Abstract
Aims
The feasibility, safety, and clinical utility of percutaneous coronary intervention (PCI) without radio-contrast medium in patients with advanced chronic kidney disease (CKD) are unknown. In this series, we investigated a specific strategy for ‘zero contrast’ PCI with the aims of preserving renal function and preventing the need for renal replacement therapy (RRT) in patients with advanced CKD.
Methods and results
A total of 31 patients with advanced CKD [creatinine = 4.2 mg/dL, inter-quartile range (IQR) 3.1–4.8, estimated glomerular filtration rate = 16 ± 8 mL/min/1.73 m2] who had clinical indication for PCI based on a prior minimal contrast coronary angiogram were included. Zero contrast PCI was performed at least 1 week after diagnostic angiography using real-time intravascular ultrasound (IVUS) guidance, with pre- and post-PCI measurements of fractional flow reserve and coronary flow reserve to confirm physiological improvement. This approach resulted in successful PCI, no major adverse cardiovascular events and preservation of renal function without the need for RRT within a follow-up time of 79 days (IQR 33–207) in all patients.
Conclusion
In patients with advanced CKD who require revascularization, PCI may safely be performed without contrast using IVUS and physiological guidance with high procedural success and without complications.
Keywords: Percutaneous coronary intervention, Chronic kidney disease, Contrast-induced nephropathy, Intravascular ultrasonography, Coronary physiology
Introduction
Contrast-induced nephropathy (CIN) is associated with increased morbidity and mortality1–3 including the need for renal replacement therapy (RRT). Various pharmacological and cardiac interventional approaches have been examined to reduce the risk of CIN,1 but no specific measures for patients with advanced chronic kidney disease (CKD) have been determined. Established approaches to prevent CIN include peri-procedural hydration4 and minimizing contrast volume (CV).1 Despite these measures, percutaneous coronary intervention (PCI) in patients with advanced CKD is associated with a high risk of CIN and requirement for RRT, leading to under-utilization of PCI in these high-risk patients.
Percutaneous coronary intervention with minimal CV using various techniques and imaging modalities has been previously described,5 and PCI with no contrast use has been reported in one patient with contrast allergy.6 Herein we describe a novel approach that involves PCI with zero contrast administration for revascularization in 31 selected consecutive patients with advanced CKD and report their clinical outcomes.
Methods
Study design and inclusion and exclusion criteria
We performed a retrospective analysis of data from patients with advanced CKD stages 4–5 [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 by the Modification of Diet in Renal Disease equation] who had a clinical indication for coronary angiography for stable coronary artery disease (CAD). The analysis is representative of data from 31 consecutive patients who were selected based on ultra-low contrast angiography to undergo zero contrast PCI at our institution. During the study period, seven patients with stage 4–5 CKD and positive functional tests for ischaemic CAD who were initially considered suitable for zero contrast PCI were subsequently excluded from the procedures due to the findings on angiography of chronic total occlusion (CTO, 3 patients) and severely calcified lesions in tortuous arteries in the presence of poor left ventricular function (4 patients). Requirement for rotational atherectomy in non-tortuous arteries was not an absolute exclusion criterion, and two such patients were included in the study.
Protocol for diagnostic angiography and zero contrast percutaneous coronary intervention
As previously recommended, modified ultra-low contrast techniques were applied for initial diagnostic angiography.5 A CV/eGFR ratio <1 was pre-determined as the maximum limit for contrast media. When the CV/eGFR <1 mandated CV <15 mL, contrast medium was diluted with saline to a higher volume to allow for injection of all coronary arteries. Iso-osmolar contrast medium (iodixanol) was used in all cases. Where indicated, staged PCI was performed at a minimum interval of 1 week to minimize the risk of CIN. Pre-procedural discussion with the patients included potential benefits of revascularization and risks of complications, in particular acute kidney injury (AKI) and the need for RRT. Details of the strategy for zero contrast PCI to minimize the risk of AKI were explained, and possible indications for radio-contrast use during the PCI, including managing procedural complications and assessing a lack of improvement in physiological indices (FFR <0.80 or CFR <2),7 were discussed. All patients provided informed written consent (Columbia University Institutional Review Board protocol AAAQ7039).
