Abstract
Aims
In type 2 diabetes, antioxidant depletion contributes to increased oxidative stress in the microvasculature. The current study was designed to assess how oxidative stress contributes to functional changes in the microvasculature, and determine the importance, and the effects of pharmacologically targeting, the transcription factor Nrf2.
Methods and results
Pressure myography was used to measure myogenic constriction in mesenteric arterioles from diabetic (db/db) and non-diabetic (db/m) mice. Compared with db/m, myogenic constriction was larger in db/db, independent of the endothelial cell layer, and directly correlated with elevated basal and pressure-induced reactive oxygen species (ROS) production. Nrf2 was depleted in db/db vessels and associated with down-regulation of Nrf2-regulated genes. Notably, expression of GCLC and GCLM, enzymes important for glutathione (GSH) synthesis, was dramatically reduced, as was total cellular GSH. Normal myogenic function was restored to db/db arterioles by incubation with cell-permeant GSH. Similarly, the db/db myogenic phenotype was recapitulated in the db/m vessels by pharmacological GSH depletion. Treatment with the Nrf2-activator sulforaphane increased Nrf2 and promoted its nuclear localization and increased GCLC and GCLM expression in both db/m and db/db. Sulforaphane dramatically lowered ROS signalling in db/db and reduced myogenic tone to levels similar to that seen in db/m vessels.
Conclusion
Depleted Nrf2 and expression of its dependent genes compromises antioxidant capacity resulting in dysfunctional myogenic tone in diabetes that is reversed by the Nrf2-activator sulforaphane.
Keywords: Type 2 diabetes, Oxidative stress, Myogenic tone, NRF2, Sulforaphane
1. Introduction
The myogenic response is the property of microcirculatory arterioles that enables them to constrict in response to increased intravascular pressure. Physiologically, this serves to set the level of basal tone, as well as regulate microvascular blood flow and capillary hydrostatic pressure.1 It is well recognized that the myogenic response is altered in type 2 diabetes and likely contributes to the broader microvascular dysfunction identified as an early manifestation of the cardiovascular complications associated with the disease.1 Several studies using both type 1 and type 2 models of diabetes have demonstrated enhanced myogenic tone in arterioles isolated from numerous vascular beds.2–6
One of the major hallmarks of diabetes is increased oxidative stress driven by increased production of cellular reactive oxygen species (ROS) and concomitant depletion of antioxidant defences.7 Redox imbalance in the diabetic vasculature is associated with overproduction of the superoxide radical (O2−)8 which rapidly converts to hydrogen peroxide (H2O2), or reacts with nitric oxide to produce additional derivatives. Both O2− and H2O2 have been implicated as modulators of smooth muscle contraction in the vasculature.9 Increased O2− has been shown to promote contractility by inhibiting endothelium-dependent relaxation.10 On the other hand H2O2 has been reported to have both vasoconstrictor11 and dilator12 effects that are highly dependent on the concentration of the stimulus, as well as the artery size and nature of pre-constriction.13,14
It is not known if redox imbalance contributes to dysfunctional myogenic tone in diabetes. There are, however, some intriguing precedents. In the healthy vasculature, an important signalling role for ROS in the generation of myogenic tone has been established.15,16 In addition, deletion of the antioxidant enzyme superoxide dismutase increases myogenic tone in arterioles isolated from knockout mice.17 On the basis of these data, we now hypothesize that altered ROS levels in diabetes impinge on the regulation of myogenic tone.
The overproduction of ROS strains endogenous antioxidant defences, a process that is further compounded in diabetes through transcriptional down-regulation of key antioxidant enzymes. Many antioxidant enzymes are regulated by the transcription factor nuclear factor NF-E2-related factor 2 (Nrf2). Nrf2 normally resides in a cytoplasmic protein complex that senses and responds to increased ROS levels by promoting Nrf2 nuclear translocation. Nrf2 binds to target antioxidant genes to increase their expression and restore redox balance. This pathway is dysregulated in diabetes through mechanisms that result in reduced Nrf2 levels and impaired Nrf2 translocation.18,19
The purpose of the present study was to determine whether oxidative stress contributes to the dysregulated myogenic response in diabetes, to assess Nrf2-regulated gene expression in the diabetic microvasculature, and to determine whether diabetes-disrupted redox balance and myogenic response can be reversed by treatment with the Nrf2-activator sulforaphane.20
2. Methods
2.1. Animals
Male control db/m (Dock7m +/+Lepdb) mice and diabetic db/db (BKS.Cg-Dock7m +/+Leprdb/J) littermates, aged 8–10 weeks, were purchased from the Jackson Laboratory and housed in the biological resource facility at Rosalind Franklin University. For the study duration, all mice were housed with a 12-h light/dark cycle in 12 × 6.25 in. cages with standard enrichment and ad libitum access to food and water. The db/m and db/db animals were housed separately up to maximum of four animals per cage. At the age of 12–14 weeks, mice were euthanized by inhalation of a lethal dose of CO2 followed by cervical dislocation. The abdomen was immediately opened, the mesenteric arcade removed and placed in a dissecting dish filled with physiological saline maintained at 4°C and 3rd order arterioles isolated, as described previously.21 For myography studies on freshly isolated tissue, vessels were kept at 4°C and used within 6 h. For drug treatment protocols, vessels were placed in tissue culture overnight and myography performed the following day. All animal procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee of Rosalind Franklin University of Medicine and Science.
