Abstract
Objective
Previous studies have documented memory impairment in several chronic pain syndromes. However, the potential link between memory loss and osteoarthritis (OA), the second most common cause of chronic pain, remains little explored. In this cross-sectional study, we examine the association of perceived memory loss to OA and assess the potential mediating influence of sleep and mood disturbance in a large Appalachian population.
Design
Cross-sectional.
Setting
US Ohio Valley.
Subjects
A total of 21,982 Appalachian adults age 40 years or older drawn from the C8 Health Project (N = 19,004 adults without and 2,478 adults with OA). All participants completed a comprehensive health survey between 2005 and 2006. Medical history, including physician diagnosis of OA, lifestyle factors, short- and long-term memory loss, sleep quality, and mood were assessed via self-report.
Results
After adjustment for demographic, lifestyle, health-related, and other factors, participants with OA were almost three times as likely to report frequent memory loss (adjusted odds ratios [ORs] for short- and long-term memory loss, respectively = 2.7, 95% confidence interval [CI] = 2.2–3.3, and 2.6, 95% CI = 2.0–3.3). The magnitude of these associations increased significantly with rising frequency of reported joint pain (adjusted OR for OA with frequent joint pain vs no OA = 3.3, 95% CI = 2.6–4.1, Ptrend < 0.00001). Including measures of mood and sleep impairment attenuated but did not eliminate these associations (ORs for any memory loss = 2.0, 95% CI = 1.6–2.4, and 2.1, 95% CI = 1.7–2.8, adjusted for sleep and mood impairment, respectively; OR = 1.8, 95% CI = 1.4–2.2, adjusted for both factors).
Conclusions
In this large cross-sectional study, OA and related joint pain were strongly associated with perceived memory loss; these associations may be partially mediated by sleep and mood disturbance.
Keywords: Cognition, Memory Loss, Osteoarthritis, Chronic Pain, Mood, Sleep
Introduction
Chronic pain, defined as pain lasting more than 12 weeks [1], is a common and costly condition. A 2016 meta-analysis of 19 population-based prevalence studies (N = 139,933 adults) estimated that between one-third and one-half of the UK adult population is affected by chronic pain (pooled estimate of 43.5%) [2]. In an age-standardized analysis of general population surveys of 42,248 adults from 18 countries, 37% of respondents in developed countries and 41% in developing countries reported a chronic pain condition [3]; of the 10 developed countries included in the study, the highest prevalence was reported in the United States (44%) and France (50%). Moreover, for many with chronic pain, symptoms are severe and relentless. In Europe, an estimated 19% of the adult population suffers moderate to severe chronic pain, a large proportion of which has inadequate pain control [4–6]. In a study of a nationally representative sample of more than 27,000 US adults, 31% reported experiencing chronic pain, of whom 50% indicated daily pain and 32% indicated severe pain [7]. Furthermore, as most studies exclude or underrepresent frail elderly and individuals in long-term care, these figures may reflect underestimates of true prevalence [6].
Chronic pain is associated with high direct and indirect health care costs and with substantial individual and societal burden. In the United States, excess health care costs attributable to persistent pain in adults are estimated to total $261 to $300 billion in 2010 dollars [8]. Chronic pain can lead to significant declines in productivity, physical function, quality of life, and overall health, mood, and well-being [2,3,6,9,10] and is a leading cause of disability both in the United States and globally [11,12]. In addition, chronic pain can have profound effects on neurocognitive function. Because the neural systems involved in memory and cognition are closely linked to those involved in pain processing, these systems may affect one another reciprocally [9,13], disrupting cognitive processing and contributing to a vicious cycle of continuing pain, adverse neurostructural changes, and deteriorating cognitive function. Patients with chronic pain do, in fact, show changes in brain morphology paralleling those impairment; these changes include gray matter reduction in the insular cortex, anterior cingulate cortex, thalamus, prefrontal cortex [9,13–16], and other brain regions involved not only in pain processing and emotional regulation, but in attention, memory consolidation, and cognitive processing. In addition, chronic pain has been shown to disrupt the functioning of the default mode network [16] and other brain networks [16] essential to normal cognitive function. These alterations are thought to help explain the reductions in memory and cognitive performance documented in a number of populations with chronic pain [9].
Memory impairment has been reported in several chronic pain syndromes, including migraine headaches, chronic low back pain, diabetic neuropathy, rheumatoid arthritis, and fibromyalgia [9,17]. However, the link between memory loss and osteoarthritis (OA), the most common form of arthritis, a major contributor to disability [11] and the second most common cause of chronic pain [7], remains little explored. In this cross-sectional study, we examine the association of perceived memory loss to osteoarthritis and frequency of associated joint pain in a large population of Appalachian adults.
Methods
Study Population and Data Source
The sample for this study was drawn from the C8 Health Project [18,19], which arose from the settlement of a class action lawsuit associated with perfluorooctane (PFOA) contamination of drinking water by a chemical plant in Washington, West Virginia. Baseline data on 69,030 individuals living or working in six PFOA-contaminated water districts in Ohio and West Virginia were collected from August 2005 to August 2006. As part of the C8 Health Project, participants completed a comprehensive health survey administered by trained personnel; blood samples were also collected to assess clinical biomarkers and serum levels of PFOA and other perfluorocarbons [19]. Project data collection was administered by Brookmar, Inc. (Parkersburg, WV, USA) and conducted under the authority and supervision of the Wood County, West Virginia, Circuit Court [19,20]. Participants were informed that central objectives of the Health Project were to determine levels of PFOA in the blood and to explore any potential associations between PFOA serum levels and diseases. Project details, from consent and enrollment to data collection, cleaning, and reporting, have been published elsewhere [19]. Blood processing and analytical methods, as well as quality-assurance measures, have also been previously described in detail [18,19,21]. Informed consent was obtained using a process approved by parties to the settlement and language specific to the project’s objectives and data collection procedures [19,20]. This study was based on aggregate, deidentified data and approved by the West Virginia University Institutional Review Board.
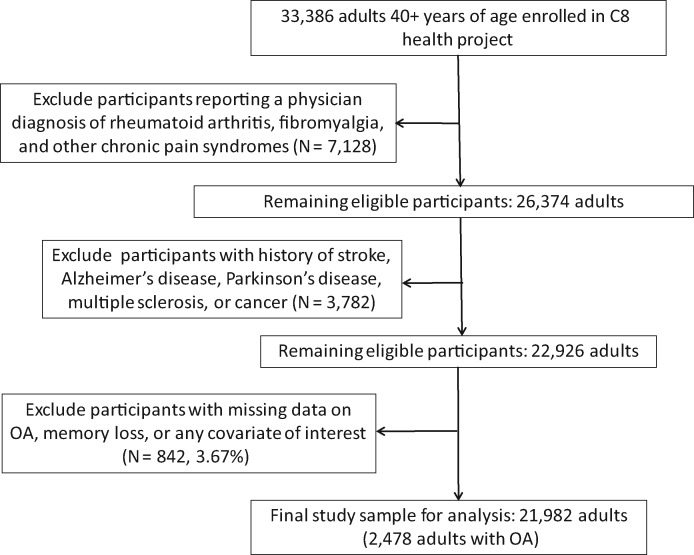
The estimated participation rate in the C8 Health Project among adult residents of the affected water districts was 81% [18]. For the current study, eligible participants included all adults age 40 years or older at the time of baseline assessment (N = 33,386 individuals). As illustrated in Figure 1, those who reported a physician diagnosis of rheumatoid arthritis, fibromyalgia, or another chronic pain syndrome other than OA (N = 7,128) were excluded from the analyses; also excluded were those diagnosed with conditions linked to impaired cognitive function (either the conditions themselves or their treatment), including stroke, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and cancer (other than nonmelanoma skin cancer, N = 3,782), leaving a total of 22,926 eligible adults. Exclusion of those with missing data on memory loss, OA joint pain severity, and/or other covariates of interest (N = 842, 3.67%) yielded a final study sample of 21,982, including 19,004 without and 2,478 adults with OA (see Figure 1). Relative to participants included in the analyses, those with missing data on any covariate were more likely to be female, older, and less educated and to indicate lower family income and a history of alcohol consumption; Participants with missing data were also less likely to be employed outside the home or to report having a regular exercise program or ever smoking (P < 0.01). There were no differences in other demographic and lifestyle characteristics, prevalence of OA, obesity, or other chronic conditions, medication use, reported memory loss, or other factors.
