Abstract
Background
Because an increase of patients who misuse opioids has been identified in our cancer clinical setting through urine drug testing (UDT) and the Screener and Opioid Assessment for Patient’s with Pain–Short Form (SOAPP-SF), we conducted this retrospective cohort study to identify patient characteristics that are associated with UDT that indicates noncompliance.
Methods
Over a two-year period, 167 of 8,727 patients (2.4%) seen in the pain clinic and who underwent UDT were evaluated to determine compliance with prescribed opioid regimens. Descriptive clinical and demographic data were collected, and group differences based on compliance with opioid therapy were evaluated.
Results
Fifty-eight percent of the patients were noncompliant with their prescribed opioid therapy. Noncompliant patients were younger than compliant patients, with a median age of 46 vs 49 years (P = 0.0408). Noncompliant patients were more likely to have higher morphine equivalent daily doses; however, the difference was not statistically significant. Patients with a history of alcohol (ETOH) (P = 0.0332), illicit drug use (P = 0.1014), and smoking (P = 0.4184) were more likely noncompliant. Univariate regression analysis showed that a history of ETOH use (P = 0.034), a history of anxiety (P = 0.027), younger age (P = 0.07), and a SOAPP-SF score of 4 or higher (P = 0.05) were associated with an abnormal UDT.
Conclusions
History of ETOH use, anxiety, high SOAPP-SF score, and younger age were associated with UDT that indicates noncompliance. Given the very small percentage of UDT testing, it is quite likely that a significant number of patients who did not undergo UDT were also nonadherent with treatment recommendations.
Keywords: Cancer Pain, Opioids, Urine Drug Testing, Opioid Compliance, Opioid Misuse
Introduction
The incidence of opioid misuse has reached epidemic proportions, and the US Centers for Disease Control and Prevention (CDC) Task Force has put forth guidelines for the primary care physicians prescribing opioids for chronic pain “outside of active cancer treatment, palliative care, and end-of-life care” [1,2]. There is a growing awareness that patients with active cancer and patients undergoing end-of life care may also misuse opioids [3–7]. The incidence of opioid addiction and misuse in the cancer population is not well studied and continues to be underreported [8], but one can estimate that it may not be much different than age-matched members of the population.
Younger age (less than 35 years), psychiatric comorbidities (anxiety, depression, psychosocial stressors, chemical coping) [9,10], history of smoking, personal and/or family history of substance abuse, history of physical or sexual abuse, history of opioid use, and MEDD greater than 120 mg put chronic pain patients at a higher risk for opioid misuse [11–16]. There is conflicting evidence on opioid misuse difference among men and women [17]. The extent to which the risk factors generalize to cancer patients with pain is unclear. Eighty-six percent of all cancers diagnosed in the United States are in patients over the age of 50 years [18], and this may shift the age that puts these patients at risk for noncompliance with opioid therapy. Patients with cancer have a high incidence of anxiety and depression, which puts them at a higher risk for opioid misuse [19]. Chemical coping remains underdiagnosed and is as high as 18% in advanced cancer patients [20]. Undiagnosed and untreated symptom burden can also lead to chemical coping and use of opioids to allay these symptoms [20,21]. Higher incidence of illegal drug use was seen in patients with advanced cancer and patients with alcoholism and smoking history [22,23]. Bruera et al. [24] elaborate on factors that place individuals with cancer at risk for overtreatment with opioids; these include long-term survival, comorbid mental health conditions, and preexisting substance use disorders, as well as limited or no financial resource. These authors also discuss pseudo-addiction, psychiatric conditions, substance use disorder, criminal intent, and inability to follow treatment plan as potential reasons for aberrant drug-taking behaviors [24]. Current oncologic therapies have improved cancer cure rates and prolonged life expectancy in most cancer types, which may also lengthen exposure to opioids and increase opioid misuse behaviors [25].
An increasing number of patients who misuse opioids have been identified in the oncologic setting through “universal precautions” including urine drug testing (UDT) and the use of other risk assessment tools including the Screener and Opioid Assessment for Patient’s with Pain–Short Form (SOAPP-SF), Opioid Risk Tool (ORT), and Screener for Opioid Assessment in Pain Patients–Revised (SOAPP-R) [5,26–28]. Evidence suggests that no single tool by itself—physician assessment, prescription monitoring programs, screening tools, or urine drug testing—is dependable enough to assess the risk of opioid abuse or misuse [10,13,29,30]. UDT is still considered the “gold standard” test to identify opioid misuse as it allows for detection of drugs with good sensitivity and specificity and ease of performance [31]. There have not been established guidelines on standard of care for the administration of this test, and there has been an overall misuse in the chronic pain population for financial gain, as well as regulatory concerns for malpractice [12,13,31–33]. With the awareness of the misuse of opioids and associated deaths, recent CDC recommendations are to get UDT on all patients with chronic pain outside of active cancer treatment, palliative care, and end-of-life care initiated on opioid therapy and at least yearly thereafter, or earlier as indicated [1]. Despite concerns for opioid misuse in the oncology setting, UDT and other tools continue to be relatively underutilized [4,7,27,34]. Available weak evidence suggests that there is a relatively high incidence of opioid misuse in oncology patients [4,27,28]. In a previous study done by the authors, 149 of the 522 (29%) consecutive cancer pain patients that had completed the SOAPP-SF had a score of 4 or higher and were considered at risk for noncompliance with their opioid therapy [5]. There are no guidelines available on risk management strategies for patients with active cancer on opioid therapy, and UDT and “pain contracts” are not standard practice in the oncology setting. The research question we sought to address in this study is what patient characteristics are associated with UDT indicating noncompliance. By addressing this question, we will identify patient characteristics that can prompt physicians to get UDT.