Procedures were performed via femoral or radial access. Baseline echocardiography was performed to check for pre-existing pericardial effusion. Interventional pharmacology included intra-procedural heparin and loading with dual anti-platelet agents. Prior to the PCI, the previous angiogram was uploaded to the monitors as a guide. A pre-procedural left ventricular end-diastolic pressure was recorded to guide intravenous hydration for the remainder of the procedure as previously described.4 Guide catheter engagement was confirmed by entry of the workhorse wire into the coronary artery. For safety, an exaggerated curve was created to form a loop once the wire was beyond the stenotic lesion. Additional guide wires were placed in the branches to silhouette the artery and branches based on the previous angiogram (Figure 1). The metallic silhouette of the coronary anatomy was used as the road map for PCI, with the position of the guide wires in major branches used as important landmarks to guide PCI and to protect the side branches when PCI was performed around branching points. Using a pressure wire (Certus, St Jude Medical, St Paul, MN, USA), baseline fractional flow reserve (FFR) and coronary flow reserve (CFR) were recorded.
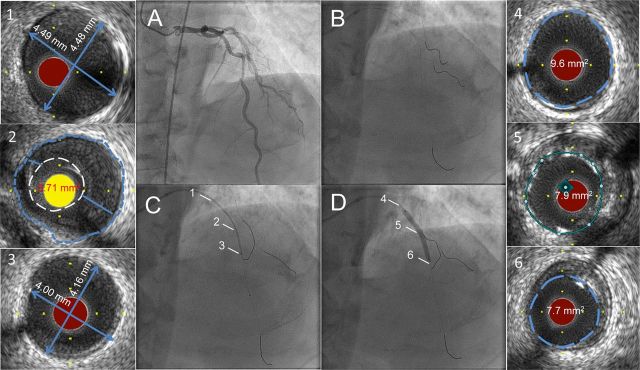
Figure 1.
Ultra-low contrast coronary angiography followed by staged percutaneous coronary intervention with zero contrast. Cine images recorded at the initial angiography using ultra-low contrast volume are displayed on adjoining screen during the staged percutaneous coronary intervention (A) and used to guide catheter engagement, coronary guide wire placement in the left anterior descending artery, diagonal branch, and the circumflex artery, thus creating a metallic silhouette of the left coronary system (B). Intravascular ultrasound imaging of the left anterior descending artery is performed with proximal reference diameter (≈4.5 mm) (1), minimal luminal area (3.71 mm2) (2), and distal reference diameter (≈4.0 mm) (3) measured for selection of the appropriate pre-dilation balloon and stent sizes. The co-registered dry cine image of intravascular ultrasound transducer placed at the distal reference (C) is used to guide the percutaneous coronary intervention. Following preparation of the lesion and deployment of a 3.5 × 38 mm drug-eluting stent (D), intravascular ultrasound is repeated to assess the result, to determine the proximal (9.6 mm2) (4) and distal (7.7 mm2) (6) reference areas, and to guide post-dilation of under-expanded segments to achieve the pre-determined MSA, defined as >90% of the mean of the proximal and distal reference areas, (7.9 mm2) (5).
Procedural planning was performed with intravascular ultrasound (IVUS) guidance. An automated IVUS pullback was performed in the target vessel to identify the proximal and distal landing zones, aiming for segments with plaque burden <55% to minimize the risk of stent edge complications.8 The reference diameters were determined by the mean of at least two measurements from external elastic lamina (EEL) to EEL taken both proximally and distally. The smaller of the two mean measurements was used to select the diameter of the pre-dilation balloons and stents. The stent length was based on the distance between the two reference areas. The IVUS catheter was then re-advanced manually to the distal and proximal landing zones and ‘dry’ cine angiograms performed to allow co-registration of the reference segments for stent placement. The distal reference on dry cine projection was marked and used as the reference on the monitors. If pre-dilation was indicated, a compliant balloon was used and escalated to a same-sized non-compliant (NC) balloon if necessary. Following stent implantation, IVUS was repeated to identify under-expansion defined as minimum stent area <90% of the mean of the proximal and distal stent reference lumen areas. An NC balloon matched to the respective proximal or distal reference diameters was used for post-dilation of under-expanded segments. A final IVUS was performed to assess for stent expansion, edge dissection, or intramural haematoma followed by repeated physiological measurements to confirm improvement in FFR (>0.80) and CFR (>2).
When femoral access was used, femoral angiography was not performed and haemostasis was achieved by manual compression. Post PCI, echocardiography was repeated in the cardiac catheterization laboratory to check for new or enlarging pericardial effusion. Pre-determined absolute indications for angiography included chest pain, ECG changes consistent with ischaemia, failure of improvement in physiological indices, or new or enlarging pericardial effusion. In these cases, angiography aids in identification and management of undetected coronary perforation, and combined with repeat IVUS where necessary, the underlying cause for lack of physiological improvement including significant edge dissection or haematoma, distal embolization, and suboptimal stent expansion. Following discharge, a metabolic panel was checked within 1 week and repeated within the following 6 months.