Figure 1.
Study flow diagram. OA = osteoarthritis.
Outcome and Exposure Measurements
Primary Outcome
Recent and long-term memory loss was ascertained via responses to two Likert scale questions: 1) “Have you experienced short-term memory loss?” and 2) “Have you experienced long-term memory loss?” Response choices were “never,” “rarely,” “sometimes,” and “frequently.” Short-term memory loss was scored as present (1) if the response was “frequently” to question 1; long-term memory loss was considered present if the participant responded “frequently” to question 2. All other responses were coded as 0. Any perceived memory loss, the primary outcome variable, was scored as positive if either short- or long-term memory loss was coded as present (1).
Key Exposure Variables
Physician diagnosis of osteoarthritis was assessed via self-report questionnaires. While self-reported diagnosis of osteoarthritis was not externally verified, a previous validation study demonstrated more than 80% agreement between self-reported and clinically confirmed diagnosis of OA [22], comparable with the 74% concordance observed between self-report and medical record–verified data on another common chronic disorder (diabetes) in the C8 Health Study population [23]. OA symptom frequency was evaluated using responses to a single Likert scale question regarding the participant’s experience of joint pain (“never,” “rarely,” “sometimes,” and “frequently”).
Other Explanatory Variables
Demographics (age, sex, education, race/ethnicity, marital status, income, employment), lifestyle factors (physical activity, alcohol consumption, smoking), medication use, and health characteristics (medical and reproductive history, weight, height) were also determined via self-report; demographic data and health survey completion were verified by trained project staff. Reported physician diagnoses of certain disorders, including cancer, diabetes, and cardiovascular disease, were further verified via chart review. Sleep quality and mood disturbance were assessed via a series of Likert scale questions. A composite sleep quality variable, with higher scores indicating poorer sleep quality, was derived from responses to four items regarding the frequency of short sleep, fitful sleep, insomnia, and daytime somnolence (with each item scored as follows: 3 = “frequently,” 2 = “sometimes,” 1 = “rarely,” 0 = “never”). Mood disturbance was also assessed as a composite variable derived from responses to three questions regarding mood swings, irritability, and inability to concentrate; items were scored using a similar scoring system (3 = “frequently,” 2 = “sometimes,” 1 = “rarely,” 0 = “never”).
Statistical Analysis
Data were analyzed using IBM SPSS Statistics version 23. We used logistic regression analysis to evaluate the associations of OA to reported frequency of memory loss (short-term memory loss, long-term memory loss, and any memory loss); to assess the influence of potential confounders, and to evaluate potential mediators and effect modifiers. Linear trends were assessed using polynomial contrasts. Potential differences between participants with and without missing data were evaluated using the Student’s t test or Mann-Whitney U test for continuous or ordinal variables and the chi-square test for categorical variables. The primary explanatory variable of interest, OA, was analyzed as both a dichotomous variable (yes/no) and by reported frequency of joint pain (OA with joint pain never/rarely, sometimes, and frequently), with no OA used as the referent category. All P values presented are two-sided.
Factors on which adequate data were available and which have been previously linked to either OA and/or memory loss were selected a priori as covariates. Associations of OA to memory were initially adjusted for age and gender, factors strongly related to both pain and OA. Unless stated otherwise, all other multivariable models were adjusted for the following: age, gender, race/ethnicity, marital status, socioeconomic status ([SES] including years of education, average family income, and employment status/disability); lifestyle factors (participation in a regular exercise program [yes/no], smoking [never, former, current], history of alcohol consumption [yes/no]) menopausal status; and use of hormone replacement therapy (women), body mass index (BMI); medical comorbidity (reported physician diagnosis of other medical conditions, including heart, kidney, liver, thyroid, immune, and connective tissue disease, stroke, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, or asthma); current treatment for hypertension or hyperlipidemia, hormone replacement therapy, and other prescription medications. While the latter category includes analgesic medications, information available in the data set did not allow adjustment for nonsteroidal anti-inflammatory (NSAID) or other individual analgesics. Additional analyses adjusted for serum levels of PFOA (mg/L) and for military service and associated exposures to harmful chemicals.
To evaluate the potential modifying effects of gender, age, and obesity on the association of perceived memory loss to history of OA, we conducted multivariable analyses stratified by each potential effect modifier. We tested the strength of each interaction by including the corresponding multiplicative interaction term in the main adjusted statistical model and evaluating the coefficient using the Wald test. We also assessed potential mediating influences of sleep impairment and mood disturbance, defined as detailed above.
Results
Table 1 illustrates the distribution of study population characteristics by presence of perceived memory loss. Participants were predominantly non-Hispanic white (97%), ranging in age from 40 to 97 years (mean = 54.21 years, SD = 10.77 years). Fifty-one percent were female, 56% had received only 12 years of schooling or less, and 30% reported a mean annual household income of less than $30,000. Sixty-one percent were employed, and approximately 6% were disabled. More than 50% reported smoking currently (22%) or previously (29%), and only 33% indicated engagement in a regular exercise program. More than 30% of the adults in this population were obese (BMI ≥ 30), with a mean BMI of 28.87 (5.93).
Table 1.
Characteristics of adults ≥ 40 years of age from 6 Ohio Valley water districts, stratified by reported history of frequent memory loss
| Frequent Memory Loss |
Adjusted OR* (95% CI) | P† | ||||
|---|---|---|---|---|---|---|
| No (N = 19,004) |
Yes (N = 719) |
|||||
| N | % | N | % | |||
| Demographics | ||||||
| Age, y | ||||||
| Per year increment | 1.00 (0.99–1.01) | 0.58 | ||||
| Age, mean (SD), y | 54.20 (10.76) | 54.24 (11.20) | 0.61 | |||
| Gender | 0.001 | |||||
| Male | 11,027 | 51.86 | 278 | 38.66 | 1.00 (referent) | |
| Female | 10,236 | 48.14 | 441 | 61.34 | 1.47 (1.16–1.87) | |
| Ethnicity | 0.97 | |||||
| White | 20,689 | 97.30 | 699 | 97.22 | 1.00 (referent) | |
| Minority | 574 | 2.70 | 20 | 2.78 | 0.99 (0.63–1.57) | |
| Marital status | 0.002 | |||||
| Married/cohabiting | 16,765 | 78.85 | 496 | 68.98 | 1.00 (referent) | |
| Single | 1,069 | 5.03 | 36 | 5.01 | 0.90 (0.63–1.28) | |
| Divorced/separated | 2,331 | 10.96 | 140 | 19.47 | 1.48 (1.21–1.82) | |
| Widowed | 1,098 | 5.16 | 47 | 6.54 | 1.09 (0.78–1.53) | |
| Years of education | 0.015 | |||||
| <12 | 2,237 | 10.52 | 123 | 17.11 | 1.00 (referent) | |
| High school/GED | 9,589 | 45.10 | 283 | 39.36 | 0.78 (0.62–0.99) | |