2.2. Pressure myography
Arterioles were mounted in a myograph chamber (Living Systems Instrumentation, Burlington, VT, USA) as described previously.21 Intraluminal pressure was increased stepwise from 20 to 120 mmHg and the vessel diameter measured. Myogenic constriction was calculated as follows: myogenic constriction (% passive diameter) = (active diameter − passive diameter)/passive diameter × 100. In some experiments, the endothelium was mechanically denuded by gentle insertion and removal of a human hair. The failure of acetylcholine (1 µM) to induce dilatation in arterioles pre-contracted with phenylephrine (1 µM) was taken as confirmation of endothelium removal.
2.3. Quantitative real-time PCR and western blot
Quantitative real-time PCR (qRT–PCR) and western blot were performed as described previously.21 For qRT–PCR, a Sybr Green PCR Master Mix was used. The relative quantitative evaluation of gene levels was performed using the 2−ΔΔCt method with the 18 s RNA as an internal reference.
2.4. Cell culture and immunocytochemistry
Primary smooth muscle cells from the aortas of db/m and db/db mice were dissociated and cultured as described previously.21 Cells were treated with either sulforaphane (0.5 μM) or vehicle control (DMSO) overnight before being prepared for immunocytochemistry as described.21 Nrf2 was labelled with polyclonal antibody (1:25 dilution; Santacruz Biotechnology, Inc., TX, USA) and detected with Alexa Fluor 488 IgG (1:1000, Life Technologies, Grand Island, NY, USA). Cells were counterstained with DAPI and visualized using a PlanApo ×60, 1.42 numerical aperture oil immersion objective and confocal images acquired using a VT-Infinity 3 (VisiTech International, Sunderland, UK).
2.5. ROS measurement
Mesenteric arterioles mounted in the myography chamber and producing myogenic tone were labelled with the ROS indicator 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (H2DCFDA; Life Technologies, Gaithersburg, MD, USA). Individual myocytes were visualized using confocal microscopy.
2.6. Glutathione measurement
The total glutathione (GSH) content in mesenteric arterioles was measured using the well-established colorimetric kinetic assay employing 5,5′-dithiobis-(2-nitrobenzoic) acid. Absolute values were determined from a standard curve and GSH content expressed as nanomoles per milligram protein.
2.7. Analysis and statistics
In all experiments, data were pooled from multiple trials carried out on arterioles originating from at least three animals from independent litters and summarized as means ± SEM. No animals were excluded from the study as outliers. Differences between means were assessed using Student's t-test for unpaired comparisons. For multiple comparisons, one or two-way ANOVA with Fisher's LSD (least significant difference) post hoc analysis was employed. For all tests, the differences between means were accepted as statistically significant at the 95% level (P < 0.05).
3. Results
3.1. Myogenic constriction is increased in the db/db arterioles by a mechanism intrinsic to vascular smooth muscle cells
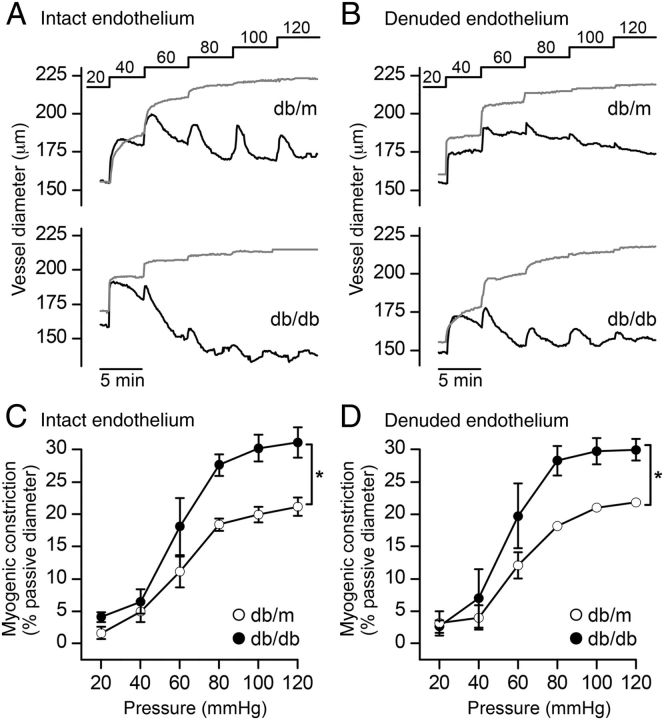
Mesenteric arterioles were mounted in a myography chamber and intraluminal pressure increased stepwise from 20 to 120 mmHg under zero flow conditions. In normal Ca2+-containing buffer, a rapid increase in arteriolar diameter was associated with each pressure rise, after which, active tension development slowly decreased the vessel diameter. This active tension development, or myogenic response, is absent in vessels incubated in zero Ca2+-containing buffer and provides a measure of the passive properties of the arteriole.1 Both the active (Figure 1A; black traces) and passive diameters (Figure 1A; grey traces) were recorded in db/m and db/db arterioles. The myogenic response developed in both db/m and db/db arterioles at pressures >40 mmHg and was maximal by 100 mmHg. In vessels producing myogenic constriction, the diameter at each pressure was normalized to the corresponding passive diameter, and summary data plotted (Figure 1C). The magnitude was significantly greater in diabetic db/db vessels compared with control db/m (Figure 1C). Of note, db/m and db/db vessels were structurally identical and exhibited the same passive pressure–diameter relationships (Table 1). Consistent with previous studies,5 the myogenic response persisted in endothelium-denuded arterioles (Figure 1B) and endothelium removal had no effect on the myogenic response in either db/m or db/db vessels (Figure 1C and D). The contractile response produced by 80 mM KCl was also unaffected. Expressed as the percentage change in diameter, the KCl-evoked response was 27 ± 3.1% (n = 5) in db/m vessels with the endothelium compared with 28 ± 4.7% (n = 7) in denuded arterioles (P > 0.05; Student's t-test). In db/db vessels the KCl-evoked response was 27 ± 1.8% (n = 4) in intact and 23 ± 2.5% (n = 7) in denuded (P > 0.05; Student's t-test). Like the high KCl-evoked contractions, the contractile response to phenylephrine in these arterioles was previously shown to be endothelium independent in both db/m and db/db.3
Figure 1.
