Abstract
Aims
Clinical observations showed a correlation between accelerated atherosclerosis in diabetes and high plasmatic level of IL-18, a pro-inflammatory cytokine. IL-18 enhances the production of inflammatory cytokines and cellular adhesion molecules contributing to atherosclerotic plaque formation and instability. Previous studies indicated that protein kinase C (PKC)-β inhibition prevented macrophage-induced cytokine expression involved in diabetic (DM) atherosclerotic plaque development. However, the role of PKC-β activation on IL-18/IL-18-binding protein (IL-18BP) pathway causing endothelial dysfunction and monocyte adhesion in diabetes has never been explored.
Methods and results
Apoe −/− mice were rendered DM and fed with western diet containing ruboxistaurin (RBX), a PKC-β inhibitor. After 20 weeks, atherosclerotic plaque composition was quantified. Compared with non-diabetic, DM mice exhibited elevated atherosclerotic plaque formation, cholestoryl ester content and macrophage infiltration, as well as reduced IL-18BP expression in the aorta which was prevented with RBX treatment. Endothelial cells (ECs) and macrophages were exposed to normal or high glucose (HG) levels with or without palmitate and recombinant IL-18 for 24 h. The combined HG and palmitate condition was required to increase IL-18 expression and secretion in macrophages, while it reduced IL-18BP expression in EC causing up-regulation of the vascular cell adhesion molecule (VCAM)-1 and monocyte adhesion. Elevated VCAM-1 expression and monocyte adherence were prevented by siRNA, RBX, and IL-18 neutralizing antibody.
Conclusion
Our study unrevealed a new mechanism by which PKC-β activation promotes EC dysfunction caused by the de-regulation of the IL-18/IL-18BP pathway, leading to increased VCAM-1 expression, monocyte/macrophage adhesion, and accelerated atherosclerotic plaque formation in diabetes.
Keywords: Atherosclerosis, Protein kinase C beta, Endothelial dysfunction, IL-18-binding protein
1. Introduction
The main long-term complications from diabetes are vascular diseases, which are in turn the main causes of morbidity and mortality in diabetic (DM) patients. Several potential links between diabetes and atherosclerosis have been identified and many clinical observations point to a correlation between risk of vascular complications in diabetes and poor glycaemic control.1 The pathogenesis of accelerated atherosclerosis in diabetes is multifactorial and involves interactions between abnormalities of apoprotein and lipoprotein particle distribution, hyperinsulinaemia, alterations in growth factor expression, and cytokine production. Moreover, a large body of evidence suggests that the inflammatory process plays a major role throughout the development of the atherosclerotic lesion, disruption, and thrombosis.2
Multiple theories have been proposed to explain the mechanism by which abnormal metabolites of glucose and lipids cause acceleration of atherosclerosis in diabetes.3 One of them is the activation of protein kinase C (PKC), specifically the β isoform. PKC activation is involved in monocytes, smooth muscle, and endothelial cell (EC) dysfunction affecting cellular activity and signalling pathways.4 Monocyte stimulation is critically dependent on PKC activation whether it is induced by cytokines or lipopolysaccharides.5 In particular, during the process of atherosclerotic plaque formation, the expression of Mac-1 and inflammatory cytokines have been shown to be regulated by PKC activation.5 Moreover, PKC activity promotes the binding of monocytes to EC, its transmigration into the arterial wall, and the release of multiple cytokines.6 These cytokines include tumour nuclear factor (TNF)-α, IFN-γ, macrophage colony stimulating factor, and multiple ILs.7,8 For EC, PKC activation increased the expression of adhesion molecules, such as selectins, intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), IL-6, IL-1, and the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and superoxide production.9,10 Schmidt and Yan's group previously published supportive data on the potential role of PKC-β in atherosclerotic plaque formation. Depletion of PKC-β gene or treatment with ruboxistaurin (RBX; LY333531) in apolipoprotein E (apoE)-deficient mice decreased atherosclerosis by inhibiting the early growth response (Egr)-1 protein, which regulated VCAM expression and MMP-2 activity preferentially in EC.11 More recently, the same group reported that inhibition of PKC-β reduced CD11c, chemokine (C–C motif) ligand 2, and IL-1β in macrophages induced by high glucose (HG) levels and diabetes.12 However, giving multiple evidences that activation of PKC-β in diabetes mainly affects EC, it is necessary to explore the potential role of PKC-β on cytokine regulation causing EC dysfunction in diabetes.
IL-18, originally described as an IFN-γ-inducing factor, is a member of the IL-1 family of cytokines and is known to contribute to the pathogenesis of chronic immunoinflammatory process.13 IL-18 is produced constitutively in many different cell types, including macrophages, vascular smooth muscle cells, and adipocytes.14,15 One of the main actions of IL-18 is to enhance the production of cytokines and cellular adhesion molecules that promote atherosclerotic plaque formation and instability.16 IL-18 activity has been shown to be associated with obesity, insulin resistance, and dyslipidaemia.17 Furthermore, circulating levels of IL-18 have been reported to be elevated in patients with type 2 diabetes mellitus in cross-sectional studies.18,19 As a negative feedback mechanism in response to elevated IL-18 production, IL-18 has a decoy receptor/inhibitor called IL-18-binding protein (IL-18BP), which is produced and associated with IL-18 to provide protection from tissue damage due to uncontrolled pro-inflammatory activity of IL-18.20 In two prospective cohorts, increased levels of IL-18 have been shown to predict the development of type 2 diabetes.21,22 Interestingly, a study demonstrated that electrotransfer of a plasmid DNA encoding for the murine IL-18BP prevented fatty streak development in the thoracic aorta of apoE-deficient mice and slowed the progression of advanced atherosclerotic plaques in the aortic sinus.23 These results suggest that the regulation of IL-18/IL-18BP pathway is involved in atherosclerosis. However, much less studied is the role of PKC-β activation on the IL-18/IL-18BP pathway in accelerated atherosclerosis in diabetes.
In the present study, we propose a new pathway by which PKC-β causes EC dysfunction and monocyte adhesion to the arterial wall, and contributes to atherosclerosis. We showed that the combination of hyperglycaemia and palmitate, a free fatty acid (FFA), increased the expression of IL-18 in macrophages/monocytes while reduced the expression of IL-18BP in EC participating at least in part to elevated VCAM-1 cell surface expression, monocyte adhesion, and atherosclerosis. Treatment with PKC-β inhibitor and siRNA restored IL-18BP expression and prevented VCAM-1 expression, monocyte adhesion, and atherosclerosis in diabetes.
2. Methods
Antibodies, reagents, lipid profile, serum levels of IL-18, cholesteryl ester content, palmitate preparation, membrane fraction isolation, siRNA transfection, quantitative PCR (qPCR), and immunoblot analyses were performed as described in Supplementary material online.
