged 5–14 years, up to 3 mL blood was tested by blood culture and an equivalent volume tested by qPCR. For children aged 14–18 years, up to 8 mL blood was tested by blood culture and an equivalent volume by qPCR. Blood culture was performed using a Bactec 9050 machine and standard microbiological techniques. Real-time PCR was performed by lysing erythrocytes, isolating DNA using a QIAamp DNA Blood Mini Kit (Qiagen) and then detecting Salmonella Typhi and Salmonella Paratyphi A by targeting the clyA gene as described above.
Statistical Analysis
Prism5 was used for most statistical analyses and graphical representation. Data were analyzed using Mann–Whitney test (2-tailed) or 1-way analysis of variance (ANOVA). Results were considered significant if P < .05. Sensitivity and specificity with 95% confidence intervals were calculated using 2 × 2 tables on the MedCalc.net website (https://www.medcalc.net/tests/diagnostic_test.php).
Research Ethics
This study was approved by the AKU Research Ethics Committee and the institutional review board of the University of Maryland School of Medicine.
RESULTS
Development of a Highly Sensitive qPCR Assay
We designed several primers and probes to detect simultaneously Salmonella Typhi and Salmonella Paratyphi A. We targeted clyA (also known as hlyE), which is conserved in Salmonella Typhi and Salmonella Paratyphi A but absent from other Salmonella serovars [31, 32]. Certain E. coli also harbor clyA [33], so we attempted to design probesets that would be specific for Salmonella Typhi and Salmonella Paratyphi A clyA, but they were not as efficient as other probesets that also bound E. coli (data not shown). We assessed the efficiency of several clyA primers and probes and determined that clyA598-probe, and primers clyA559-F and clyA722-R were as efficient as previously described Salmonella Typhi and Salmonella Paratyphi A probesets [22] (Supplementary Table 1). This probeset was used for all subsequent analyses.
Next, we optimized preparation of DNA template to enhance sensitivity. We have previously shown that by targeting lymphocytes (either by lysing erythrocytes or by isolating the buffy coat), we are able to subsequently extract DNA using a mini DNA isolation kit instead of a larger DNA isolation kit [28]. We showed that the limit of qPCR detection was decreased when erythrocyte lysis was performed prior to DNA extraction using the QIAamp Blood DNA Mini kit (Qiagen) compared with when DNA was directly isolated using the QIAamp Blood DNA Midi kit (Qiagen). In the present study, we improved our limit of detection by modifying the DNA extraction procedure as described in the Supplementary Data. A schematic diagram of the DNA extraction and qPCR procedure is shown in Supplementary Figure 1.
We also enhanced sensitivity by testing as much of the available sample as possible. Other PCR-based methods only use a fraction of available DNA template for amplification [22]. Here, we eluted DNA in 32 µL nuclease-free water and tested the majority of the sample by qPCR (3 reactions containing 9 µL of template each). We hypothesized that if there was 1 bacterium present in 3 mL blood, which is likely to occur based on results from previous studies [14], when this sample is tested by qPCR, the clyA gene target could conceivably be pipetted into a single well. As such, we interpreted a sample as being positive for qPCR if at least 1 well was positive. To maximize the chances of detecting a positive result, we used a Ct cutoff of 50 instead of 40 as is generally used for other assays. We examined the qPCR raw data closely to ensure that wells that were interpreted as positive exhibited true amplification as described in the Supplementary Data.
In Vitro Sensitivity and Specificity Using Spiked Blood
We evaluated the optimized DNA extraction and qPCR methodology in the laboratory and determined in vitro sensitivity and specificity. We isolated lymphocytes from 2–3 mL blood and then spiked with various concentrations of Salmonella Typhi, isolated DNA, and performed qPCR using the clyA probeset. As shown in Figure 1, 2 separate users were able to detect approximately 1 CFU/mL blood and even detect as few as 0.01 CFU/mL (most likely detecting dead bacteria). As expected, we observed a dose-response curve whereby higher concentrations of Salmonella Typhi yielded lower Ct values. We observed this dose-response curve for higher concentrations of bacteria (>10 CFU/mL) but not for lower concentrations (<10 CFU/mL). We believe that at concentrations <10 CFU/mL, sampling effects are occurring and one either observes detection (obtain a Ct value) or no detection (expressed by the machine as undetermined but which we have expressed as a Ct of 51 in Figure 1). Even in conditions with near-perfect reaction efficiency [34], consistent replicates for Ct are obtained when there are >10 copies of target [35], but variation in Ct is greater for low copy targets [36]. Spiking of blood with <0.01 Salmonella Typhi CFU/mL did not result in amplification.
