Abstract
Aims
The aim of this study is to simultaneously evaluate the incremental prognostic value of multiple cardiac biomarkers reflecting different underlying pathophysiological processes in a well-characterized population of patients with non-ST-segment acute coronary syndrome (NSTE-ACS).
Methods and results
We measured cardiac troponin I (cTnI), N-terminal pro B-type natriuretic peptide (NT-proBNP), C-reactive protein, and myeloperodixase (MPO) among 4352 patients with NSTE-ACS in the MERLIN-TIMI 36 (Metabolic Efficiency With Ranolazine for Less Ischaemia in Non-ST Elevation Acute Coronary-Thrombolysis In Myocardial Infarction 36) trial and followed them for a mean of 343 days. When added individually to a multivariable model adjusted for clinical characteristics, the risk of cardiovascular (CV) death rose in a stepwise fashion with increasing quartiles of each biomarker, and when using their pre-defined cut-points [HRadj 2.71 (P < 0.001) for cTnI ≥0.03 ng/mL; HRadj 3.01 (P < 0.001) for NT-proBNP ≥400 pg/mL; HRadj 1.45 (P = 0.019) for high-sensitivity (hs) C-reactive protein ≥15 mg/L; and HRadj 1.49 (P = 0.006) for MPO ≥670 pmol/L]. After including all biomarkers, only NT-proBNP and cTnI were independently associated with CV death, and only cTnI with myocardial infarction (MI). The addition of NT-proBNP to a model adjusted for TIMI risk score incorporating cTnI significantly improved both the discrimination and re-classification of the model for CV death and heart failure (HF) while there was no such improvement after the addition of either MPO or hs-C-reactive protein.
Conclusion
In this study of over 4300 patients presenting with NSTEACS, we found that both cTnI and NT-proBNP offer prognostic information beyond that achieved with clinical risk variables for CV death, MI, and HF. Myeloperoxidase and hs-C-reactive protein, while independently associated with some adverse CV outcomes, did not provide substantial incremental prognostic information when evaluated together with cTnI and NT-proBNP.
Keywords: Acute coronary syndrome, Biomarkers, Risk-stratification, Troponin, Natriuretic peptides, Myeloperoxidase
Introduction
Cardiac biomarkers are an integral component in the evaluation and risk-stratification of patients with cardiac diseases in general and acute coronary syndromes (ACS) in particular.1 Simultaneous evaluation of multiple cardiac biomarkers, which reflect different underlying pathophysiological processes, have been shown to offer complimentary prognostic information,2–5 but professional guidelines have not advocated the routine use of a multimarker strategy because of the need for additional validation in large studies of well-characterized patients with established risk indicators and the establishment of specific ties to therapy.1,6 Although prior studies have utilized different combinations of biomarkers, panels that include biomarkers representing the different underlying pathobiological processes in ACS have the greatest potential to provide incremental prognostic information. Evaluation of new biomarkers should also be conducted in the context of the two most established and studied biomarkers, troponin and natriuretic peptides.
Myeloperoxidase (MPO) is a haemoprotein, released from neutrophils and monocytes, that has been implicated in the development and subsequent instability of atherosclerotic plaques.7 Elevated levels of MPO have been shown to be independently associated with an increased risk of cardiovascular (CV) death and recurrent ischaemia in patients with ACS.4,8–10 However, the prognostic value of MPO beyond that of currently used clinical risk tools and biomarkers is still incompletely characterized. There is a need to evaluate this and other novel cardiac biomarkers in large, prospective, clinical studies to determine whether they offer independent and complimentary information when assessed together with established clinical and biochemical risk-stratification techniques.11–13
We therefore set out, first, to prospectively evaluate a ‘multimarker’ approach to risk-stratification using markers of necrosis, stress, and inflammation, employing contemporary statistical methods; and, second, to determine whether levels of MPO measured at the time of admission improve the risk-stratification of patients with non-ST-segment elevation ACS (NSTE-ACS).
Methods
Patient population
The design and primary results of the MERLIN-TIMI 36 trial have been published previously.14,15 Eligible patients had at least 10 min of ischaemic symptoms at rest and presented with one of the following: elevated biomarkers of myonecrosis, ST-depression ≥0.1 mV, history of diabetes mellitus, or an intermediate to high (≥3) TIMI risk score. Exclusion criteria included persistent ST-segment elevation, end-stage renal disease requiring dialysis, cardiogenic shock, or a life-expectancy less than 12 months. Patients were randomized in a 1:1 ratio to receive intravenous ranolazine followed by oral ranolazine or matching placebo. The protocol was approved by the relevant institutional review boards, and written consent was obtained from all patients, including for the biomarker study.
Biomarker testing
The protocol specified that blood samples be obtained at enrolment in serum-separator and EDTA-anticoagulated plastic tubes and serum/plasma isolated within 60 min of sample acquisition. Samples were stored in plastic cryovials at −20°C or colder at the enrolling site until shipped to the TIMI Clinical Trials Laboratory (Boston, MA, USA), where they were maintained at −80°C or colder. Samples were tested after one prior freeze-thaw cycle.