Methods
This study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board and granted a waiver of patient written consent. Charts of patients seen in the Cancer Pain Center from January 1, 2009, to December 31, 2011, were reviewed. A total of 211 unique patients who had UDT and had completed the SOAPP-SF questionnaire were identified. Information on pain scores based on Brief Pain Inventory (BPI), symptom burden scores using the Edmonton Symptom Assessment Scale (ESAS), and morphine equivalency daily dose (MEDD) was collected at three time points: initial visit, first follow-up visit, and second follow-up visit to the clinic. A Health Insurance Portability and Accountability Act–compliant database was created for this study.
UDT
UDT is considered a “gold standard” test for ascertaining compliance with opioid therapy. One of the primary reasons is that urinary tests allow for the presence or absence of certain drugs used in the pain patients to be evaluated with good specificity and sensitivity, ease of administration, and low cost. Drugs and their metabolites tend to stay for a longer period of time in the urine, allowing for a lengthier detection time than serum [31,35]. In our study, a urine sample (around 30 mL) was collected in the clinic. Initial immunoassay gave a qualitative analysis, followed by confirmatory gas chromatography–mass spectroscopy of positive results performed in the laboratory.
SOAPP-SF
The SOAPP version 1.0 is a 14-item self-report questionnaire designed to predict aberrant medication-related behaviors among chronic pain patients [36]. In order to reduce the time burden on patients, an abbreviated short form version of the SOAPP was used in this study (SOAPP-SF). Although there is a slight reduction in sensitivity and specificity in the five-item SOAPP-SF, the five-item version retains most of the predictive validity of the standard SOAPP version. The five questions address the following: 1) mood swings, 2) nicotine dependence, 3) noncompliance with prescribed medications, 4) history of illegal drug use, and 5) history of legal problems. A score of 4 or more is considered positive and indicative of high risk for opioid misuse, with a sensitivity of 0.86 and specificity of 0.67. There is a 33% chance of being false positive with a high SOAPP-SF score [37].
BPI
The BPI asks patients to rate the severity of their pain at its worst and at its least for the preceding week, and at the time the questionnaire is being administered. Each item is rated on an 11-point scale, where 0 is “no pain” and 10 is “pain as bad as you can imagine.” The BPI is the most widely used instrument for assessing cancer pain, is psychometrically sound, and has demonstrated validity for cancer pain assessment [38].
ESAS
The ESAS is a nine-item self-report visual analog scale originally developed for use in assessing the symptoms of patients receiving palliative care [39]. Each of nine symptoms assessed by the ESAS (fatigue, nausea, depression, anxiety, drowsiness, mental clarity, shortness of breath, poor appetite, and insomnia) is rated on an 11-point scale, with 0 being “none” and 10 being “worst.” In addition, the overall sense of well-being was assessed also using an 11-point scale, with 0 being “best” and 10 being “worst.” The scale has demonstrated validity in cancer populations, as evidenced by appropriate correlations with other measures of pain and distress. Moreover, the scale has been found to have good test-retest reliability and good internal consistency.
Sample
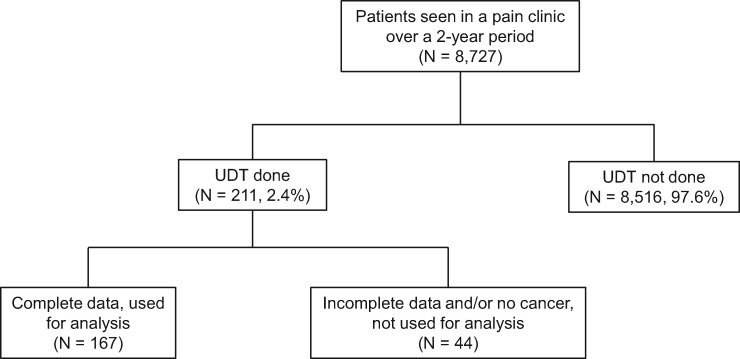
A total of 8,727 unique patients were seen in the pain management clinic during the study period. The data included 520 patient encounters collected from 211 patients who had UDT and completed the SOAPP-F questionnaire (Figure 1). Patients were included in the study even if they had UDT at consult because they were getting opioids from their oncologists within the institute and were referred to us due to poor pain control or compliance issues with their existing regimen. Thirty-five patients with missing information as well as inconclusive UDT (i.e., patient report of not taking short-acting opioid, unable to distinguish between marijuana and marinol, UDT not done for prescribed opioid) were excluded from the analysis. We also excluded nine patients without a cancer diagnosis. Hence, the sample was comprised of 167 patients with complete data.