Figure 1.

In vitro sensitivity of the Salmonella real-time polymerase chain reaction (qPCR) assay. Lymphocytes were isolated from 2–3 mL whole human blood by lysing red blood cells. Cell pellets were spiked with various concentrations of Salmonella Typhi Ty2, and DNA was isolated using a QIAamp Blood DNA Mini kit and tested using the clyA qPCR. The colored symbols represent 2 different users and the various symbols represent experiments performed on different occasions. Data are expressed as mean ± standard deviation from 3 replica wells. The maximum cycle threshold (Ct) value is 50 cycles. Wells with an undetermined Ct were assigned a Ct of 51.
We also determined whether the clyA probeset could detect other Salmonella serovars or any non-Salmonella bacteria. As expected, enteroaggregative E. coli and enteropathogenic E. coli were detected (Table 2). However, E. coli Bort, which is an invasive E. coli strain, was negative by qPCR. We confirmed that the probeset could detect 24 Salmonella Typhi and 12 Salmonella Paratyphi A strains. All of the other Salmonella serovars (Typhimurium, Enteritidis, Dublin, Paratyphi B, Paratyphi C, Choleraesuis, Newport) and non-Salmonella strains (K. pneumoniae, S. pneumoniae, H. influenzae, P. aeruginosa) tested negative by qPCR.
Evaluation of Specificity of the qPCR Diagnostic Using Healthy US Donors
We determined the specificity of our clyA qPCR-based diagnostic assay by testing blood from 97 healthy US donors. Whole human blood was lysed using erythrocyte lysis buffer and DNA was extracted from lymphocytes and tested by qPCR. Four specimens (4%) tested positive by qPCR (Table 3). For each these 4 specimens, only 1 well tested positive (out of 3) and produced high Ct values of 38.8, 38.93, 41.54, and 46.78. Therefore, using blood from healthy US donors, the specificity of the qPCR assay is 96%.
Table 3.
Specificity of the clyA Probeset Using Blood From Healthy US Donors
| Positive or Negative by qPCR | No. of Volunteers |
|---|---|
| Negative | 93 (96%) |
| Positive | 4 (4%) |
| Total | 97 |
Abbreviation: qPCR, real-time polymerase chain reaction.
Evaluation of the qPCR Diagnostic in a Typhoid-Endemic Region
We evaluated our qPCR-based diagnostic in a pediatric population in Karachi, Pakistan. We enrolled 136 children (5–18 years old) who had fever (≥38°C) for at least 3 days (cases) and 118 healthy controls. An equal volume of blood was tested by blood culture and qPCR. Twenty children tested positive for Salmonella Typhi or Salmonella Paratyphi A using standard microbiological methods (Table 4). Of these, 14 possessed Salmonella Typhi and 6 possessed Salmonella Paratyphi A. An additional 4 children tested positive for Bacillus species (2 cases), Micrococcus species (1 case), and Staphylococcus species (1 case). Of the 20 cases that were blood culture positive for Salmonella Typhi or Salmonella Paratyphi A, only 8 were also positive by qPCR. We confirmed that the clyA probeset was able to bind to DNA from all of the Salmonella Typhi and Salmonella Paratyphi A strains identified by blood culture (Table 5). Therefore, absence of detection was not due to absence of binding of primers or probe.
Table 4.
Blood Culture and Real-time Polymerase Chain Reaction Positivity for Cases and Controls in Karachi, Pakistan
| Category | qPCR Positive | qPCR Negative | Total |
|---|---|---|---|
| Clinically diagnosed with enteric fever (cases) | 23 | 113 | 136 |
| Blood culture positive for Salmonella Typhi or Salmonella Paratyphi A | 8 | 12 | 20 |
| Blood culture negative (no bacteria detected) | 15 | 101 | 116 |
| Healthy controls | 5 | 113 | 118 |
| Blood culture positive for Salmonella Typhi or Salmonella Paratyphi A | 0 | 0 | 0 |
| Blood culture negative (no bacteria detected) | 5 | 113 | 118 |
Abbreviation: qPCR, real-time polymerase chain reaction.