2.1. Experimental model and design
Male apoE-deficient (B6.129P2-Apoetm1Unc/J, stock #002052) mice were purchased from Jackson Laboratories. They were rendered DM by streptozotocin (50 mg/kg in 0.05 mol/L of citrate buffer, pH 4.5, i.p.) on five consecutive days after overnight fast at 7 weeks of age; control (non-diabetic mice, NDM) mice were injected with citrate buffer. Diabetes was confirmed by plasma glucose levels between 13.8 and 30 mmol/L. Mice were fed for 20 weeks with western diet which contained high fat (HF; 0.20%) and the PKC-β inhibitor RBX (LY333531) at the dose of 30 mg/kg body weight/day24 in the diet from 8 to 28 weeks of age. Blood glucose was measured by Glucometer (Contour, Bayer, Inc.). Throughout the period of study, animals were provided with free access to water. At the sacrifice, animals were anaesthetized with inhaled isoflurane (1–2%). All procedures were performed according to protocols approved by the Institutional Committee for Use and Care of Laboratory Animals of the Joslin Diabetes Center and University of Sherbrooke that are conformed of the NIH guidelines.
2.2. Atherosclerotic quantitation in the aorta
The whole aorta, from just distal to the aortic sinus to the iliac bifurcation, was stained for 1 h in a filtered solution containing 0.7% Oil Red O and propylene glycol, and counterstained for 1 min in 0.05% fast green. The stained aortas were placed on a glass slide and coverslipped, then photographed (QColor3 Color FireWire 3.3 MP Digital Camera, Olympus) under polarized light through a dissection microscope on a black surface. Two exposures covering the length of the aorta were merged in the imaging software (Adobe PhotoShop version CS6) and red pixels selected using an identical colour tone for all aortas. Lesion area was measured using the Threshold Adjust and Analyze Particles commands in ImageJ (NIH), and expressed as a fraction of the total surface area of the aorta.
2.3. Histology and IF
The aortic root was embedded in optimum cutting temperature medium and 6 µm cross-sections separated by 200 µm were placed on slides. Samples were blocked with 10% goat serum for 1 h and were exposed to primary antibodies (Mac-2–FITC, 1 : 50 and α-smooth muscle actin, 1 : 100) overnight. For detection of α-smooth muscle actin, an incubation of 1 h with a secondary antibody donkey anti-rabbit–FITC (1 : 500) was performed. A second set of samples were stained with Masson's trichrome according to the textbook protocol. Images were acquired with a Hamamatsu ORCA-ER digital camera attached to a Nikon Eclipse TE-2000 inverted microscope (Nikon Canada, Mississanga, Canada) equipped for epi-illumination. Analysis of images was made using the Adobe Photoshop CS6 software.
2.4. Cell culture and treatments
Bovine aortic endothelial cells (BAECs) were isolated from fresh harvested aortas that were obtained from a local abattoir. Human monocytic (THP-1), human aortic endothelial cells (HAECs), and mouse macrophage (RAW 264.7) cell lines were obtained from ATCC (Manassas, VA, USA). For the experiments, BAEC, HAEC, and RAW 264.7 were propagated in DMEM with 5.6 mmol/L of glucose and 5% fetal bovine serum (FBS) with endothelial cell growth supplement, whereas THP-1 were grown in Roswell Park Memorial Institute (RPMI) and 10% FBS. Cells were exposed to normal glucose (5.6 + 19.4 mmol/L mannitol; NG) or HG (25 mmol/L) concentration in the presence of palmitate (100 µmol/L for BAEC, HAEC, and THP-1 and 300 µmol/L for RAW 264.7) up to 24 h. The osmotic pressure was adjusted by adding proper concentration of mannitol in the NG condition. Bovine recombinant IL-18 (rIL-18, 100 ng/mL, R&D Biosystem) and neutralizing mAb (10 ng/mL) were used during HG and palmitate exposure. The selective PKC-β inhibitor, RBX (20 nmol/L), was added in the media at the beginning of the treatments.
2.5. Cell surface expression of adhesion molecules
VCAM-1 and ICAM-1 expression on BAEC monolayers were evaluated using a cell surface ELISA. Briefly, BAECs were seeded in 96-well plates. At confluence, BAECs were exposed in NG or HG in the presence or absence of palmitate (100 µmol/L) for 24 h at 37°C. In parallel, macrophages were exposed to NG, HG, and palmitate (100 µmol/L) for 24 h and the media was collected. The BAECs were exposed to either bovine rIL-18 or collected supernatants of stimulated macrophages for the last 6 h of the 24 h exposure. Neutralizing IL-18 antibody (10 ng/mL) was added in macrophages supernatant for 30 min prior exposing EC. Then, cells were fixed with 1% paraformaldehyde, washed with PBS, and then incubated with primary anti-ICAM-1 or anti-VCAM-1 antibody in PBS containing 1% BSA for 1 h at room temperature. Negative controls included omission of primary mAb. Cells were washed three times and incubated with peroxidase-conjugated goat anti-rat IgG Fab and anti-mouse IgG at 1/2000 in PBS with 1% BSA for 1 h at 4°C. Cells were washed and incubated with substrate 1.0 mg/mL of o-phenylenediamine dihydrochloride in a buffer containing citric acid (0.05 mol/L), sodium phosphate (0.05 mol/L), pH 4, and 1 µL of 30% hydrogen peroxide per 1 mL of substrate. After 10 min, 20% (v/v) of concentrated sulfuric acid was added to stop the reaction. The absorbance in the supernatant was read at 492 nm on a TECAN infinite M200 ELISA reader and normalized by the number of cells in each well counted using crystal violet solution. This procedure involved gentle washing of the plate with PBS. Then, crystal violet solution (0.1% in water, filtered) was then added to each well (50 µL/well) and incubated for 5 min at room temperature. The cells were then washed at least three times with water and dried at room temperature. Dye was released from the cells with 100 µL/well acetic acid (33% in water) at room temperature for 30 min. The absorbance in the lysates was read at 595 nm.
2.6. Static adhesion assay
Adhesion assays were performed as described elsewhere.25 Briefly, confluent BAEC and HAEC on 48-well plates were treated with NG or HG in the presence of palmitate (100 µmol/L) and RBX (20 nmol/L) for 24 h at 37°C. Recombinant IL-18 and/or neutralizing mAb were added during the last 6 h of treatment. After treatment, cells were gently washed and exposed to a suspension of unstimulated THP-1 (2 × 105 cells/well) for another 6 h. Non-adherent THP-1 cells were removed by washing three times with PBS, and adherent cells were counted in a masked manner by digital photographs using an inverted phase-contrast microscope.
2.7. Statistical analysis
The data were shown as mean ± SEM for each group. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey's test correction for multiple comparisons. All results were considered statistically significant at P < 0.05.
3. Results
3.1. RBX treatment reduced DM atherosclerosis in DM Apoe−/− mice
A previous study has shown that PKC-β induced monocyte activation which contributed to atherosclerotic development in DM mice.12 To identify new mechanisms of PKC-β activation involved in endothelial dysfunction and accelerated atherosclerosis in diabetes, apoE-deficient mice were fed with HF diet for 20 weeks and treated with a selective inhibitor of PKC-β, RBX. Atherosclerotic plaque deposition was evaluated with Oil Red O staining to visualize atherosclerotic plaques in en face preparation. As shown in Figure 1A, atherosclerotic plaque surface was significantly increased by 3.8-fold in DM mice when compared with NDM counterparts (P < 0.0001). Treatment with RBX reduced the mean atherosclerotic lesion area by 36% in DM Apoe−/− mice compared with untreated diabetic Apoe−/− mice (P = 0.0011).
