Abstract
Background
Elevated body mass index (BMI) is associated with increased risk of postmenopausal breast cancer. The underlying mechanisms, however, remain elusive.
Methods
In a nested case–control study of 621 postmenopausal breast cancer case participants and 621 matched control participants, we measured 617 metabolites in prediagnostic serum. We calculated partial Pearson correlations between metabolites and BMI, and then evaluated BMI-associated metabolites (Bonferroni-corrected α level for 617 statistical tests = P < 8.10 × 10-5) in relation to invasive breast cancer. Odds ratios (ORs) of breast cancer comparing the 90th vs 10th percentile (modeled on a continuous basis) were estimated using conditional logistic regression while controlling for breast cancer risk factors, including BMI. Metabolites with the lowest P values (false discovery rate < 0.2) were mutually adjusted for one another to determine those independently associated with breast cancer risk.
Results
Of 67 BMI-associated metabolites, two were independently associated with invasive breast cancer risk: 16a-hydroxy-DHEA-3-sulfate (OR = 1.65, 95% confidence interval [CI] = 1.22 to 2.22) and 3-methylglutarylcarnitine (OR = 1.67, 95% CI = 1.21 to 2.30). Four metabolites were independently associated with estrogen receptor–positive (ER+) breast cancer risk: 16a-hydroxy-DHEA-3-sulfate (OR = 1.84, 95% CI = 1.27 to 2.67), 3-methylglutarylcarnitine (OR = 1.91, 95% CI = 1.23 to 2.96), allo-isoleucine (OR = 1.76, 95% CI = 1.23 to 2.51), and 2-methylbutyrylcarnitine (OR = 1.89, 95% CI = 1.22 to 2.91). In a model without metabolites, each 5 kg/m2 increase in BMI was associated with a 14% higher risk of breast cancer (OR = 1.14, 95% CI = 1.01 to 1.28), but adding 16a-hydroxy-DHEA-3-sulfate and 3-methylglutarylcarnitine weakened this association (OR = 1.06, 95% CI = 0.93 to 1.20), with the logOR attenuating by 57.6% (95% CI = 21.8% to 100.0+%).
Conclusion
These four metabolites may signal metabolic pathways that contribute to breast carcinogenesis and that underlie the association of BMI with increased postmenopausal breast cancer risk. These findings warrant further replication efforts.
Obesity affects 640 million adults worldwide (1), increases the risk of 13 or more types of cancer (2), and is estimated to contribute to 9% of cancers in North America, Europe, and the Middle East (3). The biological basis by which obesity increases cancer risk, however, remains incompletely understood. Decades of research on steroid hormones, insulin resistance, and inflammation indicate that these factors explain some, but not all, of obesity’s effect on cancer risk (4,5). Other potentially relevant metabolic factors—such as dysregulated metabolism of carbohydrates, amino acids, and lipids—have received less attention, though intriguing evidence indicates that such dysregulations are important features of obesity (6–11) and possibly cancer (12,13). In recent years, technological advances in metabolomics have made it possible to quantify hundreds to thousands of metabolites in blood simultaneously, enabling more thorough explorations of metabolism.
In the current study, we applied metabolomics to prediagnostic serum from postmenopausal women to identify BMI-associated metabolites that were also associated with breast cancer risk. We proceeded as follows: 1) we identified metabolites associated with BMI; 2) we identified which of these were also associated with breast cancer risk; 3) of these, we further identified which were most statistically significantly and independently associated with risk; 4) we quantified the degree to which metabolites mediated the BMI—breast cancer association; and finally, 5) we explored whether associations were distinct from those of biomarkers previously postulated to explain the BMI–breast cancer association. Our aim is to identify metabolites that explain the association of BMI with breast cancer risk. To our knowledge, no prospective studies have used metabolomics to evaluate mechanisms that underlie obesity and breast cancer associations.
Methods
Study Population
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) is a population-based multicenter randomized screening trial of people age 55 to 74 years at baseline with no history of prostate, lung, colorectal, or ovarian cancer (NCT00339495) (14,15). This study was approved by institutional review boards at the US National Cancer Institute and the 10 centers.
