Abstract
Vaccines formulated with adjuvant have been effective against numerous infectious diseases, almost always due to induction of functional antibodies that recognizes the pathogen of interest. There is an unmet clinical need for vaccine adjuvants that induce T cells responses to potentially enhance protection against malignancies and intracellular pathogens, where a humoral response, alone, may not be adequate for protection. In this study, we demonstrate that a TLR2 ligand-based adjuvant, meningococcal PorB, has broad immunostimulatory activity with the ability to induce a robust and diverse vaccine antigen specific T cell response. We demonstrate that a vaccine formulated with PorB admixed with ovalbumin induces a wide variety of antigen specific antibody subclasses and effector molecules (MIG, MCP-1, IP-10, MIP-1α, KC & IL-2) with known roles for inducing T cell responses, along with elevated levels of Th1 and Th2 type cytokines upon antigen stimulation. We confirmed production of these cytokines by examining the antigen-specific T cells induced by PorB in vivo. After two immunizations with vaccine formulated with PorB/OVA, antigen-specific CD4 and CD8 T cells were significantly increased in numbers and produced IL-4 or IFN-γ upon ex vivo antigen re-stimulation. Finally, in a Listeria mouse infection model, vaccine formulated with PorB significantly reduced the bacterial burden upon a low dose infection and increased survival upon a high dose infection with recombinant Listeria monocytogenes engineered to express OVA (rLmOVA), a pathogen that requires OVA-antigen specific cytotoxic CD8 T cells for clearance. In summary, PorB is able to induce antigen specific broad B and T cell responses, illustrating its potential as a potent and new vaccine adjuvant.
Keywords: Adjuvant, T-cell, Vaccines, MyD88, TLR, Neisseria meningitidis, PorB
Introduction
Adjuvants are required to enhance the efficacy of vaccines, either when added to their formulations or as an inherent property of the vaccine formulation itself as in attenuated or killed whole organism vaccines (1–3). The efficacy of most, if not all, adjuvanted vaccines is by inducing a protective humoral response (4), especially for those pathogens where neutralizing or bactericidal antibodies are known to be protective, e.g. diphtheria, tetanus, polio, Haemophilus influenza type B, hepatitis A and B, rabies, measles, mumps, rubella, varicella, pneumococcus and meningococcus (5–7). Interestingly, some of the most effective vaccines contained endogenous adjuvants as components of the live or attenuated forms of the targeted pathogens. The immune system responds well to these vaccines and often mounts robust protection. The major reason for this success is that our immune system has evolved to respond to Pathogen Associate Molecular Patterns (PAMPs), which stimulates the innate immune responses through Pattern Recognition Receptors (PRRs) (8). Effective vaccines exploit this property of the immune system to enhance responses to elicit immune protection, especially vaccines made from live attenuated or killed whole organism. (9).
The development of most vaccines and adjuvants have occurred with minimal understanding of immunological mechanisms of adjuvant activity and vaccine immunity. There have been many failures to develop vaccines against pandemics such as human immune deficiency virus (HIV) infection, Mycobacterium tuberculosis (TB), Hepatitis C and Respiratory Syncytial Virus (10). Traditional vaccines that mainly induce humoral responses alone have not been as successful towards many of these pathogens. One probable reason for this finding is that protection against such pathogens, which are mainly intracellular, may require a significantly diverse set of immune responses beyond just a humoral response, including a robust set of CD4 and CD8 T cell responses (11). Previous studies have demonstrated that T cell responses, including CD8 T cell responses, have a vital role in controlling and clearing intracellular infections (12–15). This demonstrates the unmet clinical need for new novel adjuvants that can induce a strong and diverse T cell response.
To date, immunizations with specific live attenuated pathogens (such as smallpox virus, yellow fever virus and others) have been shown to be one of the only ways to induce these diverse T cell responses via vaccination (16). There are many different types of PRRs that have important roles in a vaccine induced immune responses including those in live attenuated vaccines where the endogenous adjuvant (PAMPs) are being recognized by PRRs. TOLL-like receptor 2 (TLR2) is an important PRR used in this study. TLR2 is unique among all the mammalian TLRs, as it is able to recognize the most diverse repertoire of PAMPs, such as cell walls of Gram-positive bacteria, bacterial glycolipids, mycobacterial lipoprotein, etc. (17–20). TLR2’s ability to detect a wide repertoire of PAMPs is the result of its potential to heterodimerize with either TLR 1 or 6 in mice and TLR1, 6 and 10 in humans (17–20).
Given the limitations of traditional vaccines, the success of PAMPs within live attenuated vaccines in inducing T cell responses, and the importance of TLR2, we investigated the ability of Neisseria meningitidis Porin B (PorB) protein, a TLR2 ligandbased adjuvant, to generate vaccine-induced T cell responses. PorB is the major outer membrane protein from Neisseria meningitidis (21). Meningococcal PorB has been used as an immune adjuvant for vaccines with a wide range of antigens including bacterial capsular polysaccharides, bacterial oligosaccharides and proteins (22–24). PorB is also a component of the Outer Membrane Proteins from Meningococcus (OMPC), which has been used as a carrier protein for the Haemophilus influenza type B (Hib) human vaccine (22, 23). More recently, it was demonstrated that PorB requires intact in vivo MyD88 signaling in B cells, macrophages and dendritic cells (individually) for its adjuvant activity and also has the ability to induce a robust germinal center reaction (25). The purpose of this current study is to further characterize the adjuvant activity of PorB, especially in regards to the breadth of T cell response sit induces along with its ability to induce potentially protective responses related to CD8 T cells.
Materials and Methods
Animals and Immunizations
Wild Type (WT) C57BL6J mice were purchased from Jackson Laboratories. Mice were maintained in the Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility at Boston University School of Medicine Laboratory Animal Science Center (LASC) and experiments were conducted under the approved IACUC protocol for the Wetzler Laboratory. WT mice between the ages of six to twelve weeks were immunized subcutaneously two or three times at two-week intervals. Mice were immunized with 10μg of Neisseria meningitidis Porin B (PorB) admixed with 10μg of Ovalbumin (OVA). Mice were immunized with either PBS or 10μg of OVA as controls. There were 3–4 mice per group and each experiment was repeated to obtain a total of 6–8 mice per experimental condition. PorB was purified from N. meningitidis strain H44/76 Δ−1/4 (24) using protein extraction and column chromatography as previously described (26). Activity of PorB and endotoxin content were examined by stimulation of WT, MyD88−/−, TLR2−/− and TLR4−/− BMDM and analysis of supernatant for TNF-α, silver staining and LAL Assay (Pierce Endotoxin Kit from Life Technologies), no endotoxin was found in any preparations. The amount of adjuvant PorB and OVA antigen utilized were based on previously published studies and falls within the best range of their efficacy (27). Sera were collected via tail bleed for serum cytokine measurements, pre-immune sera as well as sera from 4 hours or 12 hours post immunization were collected. Sera for antibody screening were collected two weeks after the third immunizations. These time point were selected based on our examinations of other time points and another previously published study (28).