Figure 1.
Flowchart of the screening methods. UDT = urine drug testing.
Data Collection
Demographic data, including age, gender, employment status, and cancer diagnosis, were collected at the time of initial consult on all identified patients from the medical databases. Patient medical records were also reviewed for cancer disease status, treatment status, smoking status, history of alcohol (ETOH) and/or illicit drug use, psychiatric diagnosis, and other comorbidities. UDT was reviewed, and compliance with opioid therapy was evaluated by the principal investigator. UDT was defined as abnormal when it was positive for nonprescribed opioid or illicit substance, or negative for prescribed opioid. SOAPP-SF scores were collected from clinic records at initial consultation. The patient-reported pain scores were collected at all three study time points (first visit, first follow-up visit, and second follow-up visit). Fatigue, anxiety, depression, drowsiness, difficulty thinking clearly, nausea, feeling of well-being, shortness of breath, and insomnia scores were obtained from the ESAS at all three study time points. Lastly, MEDDs were also calculated at all three time points.
Statistical Analysis
Frequencies and percentages are reported for categorical variables. Summary statistics such as number of nonmissing observations, mean, median, standard deviation, minimum, and maximum are provided for continuous data. The chi-square test and Fisher’s exact test were used to evaluate the association between categorical variables. Wilcoxon’s rank sum test was used to compare the distributions of continuous variables between study groups (i.e., compliance vs noncompliance as determined by UDT). Univariate logistic regression analysis was performed to look at compliance status for the following covariates: age, gender, pain scores, SOAPP-SF total score, MEDD, smoking, alcohol, illegal drug use, anxiety, and depression. Odds ratios and 95% confidence intervals were provided. Receiver operating characteristic (ROC) curves were used to assess discrimination of a fitted logistic model and the accuracy of prediction. The area under the ROC curve (AUC) was reported to summarize the discrimination ability of a model. Mean and standard error plots over time for each of the pain and symptom scores were provided by SOAPP-SF-based risk level and compliance status. Repeated measure models were fitted to assess the association between symptom scores and compliance status with SOAPP-SF score level over different time points. All tests were two-sided. P values of less than 0.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS, Cary, NC, USA) software.
Results
Two hundred and eleven of the 8,727 patients (2.4%) seen in the pain management clinic during the study period had UDT. One hundred and sixty-seven patients with complete data were included in the analysis. Forty-four patients (26%) had UDT at initial consult, and 123 patients (74%) at subsequent visits. Based on UDT results, 97 (58%) patients were noncompliant and 70 (42%) were compliant. Patient demographic and clinical characteristics (race, gender, employment status, disease status) were not statistically significantly different between the compliant and noncompliant patients. Patients were stratified into two groups based on their oncologic care at the time of the UDT. They were classified as active disease if they were getting oncologic therapy including chemotherapy, surgery, and/or radiation; and no evidence of disease or stable if they had no disease or indolent disease but were not receiving oncologic treatment. Noncompliant patients were younger than compliant patients, with a median age of 46 vs 59 years (P = 0.0408) (Table 1). Fifty-six percent of the patients with active cancer and 64% of the patients with indolent or no evidence of disease were noncompliant with their prescribed opioid therapy. The difference was not statistically significant (P = 0.3117).
Table 1.
Demographic and treatment status
| Variable | Level | Total (N = 167) No. (%) | Compliant (N = 70) No. (%) | Noncompliant (N = 97) No. (%) | P* |
|---|---|---|---|---|---|
| Age (mean ± SD) | 167 | 49 ± 12 | 46 ± 12 | 0.0408 | |
| Gender | F | 73 (44) | 32 (44) | 41 (56) | 0.6578 |
| M | 94 (56) | 38 (40) | 56 (60) | ||
| Race | White | 125 (75) | 54 (43) | 71 (57) | 0.7889 |
| Black | 23 (19) | 9 (39) | 14 (61) | ||
| Hispanic | 17 (10) | 6 (35) | 11 (65) | ||
| Asian | 1 (0.6) | 1 (100) | 0 | ||
| Other | 1 (0.6) | 0 | 1 (100) | ||
| Employment Status | Employed | 38 (35) | 19 (50) | 19 (50) | 0.4764 |
| Unemployed | 70 (65) | 30 (43) | 40 (57) | ||
| Disease status | Active | 122 (73) | 54 (44) | 68 (56) | 0.3117 |
| No evidence of disease | 45 (27) | 16 (36) | 29 (64) |
C = current use; H = history of use.