Testing for MPO, N-terminal pro B-type natriuretic peptide (NT-proBNP), and high-sensitivity C-reactive protein (hsCRP) was performed on the Dimension RxL (Siemens Healthcare Diagnostics, Deerfield, IL, USA) platform by personnel blinded to clinical outcomes and treatment allocation. To minimize any interaction with heparin, EDTA-anticoagulated plasma samples were used for MPO analyses16 and simultaneously collected serum samples were used for the other three biomarkers. The assay range for hs-C-reactive protein was 0.05–10 mg/L with a limit of detection of 0.03 mg/L and total imprecision (CV) of 5.1%, 2.2%, and 2.5% at hs-C-reactive protein concentrations of 0.17, 1.16, and 1.88 mg/L. The assay range for NT-proBNP was 10–30 000 pg/mL. The reported CV is 4.3%, 3.9%, and 3.8% at concentrations of 123, 462, and 5359 pg/mL, respectively. The assay range for MPO was 20–5000 pmol/L with a CV of 3.8% and 3.3% at 428 and 3644 pmol/L. Cardiac troponin I was measured using the TnI-Ultra assay (ADVIA Centaur, Siemens) which has a lower limit of detection of 0.006 μg/L and an established 99th percentile reference limit of 0.04 μg/L and a total imprecision of 10% at a concentration of 0.03 μg/L.17
Endpoints
The endpoints of CV death, fatal and non-fatal myocardial infarction (MI), and hospitalization for heart failure (HF) were adjudicated by a blinded clinical events committee. The definition of MI has been reported in detail.15 New or worsening HF was defined as re-hospitalization or prolongation of the index hospitalization (>24 h) in an acute care facility primarily for the treatment of HF along with an objective sign of HF.
Statistical analyses
Biomarkers were categorized by quartiles and then by cutpoints [MPO >670 pmol/L, hs-C-reactive protein >15 mg/L,18 NT-proBNP >400 pg/mL,19 and troponin I 0.04 μg/L17]. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using a Cox proportional-hazards regression model. Event rates are presented as Kaplan–Meier failure rates at 12 months. The relationship between biomarkers and outcomes was first evaluated by adding each biomarker individually to a clinical model that included the following clinical variables as described in the TIMI risk score—age >65 years, more than three cardiac risk factors, documented CAD, recent severe angina, ST deviation >0.5 mm, prior ASA—together with creatinine clearance <60 mL/min and a history of HF (Tables 3 and 4). We then created a fully adjusted model that incorporated all of the biomarkers together with the clinical variables (Table 5).
Table 3.
Risk of cardiovascular outcomes by cutpoints
| cTnI |
NT-proBNP |
hs-C-reactive protein |
MPO |
|||||
|---|---|---|---|---|---|---|---|---|
| <0.04 ng/mL; KM%; referent (%) | ≥0.04 ng/mL; KM%; HRadj (95%CI) | <400 pg/mL; KM%; referent (%) | ≥400 pg/mL; KM%; HRadj (95%CI) | <15 mg/L; KM%; referent (%) | ≥15 mg/L; KM%; HRadj (95%CI) | <670 pmol/L; KM%; referent (%) | ≥670 pmol/L; KM%; HRadj (95%CI) | |
| CV death | 2.0 | 5.7%; 2.72 (1.84–3.99); <0.001 | 1.8 | 9.2%; 3.01 (2.13–4.24); <0.001 | 3.9 | 6.5%; 1.45 (1.06–1.96); 0.019 | 3.6 | 5.7%; 1.49 (1.12–1.97); 0.006 |
| MI | 4.0 | 9.9%; 3.02 (2.24–4.07); <0.001 | 6.0 | 11.4%; 1.50 (1.18–1.80); 0.001 | 7.5 | 9.3%; 1.17 (0.91–1.51); 0.23 | 7.6 | 8.3%; 1. 05 (0.84–1.31); 0.63 |
| HF | 1.5 | 5.4%; 4.35 (2.72–6.94); <0.001 | 1.4 | 8.7%; 4.99 (3.38–7.37); <0.001 | 3.2 | 7.1%; 2.13 (1.56–2.91); <0.001 | 2.8 | 5.6%; 1.95 (1.43–2.64); <0.001 |
| CV death/HF | 3.1 | 8.7%; 2.97 (2.16–4.09); <0.001 | 2.7 | 13.9%; 3.55 (2.69–4.70); <0.001 | 5.9 | 10.2%; 1.62 (1.27–2.07); <0.001 | 5.5 | 8.5%; 1.49 (1.18–1.88); 0.001 |
Hazard ratio (HR) adjusted for variables in TIMI risk score (except troponin), creatinine clearance <60 mL/min, and prior HF. Each biomarker was evaluated independently with clinical model. KM, Kaplan-Meier.
Table 4.