| Some college | 6,429 | 30.24 | 244 | 33.94 | 1.01 (0.79–1.30) | |
| 4+ y college | 3,008 | 14.15 | 69 | 9.60 | 0.80 (0.57–1.13) | |
| Current employment status | <0.00001 | |||||
| Employed | 13,041 | 61.33 | 321 | 44.65 | 1.00 (referent) | |
| Homemaker | 2,397 | 11.27 | 84 | 11.68 | 1.21 (0.93–1.59) | |
| Retired | 4,036 | 18.98 | 119 | 16.55 | 1.38 (1.05–1.82) | |
| Unemployed/laid off | 586 | 2.76 | 27 | 3.76 | 1.81 (1.20–2.72) | |
| Student | 68 | 0.32 | 6 | 0.83 | 2.86 (1.22–6.74) | |
| Disabled | 1,001 | 4.71 | 150 | 20.86 | 4.46 (3.51–5.67) | |
| Other | 134 | 0.63 | 12 | 1.67 | 2.96 (1.60–5.46) | |
| Average household income | 0.04 | |||||
| <$30,000 | 6,371 | 29.96 | 306 | 42.56 | 1.00 (referent) | |
| $30,000–$70,000 | 9,208 | 43.31 | 278 | 38.66 | 0.86 (0.66–1.12) | |
| >$70,000 | 3,897 | 18.33 | 78 | 10.85 | 0.59 (0.39–0.89) | |
| Don't know/missing | 1,787 | 8.40 | 57 | 7.93 | 0.80 (0.54–1.20) | |
| Lifestyle factors | ||||||
| Alcohol consumption ever | 0.015 | |||||
| No | 7,102 | 33.40 | 211 | 29.35 | 1.00 (referent) | |
| Yes | 14,161 | 66.60 | 508 | 70.65 | 1.25 (1.04–1.49) | |
| Smoking status | 0.06 | |||||
| Never | 10,450 | 49.15 | 290 | 40.33 | 1.00 (referent) | |
| Former | 6,229 | 29.30 | 222 | 30.88 | 1.21 (1.00–1.46) | |
| Current | 4,584 | 21.56 | 207 | 28.79 | 1.23 (1.00–1.51) | |
| Regular exercise program | 0.046 | |||||
| No | 14,275 | 67.14 | 525 | 73.02 | 1.00 (referent) | |
| Yes | 6,988 | 32.86 | 194 | 26.98 | 0.84 (0.70–1.00) | |
| Anthropometrics and medical history | ||||||
| BMI, kg/m2 | 0.03 | |||||
| <30 | 13,750 | 64.67 | 409 | 56.88 | 1.00 (referent) | |
| 30+ | 6,495 | 30.55 | 310 | 43.12 | 1.20 (1.02–1.41) | |
| BM, mean (SD) | 28.85 (5.90) | 29.58 (6.63) | 0.004 | |||
| Chronic condition(s) excl OA‡ | <0.00001 | |||||
| No | 13,227 | 62.21 | 323 | 44.92 | 1.00 (referent) | |
| Yes | 8,036 | 37.79 | 396 | 55.08 | 1.53 (1.28–1.80) | |
| No. conditions, mean (SD) | 0.55 (0.85) | 0.94 (1.14) | <0.00001 | |||
| Per single comorbid condition increment | 1.25 (1.16–1.35) | <0.00001 | ||||
| On lipid-lowering or antihypertensive medication | 9,191 | 43.23 | 351 | 48.82 | 0.92 (0.76–1.11) | 0.36 |
| On other prescription medication§ | 12,201 | 57.38 | 506 | 70.38 | 1.31 (1.07–1.59) | 0.008 |
| Reproductive history (women, N = 10,236 without, 441 with memory loss) | ||||||
| Postmenopause | 0.0002 | |||||
| No | 4,045 | 39.52 | 144 | 32.65 | 1.00 (referent) | |
| Yes | 5,636 | 55.06 | 250 | 56.69 | 0.97 (0.74–1.29) | |
| Don't know | 555 | 5.42 | 47 | 10.66 | 1.96 (1.37–2.80) | |
| History of hormone replacement therapy | 0.003 | |||||
| No | 6,328 | 61.82 | 231 | 52.38 | 1.00 (referent) | |
| Yes | 3,908 | 38.18 | 210 | 47.62 | 1.40 (1.12–1.74) | |
BMI = body mass index; CI = confidence intervals; excl = excluding; OA = osteoarthritis; OR = odds ratio.
Adjusted for other factors in table.
All P values are two-sided.
Including physician diagnosis of heart, kidney, liver, immune, connective tissue, and thyroid disease, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, or asthma.
Including nonsteroidal anti-inflammatory drugs.
Of the 21,982 eligible participants, 719 (3.3%) indicated experiencing frequent short- or long-term memory loss. After adjustment for other factors in the table, women remained significantly more likely to report frequent memory loss than men (61 vs 39%, respectively, adjusted P = 0.001), as did those who were divorced or separated relative to those who were married or cohabiting (OR = 1.5, 95% CI = 1.2–1.8, adjusted P = 0.002). Perceived memory loss also retained significant positive associations with alcohol consumption, current and former tobacco smoking, obesity, and history of hormone replacement therapy, and significant negative associations with educational level, household income, and engagement in a regular exercise program (Table 1). Participants who were retired, homemakers or unemployed, who were disabled, who had been diagnosed with at least one chronic medical condition other than OA, or who were taking prescription medications other than lipid-lowering and antihypertensive drugs were also significantly more likely to report memory loss (adjusted P <0.0001).
Table 2 illustrates the associations of perceived memory loss with reported history of OA and OA symptom frequency. A total of 2,478 participants (11.3%) reported a physician diagnosis of OA, of whom 62% (N = 1532) indicated frequent joint pain. OA showed a strong, significant, positive relation to perceived memory loss in both the minimally adjusted analysis and the full models. Those reporting a diagnosis of OA were approximately four times as likely to report memory loss than those without OA (odds ratio [OR] = 3.9, 95% confidence interval [CI] = 3.2–4.6, P < 0.00001) after adjustment for age and sex. Further adjustment for race, education, marital status, income employment, and lifestyle factors slightly diminished this association (OR = 3.1, 95% CI = 2.6–3.8). OA remained strongly and positively related to perceived memory loss after additional adjustment for BMI, comorbidity, medication use, menopausal status, and use of hormone replacement therapy (OR = 2.6, 95% CI = 2.2–3.2).
Table 2.
Association of reported osteoarthritis (OA) and OA symptom severity to perceived memory loss (N = 719 with and 21,263 without reported history of memory loss) in Appalachian adults age 40 years or older (excluding those with history of stroke, Alzheimer's disease; fibromyalgia, rheumatoid arthritis, and other chronic pain syndromes, Parkinson's Disease, multiple sclerosis, or cancer)
| Reported History of Memory Loss* |
Adjusted for Age and Sex |
Adjusted for Age, Sex, Race, SES, Marital Status, Lifestyle Factors* |
Adjusted for Age, Sex, Race, SES, Marital Status, Menopausal Status, HRT, Lifestyle Factors†, BMI, Comorbidity‡, and Medications§ |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Yes | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Osteoarthritis | ||||||||
| No | 19,004 | 500 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Yes | 2,259 | 219 | 3.86 (3.23–4.62) | <0.00001 | 3.14 (2.62–3.76) | <0.00001 | 2.64 (2.18–3.20) | <0.00001 |
| OA joint pain severity | ||||||||
| No OA | 19,004 | 500 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| OA with joint pain never/rarely | 208 | 10 | 1.96 (1.02–3.74) | 0.040 | 1.80 (0.83–1.03) | 0.08 | 1.75 (0.91–3.36) | 0.10 |
| OA with joint pain sometimes | 693 | 35 | 2.04 (1.42–2.92) | 0.0001 | 1.74 (1.21–2.50) | 0.003 | 1.56 (1.08–2.24) | 0.02 |
| OA with frequent joint pain | 1,358 | 174 | 4.98 (4.11–6.03) | <0.00001 | 3.93 (3.23–4.78) | <0.00001 | 3.25 (2.64–4.01) | <0.00001 |
| Test for trend | – | – | – | <0.00001 | – | <0.00001 | – | <0.00001 |
BMI = body mass index; CI = confidence interval; HRT = hormone replacement therapy; OA = osteoarthritis; OR = odds ratio; SES = socioeconomic status (includes years of education, annual household income, and employment status/disability).
Defined as a participant report of frequent short- or long-term memory loss.
Smoking (never, former, current); current alcohol consumption (yes/no); exercise (regular exercise program [yes/no]).
Includes reported physician diagnosis of comorbid conditions (heart, kidney, liver, immune, connective tissue, and thyroid disease, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, or asthma).
Includes antihypertensive, lipid-lowering, and other prescription medications.