Measurement of Antigen Specific Antibodies
Sera were assayed for OVA-specific immunoglobulins by enzyme-linked immunosorbent assay (ELISA) as previously described (29, 30). Briefly, wells were coated with OVA (5 μg/mL) in carbonate buffer and incubated overnight at 4°C. Sera were sequentially diluted starting at 1:50 and added to the previously coated wells, and incubated overnight at 4°C. Alkaline phosphatase-conjugated anti-mouse IgG1, 2b, 2c or 3 subclasses (Sigma Aldrich, St Louis, MO) were added. After washing, the ELISA was developed with onestep p-nitrophenyl phosphate (Pierce, Rockford, IL) and the optical density (OD) at 405 nm was measured on a SpectraMax190 Microplate Reader (Molecular Devices, Sunnyvale, CA). End point titers were determined for IgG subclasses by doing serial dilution and the last dilution with detectable level of OVA IgG subclasses were multiplied by the O.D and this is reported as end point titers.
Chemokine and Cytokine Screening
Cytokine levels were measured from sera obtained 4 or 12 hours after each immunization and compared to pre-immune sera. Sera from similar immunization groups were pooled (N=8 mice), used in duplicate and screened on a MAGPIX XMAP instrument (Luminex, Austin, TX) using Mouse 20-plex cytokines kits (Life Technologies). Individual standard curves were generated for each cytokine and analyzed using the Luminex Xponent software. Unknown sample concentration was extrapolated from standard curves for single analytes. All values outside of the standard curve limit were rejected.
Antigen Specific T cells
WT mice were immunized as described above and spleens harvested on day 11 after the 2nd immunization. There were 3 to 4 mice per group and experiments were repeated to with a total of 6 to 8 mice per group. Single cell suspensions were prepared. Red blood cells were lysed with Ammonium-Chloride-Potassium lysis buffer (ACK Lysis Buffer). Cells were counted using a hemocytometer. ELISPOT plates (Cat# MAIPS4510, Merck Millipore, Ireland) were coated for 3 hours at room temperature with anti-mouse IFN-γ (5μg/ml) (mAb AN18, Mabtech, Sweden) or anti-mouse IL-4 (7.5μg/ml) (mAb 11B11, Mabtech, Sweden) or PBS. Plates were washed with PBS. Cells were plated at a density of 500,000 and 50,000 cells per well in 4 replicates per mouse per condition in RPMI 1640 media (Corning, Corning NY) supplemented with 8% Fetal Bovine Serum (Corning, Corning NY). Cells from each mouse for each immunization group were stimulated with OVA323–339 CD4 peptide (ISQAVHAAHAEINEAGR), OVA 257–264 CD8 peptide (SIINFEKL), or OVA257–254 scrambled peptide (FILKSINE) as a negative control, all supplied by ANASPEC, Fremont, CA and used at 5μg/ml. Cells from each mouse were also plated with media alone or with purified α-CD3/CD28 (1.5μg/ml) (Affymetrix bioscience, San Diego, CA) as a positive control. All stimulations were performed overnight at 37°C. Plates were then washed with PBS containing 0.1% Tween (PBST) then incubated with secondary antibodies (mAb R4–6A2 Biotin IFN-γ at 1μg/ml or mAb BVD6–24G2-Biotin IL-4 at 2 μg/ml) (Mabtech, Sweden) for 1.5 hours at room temperature. Plates were then washed and incubated with Alkaline Phosphataseconjugate streptavidin (Jackson Immuno Research Lab, West Grove, PA) (1:1000) for 30 minutes. Plates were washed and developed with Vector Blue Substrate Kit (Vector Lab, Burlingame, CA) until spots are clearly visible. Plates were read on an ImmunoSpot CTL Reader instrument and using the ImmunoSpot software (CTL Worldwide, Shaker Heights, OH) where the spots were quantitated for each well and were put through a quality control check, data analyzed and graphed using GraphPad Prism.
Listeria Murine Infection Model
WT mice were immunized as described above and infected with recombinant Listeria monocytogenes expressing OVA with resistance to erythromycin (rLmOVA) via retroorbital injection two weeks after the 3rd immunization. rLmOVA was a generous gift from Hao Shen (University of Pennsylvania) (31). rLmOVA was cultured overnight in Brain Heart Infusion (BHI) broth with erythromycin at 50μg/ml. Subculture was performed and infectious doses were prepared during bacterial log phase and used to infect mice. Colony forming units (CFU) of each injectable dose was determined by plating injectable doses of bacteria before and after injection in mice to get an accurate CFU per mouse. Mice received either a low dose of 1–5 ×105 bacteria per mouse for bacterial burden experiments or a high dose of 1×106 bacteria per mouse for survival experiments. Day 3 after a low dose infection, mice were euthanized, organs perfused with PBS and spleen and liver harvested. Single cell suspension was prepared from the spleen and the total number of cells was quantitated using a hemocytometer. The weight of each liver was recorded. The organs were permeabilized using 0.5% TritonX-100 (MP Biomedicals, France) and diluted for plating on BHI Agar (prepared in house with Erythromycin). Un-diluted, 1:10, 1:1 000, 1:10 000 and 1:100 000 diluted samples were plated in triplicate and incubated overnight at 37°C. CFU were calculated and normalized to the weight of the livers or number of cells in spleen. Mice receiving a high dose infection were monitored multiple times through the 24-hour period until they succumbed to the infection.
Statistics
Statistics were calculated using with GraphPad Prism. When comparing more than two immunization groups at time, One Way ANOVA with Tukey test were used. For comparing OVA/PorB with OVA alone for bacterial burden, the non-parametric MannWhitney U test was used. The Mantel-Cox Test was used to compare the survival distributions of OVA/PorB and OVA alone immunization group.
Results
Neisserial PorB induces antigen specific Th1/Th2 type antibody response s and effector molecules important for cellular responses in vivo.
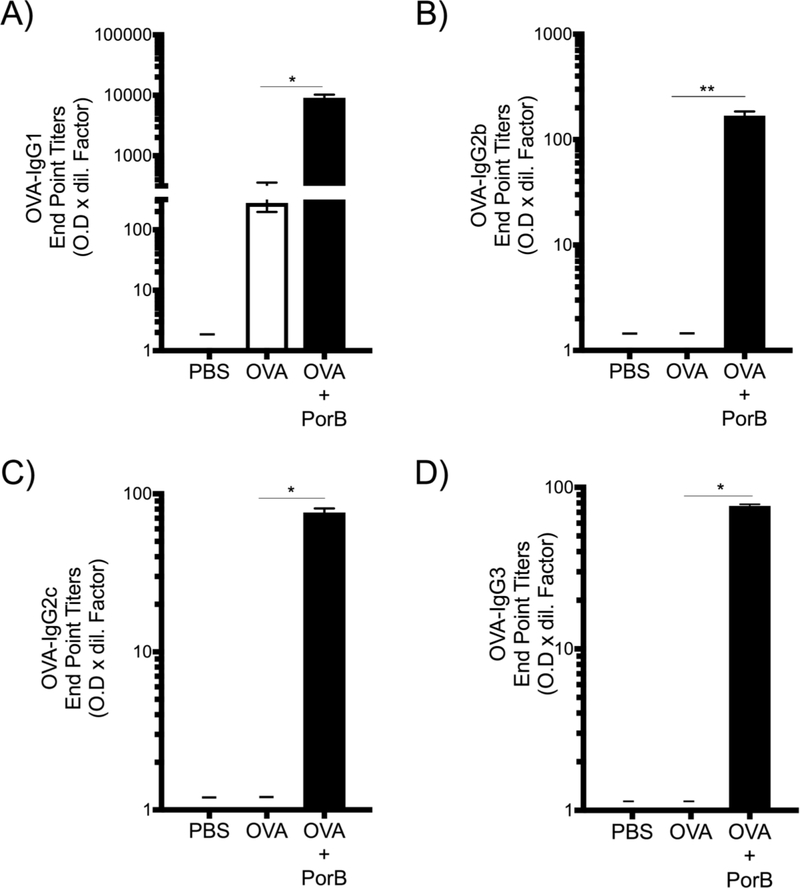
Mice were immunized three times at two-week intervals and sera were obtained either 4 hours or 12 hours after the third immunization to measure chemokines/cytokines and two weeks after the third immunization to measure antigen specific antibody subclasses. Vaccines adjuvanted with PorB induced high level of OVA specific IgG1 (Th2 type antibody response) (Figure 1A) similar to responses seen with Alum. However, PorB adjuvant activity also induced high levels of Th1 type antigen specific antibodies, IgG2b, 2c and 3 (Figure 1B-D).