Improvements in discrimination and re-classification according to the addition of biomarkers to clinical risk
| CV death |
MI |
HF |
CV death/HF |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c-index (P-value) | P-value for IDI | NRI (%) (P-value) | c-index (P-value) | P-value for IDI | NRI (%) (P-value) | c-index (P-value) | P-value for IDI | NRI (%) (P-value) | c-index (P-value) | P-value for IDI | NRI (%) (P-value) | |
| Clinical model | 0.784 | Reference | Reference | 0.654 | Reference | Reference | 0.740 | Reference | Reference | 0.749 | Reference | Reference |
| cTnI Ultra >0.04 ng/mL | 0.805 (0.005) | <0.001 | 0.389 (<0.001) | 0.698 (<0.001) | <0.001 | 0.482 (<0.001) | 0.779 (<0.001) | <0.001 | 0.410 (<0.001) | 0.776 (<0.001) | <0.001 | 0.389 (<0.001) |
| NT-ProBNP >400 pg/mL | 0.809 (0.003) | <0.001 | 0.802 (<0.001) | 0.660 (0.25) | <0.001 | 0.328 (<0.001) | 0.797 (<0.001) | <0.001 | 0.892 (<0.001) | 0.790 (<0.001) | <0.001 | 0.822 (<0.001) |
| Hs-C-reactive protein >15 mg/L | 0.789 (0.29) | 0.098 | 0.187 (0.010) | 0.655 (0.78) | 0.45 | 0.062 (0.28) | 0.760 (0.008) | 0.002 | 0.346 (<0.001) | 0.757 (0.074) | 0.009 | 0.227 (<0.001) |
| MPO >670 pmol/L | 0.791 (0.11) | 0.040 | 0.217 (0.003) | 0.654 (0.68) | 0.87 | 0.032 (0.58) | 0.751 (0.116) | 0.001 | 0.345 (<0.001) | 0.754 (0.280) | 0.007 | 0.214 (<0.001) |
| All four biomarkers | 0.818 (0.001) | <0.001 | 0.777 (<0.001) | 0.694 (<0.001) | <0.001 | 0.363 (<0.001) | 0.810 (<0.001) | <0.001 | 0.843 (<0.001) | 0.798 (<0.001) | <0.001 | −0.807 (<0.001) |
Clinical models contain variables from TIMI risk score (except troponin), creatinine clearance <60 mL/min, and history of prior HF. Each biomarker was evaluated independently with clinical model.
P-values represent comparison of differences in c-indices, IDI, and NRI between clinical models before and after the inclusion of the individual biomarkers.
Table 5.
Fully adjusted model for cardiac endpoints according to clinical characteristics and levels of biomarkers
| CV death |
MI |
HF |
CV death/HF |
|||||
|---|---|---|---|---|---|---|---|---|
| HRadj; 95% CI | P-value | HRadj; 95% CI | P-value | HRadj; 95% CI | P-value | HRadj; 95% CI | P-value | |
| cTnI Ultra >0.04 ng/mL | 1.88; 1.25–2.83 | 0.002 | 2.97; 2.12–4.06 | <0.001 | 2.41; 1.47–3.96 | <0.001 | 1.93; 1.38–2.71 | <0.001 |
| NT-ProBNP >400 pg/mL | 2.37; 1.64–3.41 | <0.001 | 1.07; 0.83–1.38 | 0.58 | 3.36; 2.22–5.10 | <0.001 | 2.73; 2.02–3.68 | <0.001 |
| hs-C-reactive protein >15 mg/L | 1.06; 0.78–1.46 | 0.70 | 1.01; 0.79–1.32 | 0.93 | 1.39; 1.01–1.91 | 0.045 | 1.15; 0.90–1.49 | 0.27 |
| MPO >670 pmol/L | 1.28; 0.96–1.71 | 0.088 | 0.94; 0.75–1.17 | 0.57 | 1.55; 1.14–2.11 | 0.005 | 1.26; 1.00–1.59 | 0.052 |
Hazard ratio adjusted for TIMI risk score, creatinine clearance <60 mL/min, and prior heart failure, MPO >670 pmol/L, NT-proBNP >400 pg/mL, cTnI ≥0.04 ng/mL, and hs-C-reactive protein >15 mg/L.
Estimates of the c-statistic for the Cox regression models were calculated and differences in the c-statistic after the addition of each individual biomarker to the clinical model were compared (Table 4). The increased discriminative value of the biomarkers was further examined with the method described by Pencina and colleagues to determine the net re-classification improvement (NRI)—the probability that patients are appropriately assigned to a higher or lower risk—and integrated discrimination improvement (IDI)—a method to quantify the differences in the probabilities for events and non-events based on the addition of the new biomarkers to the model.20
We calculated NRI using two methods. For the initial comparisons of the baseline clinical model with each biomarker, we used Harrell's technique, as programmed in R, which evaluates the change in the estimated risk as a continuous variable and therefore is not dependent on a priori categorization.21 We then categorized patients into low, moderate, or high categories based on the variables of the TIMI risk score that incorporated the cTnI Ultra results as the positive marker of necrosis, creatinine clearance, and history of HF and determined the degree of re-classification risk categories after the addition of NT-proBNP, and then when hs-C-reactive protein and MPO were added separately to the model that included NT-proBNP. With this method, it is possible to provide clinically relevant information regarding the number of patients who are more appropriately risk-stratified with the addition of the new variable.20
Analyses were performed using STATA v9.0 (STATA Corp., College Station, TX, USA) and R (version 2.10.1). The authors had full access to and take full responsibility for the integrity of the data.