Likewise, likelihood of perceived memory loss rose with increasing frequency of OA-associated joint pain. Relative to those without a reported diagnosis of OA, adults indicating a diagnosis of OA and frequent joint pain were five times more likely to report often experiencing memory loss after controlling for age and gender (OR = 5.0, 95% CI = 4.1–6.0, Ptrend < 0.00001) (Table 2). After adjustment for additional demographics, lifestyle characteristics, medical history, and other factors, OA symptom frequency remained strongly and positively associated with reported memory loss (OR for OA with frequent joint pain = 3.3, 95% CI = 2.6–4.0, Ptrend < 0.00001). Restricting analyses to include only those with self-reported OA yielded similar results (fully adjusted OR for frequent vs no joint pain = 3.6, 95% CI = 1.1–12.1, Ptrend = 0.0002).
As detailed in Table 3, analyses broken down by frequent perceived short- and long-term memory loss yielded similar findings. Relative to participants without OA, those indicating a physician diagnosis of OA were 2.7 times as likely to report frequent short-term memory loss and 2.6 times as likely to report frequent long-term memory deficits after adjustment for demographics, lifestyle factors, BMI, menopausal status, and medical history (OR = 2.7, 95% CI = 2.2–3.3, and OR = 2.6, 95% CI = 2.0–3.3) (Table 3). Likewise, compared with no OA, the likelihood of both short- and long-term memory loss increased significantly with rising frequency of reported joint pain, with those indicating frequent joint pain more than threefold as likely to indicate memory loss (ORs for short and long-term memory loss, respectively = 3.3, 95% CI = 2.6–4.1, and 3.2, 95% CI = 2.4–4.2, Ptrend < 0.00001).
Table 3.
Association of reported physician diagnosed osteoarthritis (OA) and OA symptom severity to perceived short- and long-term memory loss in 21,982 Appalachian adults age 40 years or older (excluding those with history of stroke, Alzheimer's disease, fibromyalgia, rheumatoid arthritis, and other chronic pain syndromes, Parkinson's Disease, multiple sclerosis, or cancer)
| Reported History of Memory Loss |
Adjusted for Age and Sex |
Adjusted for Age, Sex, Race, SES, Marital Status, Lifestyle Factors* |
Adjusted for Age, gender, Race, SES, Marital Status, Lifestyle Factors*, Menopausal Status, HRT, Comorbidity†, and Medications‡ |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Yes | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Short-term memory loss | ||||||||
| Osteoarthritis | ||||||||
| No | 19,105 | 399 | 1.00 (referent) | – | 1.00 (referent) | – | 1.00 (referent) | – |
| Yes | 2,293 | 584 | 3.97 (3.27–4.82) | <0.00001 | 3.20 (2.63–3.90) | <0.00001 | 2.68 (2.17–3.29) | <0.00001 |
| OA joint pain severity | ||||||||
| No OA | 19,105 | 399 | 1.00 (referent) | – | 1.00 (referent) | – | 1.00 (referent) | – |
| OA with joint pain never/rarely | 208 | 10 | 2.40 (1.25–4.59) | 0.008 | 2.21 (1.14–4.25) | 0.02 | 2.13 (1.10–4.12) | 0.02 |
| OA with joint pain sometimes | 700 | 28 | 1.99 (1.33–2.96) | 0.001 | 1.69 (1.13–2.52) | 0.01 | 1.49 (0.99–2.24 | 0.05 |
| OA with frequent joint pain | 1,385 | 147 | 5.11 (4.15–6.30) | <0.00001 | 3.99 (3.23–4.94) | <0.00001 | 3.29 (2.62–4.12) | <0.00001 |
| Test for trend | – | – | – | <0.00001 | – | <0.00001 | – | <0.00001 |
| Long-term memory loss | ||||||||
| Osteoarthritis | ||||||||
| No | 19,223 | 281 | 1.00 (referent) | – | 1.00 (referent) | – | 1.00 (referent) | – |
| Yes | 2,362 | 116 | 3.91 (3.08–4.96) | <0.00001 | 3.11 (2.44–3.96) | <0.00001 | 2.57 (1.99–3.33) | <0.00001 |
| OA joint pain severity | ||||||||
| No OA | 19,223 | 281 | 1.00 (referent) | – | 1.00 (referent) | – | 1.00 (referent) | – |
| OA with joint pain never/rarely | 217 | 1 | 0.39 (0.05–2.79) | 0.35 | 0.34 (0.05–2.47) | 0.29 | 0.33 (0.05–2.36) | 0.27 |
| OA with joint pain sometimes | 708 | 20 | 2.30 (1.43–3.68) | 0.0005 | 1.95 (1.21–3.13) | 0.006 | 1.73 (1.07–2.80) | 0.025 |
| OA with frequent joint pain | 1,437 | 95 | 5.07 (3.93–6.53) | <0.00001 | 3.92 (3.02–5.08) | <0.00001 | 3.21 (2.43–4.23) | <0.00001 |
| Test for trend | – | – | <0.00001 | <0.00001 | <0.00001 | |||
BMI = body mass index; CI = confidence interval; OA = osteoarthritis; OR = odds ratio; SES = socioeconomic status (includes years of education, annual household income, and employment status/disability).
Smoking (never, former, current); current alcohol consumption (yes/no); exercise (regular exercise program [yes/no]).
Includes reported physician diagnosis of comorbid conditions (heart, kidney, liver, immune, connective tissue, and thyroid disease, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, or asthma).
Includes antihypertensive, lipid-lowering, and other prescription medications.
Additional adjustment for PFOA levels or for military service and associated chemical exposures did not appreciably alter risk estimates (OR for reported OA diagnosis = 2.7, 95% CI = 2.2–3.3). Likewise, including in the analyses participants with a reported diagnosis of cancer, stroke, Parkinson’s disease, multiple sclerosis, fibromyalgia, and other conditions linked to memory loss and controlling for these conditions in the adjusted models did not substantively change the association of OA or OA symptom frequency to perceived memory loss (OR for OA = 2.7, 95% CI = 2.3–3.2; OR for OA with frequent joint pain = 3.4, 95% CI = 2.9–4.00, Ptrend <0.00001).
Mood disturbance and sleep impairment scores were strongly inter-related (adjusted r = 0.53, P < 0.00001) and were significantly and positively associated with reported OA diagnosis and associated frequency of joint pain (P <0.00001). For example, relative to participants scoring in the lowest mood and sleep impairment quartiles, those scoring in the highest quartiles were approximately three times as likely to report a physician diagnosis of OA (ORs for highest vs lowest quartiles of mood and sleep impairment, respectively = 3.0, 95% CI = 2.6–3.4, and 2.7, 95% CI = 2.3–3.1). Mood and sleep impairment were even more strongly related to reported history of memory loss (P <0.00001). For example, those with scores in the highest quartile of mood and sleep disturbance were approximately 25- and eightfold more likely to report a history of memory loss (ORs for highest vs lowest quartiles of mood and sleep impairment, respectively = 24.5, 95% CI = 14.9–40.4, and 7.8, 95% CI = 5.7–10.6). However, as illustrated in Table 4, while inclusion of mood and sleep impairment in the model attenuated the magnitude of the associations between perceived memory loss and OA and associated joint pain, the associations remained robust (OR for reported OA adjusted for both sleep impairment and mood swings = 1.8, 95% CI = 1.4–2.2, P < 0.00001). These findings suggest that the relation of OA to reported history of memory loss is only partially mediated by mood and sleep.
Table 4.
Association of reported osteoarthritis (OA) and OA symptom severity to perceived memory loss (N = 719 with and 21,263 without history of memory loss) in Appalachian adults age 40 years or older: Influence of sleep and mood impairment
| Reported History of Memory Loss* |
Adjusted for Demographics†, Lifestyle Factors‡, BMI, Reproductive/Medical History*, and Sleep Impairment |
Adjusted for Demographics†, Lifestyle Factors‡, BMI, Reproductive/Medical History*, and Mood Disturbance |
Adjusted for Demographics†, Lifestyle Factors‡, BMI, Reproductive/Medical History§, Sleep Impairment, and Mood Disturbance |
|||||
|---|---|---|---|---|---|---|---|---|
| N | Yes | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Osteoarthritis | ||||||||
| No | 19,004 | 500 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| Yes | 2,259 | 219 | 1.96 (1.62–2.39) | <0.00001 | 2.13 (1.74–2.80) | <0.00001 | 1.76 (1.43– 2.16) | <0.00001 |
| OA joint pain severity | ||||||||
| No OA | 19,004 | 500 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||
| OA with joint pain never/rarely | 208 | 10 | 1.80 (0.92–3.52) | 0.09 | 1.87 (0.96–3.65) | 0.07 | 1.86 (0.93–3.70) | 0.08 |
| OA with joint pain sometimes | 693 | 35 | 1.42 (0.98–2.06) | 0.06 | 1.61 (1.11–2.35) | 0.01 | 1.53 (1.05–2.22) | 0.03 |
| OA with frequent joint pain | 1,385 | 174 | 2.18 (1.76–2.70) | <0.00001 | 2.34 (1.87–2.93) | <0.00001 | 1.83 (1.45–2.29) | <0.00001 |
| Test for trend | – | – | – | <0.00001 | – | <0.00001 | – | <0.00001 |
BMI = body mass index; CI = confidence interval; OA = osteoarthritis; OR = odds ratio.