Figure 1.
PKC-β inhibition on plaque formation, complexity, and cholesteryl content in DM Apoe−/− mice. (A) En face Oil Red O staining of the whole aorta with mean atherosclerotic lesion areas values. (B) Lipids were extracted from the brachiocephalic aortic and cholesteryl ester was measured in lipid extracts by mass spectrometry. (C) Immunohistochemistry of Mac-2 (macrophage marker), α-smooth muscle cell actin, and Masson Trichrome stained in cross-sectional aortic root of NDM and DM Apoe−/− mice fed with HF diet in the presence or absence of RBX (HF+RBX) for 20 weeks. Results are shown as means ± SEM of 10 (DM) to 12 (NDM) mice/group. Scale bar, 250 µm.
3.2. PKC-β inhibitor did not affect lipid profile in DM Apoe−/− mice
The atherosclerotic plaque formation in Apoe−/− mice is driven by excess of lipids. To uncover potential mechanism by which inhibition of PKC-β reduced atherosclerosis, total cholesterol and triglyceride levels were measured. The total cholesterol (see Supplementary material online, Figure S1A) and triglycerides (see Supplementary material online, Figure S1B) levels were significantly elevated in DM vs. NDM Apoe−/− mice (P = 0.0213 and 0.0488, respectively). In contrast, we did not observe any change with RBX treatment, indicating that triglyceride and total cholesterol concentrations were not affected by the PKC-β inhibitor. Lipid profile was assessed in each group of mice. Although diabetes increased low-density lipoprotein (LDL) content, lipid profile did not change with RBX treatment, suggesting that the PKC-β inhibitor effect on atherosclerosis is not caused by the decrease in LDL content (see Supplementary material online, Figure S1C).
3.3. PKC-β inhibitor decreased macrophage infiltration and plaque complexity
It is well known that most of the cholesterol that accumulates in the arterial plaques is in ester form. Moreover, this cholesterol is derived from infiltrated plasma lipoproteins by cells of the developing plaque, such as macrophages.26 Thus, atherosclerotic lesions were assessed in all groups of mice by quantitatively extracting lipids from the brachiocephalic artery (BCA) with chloroform/methanol. In Figure 1B, cholesteryl ester content in the BCA was more than two-fold higher in DM compared with NDM Apoe−/− mice (17.68 ± 3.01 vs. 54.20 ± 10.87 mg/dL, respectively, P = 0.0006). In accordance with the surface area of the plaque, cholesteryl ester levels were reduced by RBX treatment in DM Apoe−/− mice compared with non-treated diabetic Apoe−/− mice (54.20 ± 10.87 and 23.37 ± 6.65 mg/dL, respectively, P = 0.0076). Cross-sections of the aortic root were used to analyse the complexity of the plaque. We evaluated macrophage infiltration, the presence of smooth muscle cells in the fibrous cap and the presence of necrotic cores within the atherosclerotic plaque, all important markers of advanced atherosclerosis development. Using a macrophage marker (anti-Mac-2 antibody), we showed that the area occupied by macrophages was higher in DM compared with NDM Apoe−/− mice (Figure 1C). Notably, the presence of macrophages was reduced in DM Apoe−/− mice treated with the PKC-β inhibitor. The presence of both smooth muscle cells and necrotic core (black arrows) within the plaque was increased in DM Apoe−/− mice which was prevented with RBX treatment (Figure 1C). These data indicate that DM Apoe−/− mice exhibited more atherosclerotic lesion complexity and RBX treatment diminished advanced atherosclerotic plaque formation in DM Apoe−/− mice.
3.4. DM Apoe−/− mice treated with RBX exhibited decreased IL-18BP, but not IL-18, expression in the aorta
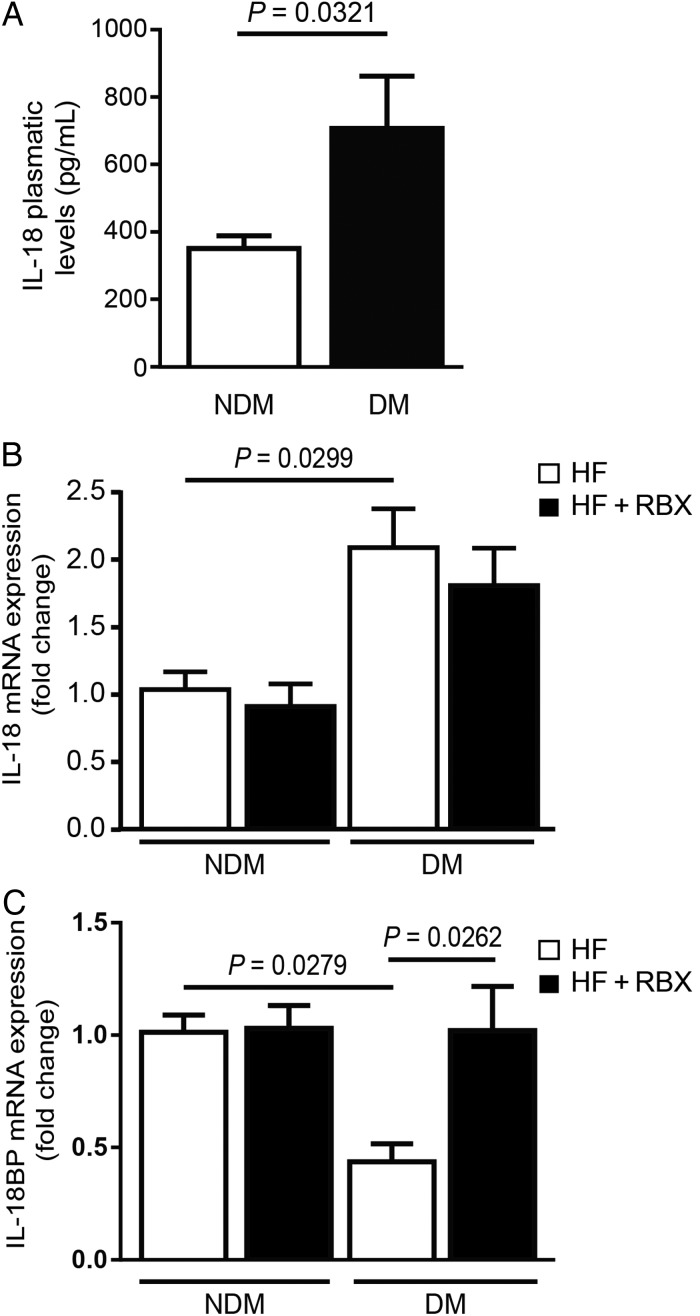
IL-18 is a pro-inflammatory and pro-atherogenic cytokine which strongly promotes macrophage infiltration. On the other hand, the IL-18 endogenous inhibitor, IL-18BP, has been shown to exert anti-atherogenic actions.27 However, little is known about the IL-18/IL-18BP regulation pathway in DM conditions. We measured serum and mRNA expression of IL-18 in all four groups of mice. We observed a significant increase of both plasmatic (Figure 2A) and mRNA levels (Figure 2B) of IL-18 in DM vs. NDM Apoe−/− mice (P = 0.0321 and 0.0299, respectively). However, RBX treatment did not affect IL-18 mRNA expression in DM mice. Interestingly, IL-18BP mRNA expression was significantly decreased by 57% in DM Apoe−/− mouse aorta compared with its NDM counterpart (P = 0.0279, Figure 2C). RBX treatment restored IL-18BP expression in DM Apoe−/− mice (P = 0.0262; Figure 2C), showing that PKC-β activation is responsible for the down-regulation of IL-18BP expression in DM aorta. We did not observe any significant change in expression of IL-18 receptor (data not shown).