The myogenic response is greater in arterioles from diabetic (db/db) compared with non-diabetic (db/m) mice. (A and B) Representative recordings of vessel diameter in mesenteric arterioles from db/m and db/db mice with intact (A) or denuded (B) endothelium in response to step increases in intraluminal pressure from 20 to 120 mmHg at 5 min intervals. The active (vessel in Ca2+-containing PSS actively producing tone) and passive (Ca2+-free PSS) traces are shown in black and grey, respectively. (C and D) Summary data of myogenic constriction plotted as a function of intraluminal pressure in intact (C) and endothelium-denuded (D) vessels. For intact endothelium experiments, data represent the means ± SEM response of n = 5 vessels from 5 db/m animals (open circles) and n = 5 vessels from 4 db/db animals (filled circles), *P < 0.05; two-way ANOVA. For denuded endothelium experiments, data represent the means ± SEM response of n = 4 vessels from 4 db/m animals (open circles) and n = 5 vessels from 3 db/db animals (filled circles), *P < 0.05; two-way ANOVA.
Table 1.
Passive diameters and structural properties of db/m and db/db arterioles at different intraluminal pressures
| Pressure (mmHg) | Passive diameter endothelium-intact
(µm) |
Passive diameter endothelium-denuded
(µm) |
Wall thickness (µm) |
Cross-sectional area
(µm2) |
Wall lumen ratio (×100) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| db/m | db/db | db/m | db/db | db/m | db/db | db/m | db/db | db/m | db/db | |
| 20 | 164 ± 6 | 162 ± 6 | 159 ± 6 | 167 ± 7 | 15.3 ± 0.9 | 15.3 ± 0.7 | 7149 ± 732 | 7410 ± 641 | 23 ± 1 | 22 ± 1 |
| 40 | 197 ± 10 | 187 ± 7 | 186 ± 9 | 193 ± 6 | 13.4 ± 0.9 | 13 ± 0.7 | 7488 ± 789 | 7539 ± 812 | 16 ± 1 | 15 ± 0.6 |
| 60 | 212 ± 8 | 210 ± 8 | 205 ± 12 | 202 ± 6 | 11.5 ± 1 | 11.6 ± 0.9 | 7329 ± 942 | 7361 ± 797 | 12 ± 0.6 | 12 ± 0.8 |
| 80 | 219 ± 7 | 215 ± 8 | 213 ± 12 | 206 ± 6 | 10.5 ± 0.5 | 11.6 ± 0.9 | 6837 ± 604 | 7612 ± 809 | 11 ± 0.4 | 11 ± 0.7 |
| 100 | 221 ± 7 | 218 ± 9 | 217 ± 12 | 208 ± 7 | 9.8 ± 0.1 | 10.7 ± 0.9 | 6456 ± 366 | 7125 ± 829 | 10 ± 0.3 | 10 ± 0.8 |
| 120 | 223 ± 7 | 219 ± 9 | 217 ± 13 | 208 ± 7 | 9.7 ± 0.2 | 10.6 ± 0.9 | 6457 ± 377 | 7079 ± 799 | 10 ± 0.3 | 10 ± 0.1 |
Each value represents the means ± SEM of n = 5 arterioles from three to five animals. Endothelium removal had no statistically significant effect on either db/m or db/db (P > 0.05; two-way ANOVA). Comparisons between db/m and db/db within each category were not statistically different (P > 0.05; two-way ANOVA).
3.2. Increased ROS production in the db/db arterioles is associated with increased myogenic constriction
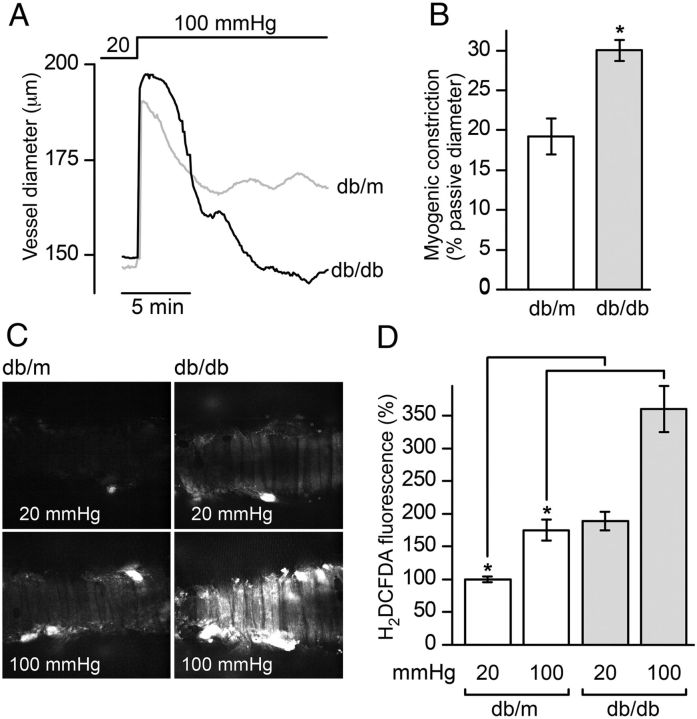
To assess the role of ROS, mesenteric arterioles were mounted in the pressure myograph chamber, loaded with the ROS-sensitive indicator H2DCFDA and imaged confocally. A single step in intraluminal pressure from 20 to 100 mmHg was used to evoke the myogenic response (Figure 2A and B) and confocal slices through the smooth muscle layer captured in the absence (at 20 mmHg) and presence (10–15 min after stepping to 100 mmHg) of myogenic constriction (Figure 2C). The fluorescence intensities from regions of interest surrounding individually resolved myocytes were collected in two to three image fields for each vessel and pooled with data from multiple vessels from at least three animals each for db/m and db/db. Intensities were normalized to the basal H2DCFDA fluorescence, defined as the mean fluorescence of db/m myocytes at 20 mmHg, and summarized in Figure 2D. The levels of ROS were significantly greater in db/db compared with db/m in vessels pressurized to 20 mmHg and not actively producing tone. Interestingly, pressurization and myogenic constriction were associated with an additional ROS production, consistent with previous studies.15 This pressure-induced ROS burst occurred in both db/m and db/db, but was notably larger in db/db (Figure 2D).