Figure 1. Adjuvant PorB induces antigen specific antibody subclasses associated with Th2/Th1 type responses.
(A) OVA-IgG1 (Th2 type associated responses) and (BD) OVA-IgG2b, OVA-IgG2c and OVA-IgG3 respectively (Th1 type associated responses) endpoint titers were measured by ELISA from sera of WT immunized three times at two-week intervals. The results shown are from samples collected two weeks after the third immunization and representative of two experiments with a total N = 8 mice per immunization group represented as standard error of the mean. One-way ANOVA with Tukey test were used (ns P>0.05, *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001). Symbol (–) indicates that the antibody levels were below detectable level.
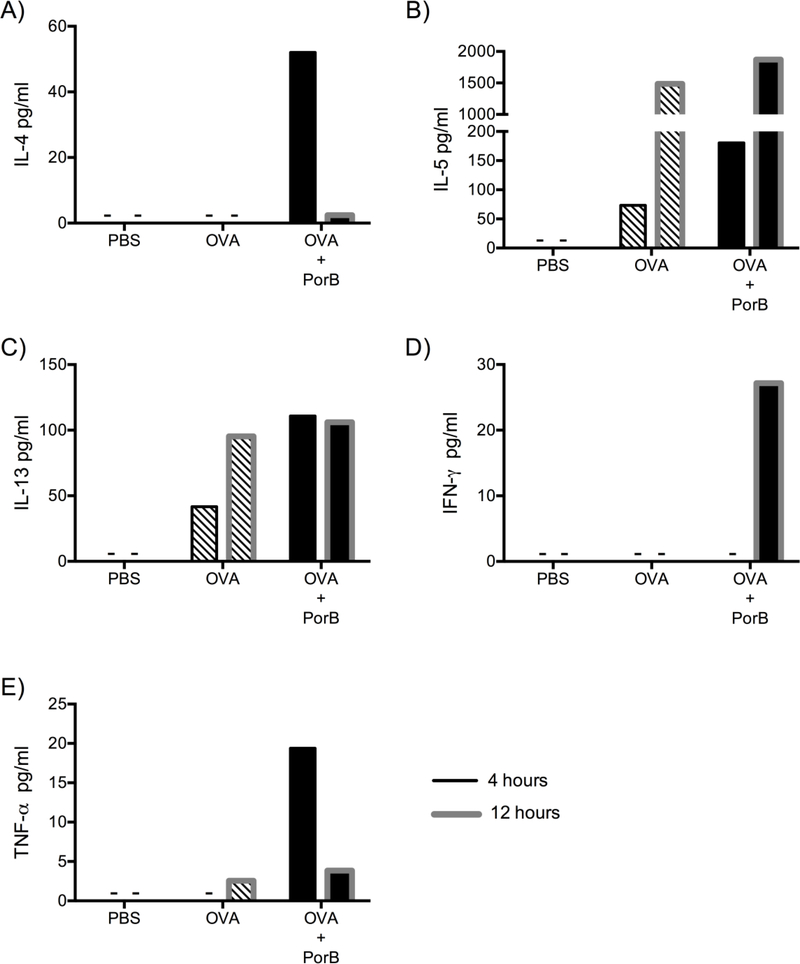
Sera were also analyzed for IL-4, IL-5, IL-13, IFN-γ and TNF-α cytokines, which are known to be produced by Th1 and Th2 cells (32–38). Mice immunized with PBS (Control) had no detectable levels of IL-4, IL-5, IL-13, IFN-γ and TNF-α cytokines in the sera 4 hours and 12 hours after immunization. Mice immunized with OVA alone had very low concentrations of IL-5 and IL-13 (Figure 2B & C) 4 hours after the 3rd immunization but higher levels of IL-5, IL-13 and very small amounts of TNF-α (Figure 2E) were seen 12 hours after the third immunization. IL-4 and IFN-γ levels were below the detection limits at both time points (Figure 2A & D). In contrast, mice immunized with PorB admixed with OVA induced higher levels of these cytokines as compared to PBS or OVA alone-immunized mice; OVA/PorB induced very high amounts of IL-4, IL-5, IL-13 and TNF-α at the earliest time point (4 hours) after the 3rd immunization as compared to mice immunized with OVA alone (Figure 2A-C & E). However, IFN-γ was below the detection limit at 4 hours after the 3rd immunization but peaked at 12 hours after the 3rd immunization with OVA/PorB (Figure 2D) and it was only induced when the vaccine contained the adjuvant. Th2 type cytokine IL-4 was noticeably lower at 12 hours compared to 4 hours after the 3rd immunization with OVA/PorB. There were small increases in the level of IL-5, IL-13 and TNF-α 12 hours after the 3rd immunization with PorB/OVA as compared to OVA alone (76). These time points (4 and 12 hours after the third immunization) were used based on observations from previous studies, as well as our own investigations, where it was demonstrated that serum T cell cytokines peak during these periods in mice upon immunization (28, 39).
Figure 2. Adjuvant PorB induces cytokines produced by T cells in vivo.
(A-C) IL-4, IL-5, and IL-13 (Th2 type cytokines), (D) IFN-γ (Th1 type cytokine) and (E) TNF-α cytokine levels in pooled sera from WT mice immunized three times at two-week intervals. Sera were collected 4 hours (black border) or 12 hours (grey border) after the 3rd immunization with PBS, OVA or OVA/PorB. These cytokines were measured by Luminex magnetic bead-based multiplex assay. The results shown are representative of two experiments with a total of 8 mice per immunization group. Sera from 8 mice were pooled and plated in duplicate. Symbol (–) indicates that effector molecules levels were below detectable level.
MIG, MCP-1, IP-10, MIP-1α and KC were measured 4 hours after each immunization. These effector molecules are known to peak early after each immunization and have been shown to play important roles in generating cellular responses in vivo, including T cell responses (39). PorB/OVA immunization induced higher levels of MIG (CXCL9), MCP-1 (CCL2), IP-10 (CXCL10), MIP-1α (CCL3) and KC (CXCL1) chemokines 4 hours after each of the three immunizations as compared to mice immunized with OVA alone or mock-immunized with PBS (Figure 3A-E). MIG and MIP-1α were highly induced 4 hours after the 1st immunization with PorB/OVA, whereas levels were decreased after the 2nd and 3rd immunizations (Figure 3A & D). MCP-1 was detected at its highest level after the 2nd immunization with the adjuvanted vaccine (Figure 3B). IP-10 and KC levels varied subtly after each immunization, with KC being induced and remaining high (~500pg/ml) in comparison to all other chemokines after each of the three immunizations (Figure 3C & E). Vaccine formulations containing PorB also increased IL-2, 4 hours after the 2nd and 3rd immunizations; there were no detectable IL-2 after the 1st immunization (Figure 3F). Control mice immunized with PBS had no detectable levels of MIG, MCP-1, IP-10, MIP-1α, KC and IL-2 in the sera (76). These chemokines levels were very low or undetectable for most analytes 12 hours after immunization.