P values were based on chi-square or Fisher’s exact test.
Employment status was available only in 108 patients, of which 70 (65%) were unemployed or disabled. Patients currently smoking or with a history of smoking or ETOH or illicit drug use were more likely noncompliant, though a statistically significant difference was seen in ETOH users only (P = 0.033). Noncompliant patients were more likely to have higher MEDD; however, the difference was not statistically significant (average daily dose = 124 vs 114, P = 0.629). The pain and symptom scores were collected at baseline, the first follow-up visit, and the second follow-up visit. The distributions of pain and symptom scores were not statistically significantly different (P values > 0.05) between compliant and noncompliant patients at each time point. A Unicovariate logistic regression analysis was done to look at variables that seemed to have a potential relevance to noncompliance with prescribed opioid therapy. Patients with a history or current use of ETOH (odds ratio [OR] = 1.96, 95% confidence interval [CI] of OR = 1.05–3.67, P = 0.034) and a SOAPP-SF score of 4 or higher (OR = 1.96, 95% CI of OR = 1.03–3.73, P = 0.041) had a statistically significant association with abnormal UDT. Younger patients (P = 0.079) and patients who had a higher anxiety score at baseline (P = 0.099) showed a marginally statistically significant association with abnormal UDT (Table 2). A majority of the patients (60%) were positive for marijuana that was not prescribed to them. Thirty-seven percent had opioids on the UDT that were not prescribed to them, and 16% were negative for prescribed opioids. Twenty-two percent were positive for other illicit drugs including amphetamines (not prescribed) and cocaine, and one patient was positive for benzodiazepines that he obtained from a family member (Table 2). The AUC was reported to summarize the discrimination ability of a model. All predictor variables showed decent discriminating ability, with all values over 0.50 (Table 3).
Table 2.
UDT results
| Variable | No. of patients | Percent |
|---|---|---|
| Positive for marijuana | 58 | 59.8 |
| Positive for not-prescribed opioids | 36 | 37.1 |
| Negative for prescribed opioids | 16 | 16.5 |
| Positive for other illicit substances | 22 | 22.7 |
UDT = urine drug testing.
Table 3.
Univariate logistic regression analysis
| Parameter | OR | 95% CI of OR | P | AUC | |
|---|---|---|---|---|---|
| Age | 0.976 | 0.951 | 1.003 | 0.079 | 0.5937 |
| Gender | 1.15 | 0.619 | 2.136 | 0.658 | 0.5172 |
| SOAPP-SF total score ≥ 4 | 1.1 | 0.999 | 1.212 | 0.052 | 0.5771 |
| Smoking | 1.381 | 0.631 | 3.022 | 0.42 | 0.5247 |
| ETOH | 1.964 | 1.052 | 3.668 | 0.034 | 0.5835 |
| Illegal drug | 1.69 | 0.9 | 3.172 | 0.102 | 0.5652 |
| Pain score | 1.013 | 0.862 | 1.19 | 0.877 | 0.5106 |
| Anxiety | 1.096 | 0.983 | 1.222 | 0.099 | 0.5805 |
| Depression | 1.052 | 0.944 | 1.173 | 0.36 | 0.5546 |
AUC = area under the receiver operating characteristic curve; CI = confidence interval; OR = odds ratio; SOAPP-SF = Screener and Opioid Assessment for Patient’s with Pain–Short Form.
A total of 108 (65%) patients had a SOAPP-SF score of 4 or higher and were in the moderate- to high-risk group for opioid noncompliance, and 59 (35%) patients were in the low-risk group. Patients who had a high SOAPP-SF score (≥ 4) were more likely noncompliant as compared with patients who had a low SOAPP-SF score (< 4; 63.9% vs 47.5%, P = 0.0397) (Table 1). The higher initial total SOAPP-SF scores and Question #4 (the item probing illegal drug use on the SOAPP-SF scores: “How often have you used illegal drugs in the past five years?”) as continuous data were statistically significantly associated with noncompliance (P values < 0.05) (Table 4). Pain and symptom scores over time in the high- vs low-risk groups based on SOAPP-SF were analyzed using repeated measures models. The higher SOAPP-SF score (≥4) was significantly associated with higher symptom burden (P values < 0.05). A score of 4 or higher was significantly associated with increased symptom scores on fatigue, anxiety, depression, difficulty thinking clearly, insomnia, and feeling of well-being, shortness of breath, and poor appetite (P values < 0.05).
Table 4.