Results
Clinical and procedural characteristics are presented in Table 1. The mean age of the patients was 66 ± 11 years and the majority (81%) were males with multiple cardiac risk factors. All patients had advanced CKD with median creatinine of 4.2 mg/dL [inter-quartile range (IQR) 3.1–4.8], and eGFR = 16 ± 8 mL/min/1.73 m2). Initial diagnostic angiography was performed with ultra-low CV (13 mL, IQR 12–15), and CV/eGFR target ratio of <1 was achieved in all cases (Table 1), with contrast dilution necessary in eight (26%) patients. More than half of the patients had single vessel disease with the majority of the stenotic lesions found in the left anterior descending artery and right coronary artery. Renal function measured within 21 h (IQR 9–36) following angiography remained stable in all patients (creatinine: 3.9 mg/dL, IQR 2.9–4.9; eGFR = 18 ± 8 mL/min/1.73 m2; P > 0.05) with no patient requiring RRT.
Table 1.
Clinical characteristics of patients and diagnostic, procedural and follow up data
| Clinical characteristics |
Angiographic data |
PCI, physiology, IVUS, and follow-up data |
|||
|---|---|---|---|---|---|
| Age (years) | 66 ± 11 | Left anterior descending artery | 16 (52) | Guide wires | 3 [3, 4] |
| Male | 25 (81) | Left circumflex artery | 5 (16) | Lesion length (mm) | 22 [16, 38] |
| Hypertension | 31 (100) | Right coronary artery | 10 (32) | Pre-FFR | 0.74 [0.70, 0.77] |
| Diabetes mellitus | 15 (48) | Proximal reference diameter (mm) | 3.5 [3, 3.5] | Pre-CFR | 1.4 [1.1, 1.9] |
| Current smoking | 0 (0) | Distal reference diameter (mm) | 3 [2.75, 3.5] | Number of stents | 1 [1, 1] |
| Dyslipidaemia | 14 (45) | Diameter stenosis (%) | 80 [70, 90] | Total stent length (mm) | 22 [16, 38] |
| Previous MI | 15 (48) | TIMI 3 flow | 31 (100) | Stent diameter (mm) | 3 [3, 3.5] |
| Previous PCI | 2 (6) | One-vessel disease | 18 (58) | Pre-dilation | 25 (81) |
| Previous CABG | 4 (13) | Two-vessel disease | 8 (26) | Post-dilation | 30 (97) |
| LVEF: | Three-vessel disease | 5 (16) | Minimal stent area (mm2) | 6.8 [5.9, 8.3] | |
| >50% | 21 (68) | Calcified lesions | 22 (71) | Expansion >90% | 27 (87) |
| 35–50% | 4 (13) | ISR | 0 (0) | Post-FFR | 0.92 [0.90, 0.93] |
| <35% | 6 (19) | Syntax score | 12 [8, 19] | Post-CFR | 2.6 [2.3, 4.2] |
| Outpatient medications | |||||
| Aspirin | 28 (90) | ACC/AHA class B2 or C lesions | 13 (42) | Procedure time (min) | 72 [61, 119] |
| Thienopyridine | 15 (48) | Contrast volume (mL) | 13 [12,15] | Fluoroscopy time (min) | 20 [16, 35] |
| Anticoagulant | 2 (6) | Contrast requiring dilution | 8 (26) | Radiation dose (mGy) | 1154 [538, 1932] |
| β-Blocker | 20 (65) | Contrast volume/eGFR | 0.8 [0.6, 0.9] | Follow-up (days) | 79 [33, 207] |
| ACEI/ARB | 9 (29) | Creatinine post angio (mg/dL) | 3.9 [2.9, 4.9] | Follow-up creatinine (mg/dL) | 3.7 [3.0, 4.5] |
| Calcium channel blocker | 22 (71) | Change in creatinine post angio (mg/dL) | 0.1 [0, 0.2] | Change in creatinine (mg/dL) | 0.1 [0.0, 0.4] |
| Nitrate | 8 (26) | eGFR post angio (mL/min/1.73 m2) | 18 ± 8 | Follow-up eGFR (mL/min/1.73 m2) | 18 [14, 22] |
| Statin | 24 (77) | Change in eGFR post angio (mL/min/1.73 m2) | −0.2 [−0.5, 0.5] | Change in eGFR (mL/min/1.73 m2) | −0.2 [−1.4, 1.8] |
| Baseline lab values | |||||
| HbA1C (%) | 6.2 [5.4, 6.7] | Time to measurement of renal function (hours) | 21 [9, 36] | Renal replacement therapy | 0 (0) |
| Creatinine (mg/dL) | 4.2 [3.1, 4.8] | Stent thrombosis | 0 (0) | ||
| eGFR (mL/min/1.73 m2) | 16 ± 8 | Revascularization | 0 (0) | ||
| MI | 0 (0) | ||||
| Death | 0 (0) | ||||
Values are n (%), mean ± SD, and/or median [Q1, Q3].
MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; TIMI, thrombolysis in myocardial infarction; ISR, in-stent restenosis; FFR, fractional flow reserve; CFR, coronary flow reserve.
During staged PCI, the haemodynamic significance of coronary lesions was documented by physiological measurements (pre-procedure FFR 0.74, IQR 0.70–0.77) and CFR (1.4, IQR 1.1–1.9). Of coronary lesions treated, 42% were class B2 or C on American College of Cardiology/American Heart Association classification system, and two lesions required rotational atherectomy. The majority of patients received a single stent, with both pre- and post-dilation (Table 1). Post-PCI median minimal stent area (MSA) was 6.8 mm2 (IQR 5.9–8.3) with the pre-determined target MSA achieved in 87% of patients. Physiological parameters improved in all patients [post-procedure FFR (0.92, IQR 0.90–0.93) and CFR (2.6, IQR 2.3–4.2)]. No contrast was used in any of these 31 selected consecutive patients.
At a median follow-up period of 79 days (IQR 33–207), neither creatinine levels (3.7, IQR 3.0–4.5; P = 0.69) nor eGFR (18, IQR 14–22 mL/min/1.73 m2; P = 0.70) significantly changed (Table 1), and no patient required RRT. Furthermore, there were no major adverse cardiovascular events, including stent thrombosis, myocardial infarction (MI), repeat revascularization, or death.
Discussion
We describe a method of sequential diagnostic angiography using ultra-low volumes of contrast followed by staged physiology- and IVUS-guided zero contrast PCI in patients with advanced CKD. This approach resulted in safe and successful PCI without CIN or the need for RRT in this extremely high-risk group of patients. To our knowledge, this is the first reported series of physiology- and imaging-guided zero contrast PCI in the literature.
Several risk factors are common to both CKD and CAD. Accordingly, patients with CKD have a higher incidence and burden of atherosclerosis.2,9 Many characteristics predictive of advanced atherosclerosis are also major risk factors for CIN,2,10 thus complicating PCI in patients with CKD. It is therefore not surprising that cardiac revascularization is under-utilized in this group of patients.9,10 In the absence of evidence from randomized trials, observational studies have shown better survival compared with medical therapy in patients with CKD and multivessel CAD who undergo revascularization across all categories of renal function.11,12 However, concerns regarding CIN and procedure-related RRT can inhibit the performance of revascularization in patients with advanced CKD, especially those approaching end-stage renal disease. Pre-existent renal disease is the strongest independent predictor for development of CIN and the requirement for RRT, which develop in 27 and 4% of patients with severe CKD, respectively.13 Contrast-induced nephropathy is independently associated with adverse events including death, MI, and bleeding,2,3,13 the rates of which dramatically increase if RRT is required, whether temporary or permanent.13,14
Our strategy of performing zero contrast PCI, staged after minimal contrast diagnostic angiography, facilitates procedural planning after the diagnostic study, and minimizes the CV that would have to be administered in a single procedure should complications develop. Staged PCI also allows time for better discussion with patients regarding the treatment options, allowing true informed consent to be obtained. We chose to perform the staged PCI no earlier than 7 days after the angiography because CIN typically occurs within the first week after the contrast exposure.1 Systemic clearance of the contrast material via renal excretion, although markedly slower in severe CKD, is expected to be almost complete within this time period.15 The 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization advocate ad hoc rather than staged PCI in patients with CKD and extensive atherosclerosis, if substantial contrast use in one single invasive procedure can be avoided, to minimize the risk of AKI secondary to atheroemboli.16 Although atheroemboli have been shown to contribute to ∼10% of acute nephropathy secondary to PCI,14 the lack of worsening renal function following staged PCI in our study strongly suggests that CIN is the major cause of acute nephropathy post PCI and that staged zero contrast PCI would be a favourable approach in this extremely high-risk group.