Figure 3. TLR2 ligand-based adjuvant PorB induced effector molecules important for cellular responses in vivo.
(A-E) MIG, MCP-1, IP-10, MIP-1α, and KC chemokines and (F) IL-2 cytokine level in pooled sera from WT mice immunized three times at two weeks interval and sera collected 4 hours after each immunization with PBS, OVA or OVA/PorB measured by Luminex magnetic bead-based multiplex assay. X-axis labeling of 1st, 2nd and 3rd represent sera collected 4 hours after each of the three different immunizations. The results shown are representative of two experiments with a total of 8 mice per immunization group. Sera from 8 mice were pooled and plated in duplicate. Symbol (–) indicates that effector molecules levels were below detectable level.
Vaccine formulated with PorB induces robust antigen specific CD4 and CD8 T cell responses in vivo.
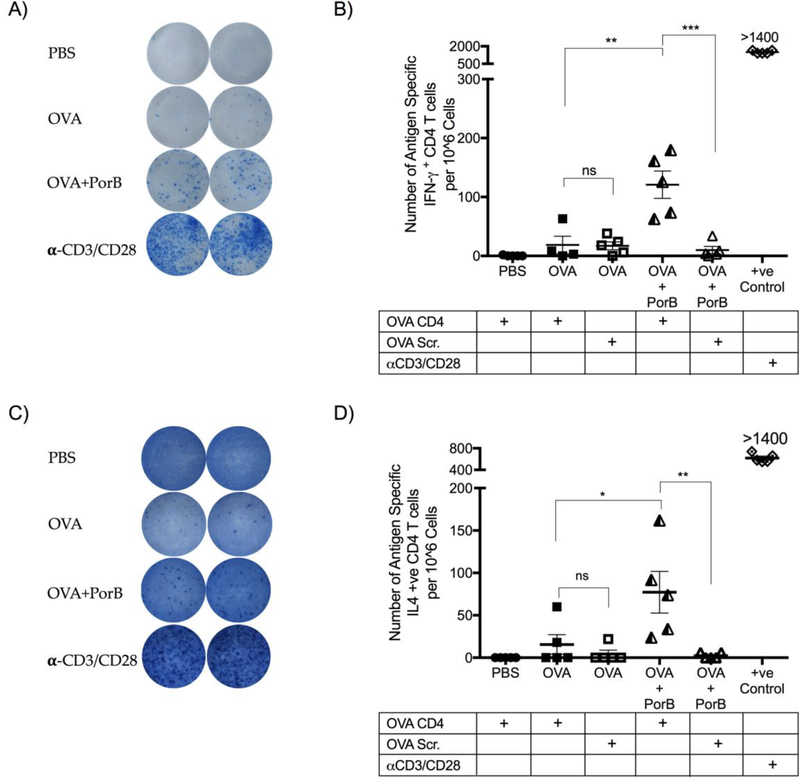
PorB has been shown to increase antigen uptake and recruitment of DC to draining lymph nodes (40). PorB’s ability to induce T cell modulating cytokines and chemokines indicates that its adjuvant activity will help initiate and improve antigen specific T cell responses. Antigen specific T cell responses were examined to corroborate this hypothesis in mice immunized with PorB/OVA, OVA alone, or PBS, (as control), twice, at two-week intervals. Eleven days after the 2nd immunization the number of splenic antigen specific CD4 T cells producing either IFN-γ or IL-4 were quantified. Single cell suspensions were prepared from the spleen and stimulated overnight with a CD4 OVA specific peptide, scrambled OVA peptide or anti-CD3/CD28 as a positive control, in an ELISPOT assay. The MHC Class II restricted OVA peptides were used to determine the relative number of OVA specific CD4 T cells induced by immunization with PorB. This assay is highly sensitive, specific and reproducible, allowing not just the quantitation but also the secretory activity of the cells being screened (41). Vaccine formulated with PorB induced a robust antigen specific CD4 T cells to OVA (Figure 4A,B). The number of OVA specific CD4 T cells was significantly greater as compared to mice immunized with OVA alone (Figure 4B). CD4 T cells from PorB/OVA immunized mice produced significantly more IFN-γ (OVA Th1 Cells) upon stimulation with OVA specific CD4 peptide as compared to mice that received OVA alone. The use of scrambled peptide as a control did not induce any T cell responses, highlighting the specificity of the measured responses while the positive control. The use of PorB also induced significantly more OVA specific CD4 T cells producing IL-4 (OVA Th2 Cells) compared to mice immunized with OVA alone (Figure 4C-D). OVA Th2 cells were at a lower frequency as compared to OVA Th1 cells (76).
Figure 4. Vaccine formulated with adjuvant PorB induced robust antigen specific CD4 T cell responses in vivo.
WT mice were immunized two times at two-week intervals. Eleven days after the 2nd immunization single cell suspensions were prepared from the spleen and stimulated overnight with an OVA CD4 peptide, OVA scrambled peptide or anti-CD3/CD28 (αCD3/CD28) as a positive control. An ELISPOT assay, as described in Methods above, was used to quantitate the number of antigen specific CD4 T cells producing either A) IFN-γ positive spots and B) quantitation of CD4 T cells producing IFN-γ or C) IL-4 positive spots and D) quantitation of CD4 T cells producing IL-4 from the spleens of immunized mice. The results shown are representative of two experiments with a total N = 5 mice per immunization group represented as standard error of the mean. CD8 responses in the same mice were analyzed in Figure 5. One-way ANOVA with Tukey test were used (ns P>0.05, *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
We examined PorB’s ability to induce antigen specific CD8 T cell response in vivo, similar to analysis of antigen specific CD4 T cells described above. We used an OVA specific CD8 MHC Class I restricted peptide to determine the level of the antigen specific CD8 T cells induced by OVA/PorB immunization. The vaccine formulated with adjuvant PorB induced a significantly larger number of antigen specific IFN-γ producing CD8 T cells as compared to mice immunized with OVA alone (Figure 5A-B). Minimal production of IFN-γ was seen when the OVA CD8 scrambled peptide was used. This demonstrates that the IFN-γ positive spots from the ex vivo assay produced by the cells from immunized mice after stimulation with OVA specific CD8 epitopes were being produced by OVA specific CD8 T cells induced by the vaccine.
Figure 5. Vaccine formulated with adjuvant PorB induced a robust antigen specific CD8 T cell response in vivo.