Our nested case–control study included 621 incident invasive primary breast cancer cases (ICD-9 174.0-174.9) who were not using hormone therapy at PLCO year 1 or who had an estrogen receptor (ER)– and/or progesterone receptor (PR)–negative status (Supplementary Methods, available online). Using incidence density sampling, we matched 621 controls based on age at random assignment to the study arm (+/-2 years), date of blood collection (+/-3 months), and menopausal hormone therapy use (current, former, never) at year 1. All controls were alive and had no history of cancer as of the date of diagnosis for the matched case.
Body Weight and Other Characteristics
At PLCO baseline, participants completed a self-administered questionnaire that inquired about current height and weight. BMI was calculated as weight in kilograms divided by height in meters squared. The questionnaire also ascertained family history of breast cancer, demographics, and other health-related factors (eg, smoking status).
Metabolite Assessment, Normalization, and Reliability
Serum samples were collected at the first follow-up visit, approximately one year postbaseline, and predated breast cancer diagnosis by a median 6.7 years. Metabolon Inc. (16) quantified levels of 1057 serum metabolites, of which 617 were identified and met our threshold for the percent of values above the limit of detection (Supplementary Methods, available online). Over the 617 metabolites, the median proportion of below-limit-of-detection values was 0% (Supplementary Table 1, available online). Metabolite peak intensities were run-day-normalized and log-transformed for analysis. Metabolite measurements were highly reliable in masked replicates. Over the 617 metabolites, the median intraclass correlation coefficient (ICC) was 0.94 (Supplementary Methods), similar to prior studies (17).
Statistical Analysis
We estimated correlations between the 617 identified metabolites and BMI using partial Pearson correlation, adjusted for age at blood draw, case–control status, and smoking history. Heterogeneity of associations by case status (multiplicative scale) was evaluated using the Wald test. We carried forward to breast cancer analyses the metabolites with P values of 8.10×10-5 or less (Bonferroni-corrected α level for 617 statistical tests) and correlations with a BMI of at least moderate magnitude (absolute value of r ≥ 0.15).
For BMI-associated metabolites, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) of breast cancer risk using conditional logistic regression. Odds ratios represent risk at the 90th percentile as compared with the 10th percentile of log metabolite intensity (OR = eβ(X90-X10) where β is the coefficient for the metabolite modeled continuously and X90 and X10 are metabolite values at the 90th and 10th percentiles). Associations were evaluated separately for ER+ and ER- cancers, as BMI associations have varied by ER subtype (18,19). We did not evaluate associations by PR status.
Multivariable models included well-established breast cancer risk factors, demographic factors, history of diabetes, weekly hours of vigorous physical activity, and BMI. We adjusted for BMI because our aim was to identify mechanistic mediators, which, by definition, requires metabolites to be associated with breast cancer even after adjusting for BMI (20). We set a false discovery rate (21,22) of less than 0.2 as the threshold for statistical significance, calculated separately for overall and ER+ breast cancers.
To determine the BMI-associated metabolites that were independently related to breast cancer risk, we used forward selection. That is, we modeled each BMI-associated metabolite in relation to breast cancer, retained the metabolite with the lowest P value in the model, modeled the remaining metabolites, again retained the one with the lowest P value, and repeated until reaching the false discovery threshold.
To assess mediation, we decomposed the “total effect” of BMI into an “indirect effect” (ie, through metabolites) and a “direct effect” (ie, through other mechanisms) (20). We report the total effect and direct effect as the estimated odds ratios for BMI in breast cancer models, respectively, without and with metabolites included as covariates. Under standard assumptions (20), we report the indirect effect as the ORtotal effect/ORdirect effect. Attenuation was defined as [logORtotal effect-logORdirect effect]/logORtotal effect. The 95% confidence intervals for the indirect effect and attenuation were estimated by bootstrap. The association of BMI with breast cancer risk was confirmed to be approximately linear using cubic splines and likelihood ratio tests.
Finally, we explored whether the metabolite–breast cancer associations were likely to be distinct from those of other biomarkers previously postulated to explain the BMI–breast cancer association. We adjusted metabolite–breast cancer associations for levels of select steroid hormone and insulin resistance–related metabolites from the metabolomics panel to determine if associations were independent. We also evaluated correlations of select metabolites with circulating estradiol from baseline (one year prior to the serum used in the current analysis), previously measured in a subset of 260 participants (245 cases, 15 controls) (23). In postmenopausal women, circulating estradiol is relatively stable over several years (five-year ICC = 0.73) (24).