Given the potential risk of undetected coronary perforation, we closely monitored intra-procedural invasive haemodynamics with an upstream strategy of pre- and post-PCI echocardiography in the catheterization laboratory. Although final angiography with minimum contrast can in principle be performed to assess the final results, successful PCI as assessed by physiology and imaging obviates the need for contrast exposure, which even in minimum amounts can cause CIN in patients with advanced CKD.17 To assess improvement in physiological parameters, we used a dedicated pressure wire to measure both FFR and CFR (Certus, St Jude Medical, St Paul, MN, USA). While physiological assessment using this technique requires repeated coronary wiring pre and post PCI, use of a rapid-exchange microcatheter-based pressure measurement system (ACIST RXi System, ACIST Medical Systems, Eden Prairie, MN, USA) could eliminate this need, potentially providing a safer and more rapid assessment, albeit of FFR only. Data to support the utility of post-PCI FFR for successful revascularization is mounting,18 supporting the potential for integration of a microcatheter-based pressure measurement approach into our zero contrast strategy. Indeed, in our cohort FFR and CFR improved concordantly in all cases (Table 1).
For intravascular imaging, we utilized IVUS as it is the imaging modality of choice in patients with advanced CKD because it does not require blood clearance by contrast media. Nevertheless, as we have recently reported, zero contrast PCI guided by automated optical coherence tomography angiography co-registration using non-contrast flush media can safely be performed19 and may confer a major advantage given the angiography co-registration feature. The use of microcatheter-based FFR measurement and OCT may also help in shortening the zero contrast procedure times (Table 1), which due to repeated intravascular imaging and physiological measurements are longer than conventional PCI in centres with similar level of case load and expertise.20
This study has several limitations. The present report is a small, non-randomized study performed at a high-volume, single centre. The methods described are tailored for patients with stable CAD and not ACS. Patients with CTO and highly complex lesions, including heavily calcified plaques within tortuous arteries requiring rotational atherectomy in the presence of poor left ventricular function, were excluded. However, our current experience does indicate that zero contrast PCI with atheroblation may also be safe and feasible in selected patients. Patients with complex multivessel disease and advanced CKD may be better managed with bypass graft surgery rather than PCI if substantial contrast use cannot be avoided. Finally, successfully performing our technique requires expertise with IVUS and coronary physiology, which may not be widely utilized in many laboratories.
In conclusion, we report a strategy for zero contrast PCI that can be utilized in experienced centres with expertise with intravascular imaging and physiology to safely revascularize selected high-risk patients with advanced CKD.
Authors’ contributions
Z.A.A. performed the procedures. Z.A.A. and K.K.G. performed statistical analysis. Z.A.A., T.N., A.M., M.A.H., D.J.C., L.E.R., M.B.C., J.W.M., A.J.K., G.W.S., D.K., and M.B.L. handled funding and supervision. Z.A.A., K.K.G., T.N., A.M., D.J.C., L.E.R., M.B.C., J.W.M., A.J.K., G.W.S., D.K., and M.B.L. acquired the data. Z.A.A., K.K.G., T.N., A.M., M.A.H., D.J.C., L.E.R., J.W.M., A.J.K., G.W.S., D.K., and M.B.L. conceived and designed the research. K.K.G. and Z.A.A. drafted the manuscript. Z.A.A., K.K.G., T.N., A.M., M.A.H., D.J.C., L.E.R., M.C., J.W.M., A.J.K., G.W.S., D.K., and M.B.L. made critical revision of the manuscript for key intellectual content.
Conflict of interest: none declared.