Results
A total of 4352 patients had baseline concentrations of NT-proBNP, hs-C-reactive protein, cTnI, and MPO measured at randomization. Baseline characteristics of the patients with biomarker data are presented in Table 1. The median (25th, 75th percentile) time from symptom onset to randomization was 23 h (13 h, 33 h). The median (25th, 75th percentile) concentration of MPO was 592 pmol/L (401 pmol/L, 907 pmol/L); the median NT-proBNP was 214.8 pg/mL (59.5 pg/mL, 677.2 pg/mL); the median hs-C-reactive protein was 5.5 mg/L (2.5 mg/L, 12.8 mg/L). The correlations between the four biomarkers are presented in Table 2. Myeloperoxidase correlated weakly with the other biomarkers. N-terminal pro B-type natriuretic peptide and cTnI were moderately correlated (ρ = 0.51, P < 0.001).
Table 1.
Baseline characteristics of patients in biomarker assessment
| Characteristics | |
|---|---|
| Age (mean), years | 64 |
| ≥75 years | 16.4% |
| Male | 64.9% |
| Weight (mean), kg | 81.8 |
| History of diabetes | 32.4% |
| History of hypertension | 74.6% |
| Dyslipidaemia | 67.9% |
| History of revascularization | 26.6% |
| History of HF | 21.1% |
| Creatinine clearance <60 mL/min | 20.2% |
| Current smoker | 25.0% |
| Prior MI | 35.8% |
|
| |
| TIMI risk score | |
| Low (0–2) | 24.5% |
| Moderate (3–4) | 43.8% |
| High (≥5) | 21.7% |
|
| |
| Index diagnosis | |
| Unstable angina | 49.2% |
| Non-ST-elevation MI | 48.3% |
| Other | 2.5% |
|
| |
| Left ventricular EF (median),% (n= 2897) | 55 |
| Symptoms to time of blood sampling (median), h | 22.4 |
|
| |
| Baseline biomarker levels | |
| Troponin I ≥0.04 ng/mL | 64.7% |
| NT-proBNP >400 pg/mL | 36.3% |
| C-reactive protein >15 mg/L | 21.6% |
| MPO >670 pmol/L | 42.4% |
| Assigned to ranolazine | 50.8% |
| Assigned to placebo | 49.2% |
Table 2.
Correlation of biomarkers
| NT-ProBNP | cTnI | hs-C-reactive protein | |
|---|---|---|---|
| MPO | 0.13 | 0.17 | 0.14 |
| NT-proBNP | 0.51 | 0.31 | |
| cTnI | 0.29 |
P < 0.001 for Spearman's rank correlation coefficient for all comparisons.
Adjusted risk relationships
All analyses were first adjusted for a clinical model that included creatinine clearance, history of HF, and the components of the TIMI risk score (except for the marker of necrosis). The adjusted relationships between each individual biomarker and CV outcomes are shown in Table 3 and Supplementary Data. When each biomarker was added individually to a multivariable model that adjusted for clinical characteristics, the risk of CV death or HF rose in a stepwise fashion with increasing quartiles of each biomarker (see Supplementary Data). Also, using their pre-defined cut-points, each biomarker identified patients with a significantly higher risk of an adverse CV event (Table 3). Notably, an elevated concentration of each of the biomarkers was associated with a significantly higher risk of CV. The relationship between the elevated biomarkers and death or HF was the most robust among the endpoints examined, with more than a two-fold increase in risk associated with each biomarker, including MPO. The relationship with MI was less consistent; only NT-proBNP and troponin were independently associated with the risk of MI.
Effect of left ventricular function and timing of samples
Left ventricular function was known in 2897 subjects and an ejection fraction ≤40% was independently associated with an increased risk of each endpoint. However, even after adjusting for left ventricular function, the association between each of the four biomarkers and each endpoint was similar to the results from the entire cohort (see Supplementary Data). There were similarly no differences in the relationship between each biomarker and outcomes based on whether the biomarker was obtained before or after 24 h from symptom onset (see Supplementary Data).
Discrimination and re-classification for individual biomarkers
Addition of each of the four biomarkers individually improved the discrimination of the clinical model for CV death or HF as calculated by improvements in the c-index or IDI. Only cTnI and NT-proBNP improved the discrimination of the model for MI (Table 4). Similarly, all four biomarkers improved re-classification of risk for CV death or HF, though only cTnI and NT-proBNP improved the re-classification of MI (Table 4). The addition of each biomarker (using either quartiles or cutpoints) significantly improved the global fit of the clinical models as assessed by likelihood-ratio tests for CV death or HF. Only models with NT-proBNP or troponin significantly improved the global fit for MI when compared with the clinical model (data not shown).