WT mice were immunized two times at two weeks interval and 11 days after the 2nd immunization single cell suspensions were prepared from the spleen and stimulated overnight with an OVA CD8 peptide, OVA scrambled peptide or anti-CD3/CD28 as positive control. An ELISPOT assay, as described in Methods, was used to quantitate the number of antigen specific CD4 T cells producing either A) IFN-γ positive spots and B) quantitation of CD8 T cells producing IFN-γ or C) IL-4+ spots and D) quantitation of CD8 T cells producing IL-4 from the spleens of immunized mice. The results shown are representative of two experiments with a total N = 5 mice per immunization group represented as standard error of the mean. CD4 responses in the same mice were analyzed in Figure 4. One-way ANOVA with Tukey test were used (ns P>0.05, *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
Vaccines prepared with PorB admixed with OVA induced a small but significant population of OVA specific CD8 T cells producing IL-4 (Tc2 cells) in vivo 11 days after the 2nd immunization compared to mice vaccinated with OVA alone (Figure 5C-D). Nonspecific signal was ruled out using a scrambled OVA peptide (as a negative control) to stimulate the cells (Figure 5D) as well as cells stimulated without any peptide.
OVA CD8 T cells secreting IFN-γ (OVA Tc1 cells) (Figure 5B) were the largest population, quantified per 106 splenocytes, when compared to the OVA Tc2 (Figure 5D), OVA Th1 (Figure 4B) and OVA Th2 cells (Figure 4D).
PorB as an adjuvant enhances protection of mice towards Listeria monocytogenes infection.
Adjuvant PorB induced a robust antigen specific CD8 T cell responses as demonstrated above. The functionality of these OVA specific CD8 T cells induced by PorB and their ability to mitigate a bacterial infection in vivo was investigated. Mice were immunized similarly to above experiments and were challenged with either low doses (~1–5 ×105 bacteria per mouse) or a high dose (~1×106 bacteria per mouse) of rLmOVA two weeks after the third immunization in order to assess bacterial burden and survival respectively. Upon infection with rLmOVA, mice vaccinated with PorB/OVA had less CFUs in the liver or spleen as compared to OVA-immunized mice. When quantitated, it was clear that the PorB adjuvanted vaccine caused a non-significant decrease in the bacterial load from the liver (Figure 6A) and a significant decrease in the bacterial load from the spleen (Figure 6B), three days after infection with 5×105 rLmOVA. Mice immunized with OVA alone (or PBS) had very high numbers of bacteria in both the liver and spleen. Similar results were seen when mice were infected with 1×105 CFU of rLmOVA per mouse (Figures 6C and D)
Figure 6. TLR2-ligand based adjuvant PorB decreases bacterial burden in mice infected with Listeria monocytogenes.
WT mice were immunized three times at two weeks interval with PBS, OVA or OVA/PorB. Two weeks after the last immunization, the mice were challenged with 5.4×105 CFU of rLMOVA per mouse and bacterial burden in A) Liver and B) Spleen were measured 3 days after the infection. Bacterial burden from mice challenged with 1×105 CFU of rLMOVA per mouse were measured in the C) Liver and D) Spleen. The results shown are representative of two experiments with an N = 4 mice per immunization group per experiment represented as standard error of the mean. Total N = 8 mice. Mann Whitney U test were used (ns P>0.05 and *P<0.05) to compare OVA/PorB with OVA alone.
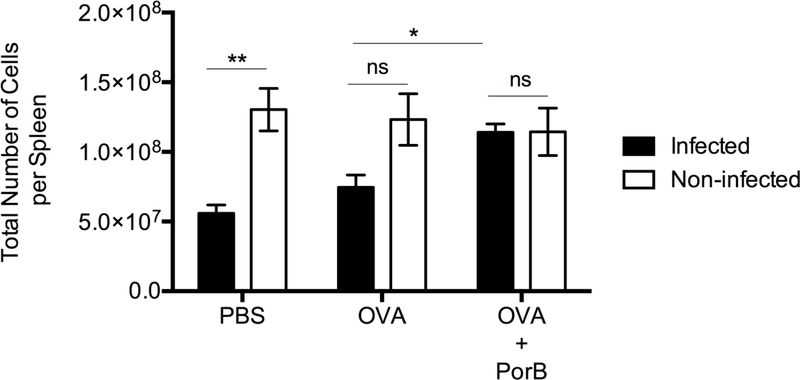
Listeria monocytogenes infection in mice is known to cause a decrease in the number of cells in the spleen (42). Two weeks after the 3rd immunization the change in the total number of cells in the spleen of non-infected mice remained unchanged (Figure 7). However, when these mice were challenged with low dose CFUs of rLmOVA two weeks after the 3rd immunization, there was a significant decrease in the number of cells in the spleen of mice immunized with OVA alone or PBS three days after the infection (Figure 7). However, mice immunized with PorB/OVA did not have a decrease in the number cells in the spleen, as compared to infected OVA or PBS immunized mice or uninfected OVA/PorB immunized mice. (Figure 7).
Figure 7. Infection with Listeria monocytogenes did not affect the total number of cells in the spleen of mice immunized with PorB adjuvanted vaccine.
WT mice were immunized three times at two weeks interval with PBS, OVA or OVA/PorB. Two weeks after the last immunization, the mice were challenged with 1×105 CFU of rLMOVA per mouse. Cell numbers in spleen were quantitated 3 days after the infection represented as standard error of the mean. The results shown are representative of two experiments with an N = 4 to 6 mice per immunization group. One-way ANOVA with Tukey test were used (ns P>0.05, *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
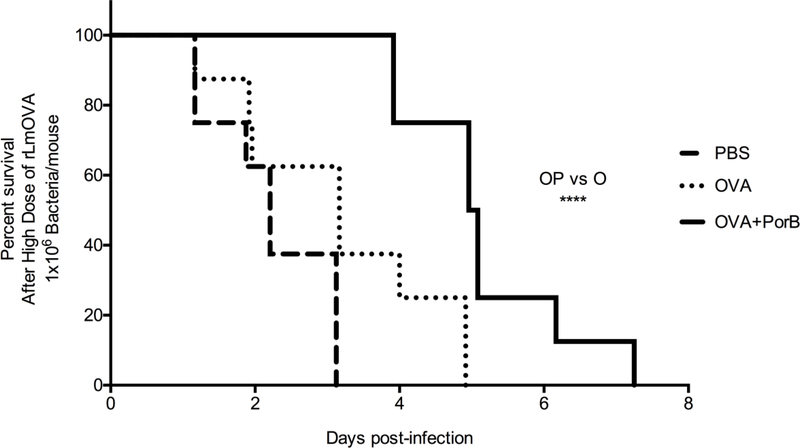
Next, survival of immunized mice infected with a high dose of rLmOVA was examined. All of these mice perished within three days after the high dose infection, many within the first 24 hours (Figure 8A). Some of the mice immunized with OVA alone and challenged with a high dose of rLmOVA succumbed to the infection after 24 hours. About 60% of these mice died within three days of the infection. However, mice immunized PorB/OVA survived longer, the first mouse died 4 days after infection and approximately 70% of the mice were still alive five days after the high dose infection. The last mouse died 7 days post infection. Survival of mice immunized with vaccine formulated with PorB were significantly greater as compared to mice immunized with OVA alone as determine by Mantel-Cox test with 95% confidence interval (Figure 8A).
Figure 8. Vaccine formulation containing PorB increases mice survival upon a high dose infection with Listeria monocytogenes.
WT mice were immunized three times at two weeks interval with PBS, OVA or OVA/PorB. Two weeks after the last immunization, the mice were challenged with high dose (1×106 CFU) of rLMOVA per mouse. A) Survival of infected mice was monitored multiple times a day until the mice succumbed to the infection. The results shown are representative of two experiments with a total N = 8 mice per immunization group. The Mantel-Cox Test was used to compare the survival distributions of OVA/PorB and OVA alone immunization group (*** P = 0.0001).