Table 5.
Bacteria Detected From Positive Blood Cultures of Cases in Karachi, Pakistan
| Bacteria | No. of Strains | Confirmed to Bind to clyA Probeset |
|---|---|---|
| Salmonella Typhi | 14 | 14 |
| Salmonella Paratyphi A | 6 | 6 |
| Bacillus spp | 2 | ND |
| Micrococcus spp | 1 | ND |
| Staphylococcus spp | 1 | ND |
Abbreviation: ND, not determined.
We determined sensitivity and specificity of the clyA qPCR and blood culture using each other as the comparator (Table 6). The clyA qPCR diagnostic was 40% as sensitive as blood culture. When qPCR-positive specimens were considered to be true positives, blood culture only exhibited 28.57% sensitivity. Specificity was ≥91%.
Table 6.
Sensitivity and Specificity for Blood Culture and Real-time Polymerase Chain Reaction Assays Compared to Each Other Using Cases and Controls From Karachi, Pakistan
| Test Assay | Comparator (True Positive) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|
| qPCR | Blood culture | 40% (19.12%–63.95%) | 91.45% (87.11%–94.7%) |
| Blood culture | qPCR | 28.57% (13.22%–48.67%) | 94.69% (90.91%–97.23%) |
Abbreviations: CI, confidence interval; qPCR, real-time polymerase chain reaction.
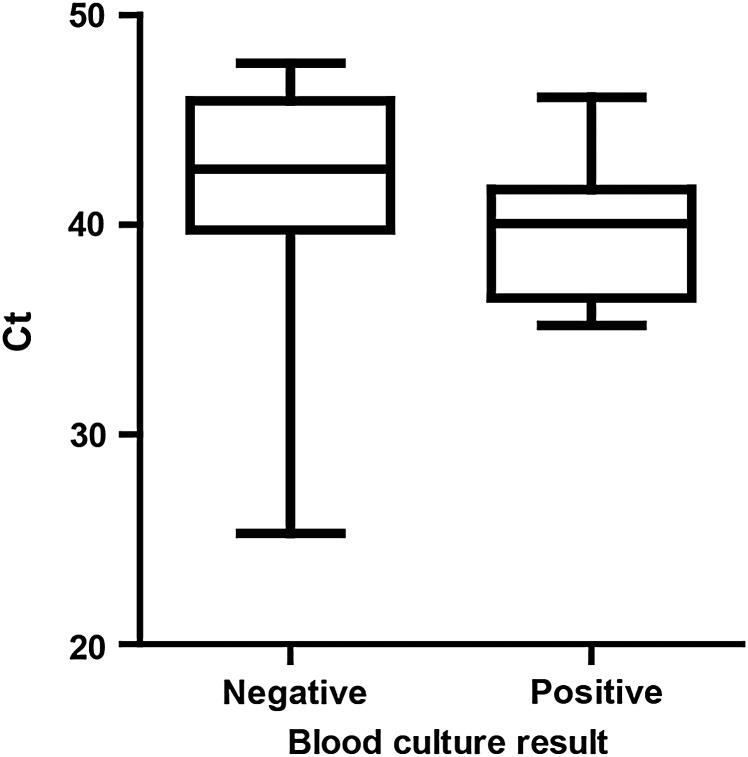
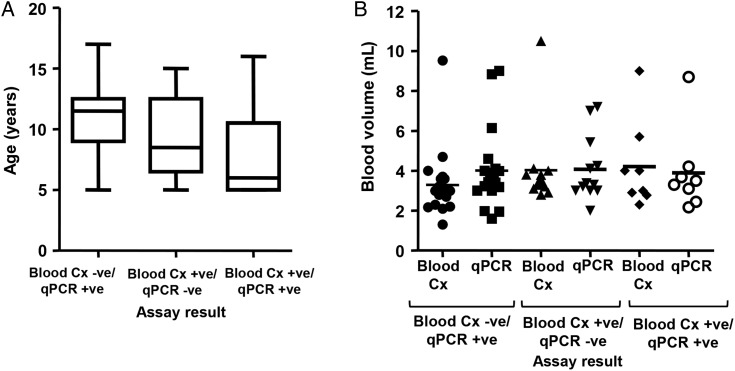
We examined the qPCR-positive specimens further to determine whether there were any differences between blood culture–positive and blood culture–negative specimens. We observed a lower Ct for blood culture–positive specimens than blood culture–negative specimens (mean ± standard deviation [SD], 39.89 ± 3.51 Ct vs 41.72 ± 5.13 Ct) but the difference was not significant (P = .1335, Mann–Whitney test; Figure 2). We also examined the ages of all of the children (cases and controls) that were either blood culture positive or qPCR positive or both (Figure 3A). There were no significant differences in age by 1-way ANOVA. Likewise, we also examined blood volumes tested by blood culture and qPCR for