Defined as a participant report of frequent short- or long-term memory loss.
Includes age, sex, race/ethnicity, years of education, annual household income, marital status, and employment status/disability.
Smoking (never, former, current); current alcohol consumption (yes/no); exercise (regular exercise program [yes/no]).
Includes reported physician diagnosis of comorbid conditions (heart, kidney, liver, immune, connective tissue, and thyroid disease, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, or asthma) and use of antihypertensive, lipid-lowering, and other prescription medications; menopausal status and use of hormone replacement therapy.
Discussion
A growing body of literature suggests that chronic pain can have significant negative effects on neurocognitive function. Previous studies have documented memory impairment in a number of chronic pain syndromes [9]. However, the potential link between memory loss and OA, a leading cause of chronic pain, remains little studied. In this large cross-sectional study of older Appalachian adults, self-reported history of memory loss was strongly and positively associated with self-reported physician diagnosis of OA and associated joint pain. After adjustment for demographics, lifestyle factors, BMI, medical history, medication use, and other factors, participants indicating a physician diagnosis of OA were 2.6 times as likely to report experiencing frequent memory loss. The magnitude of this association increased significantly with rising frequency of reported joint pain. Mood and sleep impairment were strongly and positively associated with both perceived memory loss and with reported OA diagnosis and associated frequency of joint pain; inclusion of these factors in the adjusted models attenuated but did not eliminate these associations, suggesting that mood and sleep disturbance may in part mediate the observed relationships between OA and perceived memory loss in this population.
The strong, independent association between OA and reported memory loss observed in this study is consistent with the findings reported in most clinical studies of other chronic pain syndromes, including migraine headaches, chronic low back pain, diabetic neuropathy, rheumatoid arthritis, and fibromyalgia [9,24]. Likewise, OA symptom frequency showed a strong, linear relationship to perceived memory loss in our study population, in agreement with the significant correlations between pain and cognitive performance documented in most, although not all, previous studies of chronic pain syndromes [9,25]. These findings also parallel those from a cross-sectional survey study of older British primary care patients indicating a dose-response association between reported recent pain and cognitive complaints that was not explained by co-occurring affective disorders [26]. The significant positive association of OA and associated joint pain to perceived memory loss observed in this study was independent of demographic, lifestyle, and health-related factors, including comorbidity and medication use.
Mood, Sleep, Pain, and Memory Loss
Pain is often accompanied by disruption of sleep and mood; for example, depression has been documented in 30% to 50% of chronic pain patients [27]. Current evidence from existing experimental, clinical, and epidemiologic studies suggests that the relationships between musculoskeletal and other chronic pain, inadequate sleep, and psychological distress are strongly reciprocal [27–31]. Chronic pain can lead to significant disruption of both sleep and mood [27,31–33]; conversely, accumulating research suggests that sleep deficits are known to increase sensitivity to noxious stimuli and to exacerbate both pain and affective symptoms [28,30,31,34,35]. Similarly, depression, anxiety, and other distressful states can lead to disordered sleep, as well as increased pain [27,35–38]. In addition, a substantial body of evidence indicates that both affective disturbance and sleep deficits can significantly and adversely influence memory and cognitive functioning [39–48]. Disruption of mood and sleep may thus in part mediate the documented negative effects of pain on memory and cognitive performance. Consistent with findings from these prior investigations, measures of sleep and mood impairment were significantly inter-related in the current study and were strongly and positively associated with both OA and symptom frequency and with history of perceived memory loss. That adjustment for these factors attenuated the association of OA and associated joint pain to perceived memory loss in our study population, albeit modestly, suggests that the adverse changes in mood and sleep may in part explain this relationship.
In agreement with the findings of previous studies [49–54], reported history of memory loss in this study was significantly and positively associated with smoking, unemployment, and obesity, and was inversely associated with educational attainment, household income, and engagement in regular physical activity. In addition, both menopause and history of hormone replacement therapy were positively associated with perceived memory loss among women in this study after adjustment for other demographic, health, and lifestyle factors, consistent with the findings of most recent prospective investigations [55–57]. Consistent with the findings of some [58–61], but not other, studies [61,62], reported memory loss was also independently and positively associated with female gender and retirement in our study population; recent studies suggest that these positive associations are stronger in those with lower cognitive reserve [61,62], perhaps in part helping to explain the relationships observed in this study of Appalachian adults. In contrast to the findings of most [63–67], but not all, studies [66,68,69], perceived memory loss showed a modest positive association with alcohol consumption in the current study. Finally, the number of chronic conditions demonstrated a significant, positive linear association with reported memory loss in our study population. While the relation of multimorbidity to cognitive decline and cognitive impairment is complex, co-occurrence of chronic conditions, especially those independently linked to dementia, has been associated with subjective memory complaints [70] and with significantly greater risk of incident mild cognitive impairment and dementia in several longitudinal studies [71–74], with some studies indicating a significant dose-response association between number of conditions and risk increase [71,72]. Multimorbidity has also been linked to subjective memory complaints in nondemented adults [75,76].
Strengths and Limitations
This study has several strengths, including the population-based design, high participation rates, and large sample size. Additional strengths include our ability to evaluate many potential confounders and modifiers, to assess the potential mediating influence of sleep and mood disturbance on the relation between perceived memory loss and OA, and to examine potential dose-response associations between OA pain frequency and reported memory loss.
Our study has several limitations as well. Most important, the cross-sectional nature of our data precludes determination of temporal or causal relationships. Our study population was restricted to a largely non-Hispanic white sample of Appalachian adults, potentially limiting generalizability. We lacked information on certain risk factors for memory loss, including history of head trauma, as well as duration of memory loss. As the C8 Health Survey did not include cognitive testing, ascertainment of memory loss was reliant on self-report. A potential concern relates to participant understanding of short- vs long-term memory loss. While staff were on hand to elaborate and/or answer questions on any of the survey items, it is possible that some participants did not discriminate accurately between short- and long-term memory loss. An additional concern is the potential discrepancy between reported memory loss and objective cognitive functioning. However, while subjective memory complaints do not always correlate with deficits in objective cognitive performance [77], some studies have shown significant relationships between the two [77–79]. Moreover, although cognitive function is in the normal range in those with subjective cognitive decline [80], population-based studies have demonstrated significant decrements in cognitive performance in adults with memory complaints relative to those without memory complaints [81,82]. Perhaps most important, prospective studies have shown subjective memory complaints to be strongly predictive of accelerated cognitive decline and of incident mild cognitive impairment and Alzheimer’s Disease independent of demographics, lifestyle factors, depression, and other risk factors for cognitive impairment [41,83–88]. Similarly, perceived memory loss has been linked to neuropathological changes consistent with Alzheimer’s disease [82,89–101], again suggesting that memory complaints may represent a meaningful and potentially sensitive marker of risk.
In addition, OA was determined based on participant-reported physician diagnosis and was not confirmed by chart review. While at least one previous clinical validation study has shown self-report of general OA to be reliable [22], consistent with the agreement between self-report and medical record–verified data on another common disorder, diabetes, in this study population [23], the accuracy of using self-reported diagnosis of OA for estimating OA prevalence is unknown. The estimated crude OA prevalence of 11.3% in this study was considerably lower than prevalence estimates from other contemporaneous population-based studies of older adults [102–106], suggesting that OA may have been underreported in this study. However, such underascertainment would be expected to bias the observed associations toward the null, and thus is unlikely to explain our findings. Likewise, the prevalence of memory complaints reported in this study was substantially lower than age-matched estimates documented in other population-based investigations [26,107–111]. Thus, perceived memory loss may, like OA, have been underreported, potentially attenuating the relationships observed in this study.