Discussion
Adjuvants enhance vaccines to induce protective immune responses, mainly through the induction of pathogen specific antibody responses (43). Many of the current adjuvanted vaccines contain Alum, which is very good at inducing a Th2 associated IgG1 type response (44). However, many diseases such as TB, HIV, malaria and cancer require a broader antibody response (Th1 type antibody responses) and, likely, a concomitant CD8 T cell responses (43) emphasizing the unmet clinical need for new novel adjuvants. Previous studies have demonstrated the potential for PAMPs to be used as adjuvants in vaccine formulation. In our previous work, we have shown that the TLR2-ligand Neisseria meningitidis PorB has potent adjuvant abilities, increasing the antibody response to a wide range of antigens, including polysaccharides, proteins, and LPS (22–24). This adjuvant activity was dependent on PorB’s signaling through TLR2/1 heterodimers (27), and MyD88 adaptor protein (45, 46). In addition, PorB has been shown to enhance antigen uptake in vivo and induce robust germinal center formation and diverse types of antigen specific antibody subclasses (25, 40).
We demonstrated that the TLR2-ligand based adjuvant PorB induced a number of T cell modulating cytokines including high levels of Th1 type (IFN-γ), Th2 type (IL-4, IL-5 and IL-13) and TNF-α T cell cytokines 4 or 12 hours after the 3rd immunization. The induction of Th2 and Th1 type cytokines are consistent with the induction by PorB antigen specific IgG1 (Th2 type) and IgG2b, 2c and 3 (Th1 type) responses. Interestingly, CD8 T cells have been shown to produce TNF-α within 5 hours of TCR engagement and this promotes DC maturation, CD8 T cell differentiation and proliferation (35–37, 47) and mediate the clearance of this infection in vivo (48, 49) which in our study may be promoted by the use of PorB as an adjuvant.
In addition, vaccines formulated with PorB induced an increase in effector molecules (MIG, MCP-1, IP-10, MIP-1α, KC and IL-2) that are also vital for the induction of T cell responses. PorB increases the level of these chemokines early (4 hours) after each of the three immunizations. MIG and IP-10 chemokines have been shown to act directly on CD8 T cells, which express high level of CXCR3, the receptor for these chemokines (50). Other studies have shown that cells producing IFN-γ, including CD4 T cells, have an important role in controlling the level of MIG and IP-10 and subsequent in vivo recruitment of CD8 T cells (39, 51). IP-10 is also known to promote the retention of T cells in the draining lymph nodes to enhance APC-T cell interactions and clonal expansion (52). Induction of these chemokines in vivo suggest that they may be playing a role in generating PorB’s vaccine-induced T cell responses. MIP-1α, has been shown to be able to increase the number of antigen specific CD8 T cells (53) and to induce macrophages and NK cell migration, which can lead to enhanced T cell-DC interactions (54). MIP-1α signaling on CD8 T cells induces these cells to migrate towards CD4/APC clusters, enhancing contact of CD8 T cells with CD4 T helper cells-licensed DCs (55) which could promote the increase in number and quality of memory CD8 T cells (56). Vaccines formulated with PorB induced MIP-1α after each of the three immunizations, further fueling our interest in characterizing these antigen specific T cells induced by PorB. Other investigators have shown that biglycan ligands (TLR2 and 4 agonists) can increase the level of KC (CXCL1), which in turns increase cells recruitment including T cells to the site of inflammation in mice (57). MCP-1 (CCL2) signals through CCR2 which is expressed on monocyte, macrophages and Th1 type cells, enhancing Th1 type adaptive immunity (54). PorB induction of these chemokines likely ensures recruitment of specific cells enhancing cross-presentation and APC-T cell intera ctions. PorB’s ability to induce these specific chemokines and T cell cytokines in vivo suggest that it’s adjuvant activity also involves direct enhancement of T cell responses, in addition to increased germinal center formation (46).
We further identified the source of some of these Th2 and Th1 types cytokines. PorB induced significantly more OVA specific CD4 T cells producing IL4 or IFN-γ as compared to OVA alone. Previous studies showed that the presence of CD4 T cells (Th1 type cells) producing IFN-γ enhance the differentiation of CD8 T cells into Tc1 type cells (58). This type of cellular response is crucial to prevent and delay the progression of infectious diseases (especially intracellular pathogens) as well as other pathologies, including cancer (59). The increase in the level of IFN-γ, IL-2 and the various chemokines upon immunization with PorB, likely generated an environment conducive for the robust induction of OVA specific CD8 T cells producing IFN-γ by this adjuvant. We investigated all the different types of T cell responses induced by PorB and demonstrated that PorB induced antigen specific CD8 T cells producing IL-4 (Tc2 type response).
The number of OVA specific Tc2 cells (IL-4 producing cells) induced by PorB/OVA was much smaller as compared to the other type of T cell responses measured. However, it was still significantly higher than mock-immunized or OVA-alone immunized mice. These types of antigen specific Tc2 cells have been shown to be able to provide B cell help (60). In mice, they are able to be cytotoxic via the perforin pathway, however they are less cytotoxic than Tc1 type cells (58, 61, 62). In humans, Tc2 type cells have been shown to counteract the overproduction of pro-inflammatory cytokines in old age and are important for induction of humoral response upon immunization (63). Older adults who fail to mount a protective humoral response upon immunization lack these Tc2 cell subset (63). The elderly population has an increased risk in acquiring infections with an increase in severity, which has been shown to be due to immune senescence (63, 64). Using an adjuvant like PorB in vaccine formulations could enhance the vaccine-induced immune responses in the elderly, including an increase in the number of antigen specific Tc2 cells producing IL-4, which will in turn aid in inducing a robust humoral response. These types of responses induced by PorB-adjuvanted vaccine would be very beneficial to the growing aging population in today’s world.
Previous studies demonstrated that the induction of CD8 T cells upon immunization or infection might be important for the control and clearance of various pathogens such as HIV, malaria, or tuberculosis (1). A vaccine that induces antigen specific CD8 T cells could aid in inducing protective immunity against intracellular pathogens. Here, we demonstrated that our adjuvant PorB induced robust antigen specific CD8 T cell responses. We showed that these OVA specific CD8 T cells are not just increased in numbers upon immunization with vaccine formulated with PorB but they are highly functional producing IFN-γ in an ex vivo ELISPOT assay upon engagement of its TCR with OVA CD8 (MHC Class I restricted) peptide presented by antigen presenting cells.
Using a bacterial infection model (recombinant Listeria monocytogenes expressing OVA, rLmOVA), we demonstrated that the antigen specific CD8 T cells induced by vaccine formulated with PorB are functional in vivo as well. Infection with the rLmOVA is a well-established model to study CD8 T cell responses in mice because of the bacterium’s unique in vivo life cycle (65). Pathogen specific (in this case, OVA, since these bacteria have been engineered to express OVA) CD8 T cell responses (and not CD4 T cells or antibody responses) have been shown to be required for clearance of this bacterium (31, 42, 65–68). Once inside the cell, rLmOVA replicates in the cytosol and spreads from cell to cell via actin polymerization, without having to expose itself to the outside of the cell (69, 70). OVA expression by the bacteria is under the control of the listeriolysin promoter in the bacteria and bacterial proteins from the cytosol are presented on MHC I & II (67). Upon infection with rLmOVA, it is known that the infection can cause lymphopenia by three days post infection (42) due to pore-forming toxin listeriolysin O production and induction of apoptosis (71, 72). In this infection model, we observed a decrease in the number of cells from the spleen of mock-immunized or OVAalone immunized mice but not in PorB/OVA immunized mice, likely due to the effect of antigen specific CD8 T cells controlling the rLmOVA infection, decreasing apoptosis of the splenocytes. Most importantly, the induction of antigen (OVA) specific CD8 T cells in vivo induced by PorB/OVA immunization was associated a decrease in bacterial load from the spleen and liver in mice infected with a low dose of rLmOVA and increased survival of mice infected with a high dose of rLmOVA and is the likely protective mechanism of this survival.