all blood culture–positive or qPCR-positive samples (Figure 3B). There were no significant differences in volumes tested by blood culture vs qPCR (blood culture negative/qPCR positive: mean ± SD, 3.29 ± 1.69 mL and 4.01 ± 1.94 mL, respectively; blood culture positive/qPCR negative: 4.03 ± 2.08 mL and 4.07 ± 1.64 mL, respectively; blood culture positive/qPCR positive: 4.21 ± 2.21 and 3.89 ± 2.05 mL, respectively; Mann–Whitney test). However, the blood culture–negative and qPCR-positive specimens were almost significantly different (P = .0578).
Figure 2.
Cycle threshold (Ct) values of real-time polymerase chain reaction–positive specimens according to blood culture result. Data are expressed as a box-and-whisker plot with whiskers representing the minimum and maximum value.
Figure 3.
Age (A) and volume of blood tested (B) of specimens that were blood culture negative and real-time polymerase chain reaction (qPCR) positive; blood culture positive and qPCR negative; and positive for both blood culture and qPCR. Age data are expressed as a box-and-whisker plot with whiskers representing the minimum and maximum value. Blood volumes are expressed as a scatter plot with the bar indicating the mean. Abbreviations: -ve, negative; +ve, positive; Cx, culture.
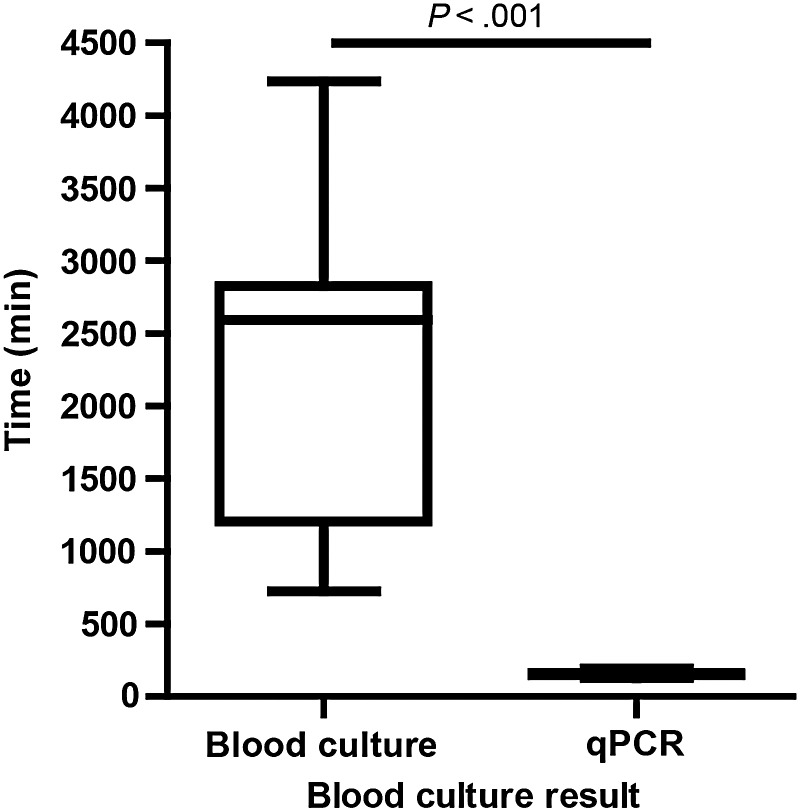
One major attribute that a new typhoid diagnostic should possess is a reduced time to detection and identification compared to blood culture. We measured the time taken to process blood samples by qPCR (erythrocyte lysis and DNA extraction and qPCR) compared with blood culture (detection by instrumentation and identification by classical clinical microbiology). As shown in Figure 4, qPCR is significantly faster (mean ± SD, 2 hours 34 minutes ± 15 minutes) than blood culture (mean ± SD, 37 hours 40 minutes ± 17 hours) in terms of detection and identification of Salmonella Typhi and Salmonella Paratyphi A (P < .0001; Mann–Whitney test, 2-tailed).