Multimarker approach
The results of full multivariable models that included all four biomarkers and clinical characteristics are presented in Table 5. Each of the biomarkers, except hs-C-reactive protein, was associated with a higher risk of CV death or HF. N-terminal pro B-type natriuretic peptide and cTnI were independently associated with CV death. Only cTnI was independently associated with the risk of MI. The addition of all four biomarkers together in the clinical model significantly improved the discrimination as determined by improved c-statistic or IDI and improved the re-classification (NRI) of risk for all endpoints (Table 3). These results were consistent when left ventricular function was included in the clinical model (see Supplementary Data) and when examining the early (≤24 h from symptom onset) and late (>24 h) samples (see Supplementary Data).
Re-classification of the TIMI risk score
We then determined whether the incremental addition of each biomarker significantly improved the risk-stratification based on tertiles of TIMI risk score. First, we first added NT-proBNP to the TIMI risk score that incorporated cTnI as the indicator for elevated marker of necrosis, as well as creatinine clearance and history of HF. N-terminal pro B-type natriuretic peptide significantly improved the c-statistic, NRI, and IDI for CV death and HF, but not MI (Figure 1). The addition of either MPO or hs-C-reactive protein to the TIMI risk score that included cTnI and NT-proBNP did not result in any consistent improvement in either discrimination (c-statistic or IDI) and re-classification (NRI) for any of the clinical endpoint (Figure 1).
Figure 1.
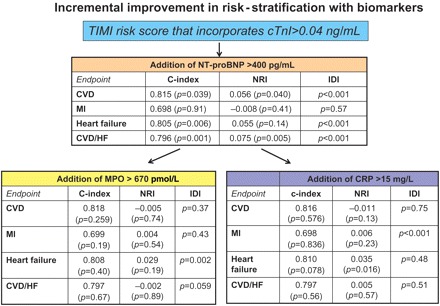
Incremental prognostic benefit of the addition of myeloperodixase and C-reactive protein to more established biomarkers. The baseline model in this figure utilized the TIMI risk score that incorporates the cardiac troponin value of ≥0.04 mg/dL as the marker of myocardial necrosis. When N-terminal pro B-type natriuretic peptide was added to the model, it significantly improved the discrimination (c-statistic and IDI) and re-classification for cardiovascular death and heart failure, but not myocardial infarction. The addition of myeloperoxidase or C-reactive protein to the model that already incorporated N-terminal pro B-type natriuretic peptide did not meaningfully improve the prognostic value for any endpoint. The TIMI risk score in this model included the standard variables as well as cardiac troponin I ≥0.04 mg/dL as the marker of myocardial necrosis, creatinine clearance <60 mL/min, and a history of heart failure.
Figure 2 graphically presents how patients were reclassified in terms of their risk of CV death or HF with the addition on NT-proBNP to the TIMI risk score that included cTnI as the marker of necrosis. The greatest degree of re-classification with the addition of NT-proBNP occurred in the moderate group, where over 30% of patients originally deemed moderate risk according to the TIMI risk score were reclassified (12.1% to low risk and 19.8% to high risk). With the addition of NT-proBNP, 13% of patients who were previously categorized as low risk were re-categorized to moderate risk. A total of 23.8% of patients originally categorized as high risk were subsequently lowered to moderate risk after NT-proBNP was included in the model (Figure 2).
Figure 2.
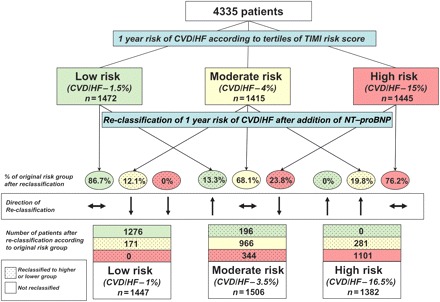
Degree of re-classification of risk for cardiovascular death or heart failure after the addition of N-terminal pro B-type natriuretic peptide to the TIMI risk score. Patients were first categorized into tertiles according to their TIMI risk score (low, moderate, or high). Each individual patient's risk was then re-calculated after including N-terminal pro B-type natriuretic peptide into the model and the proportion of patients re-classified into a higher or lower risk group are shown, For example, of the 1418 patients who were originally categorized as moderate risk for cardiovascular death or heart failure (4% risk), 171 (12.1%) were re-classified as low-risk (1.5%) and 281 (19.8%) were re-classified as high risk (15%). The TIMI risk score in this model included the standard variables as well as cardiac troponin I ≥0.04 mg/dL as the marker of myocardial necrosis, creatinine clearance <60 mL/min, and a history of heart failure.
Discussion
The development of novel biomarkers in CV disease requires rigorous evaluation in the context of established tools. In this analysis of over 4300 patients with moderate- to high-risk NSTEACS, we evaluated the prognostic performance of three established cardiac biomarkers—cTnI, NT-proBNP, and hs-C-reactive protein—together with MPO, a clinically available novel marker of macrophage and monocyte activation, using both traditional and recently proposed statistical techniques to assess their incremental contribution to risk-stratification. We found that each biomarker contributed statistically significant independent information towards risk-stratification at presentation with NSTEACS; however, only NT-proBNP and troponin substantially influenced discrimination beyond clinical risk predictors alone.