In summary, TLR2 ligand based adjuvant Neisseria meningitidis PorB has broad adjuvant activity with a wide range of antigens (22–24), can induce strong humoral responses (30) and robust germinal center formation and activates B cells, dendritic cells and macrophages, in vivo, for its adjuvant activity (25). In this study, we now demonstrate that PorB can induce a robust and diverse T cell response, that promotes antibody formation as well as strong antigen specific CD8 cytotoxic T cell response that can increase survival of mice infected with Listeria, which depends on antigen specific CD8 T cells. This data supports the premise that PorB will be useful in future vaccine development alone or in combination with other adjuvants to induce responses that’s needed for protection.
Acknowledgement
We thank Dr. Michael Reiser and Ian Francis for critically reviewing this manuscript. We thank Dr. Paola Massari for advice and help regarding the use of PorB and with Deana Toussi and Xiuping Liu for purifying PorB. We thank Dr. Jennifer Cappione from the BUSM Flow Cytometry Core for advice and help with setting ELISPOT assay. We thank Judy Yen and Dr. Ronald Corley for allowing us to use the ELISPOT reader and software in the NEIDL at Boston University. We thank Dr. Shen for providing the rLmOVA bacteria. This work was supported by Boston University School of Medicine and the National Institutes of Health/NIAID (2 R01 AI040944; to Lee Wetzler). Some of the data from the manuscript also appears in the first author’s doctoral thesis from Boston University School of Medicine. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coffman RL, Sher A, and Seder RA 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin SA 2005. Vaccines: past, present and future. Nature medicine 11: S5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plotkin SA 2005. Six revolutions in vaccinology. The Pediatric infectious disease journal 24: 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA 2010. Correlates of protection induced by vaccination. Clin Vaccine Immunol 17: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robbins JB, Schneerson R, and Szu SC 1995. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis 171: 1387–1398. [DOI] [PubMed] [Google Scholar]
- 6.Robbins JB, Schneerson R, Vann WF, Bryla DA, and Fattom A 1995. Prevention of systemic infections caused by group B streptococcus and Staphylococcus aureus by multivalent polysaccharide-protein conjugate vaccines. Annals of the New York Academy of Sciences 754: 68–82. [DOI] [PubMed] [Google Scholar]
- 7.Robbins JB, Schneerson R, and Szu SC 1996. Hypothesis: how licensed vaccines confer protective immunity. Advances in experimental medicine and biology 397: 169–182. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Uematsu S, and Takeuchi O 2006. Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 9.Makela PH 2000. Vaccines, coming of age after 200 years. FEMS microbiology reviews 24: 9–20. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, and Ahmed R 2011. Immunological mechanisms of vaccination. Nature immunology 12: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, and Wyatt RT 2004. HIV vaccine design and the neutralizing antibody problem. Nature immunology 5: 233–236. [DOI] [PubMed] [Google Scholar]
- 12.Seder RA, and Hill AV 2000. Vaccines against intracellular infections requiring cellular immunity. Nature 406: 793–798. [DOI] [PubMed] [Google Scholar]
- 13.Moore AC, and Hill AV 2004. Progress in DNA-based heterologous primeboost immunization strategies for malaria. Immunological reviews 199: 126–143. [DOI] [PubMed] [Google Scholar]
- 14.Prieur E, Gilbert SC, Schneider J, Moore AC, Sheu EG, Goonetilleke N, Robson KJ, and Hill AV 2004. A Plasmodium falciparum candidate vaccine based on a six-antigen polyprotein encoded by recombinant poxviruses. Proceedings of the National Academy of Sciences of the United States of America 101: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson HL, and Amara RR 2005. T cell vaccines for microbial infections. Nature medicine 11: S25–32. [DOI] [PubMed] [Google Scholar]
- 16.Leroux-Roels G 2010. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine 28 Suppl 3: C25–36. [DOI] [PubMed] [Google Scholar]
- 17.Dasari P, Nicholson IC, Hodge G, Dandie GW, and Zola H 2005. Expression of toll-like receptors on B lymphocytes. Cellular immunology 236: 140–145. [DOI] [PubMed] [Google Scholar]
- 18.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, and Aderem A 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proceedings of the National Academy of Sciences of the United States of America 97: 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, and Akira S 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. International immunology 13: 933–940. [DOI] [PubMed] [Google Scholar]
- 20.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, and Bates EE 2005. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. Journal of immunology 174: 2942–2950. [DOI] [PubMed] [Google Scholar]
- 21.Wetzler LM 2010. Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol 5: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiavolini D, Weir S, Murphy JR, and Wetzler LM 2008. Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the capacity of Francisella tularensis lipopolysaccharide to protect mice against experimental tularemia. Clin Vaccine Immunol 15: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latz E, Franko J, Golenbock DT, and Schreiber JR 2004. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol 172: 2431–2438. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon FG, Ho Y, Blake MS, Michon F, Chandraker A, Sayegh MH, and Wetzler LM 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. The Journal of infectious diseases 180: 755–761. [DOI] [PubMed] [Google Scholar]
- 25.Mosaheb MM, Reiser ML, and Wetzler LM 2017. Toll-Like Receptor Ligand-Based Vaccine Adjuvants Require Intact MyD88 Signaling in AntigenPresenting Cells for Germinal Center Formation and Antibody Production. Front Immunol 8: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massari P, King CA, MacLeod H, and Wetzler LM 2005. Improved purification of native meningococcal porin PorB and studies on its structure/function. Protein expression and purification 44: 136–146. [DOI] [PubMed] [Google Scholar]
- 27.Massari P, Visintin A, Gunawardana J, Halmen KA, King CA, Golenbock DT, and Wetzler LM 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J Immunol 176: 2373–2380. [DOI] [PubMed] [Google Scholar]
- 28.Valensi JP, Carlson JR, and Van Nest GA 1994. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol 153: 4029–4039. [PubMed] [Google Scholar]
- 29.Liu X, Wetzler LM, and Massari P 2008. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 26: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platt A, Macleod H, Massari P, Liu X, and Wetzler L 2013. In Vivo and In Vitro Characterization of the Immune Stimulating Activity of the Neisserial Porin PorB. PLoS One 8: e82171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, and Shen H 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. Journal of immunology 168: 1528–1532. [DOI] [PubMed] [Google Scholar]
- 32.Ohshima Y, Yang LP, Avice MN, Kurimoto M, Nakajima T, Sergerie M, Demeure CE, Sarfati M, and Delespesse G 1999. Naive human CD4+ T cells are a major source of lymphotoxin alpha. Journal of immunology 162: 3790–3794. [PubMed] [Google Scholar]