Association between compliance status and SOAPP-SF
| Variable | Compliance_2 | No. | Mean ± SD, median (min–max) | P (Wilcoxon) |
|---|---|---|---|---|
| SOAPP-SF score Q 1 | No | 97 | 1.98 ± 1.29, 2 (0–4) | 0.0631 |
| Yes | 69 | 1.59 ± 1.12, 2 (0–4) | . | |
| SOAPP-SF score Q 2 | No | 97 | 1.4 ± 1.62, 1 (0–4) | 0.7060 |
| Yes | 67 | 1.37 ± 1.62, 0 (0–4) | . | |
| SOAPP-SF score Q 3 | No | 95 | 0.94 ± 1.16, 1 (0–4) | 0.3604 |
| Yes | 69 | 0.8 ± 1.13, 0 (0–4) | . | |
| SOAPP-SF score Q 4 | No | 96 | 0.91 ± 1.16, 0 (0–4) | 0.0036 |
| Yes | 70 | 0.49 ± 1.03, 0 (0–4) | . | |
| SOAPP-SF score Q 5 | No | 96 | 0.47 ± 0.75, 0 (0–4) | 0.7126 |
| Yes | 68 | 0.49 ± 0.87, 0 (0–4) | . | |
| SOAPP-SF total score | No | 97 | 5.66 ± 3.22, 5 (0–16) | 0.0403 |
| Yes | 70 | 4.63 ± 3.46, 4.5 (0–14) | . |
Q = question; SOAPP-SF = Screener and Opioid Assessment for Patient’s with Pain–Short Form.
Discussion
The results from this retrospective cohort indicate that a very small number of patients seen at our pain management clinic had UDT, 211 out of 8,727 (2.4%) during the study period. This is consistent with other studies in oncology practices [4,5,34]. This is in contrast to what is seen in the chronic pain clinics; as high as 19% of patients in a national cohort of US veterans seen in pain clinics had UDT [40]. In spite of various guidelines on getting UDT [41,42], chronic pain practices have misused the application of UDT for financial gains, and some have been influenced by medical licensure boards and other governmental agencies [31]. Morasco et al. [40] looked at predictive factors that led physicians to get UDT when initiating chronic opioid therapy. They found that patient-level factors that predicted increased likelihood of getting UDT included male gender, black race, divorced/separated marital status, higher pain intensity, comorbid substance use disorder, post-traumatic stress disorder, bipolar disorder or schizophrenia, and a higher baseline opioid dose [40]. It is currently recommended that all patients with chronic pain get UDT prior to prescribing opioid therapy as well as at least yearly thereafter, and as clinically indicated by the health care provider [1]. There are no clear guidelines for the oncology setting, and UDT and pain agreements are not routine, but given the growing awareness of opioid misuse in the cancer patients [3,4,6,27,28], it would be prudent to apply similar guidelines. UDT is a great tool to identify patient compliance to prescribed therapy; an initial rapid immunoassay will give a qualitative analysis, and a quantitative analysis will give a confirmation. There are some pitfalls to this simple test as well; there are discrepancies in the interpretation of the test based on training. Understanding the cutoff points determined by a particular laboratory, the pharmacogenetics, the pharmacodynamics, and the pharmacokinetic properties of the opioid is necessary [43,44]. Thirty-five patients with missing information as well as inconclusive UDT (i.e., patient report of not taking short-acting opioid, unable to distinguish between marijuana vs marinol, UDT not done for prescribed opioid) had to be excluded from the analysis in our study.
A majority (58%) of this cohort was noncompliant with their prescribed opioid therapy. Patients who were noncompliant were younger (46 ± 12 years), though older than what is considered the at-risk age (older than 35 years) in the chronic pain population; this can be partially explained by the higher incidence of cancer in the elderly population [45]. Psychiatric comorbidities [9,10], history of opioid use, history of smoking, personal and/or family history of substance abuse, and history of physical or sexual abuse have shown to put chronic pain patients at risk for opioid misuse. The noncompliant patients in this cohort had a higher anxiety level and history or current use of ETOH. History of smoking and illicit drug use were seen more commonly in the noncompliant patients, although this was not statistically significant. History of physical and sexual abuse was not available on all patients in this cohort.