References
- 1. Mehran R, Nikolsky E. Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 2006;100:S11–S15. [DOI] [PubMed] [Google Scholar]
- 2. Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005;95:13–19. [DOI] [PubMed] [Google Scholar]
- 3. Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu K, Kornowski R, Brener SJ, Genereux P, Stone GW, Mehran R. Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short- and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv 2015;8:e002475. [DOI] [PubMed] [Google Scholar]
- 4. Brar SS, Aharonian V, Mansukhani P, Moore N, Shen AY, Jorgensen M, Dua A, Short L, Kane K. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet 2014;383:1814–1823. [DOI] [PubMed] [Google Scholar]
- 5. Nayak KR, Mehta HS, Price MJ, Russo RJ, Stinis CT, Moses JW, Mehran R, Leon MB, Kandzari DE, Teirstein PS. A novel technique for ultra-low contrast administration during angiography or intervention. Catheter Cardiovasc Interv 2010;75:1076–1083. [DOI] [PubMed] [Google Scholar]
- 6. Okura H, Nezuo S, Yoshida K. Successful stent implantation guided by intravascular ultrasound and a Doppler guidewire without contrast injection in a patient with allergy to iodinated contrast media. J Invasive Cardiol 2011;23:297–299. [PubMed] [Google Scholar]
- 7. Chamuleau SA, Meuwissen M, van Eck-Smit BL, Koch KT, de Jong A, de Winter RJ, Schotborgh CE, Bax M, Verberne HJ, Tijssen JG, Piek JJ. Fractional flow reserve, absolute and relative coronary blood flow velocity reserve in relation to the results of technetium-99m sestamibi single-photon emission computed tomography in patients with two-vessel coronary artery disease. J Am Coll Cardiol 2001;37:1316–1322. [DOI] [PubMed] [Google Scholar]
- 8. Kang SJ, Cho YR, Park GM, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW, Park SJ. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am J Cardiol 2013;111:1408–1414. [DOI] [PubMed] [Google Scholar]
- 9. Chertow GM, Normand SL, McNeil BJ. Renalism: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 2004;15:2462–2468. [DOI] [PubMed] [Google Scholar]
- 10. Charytan D, Mauri L, Agarwal A, Servoss S, Scirica B, Kuntz RE. The use of invasive cardiac procedures after acute myocardial infarction in long-term dialysis patients. Am Heart J 2006;152:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reddan DN, Szczech LA, Tuttle RH, Shaw LK, Jones RH, Schwab SJ, Smith MS, Califf RM, Mark DB, Owen WF Jr. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol 2003;14:2373–2380. [DOI] [PubMed] [Google Scholar]
- 12. Hemmelgarn BR, Southern D, Culleton BF, Mitchell LB, Knudtson ML, Ghali WA, Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease Investigators. Survival after coronary revascularization among patients with kidney disease. Circulation 2004;110:1890–1895. [DOI] [PubMed] [Google Scholar]
- 13. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gruberg L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, Pichard AD, Satler LF, Wu H, Leon MB. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv 2001;52:409–416. [DOI] [PubMed] [Google Scholar]
- 15. Lorusso V, Taroni P, Alvino S, Spinazzi A. Pharmacokinetics and safety of iomeprol in healthy volunteers and in patients with renal impairment or end-stage renal disease requiring hemodialysis. Invest Radiol 2001;36:309–316. [DOI] [PubMed] [Google Scholar]
- 16. Authors/Task Force Members Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 17. Kane GC, Doyle BJ, Lerman A, Barsness GW, Best PJ, Rihal CS. Ultra-low contrast volumes reduce rates of contrast-induced nephropathy in patients with chronic kidney disease undergoing coronary angiography. J Am Coll Cardiol 2008;51:89–90. [DOI] [PubMed] [Google Scholar]
- 18. Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen S-L, Di Serafino L, Domínguez-Franco AJ, Dupouy P, Esen AM, Esen ÖB, Hamilos M, Iwasaki K, Jensen LO, Jiménez-Navarro MF, Katritsis DG, Kocaman SA, Koo B-K, López-Palop R, Lorin JD, Miller LH, Muller O, Nam C-W, Oud N, Puymirat E, Rieber J, Rioufol G, Rodés-Cabau J, Sedlis SP, Takeishi Y, Tonino PAL, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NHJ, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 19. Karimi Galougahi K, Zalewski A, Leon MB, Karmpaliotis D, Ali ZA. Optical coherence tomography-guided percutaneous coronary intervention in pre-terminal chronic kidney disease with no radio-contrast administration.Eur Heart J 2016;37:1059. [DOI] [PubMed] [Google Scholar]
- 20. Michael TT, Alomar M, Papayannis A, Mogabgab O, Patel VG, Rangan BV, Luna M, Hastings JL, Grodin J, Abdullah S, Banerjee S, Brilakis ES. A randomized comparison of the transradial and transfemoral approaches for coronary artery bypass graft angiography and intervention: the RADIAL-CABG Trial (RADIAL Versus Femoral Access for Coronary Artery Bypass Graft Angiography and Intervention). JACC Cardiovasc Interv 2013;6:1138–1144. [DOI] [PubMed] [Google Scholar]