Figure 2.
Serum IL-18 levels and IL-18BP expression in the aorta on NDM and DM Apoe−/− mice. (A) Plasma circulating and (B) mRNA levels of IL-18 as well as (C) IL-18BP mRNA expression in the whole aorta of NDM and DM Apoe−/− mice fed with HF diet in the presence or absence of RBX (HF+RBX) for 20 weeks. Results are shown as means ± SEM of 10 (DM) to 12 (NDM) mice/group.
3.5. HG and palmitate levels are necessary to induce IL-18 protein expression and secretion in macrophages
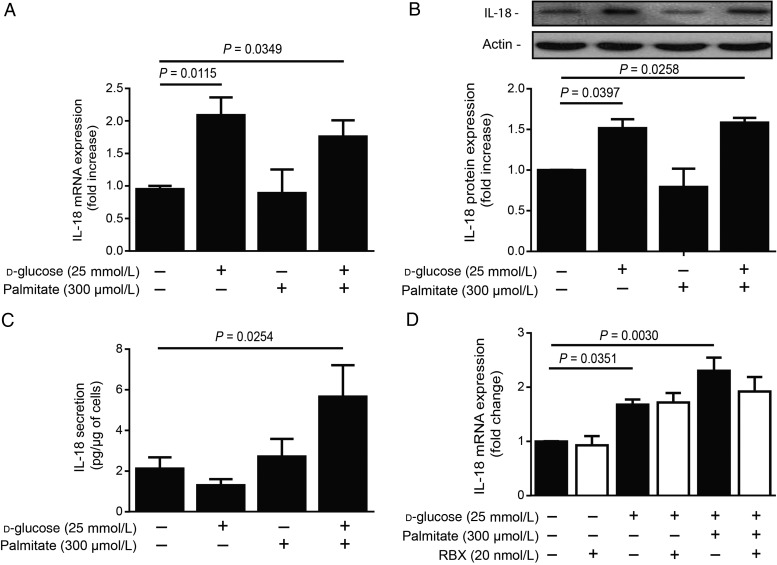
IL-18 is mainly produced by activated macrophages.16 Thus, macrophages were treated with HG in the presence of palmitate (300 µmol/L) for 24 h. IL-18 mRNA expression was significantly increased with HG alone or in combination with palmitate compared with NG conditions (Figure 3A). Immunoblot analysis confirmed elevated IL-18 protein expression in macrophages exposed to HG alone or in the presence of palmitate (Figure 3B). To asses if increased expression of IL-18 corroborated with its secretion, IL-18 levels were measured in the macrophage culture media. Interestingly, secreted IL-18 levels were significantly elevated by 37% in the media of macrophages exposed to HG + palmitate combination (P = 0.0254; Figure 3C). However, IL-18 mRNA expression did not change with RBX treatment in HG and palmitate conditions, suggesting that PKC-β did not modulate IL-18 production in macrophages (Figure 3D).
Figure 3.
IL-18 expression and release in macrophages exposed to HG levels and palmitate. (A) mRNA, (B) protein expression, and (C) secretion of IL-18 in mouse macrophage lines (RAW 267.4) treated with NG (5.6 + 19.4 mmol/L mannitol) or HG (25 mmol/L) levels in the presence of palmitate (300 µmol/L) for 24 h. (D) IL-18 mRNA expression in mouse macrophage exposed to HG and palmitate in the absence (white bars) or in the presence (black bars) of RBX (20 nmol/L) for 24 h. Protein expression was detected by western blot and densitometric quantitation was measured. Results are shown as means ± SEM of 4 (B and D) to 7 (A and C) independent experiments.
3.6. The combination of HG and FFA conditions is required for PKC-β-induced reduction of IL-18BP expression in EC
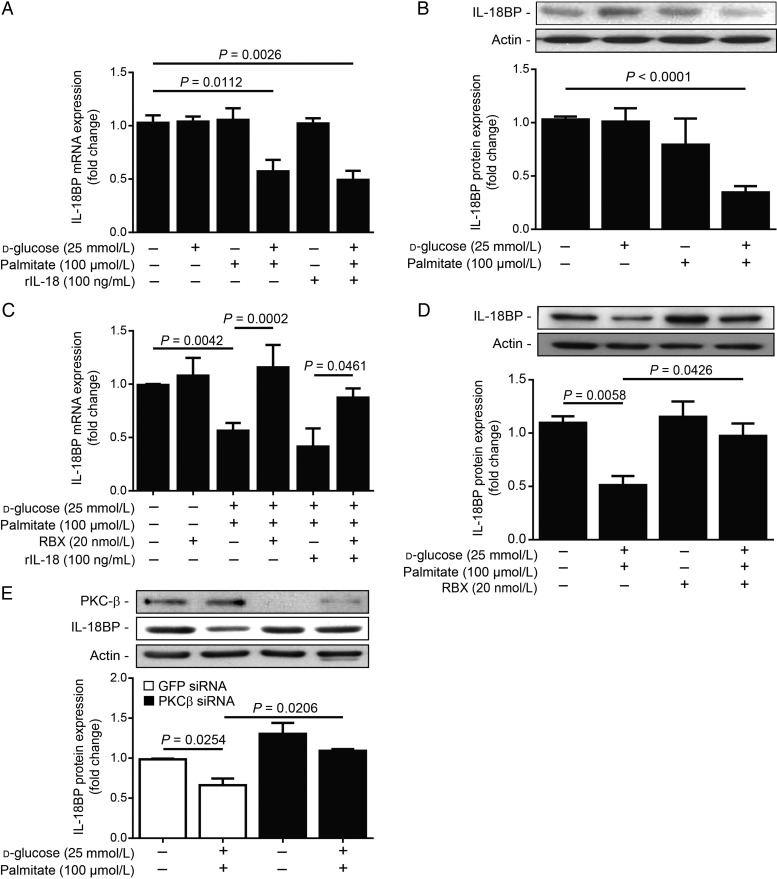
Since HG + palmitate exposure increased IL-18 production and secretion in macrophages, it may influence EC function. First, we assessed the expression of IL-18 in BAEC in the HG condition in the presence of palmitate. Treatment with HG and/or palmitate did not affect IL-18 or IL-18 receptor expression (data not shown). While no change was observed in HG or palmitate alone, the combination of HG + palmitate decreased mRNA (Figure 4A; P = 0.0112) and protein (Figure 4B; P < 0.0001) expression of IL-18BP in BAEC and HAEC (see Supplementary material online, Figure S2). Recombinant IL-18 stimulation did not influence the expression of IL-18BP in either NG or HG + palmitate (Figure 4A). Translocation of PKC isoform to the membrane fraction is an indication of its activity. As expected, exposing EC to HG + palmitate increased the translocation to the membrane of PKC- β isoform (see Supplementary material online, Figure S3). Since our in vivo data suggested that the reduction of IL-18BP in DM Apoe−/− mice fed with HF diet is regulated by PKC-β, we treated BAEC with RBX during HG and palmitate exposure. As shown in Figure 4C and D, treatment with RBX prevented the loss of IL18BP mRNA and protein expression in EC exposed to the HG + palmitate condition. Moreover, the diminution of IL-18BP in the HG + palmitate condition is prevented by PKC-β siRNA (Figure 4E). These data suggest that PKC-β activation induced by HG and palmitate exposure is required for the reduction of IL-18BP expression in EC.