Exclusion of eligible participants with missing data on covariates may have introduced selection bias. However, the percentage with missing data was small (3.7%), and those with missing data did not differ in most factors related to either OA or memory loss, rendering exclusion of these individuals unlikely to explain the observed associations. Participants were informed about the objectives of the study, which was conducted in partial fulfillment of a settlement for a class action lawsuit, potentially biasing reporting of health problems. However, as noted above, both perceived memory loss and OA were likely underascertained in this study, potentially attenuating observed associations and arguing against possible overreporting by participants concerned about the effects of PFOA. Hence, any bias introduced by participant knowledge regarding the purpose of the study is unlikely to explain our findings.
Although we adjusted for other prescribed medications, we were unable to specifically assess the role of NSAIDs or opioid medications. NSAIDS have been associated with reduced risk for incident cognitive impairment and dementia in several observational studies [112,113] and with lower risk for cognitive decline in a recent meta-analysis of 11 prospective cohort studies [114]. While findings regarding the effects of therapeutic opioid use on cognition have been inconsistent, many clinical studies, including several rigorously conducted randomized controlled trials, have found no association between cognitive functioning and long-term opioid use for nonmalignant chronic pain [115–120], with some studies showing pain relief from opioid therapy to be associated with improved cognitive performance [121]. Similarly, in a recent prospective cohort study of community-dwelling US seniors, investigators found little evidence for adverse effects of long-term opioid use on cognition [122]. As most OA patients rely on NSAIDs and other pain medications, failure to control for this factor may have biased observed risk estimates, likely toward the null. Thus, while the possibility cannot be ruled out, use of NSAIDs or opioids is unlikely to explain the strong positive associations observed between reported memory loss and OA in this study. Finally, unmeasured confounding may also help explain our findings, although our ability to control for a large number of both potential and known risk factors for memory loss renders this possibility less probable.
Conclusions
In this cross-sectional study of a large Appalachian population, history of perceived memory loss was strongly and positively associated with self-reported physician diagnosis of OA and related joint pain, associations that were only modestly attenuated by adjustment for sleep and mood disturbance. Prospective studies using validated pain scales and objective measures of cognitive performance are needed to investigate potential causal associations and determine if symptomatic OA can contribute to cognitive decline and, ultimately, to incident cognitive impairment.
Authors’ Contributions
KEI conceived and designed the study, conducted the analyses, and prepared and critically reviewed the manuscript. US provided feedback on study design, critically reviewed all analyses, provided substantive input on interpretation of findings, and critically reviewed the manuscript. Both authors discussed the results, commented on the manuscript, and approved the version submitted for publication. KEI takes responsibility for the integrity of the work as a whole, from inception to published article.
Funding sources: This work was supported, in part, by the Mitchell M. Benedict and Helen L. Benedict Endowment Fund, by the West Virginia University Health Sciences Center, by the National Institute of General Medical Sciences (U54GM104942), and by the Alzheimer’s Research and Prevention Foundation.
Conflicts of interest: The authors have no financial or personal conflicts of interest to declare.
Disclosure: The contents are solely the responsibility of the authors and do not represent the official views of the authors’ academic institution, Brookmar, Inc., the National Institutes of Health, or the Alzheimer’s Research and Prevention Foundation.
References
- 1. Taxonomy Working Group. Classification of Chronic Pain, 2nd edition.Seattle: IASP Press; 1994. [Google Scholar]
- 2. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT.. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016;66:e010364.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsang A, Von Korff M, Lee S et al. , Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008;910:883–91. [DOI] [PubMed] [Google Scholar]
- 4. Reid KJ, Harker J, Bala MM et al. , Epidemiology of chronic non-cancer pain in Europe: Narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011;272:449–62. [DOI] [PubMed] [Google Scholar]
- 5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D.. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain 2006;104:287–333. [DOI] [PubMed] [Google Scholar]
- 6. Breivik H, Eisenberg E, O'Brien T et al. , The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1229.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH.. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 2010;1111:1230–9. [DOI] [PubMed] [Google Scholar]
- 8. Gaskin DJ, Richard P.. The economic costs of pain in the United States. J Pain 2012;138:715–24. [DOI] [PubMed] [Google Scholar]
- 9. Moriarty O, McGuire BE, Finn DP.. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol 2011;933:385–404. [DOI] [PubMed] [Google Scholar]
- 10. Patel AS, Farquharson R, Carroll D et al. , The impact and burden of chronic pain in the workplace: A qualitative systematic review. Pain Pract 2012;127:578–89. [DOI] [PubMed] [Google Scholar]
- 11. Cross M, Smith E, Hoy D et al. , The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;737:1323–30. [DOI] [PubMed] [Google Scholar]
- 12. DALYs GBD, Collaborators H, Murray CJL et al. , Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015;38610009:2145–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seminowicz DA, Ceko M.. Can we exploit cognitive brain networks to treat chronic pain? Pain Manag 2015;56:399–402. [DOI] [PubMed] [Google Scholar]
- 14. Apkarian AV, Hashmi JA, Baliki MN.. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011;1523:S49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ivo R, Nicklas A, Dargel J et al. , Brain structural and psychometric alterations in chronic low back pain. Eur Spine J 2013;229:1958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cauda F, Palermo S, Costa T et al. , Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. Neuroimage Clin 2014;4:676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berryman C, Stanton TR, Jane Bowering K et al. , Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. Pain 2013;1548:1181–96. [DOI] [PubMed] [Google Scholar]
- 18. Steenland K, Tinker S, Shankar A, Ducatman A.. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect 2010;118:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisbee SJ, Brooks AP Jr, Maher A et al. , The C8 Health Project: Design, methods, and participants. Environ Health Perspect 2009;11712:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frisbee S, Shankar A, Knox S et al. , Perfluorooctanoic Acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Arch Pediatr Adolesc Med 2010;1649:860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V.. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 2009;17010:1268–78. [DOI] [PubMed] [Google Scholar]
- 22. March L, Schwarz J, Carfrae B, Bagge E.. Clinical validation of self-reported osteoarthritis. Osteoarthritis Cartilage 1998;62:87–93. [DOI] [PubMed] [Google Scholar]
- 23. MacNeil J, Steenland NK, Shankar A, Ducatman A.. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environ Res 2009;1098:997–1003. [DOI] [PubMed] [Google Scholar]
- 24. Berryman C, Stanton TR, Bowering KJ et al. , Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev 2014;347:563–79. [DOI] [PubMed] [Google Scholar]
- 25. Morone NE, Abebe KZ, Morrow LA, Weiner DK.. Pain and decreased cognitive function negatively impact physical functioning in older adults with knee osteoarthritis. Pain Med 2014;159:1481–7. [DOI] [PubMed] [Google Scholar]
- 26. Westoby CJ, Mallen CD, Thomas E.. Cognitive complaints in a general population of older adults: Prevalence, association with pain and the influence of concurrent affective disorders. Eur J Pain 2009;139:970–6. [DOI] [PubMed] [Google Scholar]
- 27. Kroenke K, Wu JW, Bair MJ et al. , Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. J Pain 2011;129:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moldofsky H. Rheumatic manifestations of sleep disorders. Curr Opin Rheumatol 2010;221:59–63. [DOI] [PubMed] [Google Scholar]