Figure 2.
ROS levels are elevated in vascular smooth muscle cells of arterioles from diabetic (db/db) mice. (A) Vessel diameter recorded in response to a single step increase in pressure from 20 to 100 mmHg in db/m (grey tracing) and db/db (black tracing) arterioles. (B) Summary data (means ± SEM) of myogenic tone from db/m (n = 5 vessels from three animals) and db/db (n = 5 vessels from three animals), *P < 0.001; unpaired t-test. (C) Representative confocal sections through pressurized arterioles labelled with the ROS indicator H2DCFDA. Shown are images of db/m and db/db arterioles captured before (20 mmHg) and after (100 mmHg) development of myogenic constriction. (D) H2DCFDA fluorescence levels in single myocytes within a given slice were quantified. Summary data showing the means ± SEM of ≥250 cells pooled from multiple fields in 7 db/m and 5 db/db arterioles isolated from 3 and 4 db/m and db/db animals, respectively, and normalized to fluorescence values at 20 mmHg in db/m arterioles. Statistical comparisons were made prior to normalization (*P < 0.05; one-way ANOVA).
3.3. Antioxidant genes are down-regulated and total GSH depleted in db/db arterioles
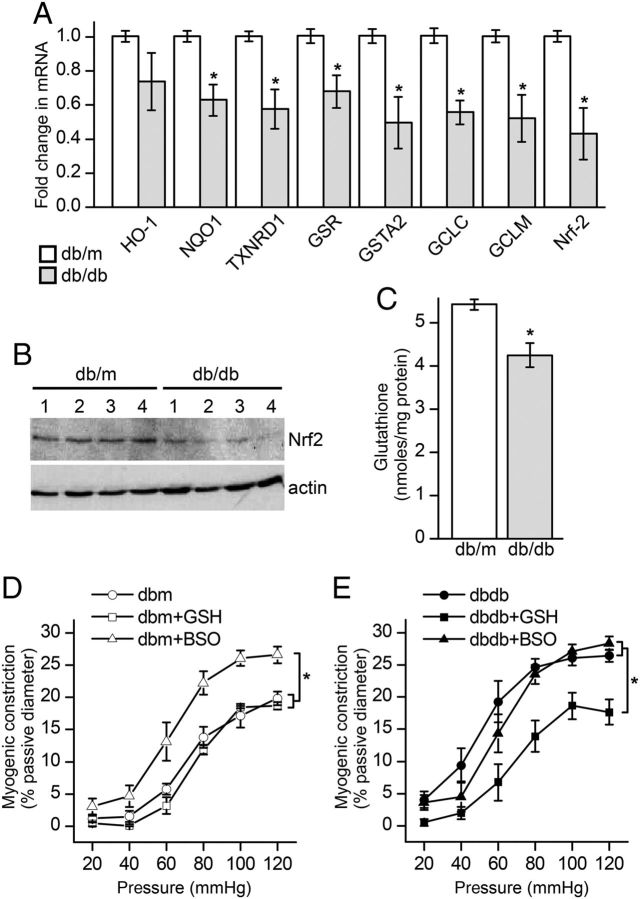
We next quantified levels of Nrf2-regulated antioxidant molecules along with Nrf2 itself in mesenteric arterioles isolated from db/m and db/db. The mRNA levels of NQO1, GSR, GSTA2, TXNDR1, GCLC, GCLM, along with Nrf2 were significantly down-regulated in vessels from the diabetics compared with controls (Figure 3A). Nrf2 levels were also assessed by western blot to confirm that protein levels were also reduced (Figure 3B). In addition to Nrf2, the most dramatically affected genes were GCLC and GCLM, enzymes critical for the regulation of GSH synthesis.22 Total cellular GSH was measured and found to be ∼20% lower in db/db mesenteric vessels compared with db/m (Figure 3C).
Figure 3.