Figure 4.
PKC-β inhibition restored IL-18BP expression in ECs exposed to HG levels and palmitate. IL-18BP (A) mRNA and (B) protein expression in BAECs exposed to NG (5.6 + 19.4 mmol/L mannitol) or HG (25 mmol/L) levels in the presence or absence of palmitate (100 µmol/L) and rIL-18 (100 ng/mL), and (C and D) treated or not treated with RBX (20 nmol/L) for 24 h. (E) IL-18BP and PKC-β protein expression after treatment with PKC-β siRNA. Protein expression was detected by western blot and densitometric quantitation was measured. Results are shown as means ± SEM of 4 (A, C, and E) to 5 (B and D) independent experiments.
3.7. Reduced IL-18BP expression is associated with increased VCAM-1 expression in EC
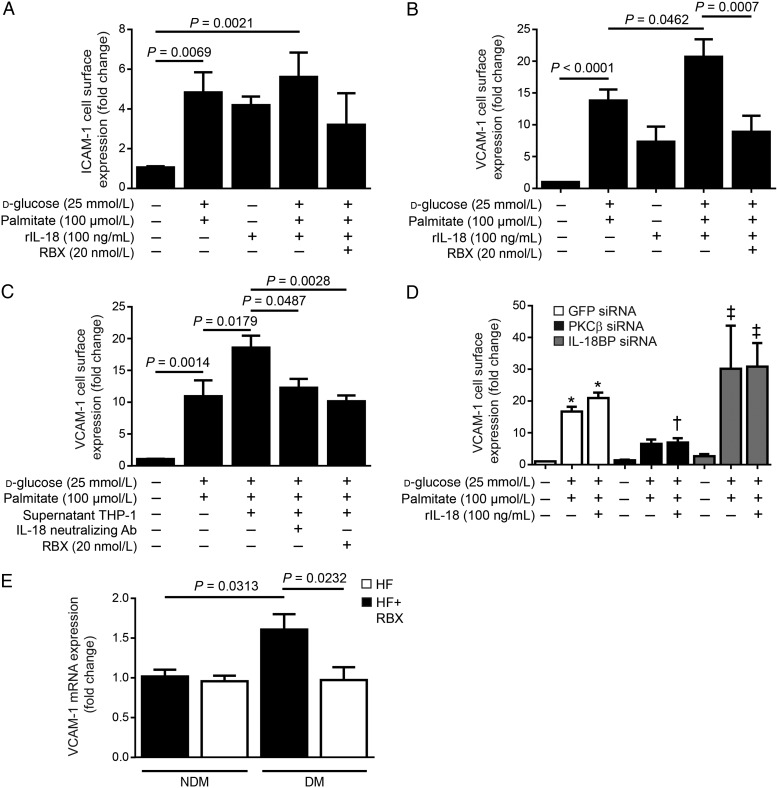
IL-18 can regulate the expression of adhesion molecules on EC.28 Therefore, we performed ELISA to evaluate if PKC-β activation increased the cell surface expression of VCAM-1 and ICAM-1, two well-known adhesion molecules to be involved in EC dysfunction and atherosclerotic initiation and development. We demonstrated that combination of both HG and palmitate elevated the expression of ICAM-1 (P = 0.0069, Figure 5A) and VCAM-1 (P < 0.0001, Figure 5B) by 3.6- and 12.6-fold, respectively. We showed that IL-18 treatment alone increased the expression of these two adhesion molecules. Interestingly, the addition of rIL-18 exacerbated VCAM-1, but not ICAM-1, surface expression in EC that were exposed to HG and palmitate (P = 0.0462, Figure 5B). Treatment of BAEC with RBX significantly reduced VCAM-1 cell surface expression in HG + palmitate conditions in the presence of IL-18 (P = 0.0007, Figure 5B) without affecting significantly ICAM-1 expression (Figure 5A). These data indicate that PKC-β activation modulated VCAM-1, but not ICAM-1, expression. Since our data demonstrated that macrophages exposed to HG + palmitate secreted more IL-18 into the media, we evaluated the role of IL-18 from activated monocytes causing elevated VCAM-1 cell surface expression in HG and palmitate conditions. BAECs were treated with HG + palmitate for 18 h. In parallel, media of THP-1 cells treated for 24 h in the presence of HG + palmitate were collected. Following this period, media of THP-1 treated with HG + palmitate were added to EC with or without a specific inhibitor of IL-18 for 6 h. As shown in Figure 5C, EC exposed to THP-1 media, which contained secreted IL-18 and other factors, exhibited elevated VCAM-1 cell surface expression when compared with EC exposed only to HG + palmitate (P = 0.0179). To determine the implication of IL-18 action in VCAM-1 expression in EC exposed to THP-1 media, neutralizing IL-18 mAb was added in the media of monocytes treated with the HG + palmitate condition. Interestingly, neutralizing IL-18 antibody reduced VCAM-1 expression to the level of the HG + palmitate condition (P = 0.0487). These data suggest that IL-18 secreted by monocytes contributed to elevated VCAM-1 cell surface expression in EC. Furthermore, EC treated with RBX and then exposed to THP-1 media exhibited significantly lower VCAM-1 cell surface expression (P = 0.0028), results that were similar to those obtained in the presence of the specific inhibitor of IL-18. Although 20 nM of RBX is selective to PKC-β isoform, siRNA targeting PKC-β was used as a complementary strategy. BAEC treated with PKC-β siRNA showed similar results as RBX treatment and prevented VCAM-1 cell surface expression (Figure 5D). Treatment with IL-18BP siRNA reduced IL-18BP expression in NG and HG + palmitate conditions (see Supplementary material online, Figure S4), which exacerbated the effects of HG + palmitate on VCAM-1 expression (Figure 5D). To verify whether VCAM-1 is regulated by PKC-β activation in vivo, we measured VCAM-1 mRNA expression in aorta of NDM and DM Apoe−/− mice. We showed that diabetes up-regulated VCAM-1 expression, which was prevented by the RBX treatment (Figure 5E).
Figure 5.