- 29. Simons LE, Elman I, Borsook D.. Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav Rev 2014;39:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okifuji A, Hare BD.. Do sleep disorders contribute to pain sensitivity? Curr Rheumatol Rep 2011;136:528–34. [DOI] [PubMed] [Google Scholar]
- 31. Koffel E, Kroenke K, Bair MJ et al. , The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol 2016;351:41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karaman S, Karaman T, Dogru S et al. , Prevalence of sleep disturbance in chronic pain. Eur Rev Med Pharmacol Sci 2014;1817:2475–81. [PubMed] [Google Scholar]
- 33. Hawker GA, Gignac MAM, Badley E et al. , A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res 2011;6310:1382–90. [DOI] [PubMed] [Google Scholar]
- 34. Lopresti AL, Hood SD, Drummond PD.. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J Affect Disord 2013;1481:12–27. [DOI] [PubMed] [Google Scholar]
- 35. Alvaro PK, Roberts RM, Harris JK.. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013;367:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dominick CH, Blyth FM, Nicholas MK.. Unpacking the burden: Understanding the relationships between chronic pain and comorbidity in the general population. Pain 2012;1532:293–304. [DOI] [PubMed] [Google Scholar]
- 37. Jordan KD, Okifuji A.. Anxiety disorders: Differential diagnosis and their relationship to chronic pain. J Pain Palliat Care Pharmacother 2011;253:231–45. [DOI] [PubMed] [Google Scholar]
- 38. Arola H-M, Nicholls E, Mallen C, Thomas E.. Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined? Eur J Pain 2010;149:966–71. [DOI] [PubMed] [Google Scholar]
- 39. Marin MF, Lord C, Andrews J et al. , Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem 2011;964:583–95. [DOI] [PubMed] [Google Scholar]
- 40. Chodosh J, Kado DM, Seeman TE, Karlamangla AS.. Depressive symptoms as a predictor of cognitive decline: MacArthur studies of successful aging. Am J Geriatr Psychiatry 2007;155:406–15. [DOI] [PubMed] [Google Scholar]
- 41. Donovan NJ, Amariglio RE, Zoller AS et al. , Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer Disease. Am J Geriatr Psychiatry 2014;2212:1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keage HAD, Banks S, Yang KL et al. , What sleep characteristics predict cognitive decline in the elderly? Sleep Med 2012;137:886–92. [DOI] [PubMed] [Google Scholar]
- 43. Blackwell T, Yaffe K, Ancoli-Israel S et al. , Poor sleep is associated with impaired cognitive function in older women: The Study of Osteoporotic Fractures. J Gerontol A Biol 2006;614:405–10. [DOI] [PubMed] [Google Scholar]
- 44. Rasch B, Born J.. About sleep's role in memory. Physiol Rev 2013;932:681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yaffe K, Falvey CM, Hoang T.. Connections between sleep and cognition in older adults. Lancet Neurol 2014;1310:1017–28. [DOI] [PubMed] [Google Scholar]
- 46. Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry 2010;345:742–55. [DOI] [PubMed] [Google Scholar]
- 47. Rock PL, Roiser JP, Riedel WJ, Blackwell AD.. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol Med 2014;4410:2029–40. [DOI] [PubMed] [Google Scholar]
- 48. Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res 2010;185:105–29. [DOI] [PubMed] [Google Scholar]
- 49. Baumgart M, Snyder HM, Carrillo MC et al. , Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 2015;116:718–26. [DOI] [PubMed] [Google Scholar]
- 50. Yaffe K, Hoang TD, Byers AL, Barnes DE, Friedl KE.. Lifestyle and health-related risk factors and risk of cognitive aging among older veterans. Alzheimers Dement 2014;10(suppl 3):S111–21. [DOI] [PubMed] [Google Scholar]
- 51. Then F, Schroeter M, Arélin K et al. , Interactions between APOE genotype and lifestyle factors on cognitive functioning: Results of the health study of the Leipzig Research Center for Civilization Diseases (LIFE). Gesundheitswesen 2016;78:A13. [Google Scholar]
- 52. Hessel P, Leist A, Riumallo-Herl CJ, Avendano M. Recessions, unemployment and the brain: Do individual and aggregate economic shocks prior to retirement leave a cognitive ‘scar’? Available at: http://paa2015princetonedu.2015.
- 53. Sellbom KS, Gunstad J.. Cognitive function and decline in obesity. J Alzheimers Dis 2012;30(suppl 2): S89–95. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen JCD, Killcross AS, Jenkins TA.. Obesity and cognitive decline: Role of inflammation and vascular changes. Front Neurosci 2014;8:375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weber MT, Maki PM, McDermott MP.. Cognition and mood in perimenopause: A systematic review and meta-analysis. J Steroid Biochem Mol Biol 2014;142:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coker LH, Espeland MA, Rapp SR et al. , Postmenopausal hormone therapy and cognitive outcomes: The Women's Health Initiative Memory Study (WHIMS). J Steroid Biochem Mol Biol 2010;118(4–5):304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmidt P. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause 2012;193:257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mazzonna F, Peracchi F.. Ageing, cognitive abilities and retirement. Eur Econ Rev 2012;564:691–710. [Google Scholar]
- 59. Bonsang SA, Eric SP.. Does retirement affect cognitive performance? J Health Econ 2012;31:490–501. [DOI] [PubMed] [Google Scholar]
- 60. Rohwedder S, Willis RJ.. Mental retirement. J Econ Perspect 2010;241:119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mielke MM, Vemuri P, Rocca WA.. Clinical epidemiology of Alzheimer's disease: Assessing sex and gender differences. Clin Epidemiol 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Grip A, Dupuy A, Jolles J, van Boxtel M.. Retirement and cognitive development in the Netherlands: Are the retired really inactive? Econ Hum Biol 2015;19:157–69. [DOI] [PubMed] [Google Scholar]
- 63. Daviglus ML, Bell CC, Berrettini W et al. , National Institutes of Health state-of-the-science conference statement: Preventing Alzheimer disease and cognitive decline. Ann Intern Med 2010;1533:176–81. [DOI] [PubMed] [Google Scholar]
- 64. Anstey KJ, Mack HA, Cherbuin N.. Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am J Geriatr Psychiatry 2009;177:542–55. [DOI] [PubMed] [Google Scholar]
- 65. Neafsey EJ, Collins MA.. Moderate alcohol consumption and cognitive risk. Neuropsych Dis Treat 2011;7:465–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lobo E, Dufouil C, Marcos G et al. , Is there an association between low-to-moderate alcohol consumption and risk of cognitive decline? Am J Epidemiol 2010;1726:708–16. [DOI] [PubMed] [Google Scholar]
- 67. Ilomaki J, Jokanovic N, Tan ECK, Lonnroos E.. Alcohol consumption, dementia and cognitive decline: An overview of systematic reviews. Curr Clin Pharmacol 2015;103:204–12. [DOI] [PubMed] [Google Scholar]
- 68. Horvat P, Richards M, Kubinova R et al. , Alcohol consumption, drinking patterns, and cognitive function in older Eastern European adults. Neurology 2015;843:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sabia S, Elbaz A, Britton A et al. , Alcohol consumption and cognitive decline in early old age. Neurology 2014;824:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pedro MC, Mercedes M-P, Ramón L-H, Borja MR.. Subjective memory complaints in elderly: Relationship with health status, multimorbidity, medications, and use of services in a population-based study. Int Psychogeriatr 2016;2811:1903–16. [DOI] [PubMed] [Google Scholar]
- 71. Lee SJ, Ritchie CS, Yaffe K, Stijacic Cenzer I, Barnes DE.. A clinical index to predict progression from mild cognitive impairment to dementia due to Alzheimer's disease. PLoS One 2014;912:e113535.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K.. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;642:277–81. [DOI] [PubMed] [Google Scholar]
- 73. Raffaitin C, Gin H, Empana JP et al. , Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: The Three-City Study. Diabetes Care 2009;321:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qiu C, Fratiglioni L.. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015;125:267–77. [DOI] [PubMed] [Google Scholar]
- 75. Caracciolo B, Gatz M, Xu W et al. , Relationship of subjective cognitive impairment and cognitive impairment no dementia to chronic disease and multimorbidity in a nation-wide twin study. J Alzheimers Dis 2013;362:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aarts S, van den Akker M, Hajema KJ et al. , Multimorbidity and its relation to subjective memory complaints in a large general population of older adults. Int Psychogeriatr 2011;234:616–24. [DOI] [PubMed] [Google Scholar]