Performance of individual biomarkers
Using standard multivariable Cox models and likelihood-ratio tests, we found that when evaluated separately in a model adjusting for clinical characteristics, MPO, NT-proBNP, cTnI, and hs-C-reactive protein were each associated with CV death and HF, and NT-proBNP and cTnI with MI. Our findings expand on the previously reported relationship between MPO and overall mortality reported in 516 patients with STEMI8 and in patients with ACS,4,9,10 and newly demonstrate an independent association determined using Cox models between MPO and new or worsening HF after ACS. By this metric, each of these biomarkers was supported as viable tools for risk-stratification.
Both hs-C-reactive protein and MPO are markers of inflammation; however, they appear to reflect different aspects of the inflammatory process. C-reactive protein, which is produced in the liver after cytokine stimulations, is one of the earliest and most non-specific acute-phase reactants, though its role as a direct causal agent in atherosclerosis remains uncertain.22 Owing to the development of highly sensitive and reliable assays and the well-documented association with CV outcomes in stable patients, it is the most commonly utilized marker of inflammation. Myeloperoxidase, in contrast, may play a direct role in the growth and destabilization of atherosclerotic plaques through oxidizing LDL cholesterol,23 activating metalloproteinases, and reducing endothelial-derived nitric oxide.24 While MPO and hs-C-reactive protein only correlate weakly in our analysis—thus illustrating the differential assessment of inflammation—the risk of cardiovascular outcomes associated with each inflammatory biomarker was similar.
Assessment of incremental discrimination and re-classification
Traditionally, Cox models and receiver-operator characteristic curves have been used to identify variables that are independently associated with outcomes or to demonstrate an improvement in a model's ability to identify patients who will or will not have an event. However, these statistical techniques may underestimate the significance of a new variable in predicting relatively infrequent events such as CV death and recurrent ischaemic episodes and is dependent on the strength of the baseline model and overall effect size. Nor, as in the case of a change in the c-statistic, do they provide clinically meaningful data. Newer statistical methods, such as NRI and IDI attempt to improve the integration of sensitivity and specificity and evaluate the proportion of patients who are reclassified to higher or lower risk categories based on new biomarkers.20
When we further evaluated the incremental value of each biomarker using tests of discrimination and re-classification, all four biomarkers improved discrimination as determined by improvements in the c-statistic or IDI for CV death and HF, but only cTnI and NT-proBNP significantly improved the discrimination of the clinical model for MI. Statistically significant improvements were more often detected using the IDI compared with improvement in c-statistics, which is consistent with the concept that IDI is a more sensitive statistical test for comparing two predictive models, because the c-statistic utilizes the rank of estimated probabilities rather than the relative contribution of each risk variable and thus may underestimate large relative risk differences.13,20,25 These results highlight the significant improvement in discrimination and re-classification of risk with the established biomarkers NT-proBNP and cTnI; contrasting with the relatively small improvements in discrimination provided by MPO or hs-C-reactive protein.
Improvement in re-classification with the addition of each biomarker followed a similar pattern, with the greatest relative re-classification observed when either cTnI or NT-proBNP was added to the clinical models. Notably, this finding is conceptually consistent with Eggers and colleagues who found that among the biomarkers cTnI, NT-proBNP, and hs-C-reactive protein, only NT-proBNP was associated with death and only elevated cTnI was associated with MI.26 However, when evaluated in context with the TIMI risk score that utilized the ultra cTnI assay, only NT-proBNP significantly improved the discrimination and re-classification of patients. Neither C-reactive protein nor MPO improved upon the TIMI risk score with the addition of NT-proBNP.
High-sensitivity-C-reactive protein has been evaluated extensively in patients with NSTEACS. Earlier reports, including large, prospective, and well-characterized populations, demonstrated an independent relationship between hs-C-reactive protein and death and MI, in particular.18,27–29 However, similar to Eggers et al.26 and observations in primary prevention populations,30,31 the incorporation of more sensitive measures of necrosis and natriuretic peptides diminished the independent contribution of hs-C-reactive protein for prediction of outcomes.
Limitations
There are several potential limitations in this study. First, left ventricular function, a robust maker of CV risk, was not assessed in all patients; though with data on LV function in nearly 3000 patients, we did not observe any differences in the relationships between the biomarkers and outcomes compared with the overall cohort, even after adjusting for LV function. We also did not measure these biomarkers at later timepoints and therefore cannot examine the relationship between dynamic changes in each biomarker and outcomes. And finally, these findings are from a clinical trial cohort and thus cannot be generalized to the overall population and should be validated in a larger population-based cohort. Moreover, our data relate to prognostic rather than diagnostic applications of these biomarkers.