Figure 4.
Time to identification for blood culture or real-time polymerase chain reaction (qPCR) positivity. Data are expressed as a box-and-whisker plot with whiskers representing the minimum and maximum value. Data were analyzed using Mann–Whitney test, 2-tailed.
DISCUSSION
Evaluation of diagnostic tests for invasive Salmonella infection is particularly challenging in light of the fact that the “gold standard” comparator diagnostic in most field trials is blood culture, which exhibits low sensitivity itself. Although we observed a higher sensitivity for detection by qPCR than blood culture, we did not detect bacteria by qPCR in 12 of 20 blood culture–positive specimens. A possible explanation is that due to sampling effects, Salmonella Typhi and Salmonella Paratyphi A in these blood samples were aliquoted into the blood culture bottles but not the tubes tested by qPCR. The average volume of blood tested in these samples was approximately 4 mL and the median concentration of Salmonella Typhi in blood is 0.3–1 CFU/mL, which means that there was 1.2–4 CFU per sample [14, 15]. Therefore, it is conceivable that for the blood culture–positive/qPCR-negative specimens, 1 CFU was detected by blood culture, but due to sampling, there were no bacteria available for detection by qPCR.
Our data suggest that the blood culture–negative but qPCR-positive specimens had a lower bacterial concentration (than the blood culture–positive specimens), which the qPCR-based assay was able to detect but which could not be cultured. However, the differences in Ct values were not statistically significant. Based on our results, we recommend that future field trials employ multiple diagnostic methodologies to define a “positive” sample. We suggest that future typhoid/paratyphoid diagnostic assays should first be tested in an adult population where larger blood volumes can be obtained and tested simultaneously in various assays. We propose that new Salmonella diagnostic assays be evaluated in clinical studies with various comparators. If possible, the novel diagnostic should be compared to bone marrow culture as this method has shown the highest sensitivity to date. If bone marrow culture is not feasible, then blood culture should be performed with at least 2 other diagnostic tests (eg, qPCR with or without preenrichment vs an immunoassay such as the TPTest). If at least 2 assays test positive, then the specimen should be considered a true positive. Once proof of principle has been established, then the assay should be validated in adults from other geographic regions and in pediatric populations.
For iNTS diagnostics, a similar approach could be used as for typhoid diagnostics but with slight differences due to the populations that are susceptible to iNTS infections. The iNTS assays could first be tested in human immunodeficiency virus–infected adults and then evaluated in infants. These studies would have to be performed in Africa.
In addition to standard quantitative metrics of performance, operational characteristics of future typhoid and iNTS assays must be considered as well. These include factors such as time to definitive diagnosis, technical simplicity, cost and stability of reagents and equipment, and level of staff training needed to reliably perform a test and interpret the results. There are many advantages of PCR over other technologies, including the ability to detect various bacterial targets on the same platform/equipment; permit speciation, detection of antibiotic resistance genes, and evolution of target selection as knowledge of circulating outbreak strains evolves; and lack of necessity for bacterial viability. However, there are several disadvantages to PCR, including that the high degree of specificity can limit the utility of specific primer sets to validated target species and requires limit of detection, sensitivity, and specificity testing on many serovars [37]; targets must be selected that are present and retained across relevant species and serovars; a centralized laboratory with skilled personnel is required [10]; and it is susceptible to contamination.