RBX treatment prevented elevated cell surface expression of VCAM-1 induced by HG levels, palmitate, and IL-18. Cell surface expression of (A) ICAM-1 and (B and C) VCAM-1 measured by ELISA in BAEC exposed to NG (5.6 + 19.4 mmol/L mannitol), HG (25 mmol/L) levels, palmitate (100 µmol/L), and RBX (20 nmol/L) for 24 h in the presence of IL-18 (100 ng/mL). (C) Macrophages were exposed to HG and palmitate for 24 h. The supernatant was collected and IL-18 neutralizing antibody (Ab) was added for 30 min. Macrophage supernatant with or without IL-18 neutralizing Ab was added to BAEC for 6 h. (D) VCAM-1 cell surface expression after treatment of BAEC with PKC-β and IL-18BP siRNA. (E) Quantitative PCR of VCAM-1 mRNA expression in the aorta of NDM and DM Apoe−/− mice fed with HF diet in the presence or absence of RBX for 20 weeks. Results are shown as means ± SEM of 4 (A, C, D, and E) to 7 (B) independent experiments. *P < 0.05 vs. NG in GFP siRNA cells; †P < 0.05 vs. HG + palmitate + rIL-18 in GFP siRNA cells; ‡P < 0.05 vs. NG in IL-18BP siRNA cells.
3.8. PKC-β activation-induced reduction of IL-18BP expression promotes monocyte adhesion on EC
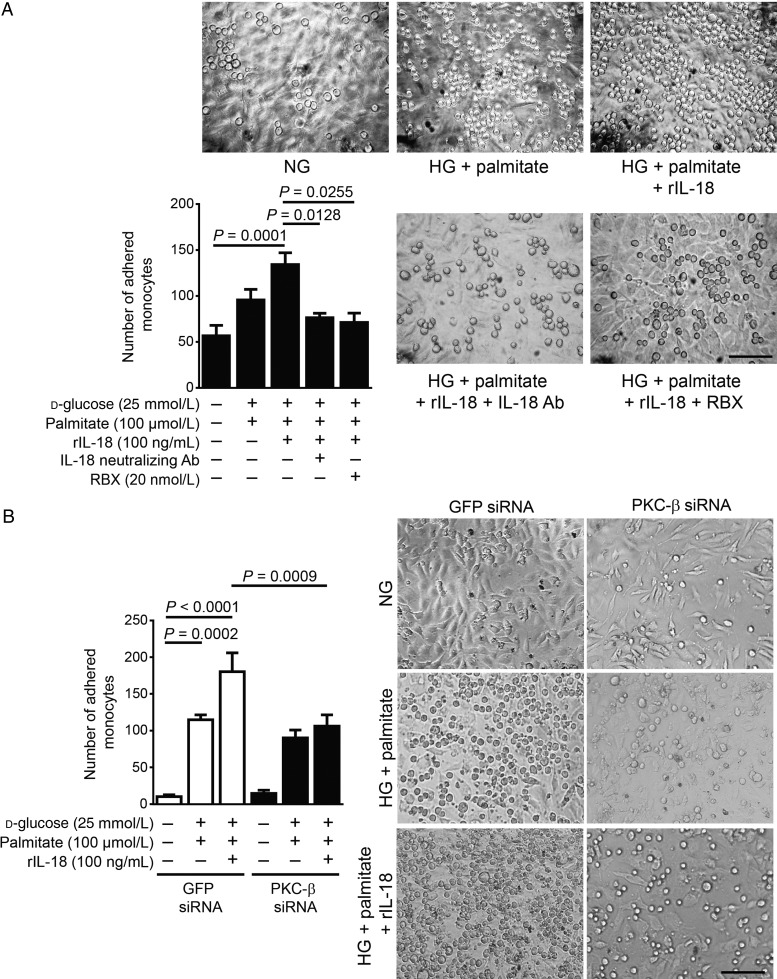
To determine whether the increased expression of VCAM-1 due to the presence of IL-18 in the HG + palmitate condition is sufficient to enhance the adhesion of monocytes on EC contributing to atherosclerotic plaque formation, we performed monocyte adhesion assay on EC. HG + palmitate exposure significantly induced monocyte adhesion when compared with the NG condition in both BAEC (Figure 6A) and HAEC (see Supplementary material online, Figure S5). The addition of rIL-18 in the media raised the number of attached monocytes when compared with HG + palmitate alone, suggesting that IL-18 contributed to the increase of monocyte adhesion on EC (P = 0.0001, Figure 6A). The use of the neutralizing IL-18 antibody, RBX, and PKC-β siRNA treatment significantly prevented monocyte adhesion on EC by 81, 74, and 64%, respectively, compared with the HG + palmitate + rIL-18 condition.
Figure 6.
Monocyte adherence to EC induced by HG, palmitate, and IL-18 is reduced by PKC-β inhibition. BAECs were exposed to NG (5.6 + 19.4 mmol/L mannitol), HG (25 mmol/L) levels, palmitate (100 µmol/L), and (A) RBX (20 nmol/L) or (B) treated with PKC-β siRNA for 24 h. Recombinant IL-18 (rIL-18, 100 ng/mL) premixed or not with IL-18 neutralizing antibody was added to the media for 6 h. Images are representative of the mean of adherent monocytes per image area. Results are shown as means ± SEM of 5 (B) to 6 (A) independent experiments. Scale bar, 100 µm.
4. Discussion
Atherosclerosis is one such clinical manifestation of pro-inflammatory state associated with the vasculature. The exact mechanism by which metabolic stress induces the pro-inflammatory milieu and promotes advanced atherogenesis in the context of diabetes remains elusive. Many biochemical pathways have been proposed to link hyperglycaemia and accelerated atherosclerosis including PKC activation. In the present study, we demonstrated that accelerated atherosclerosis and plaque complexity in DM Apoe−/− mice were significantly reduced by a treatment with a selective PKC-β inhibitor. Moreover, we uncovered a new mechanism by which PKC-β decreased IL-18BP expression in EC which enhanced IL-18 action leading to increase surface expression of VCAM-1 in EC causing monocyte adhesion, macrophage infiltration, and atherosclerotic plaque formation in diabetes.
Among the different isoforms of PKC, PKC-β has been recognized to play a major role in endothelial dysfunction of vascular tissues that are affected by diabetes including the retina, the kidney, and peripheral vessels. Not surprisingly, the use of a selective PKC-β inhibitor to treat vascular complications has been studied29 and showed positive effects to prevent retinopathy and nephropathy in DM animal models as well as to preserve visual acuity in DM patients.30–32 Yang's group has recently published that both lack of PKC-β gene and RBX treatment reduced atherosclerotic plaque formation in DM apoE-deficient mice, mainly affecting macrophage ability to promote Egr-1, chemokine (C–C motif) ligand 2, and IL-1β expression.12 However, potential benefit of RBX treatment on EC in a context of diabetes was not reported. In our study, we demonstrated that inhibition of PKC-β restored IL-18BP expression in the aorta preventing macrophage infiltration and advanced atherosclerosis. Interestingly, we have reported that overexpression of PKC-β, specifically in EC, in the absence of diabetes reduced insulin-stimulated Akt/endothelial nitric oxide synthase activation causing endothelial dysfunction and atherosclerosis.33 Overall, previous and present data suggest that PKC-β isoform is involved in endothelial and macrophage dysfunction in diabetes.