- 33.Sung SS, Bjorndahl JM, Wang CY, Kao HT, and Fu SM 1988. Production of tumor necrosis factor/cachectin by human T cell lines and peripheral blood T lymphocytes stimulated by phorbol myristate acetate and antiCD3 antibody. The Journal of experimental medicine 167: 937–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Chang JF, Parnes JR, and Fathman CG 1998. T cell receptor (TCR) engagement leads to activation-induced splicing of tumor necrosis factor (TNF) nuclear pre-mRNA. The Journal of experimental medicine 188: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal BB 2003. Signalling pathways of the TNF superfamily: a double edged sword. Nat Rev Immunol 3: 745–756. [DOI] [PubMed] [Google Scholar]
- 36.Ruddle NH 1992. Tumor necrosis factor (TNF-alpha) and lymphotoxin (TNFbeta). Current opinion in immunology 4: 327–332. [DOI] [PubMed] [Google Scholar]
- 37.Smyth MJ, and Johnstone RW 2000. Role of TNF in lymphocyte-mediated cytotoxicity. Microscopy research and technique 50: 196–208. [DOI] [PubMed] [Google Scholar]
- 38.Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, and Kedl RM 2013. T cell responses: naive to memory and everything in between. Advances in physiology education 37: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolz JC, Starbeck-Miller GR, and Harty JT 2011. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 3: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiser ML, Mosaheb MM, Lisk C, Platt A, and Wetzler LM 2017. The TLR2 Binding Neisserial Porin PorB Enhances Antigen Presenting Cell Trafficking and Cross-presentation. Scientific reports 7: 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann PV, and Zhang W 2012. Unique strengths of ELISPOT for T cell diagnostics. Methods in molecular biology 792: 3–23. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Strugnell R, Wijburg O, and Brodnicki T 2011. Measuring bacterial load and immune responses in mice infected with Listeria monocytogenes. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rappuoli R 2007. Bridging the knowledge gaps in vaccine design. Nature biotechnology 25: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 44.Brewer JM 2006. (How) do aluminium adjuvants work? Immunology letters 102: 10–15. [DOI] [PubMed] [Google Scholar]
- 45.Singleton TE, Massari P, and Wetzler LM 2005. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol 174: 3545–3550. [DOI] [PubMed] [Google Scholar]
- 46.Mosaheb MM, Reiser ML, and Wetzler LM 2017. Toll-Like Receptor Ligand-Based Vaccine Adjuvants Require Intact MyD88 Signaling in AntigenPresenting Cells for Germinal Center Formation and Antibody Production. Frontiers in immunology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brehm MA, Daniels KA, and Welsh RM 2005. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. Journal of immunology 175: 5043–5049. [DOI] [PubMed] [Google Scholar]
- 48.Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Forster I, Clausen BE, Tessarollo L, Ryffel B, Kuprash DV, and Nedospasov SA 2005. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22: 93–104. [DOI] [PubMed] [Google Scholar]
- 49.Xu L, Yoon H, Zhao MQ, Liu J, Ramana CV, and Enelow RI 2004. Cutting edge: pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. Journal of immunology 173: 721–725. [DOI] [PubMed] [Google Scholar]
- 50.Groom JR, and Luster AD 2011. CXCR3 in T cell function. Exp Cell Res 317: 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakanishi Y, Lu B, Gerard C, and Iwasaki A 2009. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 462: 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandhapudi SK, Chilton PM, and Mitchell TC 2013. TRIF is required for TLR4 mediated adjuvant effects on T cell clonal expansion. PloS one 8: e56855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oynebraten I, Hinkula J, Fredriksen AB, and Bogen B 2014. Increased generation of HIV-1 gp120-reactive CD8+ T cells by a DNA vaccine construct encoding the chemokine CCL3. PloS one 9: e104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffith JW, Sokol CL, and Luster AD 2014. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual review of immunology 32: 659–702. [DOI] [PubMed] [Google Scholar]
- 55.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, and Germain RN 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440: 890–895. [DOI] [PubMed] [Google Scholar]
- 56.Hugues S, Scholer A, Boissonnas A, Nussbaum A, Combadière C, Amigorena S, and Fetler L 2007. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol 8: 921–930. [DOI] [PubMed] [Google Scholar]
- 57.Moreth K, Frey H, Hubo M, Zeng-Brouwers J, Nastase MV, Hsieh LT, Haceni R, Pfeilschifter J, Iozzo RV, and Schaefer L 2014. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix biology : journal of the International Society for Matrix Biology 35: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosmann TR, Li L, and Sad S 1997. Functions of CD8 T-cell subsets secreting different cytokine patterns. Seminars in immunology 9: 87–92. [DOI] [PubMed] [Google Scholar]
- 59.He Y, and Falo LD 2006. Induction of T cell immunity by cutaneous genetic immunization with recombinant lentivector. Immunologic research 36: 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maggi E, Giudizi MG, Biagiotti R, Annunziato F, Manetti R, Piccinni MP, Parronchi P, Sampognaro S, Giannarini L, Zuccati G, and Romagnani S 1994. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. The Journal of experimental medicine 180: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sad S, Marcotte R, and Mosmann TR 1995. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity 2: 271–279. [DOI] [PubMed] [Google Scholar]
- 62.Sad S, Krishnan L, Bleackley RC, Kagi D, Hengartner H, and Mosmann TR 1997. Cytotoxicity and weak CD40 ligand expression of CD8+ type 2 cytotoxic T cells restricts their potential B cell helper activity. European journal of immunology 27: 914–922. [DOI] [PubMed] [Google Scholar]
- 63.Schwaiger S, Wolf AM, Robatscher P, Jenewein B, and GrubeckLoebenstein B 2003. IL-4-producing CD8+ T cells with a CD62L++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in old age. Journal of immunology 170: 613–619. [DOI] [PubMed] [Google Scholar]
- 64.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, and Grubeck-Loebenstein B 2008. Biology of immune responses to vaccines in elderly persons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 46: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 65.Edelson BT, and Unanue ER 2000. Immunity to Listeria infection. Current opinion in immunology 12: 425–431. [DOI] [PubMed] [Google Scholar]
- 66.Sanjabi S, Mosaheb MM, and Flavell RA 2009. Opposing effects of TGFbeta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, and Shen H 2003. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. Journal of immunology 170: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 68.Zenewicz LA, Foulds KE, Jiang J, Fan X, and Shen H 2002. Nonsecreted bacterial proteins induce recall CD8 T cell responses but do not serve as protective antigens. Journal of immunology 169: 5805–5812. [DOI] [PubMed] [Google Scholar]
- 69.Steffen P, Schafer DA, David V, Gouin E, Cooper JA, and Cossart P 2000. Listeria monocytogenes ActA protein interacts with phosphatidylinositol 4,5-bisphosphate in vitro. Cell motility and the cytoskeleton 45: 58–66. [DOI] [PubMed] [Google Scholar]
- 70.Gedde MM, Higgins DE, Tilney LG, and Portnoy DA 2000. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infection and immunity 68: 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrero JA, and Unanue ER 2012. Mechanisms and immunological effects of apoptosis caused by Listeria monocytogenes. Advances in immunology 113: 157–174. [DOI] [PubMed] [Google Scholar]
- 72.Unanue ER, and Carrero JA 2012. Studies with Listeria monocytogenes lead the way. Advances in immunology 113: 1–5. [DOI] [PubMed] [Google Scholar]