A significant strength of this study is that the entirety of the assay (including nucleic acid extraction, qPCR, and analysis) was performed on-site, in a relevant hospital laboratory, by intended end-user technicians. Although test optimization and validation in the laboratory environment are critical preliminary steps, performance characteristics must subsequently be evaluated in the field setting for which the assay is intended [38]. Discrepancies in performance characteristics between use in developing laboratories and field testing is particularly important for assays with increased sensitivity or technical complexity (such as nucleic acid detection tests), as numerous factors including reagent transport conditions and equipment or operator variability can substantially alter performance.
Two desirable attributes of a typhoid diagnostic are improved sensitivity compared to blood culture and reduced time to detection. Here, we clearly show that real-time PCR is significantly faster at detecting and identifying Salmonella Typhi or Salmonella Paratyphi A than classical microbiological techniques. We would expect the same to also be true for detection of iNTS.
Development of new and improved typhoid and paratyphoid diagnostics has been challenging, but with further testing and evaluation, diagnostic assays that are suitable for use in developing countries are on the horizon [39]. The first Salmonella diagnostics should target Salmonella Typhi and Salmonella Paratyphi A in preparation for imminent typhoid and paratyphoid vaccine evaluation programs [40, 41]. However, additional diagnostic assays should also target iNTS such as Salmonella Typhimurium and Salmonella Enteritidis, which are a significant cause of morbidity and mortality in sub-Saharan Africa and for which vaccines are also in development [4, 41].
Supplementary Material
Notes
Acknowledgments. We thank Dr Stephen Baker for the kind gift of the PhHV-1 gB plasmid. We also thank Dr Sandra Panchalingam for suggestions made about real-time polymerase chain reaction (qPCR) analysis and interpretation.
Financial support. This work was supported by the Bill & Melinda Gates Foundation (grant number OPP1039437 to M. M. L.).
Supplement sponsorship. This article appeared as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. During this work, A. K. Z. joined the Bill & Melinda Gates Foundation. To avoid potential conflicts of interest, F. Q. replaced A. K. Z. as the AKU Principal Investigator. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis 2015; 21:941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ 2004; 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 3. Crump JA, Mintz ED. Global trends in typhoid and paratyphoid fever. Clin Infect Dis 2010; 50:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 2012; 379:2489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arndt MB, Mosites EM, Tian M et al. . Estimating the burden of paratyphoid A in Asia and Africa. PLoS Negl Trop Dis 2014; 8:e2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. Typhoid fever. Lancet 2015; 385:1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg Infect Dis 2001; 7:302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson ML. Assuring the quality of clinical microbiology test results. Clin Infect Dis 2008; 47:1077–82. [DOI] [PubMed] [Google Scholar]
- 9. Baker S, Favorov M, Dougan G. Searching for the elusive typhoid diagnostic. BMC Infect Dis 2010; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wain J, Hosoglu S. The laboratory diagnosis of enteric fever. J Infect Dev Ctries 2008; 2:421–5. [DOI] [PubMed] [Google Scholar]
- 11. Gordon MA. Salmonella infections in immunocompromised adults. J Infect 2008; 56:413–22. [DOI] [PubMed] [Google Scholar]
- 12. Waddington CS, Darton TC, Jones C et al. . An outpatient, ambulant-design, controlled human infection model using escalating doses of Salmonella Typhi challenge delivered in sodium bicarbonate solution. Clin Infect Dis 2014; 58:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gordon MA, Kankwatira AM, Mwafulirwa G et al. . Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: an emerging disease pathogenesis. Clin Infect Dis 2010; 50:953–62. [DOI] [PubMed] [Google Scholar]
- 14. Wain J, Diep TS, Ho VA et al. . Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wain J, Pham VB, Ha V et al. . Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J Clin Microbiol 2001; 39:1571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vallenas C, Hernandez H, Kay B, Black R, Gotuzzo E. Efficacy of bone marrow, blood, stool and duodenal contents cultures for bacteriologic confirmation of typhoid fever in children. Pediatr Infect Dis 1985; 4:496–8. [DOI] [PubMed] [Google Scholar]