Clinical implications
Recent expert consensus regarding the evaluation of new biomarkers has recommended that the evaluation of emerging biomarkers should include assessments of their effect on discrimination and re-classification as well as their potential for interactions with specific therapies.13 However, the most recent professional society guidelines to directly address the application of novel biomarkers in patients with ACS were formulated prior to these recommendations.1 The MERLIN-TIMI 36 biomarker study is one of the largest assessments of biomarkers in ACS to comprehensively evaluate multiple biomarkers in ACS and incorporate newly recommended statistical techniques.13,25 Overall, our results demonstrate that a sensitive cardiac troponin and natriuretic peptide provide the most robust information for early risk-stratification of patients with ACS.1 In addition, cardiac troponin remains the most useful marker for identifying patients at high risk for recurrent MI, and natriuretic peptides the most useful for identifying those at risk for CV death and HF. These findings confirm the discriminatory capacity of these biomarkers and support present guidelines with respect to the use of troponin (Class I) and natriuretic peptides (Class IIa) for risk-stratification in ACS. The higher recommendation for measuring troponin rests, in part, on the important therapeutic implications associated with elevated levels of cardiac necrosis. Current guidelines recommend an early invasive strategy in patients at high risk based on elevated troponin. The therapeutic implications of an elevated level of natriuretic peptides have not been as clearly demonstrated, though one post hoc analysis did suggest a benefit of early invasive therapy.32
Use of hs-C-reactive protein (Class IIa) and novel inflammatory biomarkers such as MPO (Class IIb) are presently recommended as reasonable to consider for risk-stratification in patients presenting with ACS when additional prognostic information beyond clinical characteristics and troponin is desired by the clinician.1 Our finding that levels of hs-C-reactive protein and MPO offer independent information in the risk-stratification for HF support prior work in smaller studies4,33,34 and is consistent with the hypothesis that activated inflammation exacerbates HF. However, the small incremental contribution of these two markers points toward, at most, a limited clinical role early after ACS, among patients in whom troponin and natriuretic peptides have been measured. A broader role would be considered if these biomarkers were to be proven useful for therapeutic decision-making, such as using hs-C-reactive protein for monitoring the intensity of statin therapy when measured 30 days or later after ACS. These findings may be taken into account with future updates to professional guidelines for the clinical use of these biomarkers.1
Conclusion
In this study of over 4300 well-characterized patients presenting with NSTEACS, we found that both cTnI and NT-proBNP offer prognostic information beyond that achieved with clinical risk variables for CV death, MI, and HF. Myeloperoxidase and hs-C-reactive protein, while independently associated with some adverse CV outcomes, did not provide substantial incremental prognostic information when evaluated together with cTnI and NT-proBNP.
Supplementary material
Funding
MERLIN-TIMI 36 was sponsored by CV Therapeutics (now Gilead Sciences).
Conflict of interest: B.M.S. has received grants for clinical research via the TIMI Study Group and Brigham and Women's Hospital from CV Therapeutics; Novartis Pharmaceuticals Corporation; AstraZeneca Pharmaceuticals LP; Daiichi-Sankyo, Inc.; Merck & Co., Inc.; Johnson and Johnson Pharmaceutical Research & Development, L.L.C.; Bayer HealthCare Pharmaceuticals; Bristol-Myers-Squibb Company; and served as a consultant for: AstraZeneca Pharmaceuticals LP; Novartis Pharmaceuticals Corporation; CV Therapeutics; Cogentus; Shionogi & Co., Ltd; Gilead Sciences, Inc.; Merck & Co., Inc.; Schering-Plough Corporation. Dr M.S.S. has received research grants from BRAHMS, diaDexus, and Ortho-Clinical Diagnostics, and is a consultant for Singulex. Dr P.J. has received research support from Amgen, Beckman-Coulter, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics, and honoraria for educational presentations from Ortho Clinical Diagnostics. J.L.de L. reports grant support from Biosite and Roche, and consulting income from Johnson and Johnson and Tethys. E.B. is the chairman of the TIMI Study Group, who receives grant support from Beckman-Coulter, CV Therapeutics (now Gilead Sciences), and Roche Diagnostics. Dr D.A.M. has received research/educational grant support through the TIMI Study Group from Accumetrics, AstraZeneca, Bayer Healthcare, Beckman Coulter, Bristol-Myers Squibb, CV Therapeutics, Daiichi Sankyo, Eli Lilly and Co., Genentech, Johnson & Johnson, Merck and Company, Nanosphere, Novartis Pharmaceuticals, Ortho-Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi-Aventis, Siemens, Singulex, and Schering-Plough; has received honoraria for educational presentations from CV Therapeutics and Eli Lilly and Co.; and is a consultant/on the advisory board of AstraZeneca, Beckman-Coulter, Cardiokinetix, Gilead, Ikaria, Menarini, Molecular Insight, OrthoClinical Diagnostics, Sanofi-Aventis, Schering-Plough Research Institute, Siemens, and Roche Diagnostics.