- 77. Crumley JJ, Stetler CA, Horhota M.. Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychol Aging 2014;292:250–63. [DOI] [PubMed] [Google Scholar]
- 78. Shin JH, Lim JY, Kim KW et al. , Functional and physical abilities in the early continuum of cognitive decline. Dement Geriatr Cogn Disord 2015;39(1–2):41–51. [DOI] [PubMed] [Google Scholar]
- 79. Innes K, Selfe T, Khalsa D, Kandati S.. Meditation and music improve cognition in adults with subjective cognitive decline. A preliminary randomized controlled trial. J Alzheimers Dis 2017;563:899–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Reisberg B, Prichep L, Mosconi L et al. , The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement 2008;4(suppl 1):S98–S108. [DOI] [PubMed] [Google Scholar]
- 81. Dufouil C, Fuhrer R, Alperovitch A.. Subjective cognitive complaints and cognitive decline: Consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc 2005;534:616–21. [DOI] [PubMed] [Google Scholar]
- 82. Schultz SA, Oh JM, Rebecca LK et al. , Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-age adults at risk of AD. Alzheimers Dement 2015;11:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W.. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 2010;61:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koppara A, Wagner M, Lange C et al. , Cognitive performance before and after the onset of subjective cognitive decline in old age. Alzheimers Dement 2015;12:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luck T, Riedel-Heller SG, Luppa M et al. , A hierarchy of predictors for dementia-free survival in old-age: Results of the AgeCoDe study. Acta Psychiatr Scand 2014;1291:63–72. [DOI] [PubMed] [Google Scholar]
- 86. Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA.. Baseline subjective memory complaints associate with increased risk of incident dementia: The PREADVISE trial. J Prev Alzheimers Dis 2015;21:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mendonca MD, Alves L, Bugalho P.. From subjective cognitive complaints to dementia: Who is at risk?: A Systematic Review. Am J Alzheimers Dis Other Demen 2016;312:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jessen F, Wiese B, Bachmann C et al. , Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;674:414–22. [DOI] [PubMed] [Google Scholar]
- 89. Amariglio RE, Becker JA, Carmasin J et al. , Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;5012:2880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Snitz BE, Weissfeld LA, Cohen AD et al. , Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry 2015;239:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mormino E, Vannini P, Amariglio R, Schultz A et al. , Subjective cognitive concerns, amyloid burden and cognitive reserve. Alzheimers Dement 2013;9(suppl 4):P133–P4. [Google Scholar]
- 92. Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ.. Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;692:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Amariglio RE, Mormino EC, Pietras AC et al. , Subjective cognitive concerns, amyloid-beta, and neurodegeneration in clinically normal elderly. Neurology 2015;851:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rowe CC, Ellis KA, Rimajova M et al. , Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;318:1275–83. [DOI] [PubMed] [Google Scholar]
- 95. Colijn MA, Grossberg GT.. Amyloid and Tau biomarkers in subjective cognitive impairment. J Alzheimers Dis 2015;471:1–8. [DOI] [PubMed] [Google Scholar]
- 96. Visser PJ, Verhey F, Knol DL et al. , Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol 2009;87:619–27. [DOI] [PubMed] [Google Scholar]
- 97. Perrotin A, Mézenge F, Landeau B et al. , Is hippocampal atrophy in healthy elderly individuals with subjective cognitive decline related to amyloid deposition? Alzheimers Dement 2014;104:P58–9. [Google Scholar]
- 98. Saykin AJ, Wishart HA, Rabin LA et al. , Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;675:834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stewart R, Godin O, Crivello F et al. , Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry 2011;1983:199–205. [DOI] [PubMed] [Google Scholar]
- 100. Stewart R, Dufouil C, Godin O et al. , Neuroimaging correlates of subjective memory deficits in a community population. Neurology 2008;7018:1601–7. [DOI] [PubMed] [Google Scholar]
- 101. Minett TSC, Dean JL, Firbank M, English P, O'Brien JT.. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am J Geriatr Psychiatry 2005;138:665–71. [DOI] [PubMed] [Google Scholar]
- 102. Singh G, Miller JD, Lee FH, Pettitt D, Russell MW.. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: Data from the Third National Health and Nutrition Examination Survey. Am J Manag Care 2002;815:S383–S91. [PubMed] [Google Scholar]
- 103. Puenpatom RA, Victor TW.. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad Med 2009;1216:9–20. [DOI] [PubMed] [Google Scholar]
- 104. Lawrence RC, Felson DT, Helmick CG et al. , Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;581:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Helmick CG, Felson DT, Lawrence RC et al. , Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum 2008;581:15–25. [DOI] [PubMed] [Google Scholar]
- 106. Hootman JM, Helmick CG.. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 2006;541:226–9. [DOI] [PubMed] [Google Scholar]
- 107. Montejo P, Montenegro M, Fernandez MA, Maestu F.. Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging Ment Health 2011;151:85–96. [DOI] [PubMed] [Google Scholar]
- 108. Juncos-Rabadan O, Pereiro AX, Facal D et al. , Prevalence and correlates of cognitive impairment in adults with subjective memory complaints in primary care centres. Dement Geriatr Cogn Disord 2012;334:226–32. [DOI] [PubMed] [Google Scholar]
- 109. Rijs KJ, Van den Kommer TN, Comijs HC, Deeg DJ.. Prevalence and incidence of memory complaints in employed compared to non-employed aged 55–64 years and the role of employment characteristics. PLoS One 2015;103:e0119192.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Perquin M, Diederich N, Pastore J et al. , Prevalence of dementia and cognitive complaints in the context of high cognitive reserve: A population-based study. PLoS One 2015;109:e0138818.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fritsch T, McClendon MJ, Wallendal MS, Hyde TF, Larsen JD.. Prevalence and cognitive bases of subjective memory complaints in older adults: Evidence from a community sample. J Neurodegener Dis 2014;2014:176843.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gorelick PB. Role of inflammation in cognitive impairment: Results of observational epidemiological studies and clinical trials. Innate Inflamm Stroke 2010;1207:155–62. [DOI] [PubMed] [Google Scholar]
- 113. Cote S, Carmichael P-H, Verreault R et al. , Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer's disease. Alzheimers Dement 2012;83:219–26. [DOI] [PubMed] [Google Scholar]
- 114. Wang W, Sun Y, Zhang D.. Association between non-steroidal anti-inflammatory drug use and cognitive decline: A systematic review and meta-analysis of prospective cohort studies. Drugs Aging 2016;337:501–9. [DOI] [PubMed] [Google Scholar]
- 115. Jamison RN, Schein JR, Vallow S et al. , Neuropsychological effects of long-term opioid use in chronic pain patients. J Pain Symptom Manage 2003;264:913–21. [DOI] [PubMed] [Google Scholar]
- 116. Byas-Smith MG, Chapman SL, Reed B, Cotsonis G.. The effect of opioids on driving and psychomotor performance in patients with chronic pain. Clin J Pain 2005;214:345–52. [DOI] [PubMed] [Google Scholar]
- 117. Sabatowski R, Schwalen S, Rettig K et al. , Driving ability under long-term treatment with transdermal fentanyl. J Pain Symptom Manage 2003;251:38–47. [DOI] [PubMed] [Google Scholar]
- 118. McCracken LM, Iverson GL.. Predicting complaints of impaired cognitive functioning in patients with chronic pain. J Pain Symptom Manage 2001;215:392–6. [DOI] [PubMed] [Google Scholar]
- 119. Oosterman JM, Derksen LC, van Wijck AJM, Veldhuijzen DS, Kessels RPC.. Memory functions in chronic pain: Examining contributions of attention and age to test performance. Clin J Pain 2011;271:70–5. [DOI] [PubMed] [Google Scholar]
- 120. Kendall SE, Sjogren P, Pimenta CA, Hojsted J, Kurita GP.. The cognitive effects of opioids in chronic non-cancer pain. Pain 2010;1502:225–30. [DOI] [PubMed] [Google Scholar]
- 121. Haythornthwaite J, Menefee L, Quartrano-Piacenti A, Pappagallo M.. Outcome of chronic opioid therapy for non-cancer pain. J Pain Symptom Manage 1998;15:185–94. [DOI] [PubMed] [Google Scholar]
- 122. Dublin S, Walker RL, Gray SL et al. , Prescription opioids and risk of dementia or cognitive decline: A prospective cohort study. J Am Geriatr Soc 2015;638:1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]