All statistical tests were two-sided. Analyses were done in SAS (v. 9.3) and R (v. 3.1.2).
Results
Population Characteristics
Women were, on average, 64 years of age. In line with past studies, breast cancer risk factors—early age at menarche, late age at first live birth/parity, late age at menopause, a history of benign breast disease, family history of breast cancer, low physical activity, and a high BMI—were generally more prevalent among cases than controls (Supplementary Table 2, available online).
BMI and Metabolite Correlations
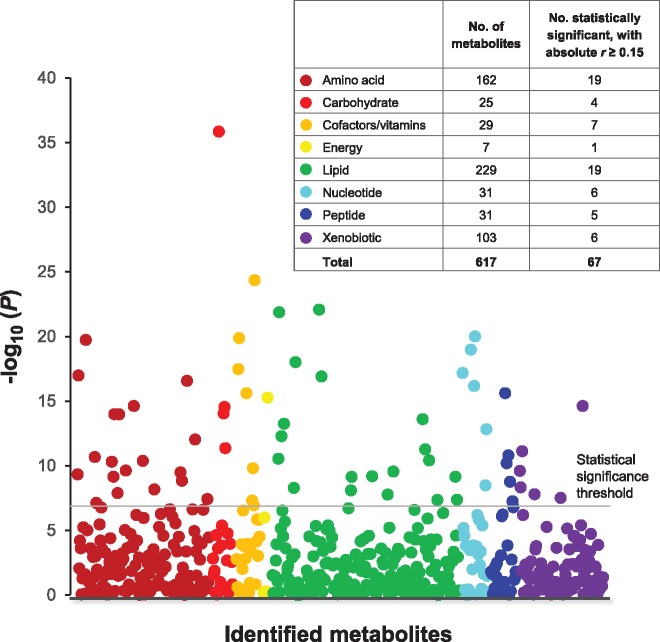
Sixty-seven of the 617 metabolites were statistically significantly and at least moderately associated with BMI (Bonferroni P ≤ 8.10×10-5, absolute r ≥ 0.15), predominantly comprising amino acids (n = 19) and lipids (n = 19) (Figure 1). Pearson correlations were positive for 46 metabolites, inverse for 21, and ranged from –0.26 (oxalate) to 0.35 (mannose) (Table 1). There was no statistically significant heterogeneity of BMI-metabolite associations by case status (a = 0.05/617 threshold).
Figure 1.
Manhattan plot displaying the P values for BMI-metabolite Pearson correlations according to metabolite chemical class. The total number of metabolites and the number with statistically significant associations (Bonferroni P < 8.10 × 10-5 and absolute correlations of r ≥ 0.15) are presented by chemical class in the key. Correlation coefficients were adjusted for age at blood draw (years), case–control status (no, yes), and smoking history (never, former, current).
Table 1.
Serum metabolites statistically significantly and at least moderately associated with body mass index (P < 8.10 × 10-5 and absolute r ≥ 0.15) among 1242 participants in a nested case–control study within the PLCO cohort*
| Metabolite | r | P |
|---|---|---|
| Amino acid | ||
| Glutamate | 0.26 | 1.88 × 10-20 |
| Asparagine | –0.24 | 1.03 × 10-17 |
| C-glycosyltryptophan | 0.24 | 2.70 × 10-17 |
| 3-methylglutarylcarnitine-1 | 0.22 | 2.41 × 10-15 |
| 3-methyl-2-oxobutyrate | 0.22 | 1.04 × 10-14 |
| Isovalerylglycine | –0.22 | 1.06 × 10-14 |
| Kynurenine | 0.20 | 9.38 × 10-13 |
| N-acetylglycine | –0.19 | 2.14 × 10-11 |
| 2-hydroxybutyrate | 0.19 | 4.18 × 10-11 |
| Allo-isoleucine | 0.19 | 4.88 × 10-11 |
| Valine | 0.18 | 2.38 × 10-10 |
| 5-methylthioadenosine | 0.18 | 3.29 × 10-10 |
| N-acetylalanine | 0.18 | 4.65 × 10-10 |
| 2-methylbutyrylcarnitine | 0.17 | 7.12 × 10-10 |
| Acisoga | 0.17 | 1.43 × 10-09 |
| Cystine | 0.16 | 6.71 × 10-09 |
| Isobutyrylglycine | –0.16 | 1.30 × 10-08 |
| N-delta-acetylornithine | –0.16 | 3.85 × 10-08 |
| Glycine | –0.15 | 7.58 × 10-08 |
| Carbohydrate | ||