Chronic inflammation is the hallmark of atherosclerosis and epidemiology studies showed strong correlation between inflammatory markers and severity or risk of cardiovascular diseases.2 ILs are considered to be a key factor in the induction and development of chronic vascular inflammatory response. ILs such as IL-1, IL-6, and IL-17 are known to contribute to atherosclerosis.34,35 However, even if serum IL-6 is significantly associated with cardiovascular risk, it did not substantially improve risk prediction above traditional risk factors in a DM cohort.36 Among the plethora of IL, high serum levels of IL-18 have been related with the metabolic syndrome, obesity, type 2 diabetes, and their consequences.37,38 Two studies have shown that high risk of diabetes correlated with elevated serum levels of IL-18.18,22 Li and collaborators demonstrated that a single intravenous dose of IL-18BP significantly decreased arterial neointimal hyperplasia, improved lumen-to-artery ratio after balloon injury, and prevented arteriosclerotic progression.27 Our data suggest that the IL-18/IL-18BP pathway is de-regulated in the aorta of DM mouse model of atherosclerosis. DM Apoe−/− mice exhibited high plasmatic levels of IL-18. Moreover, we observed that level of IL-18BP expression is significantly reduced in aorta correlating with increased plaque deposition in the arterial wall. Interestingly, the importance of sufficient amount of IL-18BP expression has been demonstrated to be a key factor in other pathogenesis. For example, pathologies such as the WG and SLE exhibit elevated expression of both IL-18BP and IL-18,39 but the level of IL-18BP was not sufficiently high to neutralize IL-18 leading to a net increase in free IL-18 compared with healthy subjects. Although most of the ILs, such as IL-1, IL-6, and IL-8, have been previously reported to be activated by PKC,6 our study showed for the first time that the reduction of the IL-18 endogenous inhibitor, IL-18BP, in EC exposed to the HG and palmitate condition is regulated by PKC-β activation and inhibition of PKC-β by RBX and siRNA treatment restored the ratio of IL-18/IL-18BP in diabetes.
Up-regulation of cellular adhesion molecule expression, such as VCAM-1 and ICAM-1, is essential to allow the recruitment of monocytes from the circulation and their transendothelial migration. Moreover, cell surface expression of VCAM-1 and ICAM-1 can play an important role in endothelial dysfunction, an early step in the development of atherosclerotic plaque. Several clinical studies clearly showed that ICAM-1 and VCAM-1 expression correlated with the degree of atherosclerosis in type 2 DM patients.40 A previous study has reported that deletion of the Prkcb gene in NDM Apoe−/− mice reduced VCAM-1 expression in ECs and lowered atherosclerosis, compared with Apoe−/− mice.11 Our results indicate that the PKC-β inhibition prevented IL-18 actions on VCAM-1 expression, probably as a consequence of the re-establishment of IL-18BP expression in EC treated with RBX and siRNA. These data unrevealed a new pathway by which PKC-β activation contributes to EC dysfunction. In contrast, surface expression ICAM-1 was not affected by the addition of IL-18 when ECs were exposed to the HG and palmitate condition. Furthermore, we found that RBX treatment did not modulate ICAM-1 expression, suggesting that IL-18/IL-18BP deregulation did not influence ICAM-1 expression. Although expression of both VCAM-1 and ICAM-1 is up-regulated in atherosclerotic lesion, Cybulsky et al.41 reported that VCAM-1 plays a dominant role in the initiation of atherosclerosis.
One of the consequences of IL-18/IL-18BP deregulation is the increase of vascular inflammation and remodelling. VCAM-1 plays an important role in regulating the binding of leukocytes to EC, and its reduction can attenuate the development of atherosclerosis. Our study demonstrated that disruption in the equilibrium between IL-18 and IL-18BP expression resulted in elevated expression of VCAM-1. A previous study reported that IL-18 increased VCAM-1 expression in EC independently of IFN-γ in the metastatic process.42 To specifically evaluate IL-18 effect on VCAM-1 expression, we exposed EC with media of monocytes treated in HG and palmitate conditions in the presence of neutralizing antibody against IL-18 or RBX. Our data indicated that IL-18 neutralizing antibody, RBX, and PKC-β siRNA treatment reduced cell surface expression of VCAM-1 induced by HG, palmitate, and IL-18 conditions preventing adherence of monocytes on EC. Monocyte adhesion in EC stimulated with the rIL-18 is abolished with RBX and PKC-β siRNA treatment and neutralizing antibody of IL-18. Our data suggest that IL-18 participated to monocyte activity and EC dysfunction which can be inhibited by PKC-β inhibitor and siRNA. The pro-atherogenic ability of IL-18 has been shown in vivo in a rat model with the metabolic syndrome where IL-18 overexpression has aggravated insulin resistance, increased vascular inflammation, and promoted remodelling by enhancing infiltration of macrophages and medial thickness in the aortic wall.43 IL-18 is not the only cytokine secreted by macrophages. Another study has shown that HG and palmitate stimulation increased IL-1 and monocyte chemoattractant protein-1 expression in THP-1 cells.44 According to our results, PKC-β inhibition did not completely prevented monocyte adhesion, suggesting that PKC-β may not regulate all ILs and cytokines involved in monocyte attachment and potentially other PKC isoforms or other glucose and lipid metabolites may contribute to monocyte adhesion and atherosclerosis in diabetes.
In summary, our results showed a novel mechanism by which PKC-β activation reduces IL-18BP expression causing endothelial dysfunction, monocyte adhesion, and accelerated atherosclerosis in DM Apoe−/− mice fed with HF diet. Our study provides a new insight of the molecular mechanisms of endothelial dysfunction caused by IL-18/IL-18BP de-regulation induced by PKC-β activation in diabetes. The inhibition of PKC-β and potentially IL-18 could be served as possible strategies for the prevention of accelerated atherosclerosis in diabetes.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study was supported by grants from the Canadian Institute of Health Research (MOP 130334 to P.G.), ‘Fonds de Recherche du Québec – Santé’ (FRQS), and the ‘Centre Hospitalier Universitaire de Sherbrooke’ (CHUS) foundation to P.G. This work was performed at the CHUS research center, funded by the FRQS. P.G. is currently the holder of the Canadian Research Chair in Vascular Complications of Diabetes and the recipient of a Scholarship Award from the Canadian Diabetes Association.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Anne Vezina for her assistance with histochemistry techniques.
Conflict of interest: M.R. is employed by Lilly Research Laboratories. Lilly Research Laboratories supported the preclinical use of ruboxistaurin and analytical work.