Nrf2-regulated genes and Nrf2 protein are down-regulated in arterioles from diabetic (db/db) mice and correlate with decreased glutathione levels and dysfunctional myogenic constriction. (A) Expression levels of Nrf2-regulated genes were determined by real-time PCR and included: haeme oxygenase-1 (HO-1); thioredoxin reductase 1 (TXNRD1); glutathione reductase (GSR); glutathione S-transferase alpha 2 (GSTA2); NAD(P)H dehydrogenase, quinone 1 (NQO1); glutamate-cysteine ligase, catalytic subunit (GCLC); glutamate-cysteine ligase, modifier subunit (GCLM), and NF-E2-related factor 2 (Nrf2). Data are represented as the fold difference between db/db and db/m relative to 18S ribosomal RNA used as an internal reference. Data were pooled from 6 db/m and db/db mice and summarized as mean ± SEM (*P < 0.05; unpaired t-test). (B) Western blot for Nrf2 protein in mesenteric arterioles. Tissue lysates prepared from 4 db/m and db/db individual animals are shown in lanes 1–4. (C) Summary data (means ± SEM) showing total glutathione levels in db/m (n = 6 animals) and db/db (n = 6 animals) mesenteric arterioles (*P < 0.01; unpaired t-test). (D and E) Summary data of myogenic constriction as a function of intraluminal pressure in db/m (D) and db/db (E) vessels treated overnight with cell permeable GSH ethyl ester (10 mM), GSH synthesis inhibitor BSO (100 µM), or vehicle control. Data from db/m animals are represented in (D) and are the means ± SEM response of n = 4 vessels from three animals (vehicle control, open circles); n = 4 vessels from three animals (GSH, open squares), and n = 5 vessels from three animals (BSO, open triangles). Data from db/db animals are represented in (E) and are the means ± SEM response of n = 5 vessels from five animals (vehicle control, filled circles); n = 6 vessels from five animals (GSH, filled squares), and n = 5 vessels from three animals (BSO, filled triangles). For both db/m and db/db groups, statistical comparisons were made between treatments (*P < 0.05; two-way ANOVA).
3.4. The myogenic response is critically dependent on total GSH levels
The myogenic response was monitored in mesenteric vessels after experimental manipulation of GSH levels. First, GSH levels were increased by incubating vessels overnight in a tissue culture incubator with cell permeable GSH ethyl ester (10 mM). Control vessels were incubated in parallel with vehicle alone. While GSH treatment had no effect on non-diabetic db/m vessels, it significantly reduced myogenic tone in the diabetic db/db (Figure 3D and E). In the reciprocal experiment, GSH levels were depleted by overnight incubation with buthionine sulfoximine (BSO; 100 µM), a GSH synthesis inhibitor. The BSO-treated db/m vessels showed increased myogenic tone compared with untreated controls but had no additional effect on the tone recorded in db/db (Figure 3D and E).
3.5. The Nrf2-activator sulforaphane increases nuclear localization of Nrf2 and expression of the GSH synthesis enzymes GCLC and GCLM
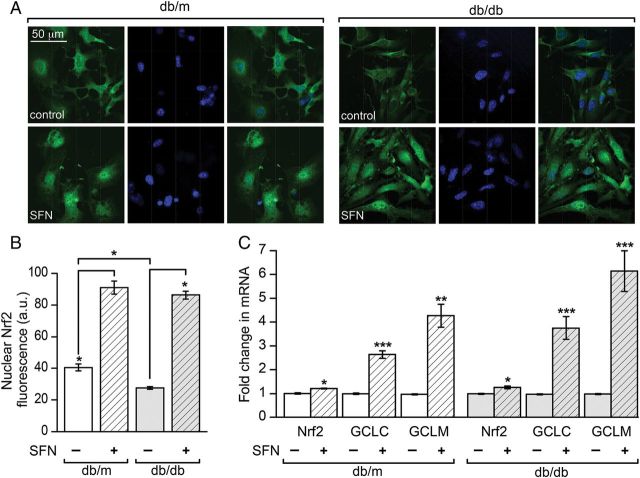
The compound sulforaphane has been shown to increase expression of GCLC and GCLM and increase GSH synthesis by promoting Nrf2 nuclear translocation.23 To assess the effect of Nrf2 translocation, single smooth muscle cells isolated from the aortas of db/m and db/db mice were placed into culture and incubated overnight with sulforaphane or vehicle control. Nrf2 was then immunofluorescently labelled and imaged using confocal microscopy (Figure 4A). Under control conditions nuclear-localized Nrf2 levels were significantly lower in db/db compared with db/m (Figure 4B). Sulforaphane treatment dramatically increased the nuclear Nrf2 in both db/m and db/db cells (Figure 4A and B). To determine whether this could translate into increased Nrf2-dependent gene expression, mesenteric arterioles were treated overnight with sulforaphane or vehicle control. A robust increase in the mRNA levels of the rate-limiting GSH synthesis enzymes GCLC and GCLM was observed in vessels from both the db/m and db/db animals (Figure 4C). Sulforaphane also produced a modest, yet significant, increase in Nrf2 expression in both non-diabetic db/m and diabetic db/db vessels (Figure 4C), suggesting that it promotes both increased expression and nuclear translocation, consistent with its documented effects in other tissues.23
Figure 4.
Sulforaphane increases nuclear Nrf2 levels and drives increased expression of the glutathione synthesis enzymes GCLC and GCLM. (A) Primary smooth muscle cells isolated from the aortas of db/m and db/db animals were treated overnight with sulforaphane (SFN; 0.5 μM) or vehicle control and prepared for immunofluorescent labelling the following day. Representative confocal slices are shown depicting the localization of Nrf2 (green) and cell nuclei (blue) in control and SFN-treated db/m and db/db cells. (B) The fluorescence intensity of Nrf2 label (green) localized to nuclear regions, as defined by DAPI staining (blue), was quantified and summarized as the means ± SEM of ≥450 cells pooled from multiple coverslips, and independent primary cultures, derived from the aortas of 3 db/m and 3 db/db animals (*P < 0.05; one-way ANOVA). (C) Mesenteric arterioles from db/m or db/db animals were isolated and separated into two groups, one of which was treated overnight with SFN (0.5 μM), and the other vehicle control. Levels of Nrf2 and the glutathione synthesis enzymes GCLC and GCLM were determined by quantitative real-time PCR and represented as the fold difference between control and sulforaphane-treated (mean ± SEM; n = 3 animals; *P < 0.05, **P < 0.01, ***P < 0.001; unpaired t-test) in db/m and db/db.