| Mannose | 0.35 | 1.44 × 10-36 |
| Glucose | 0.22 | 2.85 × 10-15 |
| Glycerate | –0.22 | 8.88 × 10-15 |
| Pyruvate | 0.20 | 4.44 × 10-12 |
| Cofactors and vitamins | ||
| Gamma-tocopherol | 0.29 | 4.50 × 10-25 |
| Oxalate | –0.26 | 1.36 × 10-20 |
| Threonate | –0.25 | 3.42 × 10-18 |
| Quinolinate | 0.23 | 2.43 × 10-16 |
| Delta-tocopherol | 0.18 | 1.58 × 10-10 |
| Gamma-CEHC glucuronide | 0.15 | 5.05 × 10-08 |
| Alpha-tocopherol | –0.15 | 1.13 × 10-07 |
| Energy | ||
| Alpha-ketoglutarate | 0.23 | 5.36 × 10-16 |
| Lipid | ||
| Glycerol | 0.28 | 8.37 × 10-23 |
| Butyrylcarnitine | 0.27 | 1.33 × 10-22 |
| 2-aminoheptanoate | 0.25 | 9.71 × 10-19 |
| Scyllo-inositol | –0.24 | 1.25 × 10-17 |
| Oleoyl sphingomyelin | 0.22 | 2.48 × 10-14 |
| Hexanoylcarnitine | 0.21 | 5.57 × 10-14 |
| Hydroxybutyrylcarnitine | 0.20 | 5.26 × 10-13 |
| Palmitoleoyl sphingomyelin | 0.20 | 5.38 × 10-12 |
| Propionylglycine | –0.19 | 2.83 × 10-11 |
| 16a-hydroxy DHEA 3-sulfate | 0.19 | 3.84 × 10-11 |
| Phosphoethanolamine | –0.18 | 2.80 × 10-10 |
| 1-linoleoylglycerophosphocholine | –0.18 | 6.44 × 10-10 |
| Lathosterol | 0.18 | 7.06 × 10-10 |
| 1-linolenoylglycerophosphocholine | –0.17 | 7.42 × 10-10 |
| Malonylcarnitine | 0.17 | 5.22 × 10-09 |
| Palmitoyl-linoleoyl-glycerophosphoinositol | 0.16 | 8.14 × 10-09 |
| 1-dihomo-linolenylglycerol | 0.16 | 1.69 × 10-08 |
| 7-HOCA† | 0.16 | 4.23 × 10-08 |
| 4-androsten-3beta,17beta-diol disulfate (2) | 0.16 | 4.36 × 10-08 |
| Nucleotide | ||
| N2,N2-dimethylguanosine | 0.26 | 1.00 × 10-20 |
| N6-carbamoylthreonyladenosine | 0.26 | 1.05 × 10-19 |
| Urate | 0.24 | 6.71 × 10-18 |
| N1-methylguanosine | 0.24 | 6.84 × 10-17 |
| Pseudouridine | 0.21 | 1.51 × 10-13 |
| 5,6-dihydrouracil | 0.17 | 3.37 × 10-09 |
| Peptide | ||
| Gamma-glutamylvaline | 0.23 | 2.47 × 10-16 |
| Gamma-glutamylisoleucine | 0.19 | 1.58 × 10-11 |
| Gamma-glutamylglutamine | –0.19 | 6.37 × 10-11 |
| Gamma-glutamylphenylalanine | 0.17 | 1.75 × 10-09 |
| Gamma-glutamyltyrosine | 0.15 | 5.54 × 10-08 |
| Xenobiotics | ||
| Methyl glucopyranoside | –0.22 | 2.43 × 10-15 |
| Propyl 4-hydroxybenzoate sulfate | –0.19 | 7.59 × 10-12 |
| Tartronate | –0.18 | 2.51 × 10-10 |
| Methyl-4-hydroxybenzoate sulfate | –0.17 | 4.72 × 10-09 |
| Catechol sulfate | –0.16 | 1.60 × 10-08 |
| Hydrochlorothiazide | 0.16 | 3.05 × 10-08 |
Metabolites were sorted by chemical class, then P values within chemical class. Pearson correlation coefficients were adjusted for age at blood draw (years), case–control status (no, yes), and smoking history (never, former, current). PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
Full metabolite name is 7-alpha-hydroxy-3-oxo-4-cholestenoate.