Supplementary Material
References
- 1.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–1763. doi: 10.1161/01.CIR.0000015464.18023.0A. [DOI] [PubMed] [Google Scholar]
- 3.Apple FS, Pearce LA, Chung A, Ler R, Murakami MM. Multiple biomarker use for detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2007;53:874–881. doi: 10.1373/clinchem.2006.080192. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, Sabatine MS, Brennan ML, de Lemos JA, Murphy SA, Ruff CT, Rifai N, Cannon CP, Hazen SL. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J. 2008;29:1096–1102. doi: 10.1093/eurheartj/ehn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 6.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349:1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 10.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 11.McGeechan K, Macaskill P, Irwig L, Liew G, Wong TY. Assessing new biomarkers and predictive models for use in clinical practice: a clinician's guide. Arch Intern Med. 2008;168:2304–2310. doi: 10.1001/archinte.168.21.2304. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 13.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC, Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk. a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E. Effects of ranolazine on recurrent cardiovascular events in patients with non-st-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 15.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Skene A, McCabe CH, Braunwald E. Evaluation of a novel anti-ischemic agent in acute coronary syndromes: design and rationale for the metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes (MERLIN)-TIMI 36 trial. Am Heart J. 2006;151:1186 e1–1186 e9. doi: 10.1016/j.ahj.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Shih J, Datwyler SA, Hsu SC, Matias MS, Pacenti DP, Lueders C, Mueller C, Danne O, Mockel M. Effect of collection tube type and preanalytical handling on myeloperoxidase concentrations. Clin Chem. 2008;54:1076–1079. doi: 10.1373/clinchem.2007.101568. [DOI] [PubMed] [Google Scholar]
- 17.Bonaca M, Scirica B, Sabatine M, Dalby A, Spinar J, Murphy SA, Jarolim P, Braunwald E, Morrow DA. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55:2118–2124. doi: 10.1016/j.jacc.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, Braunwald E. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1998;31:1460–1465. doi: 10.1016/S0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 19.Scirica BM, Morrow DA, Bode C, Ruzyllo W, Ruda M, Oude Ophuis AJ, Lopez-Sendon J, Swedberg K, Ogorek M, Rifai N, Lukashevich V, Maboudian M, Cannon CP, McCabe CH, Braunwald E. Patients with acute coronary syndromes and elevated levels of natriuretic peptides: the results of the AVANT GARDE-TIMI 43 Trial. Eur Heart J. 2010;31:1993–2005. doi: 10.1093/eurheartj/ehq190. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–212) [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE., Jr . Harrell Miscellaneous Package for R. 2008. 3.7–0. http://biostat.mc.vanderbilt.edu/s/Hmisc. (1 July 2010) [Google Scholar]
- 22.Scirica BM, Morrow DA. Is C-reactive protein an innocent bystander or proatherogenic culprit? The verdict is still out. Circulation. 2006;113:2128–2134. doi: 10.1161/CIRCULATIONAHA.105.611350. discussion 2151. [DOI] [PubMed] [Google Scholar]
- 23.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/S0891-5849(00)00229-X. [DOI] [PubMed] [Google Scholar]
- 24.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 25.Cook NR. Statistical evaluation of prognostic versus diagnostic models: beyond the ROC curve. Clin Chem. 2008;54:17–23. doi: 10.1373/clinchem.2007.096529. [DOI] [PubMed] [Google Scholar]
- 26.Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2009;54:357–364. doi: 10.1016/j.jacc.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 27.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. N Engl J Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 28.James SK, Armstrong P, Barnathan E, Califf R, Lindahl B, Siegbahn A, Simoons ML, Topol EJ, Venge P, Wallentin L. Troponin and C-reactive protein have different relations to subsequent mortality and myocardial infarction after acute coronary syndrome: a GUSTO-IV substudy. J Am Coll Cardiol. 2003;41:916–924. doi: 10.1016/S0735-1097(02)02969-8. [DOI] [PubMed] [Google Scholar]
- 29.Scirica BM, Morrow DA, Cannon CP, de Lemos JA, Murphy S, Sabatine MS, Wiviott SD, Rifai N, McCabe CH, Braunwald E for the Thrombolysis in Myocardial Infarction Study G. Clinical application of C-reactive protein across the spectrum of acute coronary syndromes. Clin Chem. 2007;53:1800–1807. doi: 10.1373/clinchem.2007.087957. [DOI] [PubMed] [Google Scholar]
- 30.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. Jama. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 32.Jernberg T, Lindahl B, Siegbahn A, Andren B, Frostfeldt G, Lagerqvist B, Stridsberg M, Venge P, Wallentin L. N-terminal pro-brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. J Am Coll Cardiol. 2003;42:1909–1916. doi: 10.1016/j.jacc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Scirica BM, Morrow DA, Cannon CP, de Lemos JA, Murphy S, Sabatine MS, Wiviott SD, Rifai N, McCabe CH, Braunwald E. Clinical application of C-reactive protein across the spectrum of acute coronary syndromes. Clin Chem. 2007;53:1800–1807. doi: 10.1373/clinchem.2007.087957. [DOI] [PubMed] [Google Scholar]
- 34.Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction: predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47:962–968. doi: 10.1016/j.jacc.2005.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