Almost 60% of the noncompliant patients were positive for THC, which is currently illegal in Texas. There continues to be an ongoing debate on the use of “medical marijuana,” and currently a total of 28 states, the District of Columbia, Guam, and Puerto Rico have legalized it in some form [46]. With increased access to marijuana, it has become common for patients on chronic opioid therapy to use concurrent marijuana. Pain, nausea or lack of appetite, and weight stabilization are some of the indications for use of medical cannabis in the oncology setting, and available evidence is inconclusive for the use of cannabinoids over traditional anti-emetics [47]. There is a dearth of high-quality studies to support the use of cannabinoids for cancer pain [48]. Medical cannabis has potential adverse effects, such as acute impairment of memory, coordination, and judgment, a concern with concurrent chronic opioid therapy. There is scant literature on the benefits and risks, including cognitive changes associated with concurrent use of opioids and marijuana [49]. Physicians cannot dismiss the concern for potential interactions of cannabis with other parallel pharmacotherapy. Until further evidence-based research on the medical use of cannabis is available, clinicians must use current evidence and expert opinion to guide their practices in states where cannabis is legal [50]. Almost 44% of patients were positive for opioids not prescribed to them or negative for prescribed opioids; 22% were positive for amphetamines or benzodiazepines (one patient) not prescribed to them, or cocaine.
There are several screening tools for prediction and identification of aberrant drug-related behavior, and there is weak evidence for their accuracy [30]. None of the available tools have been validated in the oncology setting. Sixty-five percent of the patients had a SOAPP-SF score of 4 or higher and were in the moderate- to high-risk group for opioid misuse. The higher initial total SOAPP-SF scores and Question #4 (the item probing illegal drug use on the SOAPP-SF scores: “How often have you used illegal drugs in the past five years?”) as continuous data were statistically significantly associated with noncompliance (P values < 0.05). This raises the question as to whether a physician and/or psychologist probing for illicit drug use is a sufficient screening tool by itself.
Cancer pain is a biopsychosocial experience, with significant contributions coming from sensory, emotional, and cognitive components. In the case of patients with pain from cancer, there is a greater effort expended and leniency to help a patient become more compliant to appropriate use of pain medications. Opioid therapy is but one modality in the context of other concurrent multidisciplinary treatment approaches, each targeting specific aspects of the overall pain experience. Safe and effective opioid therapy should be based upon rational clinical decision-making and practice, consistent with established evidence-based strategies, grounded in practical ethical principles, all aimed at optimal patient outcomes and quality of life. The ethical pain physician should adhere to professional behaviors including altruism, accountability, excellence, duty, respect, honor, and integrity [51]. Likewise, patient responsibilities include adherence to the agreed-upon plan of care, compliance with treatments as prescribed, regular transparent communication of treatment effects and side effects, and reporting therapeutic benefits or unintended consequences of therapy. Patients should be accountable for their responsibilities in their care.
We are faced with the ethical dilemma of denying opioids for patients with active cancer and ongoing opioid misuse. It is optimal to treat the pain and addiction as well psychiatric comorbidities and chemical coping concurrently in this population. Ethical opioid therapy management should include multidisciplinary evaluation, appropriate patient selection, risk management using stratification tools (ORT, SOAPP, SOPP-SF, etc.), initial and follow-up toxicology screening, review of the state electronic prescription monitoring program, patient education, informed consent for opioid therapy along with a treatment agreement, opioid trial period with exit strategy, and follow-up assessments at appropriate intervals. Ongoing opioid treatment necessitates regular follow-up visits for patient reassessment, indicated toxicology and e-PMP data review, side effect management, indicated dosage adjustments, and adjuvant agents. Good clinical practice, as well as legal and regulatory requirements, mandate complete documentation of care, including the above parameters along with evolving treatment plans based on patient outcomes [6,24,52,53]. Cohen and Jangro [52] describe a rational six-step approach to ethical decision-making for opioid treatment that includes the patient’s narrative history with contextual and collateral facts, relevant pain pathophysiology, individual and collaborative goals of care, and outcomes-based reassessment to adjust goals and treatment plans [52].
Limitations
This is a retrospective cohort study with a relatively small sample size. UDT was performed at the discretion of the physician based on their concern for opioid misuse in this small sample of patients.
Conclusions
A very small proportion (2.4%) of patients seen in the pain clinic and prescribed chronic opioid therapy had UDT. A majority (58%) of the patients in this retrospective cohort of cancer patients were noncompliant with their prescribed opioid therapy. Noncompliant patients were younger, more anxious, had a history of or were current users of ETOH, and used higher doses of opioids. SOAPP-SF total score and the response to the item probing illegal drug use were statistically significantly correlated with opioid misuse based on UDT. Given the very small percentage of UDT completed, it is quite likely that a significant number of patients who did not undergo UDT were also nonadherent with treatment recommendations. More research is needed to determine in more detail the overall frequency of inappropriate opioid use among this population.
Disclosures: None pertaining to this manuscript.