BMI-Associated Metabolites and Breast Cancer Risk
Of 67 BMI-associated metabolites, seven were statistically significantly (false discovery rate < 0.2) associated with breast cancer risk (Table 2), and 23 were statistically significantly associated with ER+ breast cancer risk (Table 3). All associations were positive in direction, except for an inverse association with ER+ disease for alpha tocopherol. Breast cancer odds ratios comparing high vs low metabolite levels (90th vs 10th percentile) ranged from 1.45 to 1.84, while ER+ breast cancer odds ratios for the same contrast ranged from 1.50 to 2.49 (multivariable models that included BMI). The associations were slightly stronger in the models without BMI than in models with BMI (ORs shifted 0.01–0.08 away from the null) and were consistent with linearity in cubic spline models (all Pnonlinearity > .05). There was no statistically significant (α = 0.05/67 threshold) heterogeneity when examining cases with shorter vs longer times to diagnosis (≤ vs > 6.7 years). Results for metabolites that were not statistically significantly associated with breast cancer or ER+ breast cancer risk can be found in Supplementary Tables 3 and 4 (available online).
Table 2.
Multivariable ORs and 95% CIs for postmenopausal invasive breast cancer when comparing the 90th with the 10th percentile levels of BMI-associated metabolites*
| Multivariable-adjusted† |
Multivariable-adjusted including BMI‡ |
|||||
|---|---|---|---|---|---|---|
| Metabolite | Chemical class | OR (95% CI) | P | OR (95% CI) | P | Q§ |
| 16a-hydroxy DHEA 3-sulfate | Lipid | 1.82 (1.36 to 2.43) | 5.75 × 10-05 | 1.76 (1.31 to 2.37) | 1.58 × 10-04 | 0.01 |
| 3-methylglutarylcarnitine | Amino acid | 1.84 (1.34 to 2.52) | 1.66 × 10-04 | 1.80 (1.31 to 2.48) | 2.89 × 10-04 | 0.01 |
| Hydroxybutyrylcarnitine | Lipid | 1.61 (1.19 to 2.18) | .002 | 1.57 (1.16 to 2.14) | .004 | 0.08 |
| Allo-isoleucine | Amino acid | 1.52 (1.16 to 1.98) | .002 | 1.47 (1.13 to 1.92) | .005 | 0.08 |
| 2-methylbutyrylcarnitine | Amino acid | 1.53 (1.13 to 2.08) | .006 | 1.47 (1.08 to 2.01) | .01 | 0.19 |
| 3-methyl-2-oxobutyrate | Amino acid | 1.57 (1.13 to 2.17) | .007 | 1.50 (1.08 to 2.09) | .02 | 0.19 |
| 4-androsten-3beta,17beta-diol‖ | Lipid | 1.50 (1.11 to 2.02) | .008 | 1.45 (1.07 to 1.96) | .02 | 0.19 |
Only metabolites with a false discovery rate <0.2 are presented. BMI = body mass index; CI = confidence interval; OR = odds ratio.
Odds ratios were estimated with conditional logistic regression and adjusted for age at blood draw (years), age at menarche (≤12 years, 12–13 years or missing, ≥14 years), age at first live birth and number of live births (nulliparous, age ≤19 years and one or more live births, age 20–29 years with one or two live births, age 20–29 with three or more live births or missing, age 30+ with one or more live births), type of menopause and age at menopause (natural and <45 years, natural and 45–49 years, natural and 50–54 years, natural and ≥55 years, bilateral oophorectomy/surgery, drugs/radiation, hysterectomy or missing), menopausal hormone therapy use at blood draw (never, former, current), history of benign breast disease (no or missing, yes), first-degree family history of breast cancer (no or missing, yes), race/ethnicity (non-Hispanic white or missing, other), education (high school or less, post–high school training besides college, some college or missing, completed college, postgraduate), smoking history (never, former, current), diabetes history (no or missing, yes), and leisure time physical activity (none, less than one hour/week, one hour/week, two hours/week, three hours/week, four or more hours/week, missing).
Adjusted for the variables above plus body mass index (<25 kg/m2, 25.0–<30 kg/m2 or missing, >30 kg/m2).