- 17. Wain J, Diep TS, Bay PV et al. . Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries 2008; 2:469–74. [DOI] [PubMed] [Google Scholar]
- 18. Communicable Disease Surveillance and Response Vaccines and Biologicals, World Health Organization. The diagnosis, treatment and prevention of typhoid fever. Geneva, Switzerland: WHO, 2003. [Google Scholar]
- 19. Er J, Wallis P, Maloney S, Norton R. Paediatric bacteraemias in tropical Australia. J Paediatr Child Health 2015; 51:437–42. [DOI] [PubMed] [Google Scholar]
- 20. Fan F, Du P, Kan B, Yan M. The development and evaluation of a loop-mediated isothermal amplification method for the rapid detection of Salmonella enterica serovar Typhi. PLoS One 2015; 10:e0124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nasstrom E, Vu Thieu NT, Dongol S et al. . Typhi and Paratyphi A elaborate distinct systemic metabolite signatures during enteric fever. Elife 2014; 3:e03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nga TV, Karkey A, Dongol S et al. . The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis 2010; 10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tennant SM, Zhang Y, Galen JE, Geddes CD, Levine MM. Ultra-fast and sensitive detection of non-typhoidal Salmonella using microwave-accelerated metal-enhanced fluorescence (“MAMEF”). PLoS One 2011; 6:e18700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou L, Pollard AJ. A fast and highly sensitive blood culture PCR method for clinical detection of Salmonella enterica serovar Typhi. Ann Clin Microbiol Antimicrob 2010; 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keddy KH, Sooka A, Letsoalo ME et al. . Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ 2011; 89:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khanam F, Sheikh A, Sayeed MA et al. . Evaluation of a typhoid/paratyphoid diagnostic assay (TPTest) detecting anti-Salmonella IgA in secretions of peripheral blood lymphocytes in patients in Dhaka, Bangladesh. PLoS Negl Trop Dis 2013; 7:e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheikh A, Bhuiyan MS, Khanam F et al. . Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol 2009; 16:1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyd MA, Tennant SM, Melendez JH et al. . Adaptation of red blood cell lysis represents a fundamental breakthrough that improves the sensitivity of Salmonella detection in blood. J Appl Microbiol 2015; 118:1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Untergasser A, Cutcutache I, Koressaar T et al. . Primer3—new capabilities and interfaces. Nucleic Acids Res 2012; 40:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007; 23:1289–91. [DOI] [PubMed] [Google Scholar]
- 31. Oscarsson J, Westermark M, Lofdahl S et al. . Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect Immun 2002; 70:5759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Rhein C, Bauer S, Lopez Sanjurjo EJ, Benz R, Goebel W, Ludwig A. ClyA cytolysin from Salmonella: distribution within the genus, regulation of expression by SlyA, and pore-forming characteristics. Int J Med Microbiol 2009; 299:21–35. [DOI] [PubMed] [Google Scholar]
- 33. Ludwig A, von Rhein C, Bauer S, Huttinger C, Goebel W. Molecular analysis of cytolysin A (ClyA) in pathogenic Escherichia coli strains. J Bacteriol 2004; 186:5311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Booth CS, Pienaar E, Termaat JR, Whitney SE, Louw TM, Viljoen HJ. Efficiency of the polymerase chain reaction. Chem Eng Sci 2010; 65:4996–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stenman J, Orpana A. Accuracy in amplification. Nat Biotechnol 2001; 19:1011–2. [DOI] [PubMed] [Google Scholar]
- 36. Peccoud J, Jacob C. Theoretical uncertainty of measurements using quantitative polymerase chain reaction. Biophys J 1996; 71:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ben Hassena A, Barkallah M, Fendri I et al. . Real time PCR gene profiling and detection of Salmonella using a novel target: the siiA gene. J Microbiol Methods 2014; 109C: 9–15. [DOI] [PubMed] [Google Scholar]
- 38. Parry CM, Wijedoru L, Arjyal A, Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther 2011; 9:711–25. [DOI] [PubMed] [Google Scholar]
- 39. Andrews JR, Ryan ET. Diagnostics for invasive Salmonella infections: current challenges and future directions. Vaccine 2015; 33(suppl 3):C8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin LB. Vaccines for typhoid fever and other salmonelloses. Curr Opin Infect Dis 2012; 25:489–99. [DOI] [PubMed] [Google Scholar]
- 41. Tennant SM, Levine MM. Live attenuated vaccines for invasive Salmonella infections. Vaccine 2015; 33(suppl 3):C36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.