References
- 1. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;651:1–49. [DOI] [PubMed] [Google Scholar]
- 2. Dowell D, Haegerich TM, Chou R.. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;31515:1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arthur JA, Haider A, Edwards T et al. , Aberrant opioid use and urine drug testing in outpatient palliative care. J Palliat Med 2016;197:778–82. [DOI] [PubMed] [Google Scholar]
- 4. Arthur JA, Edwards T, Lu Z et al. , Frequency, predictors, and outcomes of urine drug testing among patients with advanced cancer on chronic opioid therapy at an outpatient supportive care clinic. Cancer 2016;12223:3732–9. [DOI] [PubMed] [Google Scholar]
- 5. Koyyalagunta D, Bruera E, Aigner C et al. , Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med 2013;145:667–75. [DOI] [PubMed] [Google Scholar]
- 6. Koyyalagunta D, Burton AW, Toro MP, Driver L, Novy DM.. Opioid abuse in cancer pain: Report of two cases and presentation of an algorithm of multidisciplinary care. Pain Physician 2011;144:E361–71. [PubMed] [Google Scholar]
- 7. Childers JW, King LA, Arnold RM.. Chronic pain and risk factors for opioid misuse in a palliative care clinic. Am J Hosp Palliat Care 2015;326:654–9. [DOI] [PubMed] [Google Scholar]
- 8. Anghelescu DL, Ehrentraut JH, Faughnan LG.. Opioid misuse and abuse: Risk assessment and management in patients with cancer pain. J Natl Compr Canc Netw 2013;118:1023–31. [DOI] [PubMed] [Google Scholar]
- 9. Wasan AD, Butler SF, Budman SH et al. , Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain 2007;234:307–15. [DOI] [PubMed] [Google Scholar]
- 10. Turk DC, Swanson KS, Gatchel RJ.. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin J Pain 2008;24:497–508. [DOI] [PubMed] [Google Scholar]
- 11. White AG, Birnbaum HG, Schiller M, Tang J, Katz NP.. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care 2009;1512:897–906. [PubMed] [Google Scholar]
- 12. Rice JB, White AG, Birnbaum HG et al. , A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med 2012;139:1162–73. [DOI] [PubMed] [Google Scholar]
- 13. Sehgal N, Manchikanti L, Smith HS.. Prescription opioid abuse in chronic pain: A review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 2012;15(suppl 3):ES67–92. [PubMed] [Google Scholar]
- 14. Savage SR. Management of opioid medications in patients with chronic pain and risk of substance misuse. Curr Psychiatry Rep 2009;115:377–84. [DOI] [PubMed] [Google Scholar]
- 15. Savage SR. Assessment for addiction in pain-treatment settings. Clin J Pain 2002;18(suppl 4):S28–38. [DOI] [PubMed] [Google Scholar]
- 16. Sullivan MD, Edlund MJ, Fan MY et al. , Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and Medicaid insurance plans: The TROUP Study. Pain 2010;1502:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamison RN, Butler SF, Budman SH, Edwards RR, Wasan AD.. Gender differences in risk factors for aberrant prescription opioid use. J Pain 2010;11(4):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Cancer Society. Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 19. Delgado-Guay M, Parsons HA, Li Z, Palmer JL, Bruera E.. Symptom distress in advanced cancer patients with anxiety and depression in the palliative care setting. Support Care Cancer 2009;175:573–9. [DOI] [PubMed] [Google Scholar]
- 20. Kwon JH, Tanco K, Park JC et al. , Frequency, predictors, and medical record documentation of chemical coping among advanced cancer patients. Oncologist 2015;206:692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon JH, Tanco K, Hui D, Reddy A, Bruera E.. Chemical coping versus pseudoaddiction in patients with cancer pain. Palliat Support Care 2014;125:413–7. [DOI] [PubMed] [Google Scholar]
- 22. Dev R, Parsons HA, Palla S et al. , Undocumented alcoholism and its correlation with tobacco and illegal drug use in advanced cancer patients. Cancer 2011;11719:4551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim YJ, Dev R, Reddy A et al. , Association between tobacco use, symptom expression, and alcohol and illicit drug use in advanced cancer patients. J Pain Symptom Manage 2016;514:762–8. [DOI] [PubMed] [Google Scholar]
- 24. Bruera E, Paice JA.. Cancer pain management: Safe and effective use of opioids. Am Soc Clin Oncol Educ Book 2015;e593–9. [DOI] [PubMed] [Google Scholar]
- 25. Edlund MJ, Martin BC, Russo JE et al. , The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: The role of opioid prescription. Clin J Pain 2014;307:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peck KR, Ehrentraut JH, Anghelescu DL.. Risk factors for opioid misuse in adolescents and young adults with focus on oncology setting. J Opioid Manag 2016;123:205–16. [DOI] [PubMed] [Google Scholar]
- 27. Barclay JS, Owens JE, Blackhall LJ.. Screening for substance abuse risk in cancer patients using the Opioid Risk Tool and urine drug screen. Support Care Cancer 2014;227:1883–8. [DOI] [PubMed] [Google Scholar]
- 28. Reyes-Gibby CC, Anderson KO, Todd KH.. Risk for opioid misuse among emergency department cancer patients. Acad Emerg Med 2016;232:151–8. [DOI] [PubMed] [Google Scholar]
- 29. Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK.. Opioids for chronic noncancer pain: Prediction and identification of aberrant drug-related behaviors: A review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 2009;10:131–46. [DOI] [PubMed] [Google Scholar]
- 30. Solanki DR, Koyyalagunta D, Shah RV, Silverman SM, Manchikanti L.. Monitoring opioid adherence in chronic pain patients: Assessment of risk of substance misuse. Pain Physician 2011;142:E119–31. [PubMed] [Google Scholar]
- 31. Christo PJ, Manchikanti L, Ruan X et al. , Urine drug testing in chronic pain. Pain Physician 2011;142:123–43. [PubMed] [Google Scholar]
- 32.Divito N. Allstate calls urine test $1 million scam. Courthouse News Service. 2016.