3.6. Sulforaphane decreases ROS production and restores the myogenic response to normal in db/db arterioles
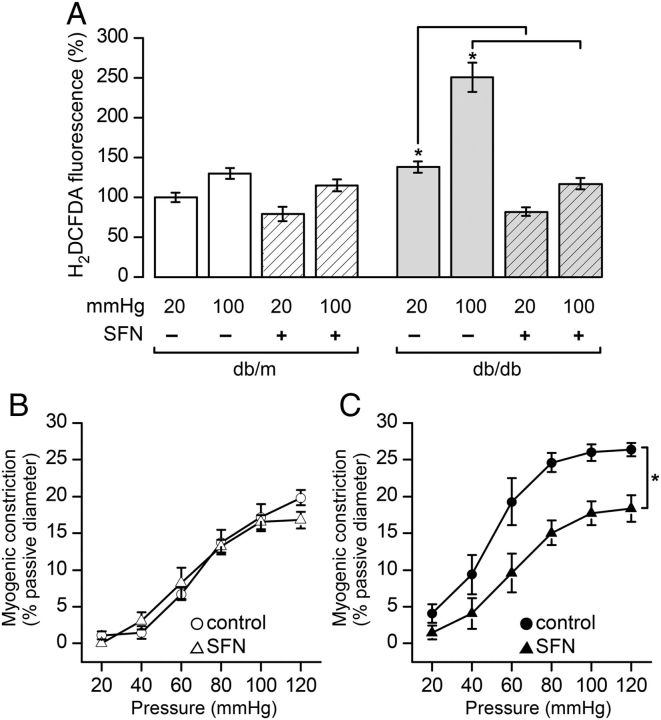
We next asked if up-regulation of the GSH synthesis pathway affected the ROS levels measured in smooth muscle cells of intact pressurized arterioles. In db/db vessels sulforaphane treatment significantly reduced ROS recorded at 20 mmHg (Figure 5A). In the same vessels, however, sulforaphane treatment dramatically attenuated the ROS elevation normally associated with the step increase in intraluminal pressure from 20 to 100 mmHg in the db/db. Interestingly, sulforaphane did not significantly affect ROS levels in the db/m arterioles (Figure 5A) despite significantly up-regulating GCLC and GCLM expression (Figure 4C). Not surprisingly then, the myogenic response in the db/m vessels was also unaffected by sulforaphane treatment (Figure 5B). This was in stark contrast to the effect of sulforaphane on db/db arterioles where it reduced myogenic contractility to a level comparable with that seen in non-diabetic controls (compare Figure 5B db/m control with Figure 5C db/db + sulforaphane).
Figure 5.
Sulforaphane restores normal redox balance and myogenic response to arterioles from diabetic (db/db) mice. (A) Isolated arterioles that had been treated overnight with sulforaphane (SFN; 0.5 μM) or vehicle control were pressurized and labelled with the ROS indicator H2DCFDA and imaged confocally. Fluorescence was quantified in smooth muscle cells at intraluminal pressures 20 and 100 mmHg and summarized as the means ± SEM of ≥150 cells pooled from multiple fields in db/m (three control and three SFN treated) and db/db (four control and four SFN treated) arterioles isolated from 3 and 4 db/m and db/db animals, respectively, and normalized to fluorescence values at 20 mmHg in db/m arterioles. Statistical comparisons were made prior to normalization (*P < 0.05; one-way ANOVA). (B and C) Summary data of the myogenic response plotted as a function of intraluminal pressure in db/m (C) and db/db (D) control and SFN-treated vessels. Data from db/m animals are represented in (C) and are the means ± SEM response of n = 7 vessels from seven animals (vehicle control and open circles); n = 4 vessels from three animals (SFN, open triangles). Data from db/db animals are represented in (D) and are the means ± SEM response of n = 8 vessels from seven animals (vehicle control and filled circles); n = 4 vessels from three animals (SFN and filled triangles), *P < 0.05; two-way ANOVA.
4. Discussion
The current study examined the role of oxidative stress in modulating the myogenic response in the db/db model of type 2 diabetes. These mice develop type 2 diabetes due to a point mutation in the leptin receptor gene, a key regulator of food intake and body weight.24 While mutated leptin is not a common cause of diabetes in humans, the development of obesity, hyperglycaemia, hyperinsulinaemia, and eventual loss of pancreatic function in these animals are also characteristics of human disease progression. This model also shares many cardiovascular phenotypes with human type 2 diabetes including abnormal vascular reactivity and hypertension.24,25 Importantly, both human and db/db exhibit increased myogenic tone in the microvasculature.2,26 Therefore, the similarity to humans in terms of metabolic state and vascular complications justifies the use of db/db as a model.
We show that the myogenic response is greater in mesenteric arterioles isolated from db/db mice compared with db/m controls. These data are in agreement with previous observations made in both mesenteric and skeletal muscle arterioles from the same animal model.2,3,6 The data also confirm that removal of the endothelium does not affect the myogenic response. Our interpretation, therefore, is that mechanisms intrinsic to the smooth muscle must be altered by diabetes. One limitation of the current study, however, was our inability to remove the adventitia. It is well recognized that adipocytes and macrophages within this layer undergo extensive remodelling in obesity and diabetes.27,28 This includes changes to the profile of signalling molecules they release, which has been linked to increased inflammation and recruitment of pericytes, both recognized as contributors to obesity-related cardiovascular disease.28,29 Such signalling pathways could be involved in driving the underlying molecular changes that cause smooth muscle in the diabetic to respond differently to pressure changes. If that were the case, then the redox pathways manipulated in the current study may not be targeting smooth muscle cells directly.