The Q value is the estimated probability of a false discovery. Results are shown only for those associations with a Q value of less than 0.20.
Full metabolite name is 4-androsten-3beta,17beta-diol disulfate 2.
Table 3.
Multivariable ORs and 95% CIs for estrogen receptor–positive breast cancer when comparing the 90th with the 10th percentile levels of BMI-associated metabolites*
| Multivariable-adjusted† |
Multivariable-adjusted including BMI‡ |
|||||
|---|---|---|---|---|---|---|
| Metabolite | Chemical class | OR (95% CI) | P | OR (95% CI) | P | Q§ |
| 3-methylglutarylcarnitine | Amino acid | 2.52 (1.66 to 3.83) | 1.41 × 10-05 | 2.49 (1.64 to 3.79) | 2.03 × 10-05 | 0.001 |
| 16a-hydroxy DHEA 3-sulfate | Lipid | 2.10 (1.47 to 3.00) | 4.10 × 10-05 | 2.07 (1.45 to 2.97) | 7.19 × 10-05 | 0.002 |
| 4-androsten-3beta,17beta-diol‖ | Lipid | 2.11 (1.44 to 3.09) | 1.18 × 10-04 | 2.08 (1.41 to 3.05) | 1.94 × 10-04 | 0.004 |
| 2-methylbutyrylcarnitine | Amino acid | 2.21 (1.47 to 3.32) | 1.28 × 10-04 | 2.19 (1.45 to 3.32) | 1.97 × 10-04 | 0.004 |
| Gamma-glutamylvaline | Peptide | 2.35 (1.52 to 3.64) | 1.28 × 10-04 | 2.35 (1.50 to 3.68) | 2.05 × 10-04 | 0.004 |
| Allo-isoleucine | Amino acid | 1.91 (1.35 to 2.69) | 2.22 × 10-04 | 1.89 (1.34 to 2.68) | 3.23 × 10-04 | 0.004 |
| Urate | Nucleotide | 1.92 (1.35 to 2.73) | 2.95 × 10-04 | 1.89 (1.32 to 2.71) | 5.03 × 10-04 | 0.005 |
| 3-methyl-2-oxobutyrate | Amino acid | 2.01 (1.36 to 2.99) | 5.21 × 10-04 | 1.98 (1.32 to 2.95) | 8.55 × 10-04 | 0.01 |
| N-acetylalanine | Amino acid | 1.86 (1.29 to 2.70) | 9.53 × 10-04 | 1.83 (1.26 to 2.66) | .002 | 0.01 |
| C-glycosyltryptophan | Amino acid | 1.74 (1.21 to 2.49) | .002 | 1.70 (1.17 to 2.47) | .005 | 0.03 |
| Valine | Amino acid | 1.88 (1.23 to 2.85) | .005 | 1.84 (1.20 to 2.82) | .005 | 0.03 |
| Gamma-glutamylisoleucine | Peptide | 1.78 (1.19 to 2.64) | .006 | 1.75 (1.17 to 2.63) | .006 | 0.04 |
| Alpha-tocopherol | Cofactors¶ | 0.59 (0.40 to 0.89) | .01 | 0.60 (0.40 to 0.90) | .01 | 0.07 |
| N1-methylguanosine | Nucleotide | 1.65 (1.13 to 2.42) | .01 | 1.61 (1.09 to 2.39) | .02 | 0.09 |
| Hexanoylcarnitine | Lipid | 1.65 (1.09 to 2.48) | .02 | 1.60 (1.06 to 2.43) | .03 | 0.12 |
| Hydroxybutyrylcarnitine | Lipid | 1.56 (1.07 to 2.26) | .02 | 1.52 (1.05 to 2.22) | .03 | 0.12 |
| 2-hydroxybutyrate | Amino acid | 1.56 (1.07 to 2.29) | .02 | 1.54 (1.04 to 2.26) | .03 | 0.12 |
| Delta-tocopherol | Cofactors | 1.64 (1.08 to 2.51) | .02 | 1.59 (1.04 to 2.45) | .03 | 0.13 |
| Glutamate | Amino acid | 1.55 (1.06 to 2.27) | .02 | 1.52 (1.03 to 2.24) | .04 | 0.13 |
| Gamma-tocopherol | Cofactors | 1.54 (1.05 to 2.25) | .03 | 1.49 (1.01 to 2.20) | .04 | 0.14 |
| 1-linolenoylglycerophosphocholine | Lipid | 1.43 (0.98 to 2.09 | .07 | 1.48 (1.01 to 2.17) | .05 | 0.15 |
| Gamma-glutamylphenylalanine | Peptide | 1.54 (1.04 to 2.29) | .03 | 1.50 (1.01 to 2.25) | .05 | 0.14 |
| Quinolinate | Cofactors | 1.55 (1.05 to 2.30) | .03 | 1.50 (1.00 to 2.26) | .05 | 0.15 |
Only metabolites with a false discovery rate <0.2 are presented. BMI = body mass index; CI = confidence interval; OR = odds ratio.