- 33. Gilbert JW, Wheeler GR, Mick GE et al. , Urine drug testing in the treatment of chronic noncancer pain in a Kentucky private neuroscience practice: The potential effect of Medicare benefit changes in Kentucky. Pain Physician 2010;132:187–94. [PubMed] [Google Scholar]
- 34. Tan PD, Barclay JS, Blackhall LJ.. Do palliative care clinics screen for substance abuse and diversion? Results of a National Survey. J Palliat Med 2015;189:752–7. [DOI] [PubMed] [Google Scholar]
- 35. Moeller KE, Lee KC, Kissack JC.. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc 2008;831:66–76. [DOI] [PubMed] [Google Scholar]
- 36. Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN.. Cross-validation of a Screener to Predict Opioid Misuse in Chronic Pain Patients (SOAPP-R). J Addict Med 2009;32:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inflexxion. Screener and Opioid Assesment for Patients with Pain (SOAPP), Version 1.0 SF. 2008. Available at: painedu@inflexxion.com.
- 38. Cleeland CS, Ryan KM.. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;232:129–38. [PubMed] [Google Scholar]
- 39. Chang VT, Hwang SS, Feuerman M.. Validation of the Edmonton Symptom Assessment Scale. Cancer 2000;889:2164–71. [DOI] [PubMed] [Google Scholar]
- 40. Morasco BJ, Peters D, Krebs EE et al. , Predictors of urine drug testing for patients with chronic pain: Results from a national cohort of U.S. veterans. Subst Abus 2016;371:82–7. [DOI] [PubMed] [Google Scholar]
- 41. Owen GT, Burton AW, Schade CM, Passik S.. Urine drug testing: Current recommendations and best practices. Pain Physician 2012;15(suppl 3):Es119–33. [PubMed] [Google Scholar]
- 42. Chou R, Fanciullo GJ, Fine PG et al. , Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;10:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Melanson SE, Ptolemy AS, Wasan AD.. Optimizing urine drug testing for monitoring medication compliance in pain management. Pain Med 2013;1412:1813–20. [DOI] [PubMed] [Google Scholar]
- 44. Wasan AD, Michna E, Janfaza D et al. , Interpreting urine drug tests: Prevalence of morphine metabolism to hydromorphone in chronic pain patients treated with morphine. Pain Med 2008;97:918–23. [DOI] [PubMed] [Google Scholar]
- 45. Starr TD, Rogak LJ, Passik SD.. Substance abuse in cancer pain. Curr Pain Headache Rep 2010;144:268–75. [DOI] [PubMed] [Google Scholar]
- 46.State Medical Marijuana Laws. 2016. Available at: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed December 1, 2016).
- 47. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S.. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev 2015;(11):CD009464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tateo S. State of the evidence: Cannabinoids and cancer pain—a systematic review. J Am Assoc Nurse Pract 2016;29(2):94–103. [DOI] [PubMed] [Google Scholar]
- 49. Becker WC, Tetrault JM.. Medical marijuana in patients prescribed opioids: A cloud of uncertainty. Mayo Clin Proc 2016;917:830–2. [DOI] [PubMed] [Google Scholar]
- 50. Choo EK, Feldstein Ewing SW, Lovejoy TI.. Opioids out, cannabis in: Negotiating the unknowns in patient care for chronic pain. JAMA 2016;31617:1763–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robins LS, Braddock CH 3rd, Fryer-Edwards KA.. Using the American Board of Internal Medicine's “Elements of Professionalism” for undergraduate ethics education. Acad Med 2002;776:523–31. [DOI] [PubMed] [Google Scholar]
- 52. Cohen MJ, Jangro WC.. A clinical ethics approach to opioid treatment of chronic noncancer pain. AMA J Ethics 2015;176:521–9. [DOI] [PubMed] [Google Scholar]
- 53. Gourlay DL, Heit HA.. Pain and addiction: Managing risk through comprehensive care. J Addict Dis 2008;273:23–30. [DOI] [PubMed] [Google Scholar]