One of the key signalling events in the initiation of myogenic constriction is the generation of a superoxide (O2−) burst evoked by an increase in intraluminal pressure. This likely originates from the NAD(P)H oxidase, however, the mechanisms coupling pressure changes to its activation are not understood.15,16,30 We too observe a dynamic change in ROS in response to a step increase in intraluminal pressure. We cannot say definitively, however, that the ROS burst observed in our study is caused by O2− because the fluorescent probe used is not specific for this reactive species. We also report that arterioles from the db/db have greater steady-state ROS levels compared with db/m when equilibrated at low (20 mmHg) pressure. This is not surprising since elevated ROS is a well-defined characteristic of diabetes.7 What is novel is our observation that the ROS burst evoked by stepping intraluminal pressure from 20 to 100 mmHg is profoundly greater in diabetic arterioles. Although not addressed in the current study, we speculate that this is due to exaggerated O2− production by the NAD(P)H oxidase, which was previously shown to have increased activity in the diabetic vasculature.8,31
But how does increased ROS produce an exaggerated myogenic response? This is not known exactly but there are several possibilities. The enhanced production of constrictor prostaglandins as a result of increased cyclooxygenase-2 (cox-2) expression in diabetes has been shown to increase vascular reactivity and contribute to the increased myogenic response.3,6,25 It is conceivable that ROS signalling could impinge on this pathway, either by regulating cox-2 expression32 or prostaglandin metabolism.2 In addition, ROS have also been shown to directly increase the Ca2+-sensitivity of the contractile proteins by acting on the Rho/Rho kinase signalling pathway,33 as well as induce Ca2+ and myosin light chain phosphorylation-independent contraction.11
Levels of the major cellular antioxidant GSH are profoundly reduced in the plasma and erythrocytes in diabetes.34,35 We now extend these observations to include GSH depletion in the microvasculature. We show that pharmacologically depleting GSH in the db/m vessels recapitulates a diabetic db/db phenotype, and that supplementing GSH to db/db vessels completely restores normal physiological function to these arterioles. These data illustrate the physiological significance of GSH depletion and suggest that redox imbalance is causative of dysfunctional myogenic tone in the diabetic microvasculature. The mechanism for GSH depletion in diabetes is not fully understood, but the observation that GCLC expression in cultured vascular cells is decreased by exposure to high glucose suggests that GSH synthesis is affected.36 Recent attention has focused on the transcription factor Nrf2 as a powerful regulator of GSH synthesis that acts by controlling expression of GCLC and GCLM.22 Of note, reduced levels of Nrf2 have been observed in diabetic rat fibroblasts,19 as well as diabetic rat and human cardiac tissues.18 Consistent with these studies, we found lower Nrf2 mRNA and protein in arterioles from the diabetic. In addition, we observed lower Nrf2 in the nuclei of primary smooth muscle cells isolated from the db/db compared with db/m. The mechanism of Nrf2 reduction is not known and its elucidation is beyond the scope of the current study; nevertheless, our data demonstrate that disruption of Nrf2 signalling occurs in the diabetic vasculature. Not surprisingly, the expression levels of an array of key antioxidant enzymes under the control of Nrf2 were also reduced, with GCLC and GCLM being the most dramatically affected, suggesting that GSH depletion is caused by altered Nrf2 expression and localization.
The effects of sulforaphane on Nrf2-dependent gene expression are well documented.20 We now show that sulforaphane treatment of isolated mesenteric arterioles increases Nrf2, GCLC, and GCLM mRNA levels in both the db/m and db/db. In addition, sulforaphane increases Nrf2 levels in the nuclei of smooth muscle cells. These data are entirely consistent with previous studies reporting that sulforaphane increased GCLC expression and GSH synthesis by promoting Nrf2 expression and nuclear translocation.23 Sulforaphane significantly lowers ROS levels in db/db vessels pressurized and equilibrated at 20 mmHg, but produces a less dramatic reduction in db/m vessels despite similar fold inductions of GCLC and GCLM. More importantly, sulforaphane treatment in db/m does not change the magnitude of the ROS burst induced by a step change in intraluminal pressure. In contrast, sulforaphane profoundly reduces pressure-induced ROS in the db/db, essentially restoring the levels seen in db/m vessels. The lack of the effect of sulforaphane treatment on the magnitude of the ROS burst in db/m vessels correlates with a lack of effect on the myogenic response in these arterioles. This is consistent with the hypothesis that the size of the ROS burst is central in determining the magnitude of the myogenic constriction. This hypothesis is further strengthened by the observation that sulforaphane treatment in the db/db restores the myogenic response phenotype to that of the db/m.
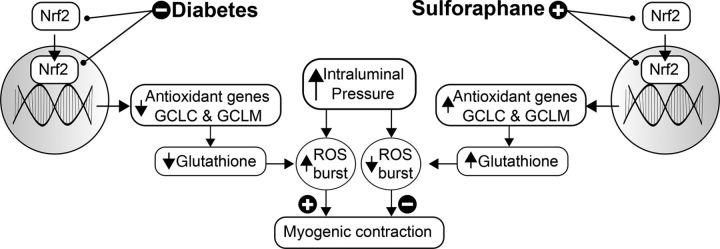
In conclusion, we demonstrate that redox imbalance contributes to dysregulation of the myogenic response in the diabetic vasculature. Our data support a model in which ROS steady-state levels and pressure-induced bursting are amplified in the smooth muscle by a mechanism involving an Nrf2-dependent reduction in the antioxidant capacity provided by GSH (summarized in Figure 6). The potential for harnessing Nrf2-regulation as a treatment modality for a variety of conditions has gained interest in recent years.20 We now demonstrate, in vitro, that targeting the Nrf2 pathway with sulforaphane effectively restores normal redox balance and myogenic responsiveness to resistance arterioles in the diabetic microvasculature.