References
- 1. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 3. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002;287:2570–2581. [DOI] [PubMed] [Google Scholar]
- 4. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 2010;106:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J 2003;376:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kontny E, Ziolkowska M, Ryzewska A, Maslinski W. Protein kinase C-dependent pathway is critical for the production of pro-inflammatory cytokines (TNF-alpha, IL-1beta, IL-6). Cytokine 1999;11:839–848. [DOI] [PubMed] [Google Scholar]
- 7. Aksoy E, Amraoui Z, Goriely S, Goldman M, Willems F. Critical role of protein kinase C epsilon for lipopolysaccharide-induced IL-12 synthesis in monocyte-derived dendritic cells. Eur J Immunol 2002;32:3040–3049. [DOI] [PubMed] [Google Scholar]
- 8. Kamanna VS, Pai R, Bassa B, Kirschenbaum MA. Activation of mesangial cells with TNF-alpha stimulates M-CSF gene expression and monocyte proliferation: evidence for involvement of protein kinase C and protein tyrosine kinase. Biochim Biophys Acta 1996;1313:161–172. [DOI] [PubMed] [Google Scholar]
- 9. Booth G, Stalker TJ, Lefer AM, Scalia R. Mechanisms of amelioration of glucose-induced endothelial dysfunction following inhibition of protein kinase C in vivo. Diabetes 2002;51:1556–1564. [DOI] [PubMed] [Google Scholar]
- 10. Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res 2002;90:1012–1019. [DOI] [PubMed] [Google Scholar]
- 11. Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. Faseb J 2009;23:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kong L, Shen X, Lin L, Leitges M, Rosario R, Zou YS, Yan SF. PKCbeta promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler Thromb Vasc Biol 2013;33:1779–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol 2013;4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellora F, Castriconi R, Doni A, Cantoni C, Moretta L, Mantovani A, Moretta A, Bottino C. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol 2012;42:1618–1626. [DOI] [PubMed] [Google Scholar]
- 15. Reddy VS, Valente AJ, Delafontaine P, Chandrasekar B. Interleukin-18/WNT1-inducible signaling pathway protein-1 signaling mediates human saphenous vein smooth muscle cell proliferation. J Cell Physiol 2011;226:3303–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001;104:1598–1603. [DOI] [PubMed] [Google Scholar]
- 17. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 2005;117:152–160. [DOI] [PubMed] [Google Scholar]
- 19. Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care 2003;26:2622–2627. [DOI] [PubMed] [Google Scholar]
- 20. Wheeler RD, Young EA, Rothwell NJ, Hall MD, Luheshi GN. Up-regulation of IL-18BP, but not IL-18 mRNA in rat liver by LPS. Cytokine 2003;21:161–166. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura A, Shikata K, Hiramatsu M, Nakatou T, Kitamura T, Wada J, Itoshima T, Makino H. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 2005;28:2890–2895. [DOI] [PubMed] [Google Scholar]
- 22. Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB. Circulating IL-18 and the risk of type 2 diabetes in women. Diabetologia 2009;52:2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, Humbert Y, Chvatchko Y, Tedgui A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res 2001;89:E41–E45. [DOI] [PubMed] [Google Scholar]
- 24. Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 2006;55:691–698. [DOI] [PubMed] [Google Scholar]
- 25. Huber J, Furnkranz A, Bochkov VN, Patricia MK, Lee H, Hedrick CC, Berliner JA, Binder BR, Leitinger N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J Lipid Res 2006;47:1054–1062. [DOI] [PubMed] [Google Scholar]
- 26. Smith EB. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res 1974;12:1–49. [DOI] [PubMed] [Google Scholar]
- 27. Li JM, Eslami MH, Rohrer MJ, Dargon P, Joris I, Hendricks G, Baker S, Cutler BS. Interleukin 18 binding protein (IL18-BP) inhibits neointimal hyperplasia after balloon injury in an atherosclerotic rabbit model. J Vasc Surg 2008;47:1048–1057. [DOI] [PubMed] [Google Scholar]
- 28. Meager A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev 1999;10:27–39. [DOI] [PubMed] [Google Scholar]
- 29. Joy SV, Scates AC, Bearelly S, Dar M, Taulien CA, Goebel JA, Cooney MJ. Ruboxistaurin, a protein kinase C beta inhibitor, as an emerging treatment for diabetes microvascular complications. Ann Pharmacother 2005;39:1693–1699. [DOI] [PubMed] [Google Scholar]
- 30. Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 1996;272:728–731. [DOI] [PubMed] [Google Scholar]
- 31. PKC-DRS Study Group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes 2005;54:2188–2197. [DOI] [PubMed] [Google Scholar]
- 32. Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005;28:2686–2690. [DOI] [PubMed] [Google Scholar]
- 33. Li Q, Park K, Li C, Rask-Madsen C, Mima A, Qi W, Mizutani K, Huang P, King GL. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circ Res 2013;113:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb 2010;17:332–341. [DOI] [PubMed] [Google Scholar]
- 35. Kumar P, Natarajan K, Shanmugam N. High glucose driven expression of pro-inflammatory cytokine and chemokine genes in lymphocytes: molecular mechanisms of IL-17 family gene expression. Cell Signal 2014;26:528–539. [DOI] [PubMed] [Google Scholar]
- 36. Herder C, Schottker B, Rothenbacher D, Roden M, Kolb H, Muller H, Brenner H. Interleukin-6 in the prediction of primary cardiovascular events in diabetes patients: results from the ESTHER study. Atherosclerosis 2011;216:244–247. [DOI] [PubMed] [Google Scholar]
- 37. Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol 2007;157:465–471. [DOI] [PubMed] [Google Scholar]
- 38. Zirlik A, Abdullah SM, Gerdes N, MacFarlane L, Schonbeck U, Khera A, McGuire DK, Vega GL, Grundy S, Libby P, de Lemos JA. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: results from the Dallas Heart Study. Arterioscler Thromb Vasc Biol 2007;27:2043–2049. [DOI] [PubMed] [Google Scholar]
- 39. Novick D, Elbirt D, Dinarello CA, Rubinstein M, Sthoeger ZM. Interleukin-18 binding protein in the sera of patients with Wegener's granulomatosis. J Clin Immunol 2009;29:38–45. [DOI] [PubMed] [Google Scholar]
- 40. Rubio-Guerra AF, Vargas-Robles H, Serrano AM, Vargas-Ayala G, Rodriguez-Lopez L, Escalante-Acosta BA. Correlation between the levels of circulating adhesion molecules and atherosclerosis in hypertensive type-2 diabetic patients. Clin Exp Hypertens 2010;32:308–310. [DOI] [PubMed] [Google Scholar]
- 41. Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 2001;107:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carrascal MT, Mendoza L, Valcarcel M, Salado C, Egilegor E, Telleria N, Vidal-Vanaclocha F, Dinarello CA. Interleukin-18 binding protein reduces b16 melanoma hepatic metastasis by neutralizing adhesiveness and growth factors of sinusoidal endothelium. Cancer Res 2003;63:491–497. [PubMed] [Google Scholar]
- 43. Tan HW, Liu X, Bi XP, Xing SS, Li L, Gong HP, Zhong M, Wang ZH, Zhang Y, Zhang W. IL-18 overexpression promotes vascular inflammation and remodeling in a rat model of metabolic syndrome. Atherosclerosis 2010;208:350–357. [DOI] [PubMed] [Google Scholar]
- 44. Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011;300:E145–E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.