Figure 6.
A model for Nrf2 modulation of the myogenic response. In type 2 diabetes increased arteriolar myogenic constriction has been observed in the microvasculature.2–6 Redox imbalance is well described in diabetes and reactive oxygen species (ROS) have been implicated as modulators of contractility7,15–17; however, the relationship between oxidative stress and myogenic contractility is poorly understood. The data presented in the current study are consistent with a model in which nuclear Nrf2 is reduced in smooth muscle cells from diabetic animals contributing to a depleted antioxidant capacity. The reactive oxygen species (ROS) burst that accompanies a step increase in intraluminal pressure is exacerbated and promotes enhanced myogenic constriction. Sulforaphane promotes Nrf2 nuclear localization and antioxidant gene expression to restore redox balance and a normal myogenic response.
Funding
This work was supported by funding from Rosalind Franklin University of Medicine & Science and The American Heart Association (to C.W.).
Acknowledgements
Work was carried out, in part, with the support of the Calcium Imaging Research Support Laboratory, Rosalind Franklin University of Medicine & Science.
Conflict of interest: none declared.
References
- 1.Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Erdei N, Bagi Z, Edes I, Kaley G, Koller A. H2O2 increases production of constrictor prostaglandins in smooth muscle leading to enhanced arteriolar tone in Type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2007;292:H649–H656. doi: 10.1152/ajpheart.00596.2006. [DOI] [PubMed] [Google Scholar]
- 3.Lagauda GJL, Masih-Khana E, Kaia S, Van Breemena C, Dubéb GP, Lagaud GJ, et al. Influence of type II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries. J Vasc Res. 2001;38:578–589. doi: 10.1159/000051094. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Pacher P, Kecskemeti V, Papp G, Szollar L, Koller A, et al. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc Res. 1999;43:1018–1028. doi: 10.1016/s0008-6363(99)00106-6. [DOI] [PubMed] [Google Scholar]
- 6.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, et al. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol. 2005;25:1610–1616. doi: 10.1161/01.ATV.0000172688.26838.9f. [DOI] [PubMed] [Google Scholar]
- 7.Giacco F, Brownlee M. Oxidative Stress and Diabetic Complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 9.Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J Vasc Res. 2002;39:191–207. doi: 10.1159/000063685. [DOI] [PubMed] [Google Scholar]
- 10.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 11.Pelaez NJ, Braun TR, Paul RJ, Meiss RA, Packer CS. H2O2 mediates Ca2+ and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1185–H1193. doi: 10.1152/ajpheart.2000.279.3.H1185. [DOI] [PubMed] [Google Scholar]
- 12.Iesaki T, Gupte SA, Kaminski PM, Wolin MS. Inhibition of guanylate cyclase stimulation by NO and bovine arterial relaxation to peroxynitrite and H2O2. Am J Physiol Heart Circ Physiol. 1999;277:H978–H985. doi: 10.1152/ajpheart.1999.277.3.H978. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y-J, Hirota S, Zhang D-W, Janssen LJ, Lee RMKW. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol. 2003;138:1085–1092. doi: 10.1038/sj.bjp.0705147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 15.Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, et al. Redox signaling of the arteriolar myogenic response. Circ Res. 2001;89:114–116. doi: 10.1161/hh1401.094367. [DOI] [PubMed] [Google Scholar]
- 16.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veerareddy S, Cooke C-LM, Baker PN, Davidge ST. Gender differences in myogenic tone in superoxide dismutase knockout mouse: animal model of oxidative stress. Am J Physiol Heart Circ Physiol. 2004;287:H40–H45. doi: 10.1152/ajpheart.01179.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitar MS, Al-Mulla F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;301:E1119–E1129. doi: 10.1152/ajpendo.00047.2011. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64:503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Velmurugan GV, White C. Calcium homeostasis in vascular smooth muscle cells is altered in type 2 diabetes by Bcl-2 protein modulation of InsP3R calcium release channels. Am J Physiol Heart Circ Physiol. 2012;302:H124–H134. doi: 10.1152/ajpheart.00218.2011. [DOI] [PubMed] [Google Scholar]
- 22.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2—related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, et al. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res. 2005;67:723–735. doi: 10.1016/j.cardiores.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Jaap AJ, Shore AC, Tooke JE. The influence of hypertension on microvascular blood flow and resistance to flow in the skin of patients with type 2 (non-insulin-dependent) diabetes. Diabet Med. 1994;11:883–887. doi: 10.1111/j.1464-5491.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 27.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148–H2165. doi: 10.1152/ajpheart.00907.2011. [DOI] [PubMed] [Google Scholar]
- 29.Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A, et al. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 31.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 32.Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, et al. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52:2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 33.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- 35.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Reed RL, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 36.Powell LA, Nally SM, McMaster D, Catherwood MA, Trimble ER. Restoration of glutathione levels in vascular smooth muscle cells exposed to high glucose conditions. Free Radic Biol Med. 2001;31:1149–1155. doi: 10.1016/s0891-5849(01)00648-7. [DOI] [PubMed] [Google Scholar]