Odds ratios were estimated with conditional logistic regression and adjusted for age at blood draw (years), age at menarche (≤12 years, 12–13 years or missing, ≥14 years), age at first live birth and number of live births (nulliparous, age ≤19 years and one or more live births, age 20–29 years with one or two live births, age 20–29 with three or more live births or missing, age 30+ with one or more live births), type of menopause and age at menopause (natural and <45 years, natural and 45–49 years, natural and 50–54 years, natural and ≥55 years, bilateral oophorectomy/surgery, drugs/radiation, hysterectomy or missing), menopausal hormone therapy use at blood draw (never, former, current), history of benign breast disease (no or missing, yes), first-degree family history of breast cancer (no or missing, yes), race/ethnicity (non-Hispanic white or missing, other), education (high school or less, post–high school training besides college, some college or missing, completed college, postgraduate), smoking history (never, former, current), diabetes history (no or missing, yes), and leisure time physical activity (none, less than one hour/week, one hour/week, two hours/week, three hours/week, four or more hours/week, missing).
Adjusted for the variables above plus body mass index (<25 kg/m2, 25.0–<30 kg/m2 or missing, >30 kg/m2).
The Q value is the estimated probability of a false discovery. Results are shown only for those associations with a Q value of less than 0.20.
Full metabolite name is 4-androsten-3beta,17beta-diol disulfate 2.
Cofactors and vitamins.
We also examined associations with ER- breast cancers, but had no statistically significant findings (data not shown). Our top result was for gamma-glutamyltyrosine, which had an odds ratio of 0.32 (95% CI = 0.12 to 0.81) and a P value of .02 that was not statistically significant after adjusting for multiple testing.
To determine whether our primary findings were robust to alternate corrections for multiple testing, we recalculated false discovery rates as if all 617 metabolites had been analyzed. Four of seven metabolites associated with breast cancer risk retained statistical significance, as did 20 of 23 metabolites associated with ER+ breast cancer risk (Supplementary Tables 5 and 6, available online).
Independent Associations With Breast Cancer Risk
Because breast cancer–associated metabolites were intercorrelated (Supplementary Figure 1, available online), with potentially redundant information, we used forward selection to identify a parsimonious set of metabolites independently associated with risk. Two metabolites were independently associated with overall breast cancer risk (Table 4): 16a-hydroxy-DHEA-3-sulfate (OR = 1.65, 95% CI = 1.22 to 2.22) and 3-methylglutarylcarnitine (OR = 1.67, 95% CI = 1.21 to 2.30). Four metabolites were independently associated with estrogen receptor–positive (ER+) breast cancer risk: 16a-hydroxy-DHEA-3-sulfate (OR = 1.84, 95% CI = 1.27 to 2.67), 3-methylglutarylcarnitine (OR = 1.91, 95% CI = 1.23 to 2.96), allo-isoleucine (OR = 1.76, 95% CI = 1.23 to 2.51), and 2-methylbutyrylcarnitine (OR = 1.89, 95% CI = 1.22 to 2.91).
Table 5.
Multivariable ORs and 95% CIs for postmenopausal breast cancer per 5 kg/m2 unit increase in BMI, without and with adjustment for metabolites associated with breast cancer or ER+ breast cancer
| Breast cancer |
ER+ breast cancer |
|||
|---|---|---|---|---|
| Model | OR per 5 kg/m2 (95% CI) | Attenuation of logOR | OR per 5 kg/m2 (95% CI) | Attenuation of logOR |
| Base model* | 1.14 (1.01 to 1.28) | – | 1.07 (0.92 to 1.23) | – |
| Base + 16a-hydroxy DHEA 3-sulfate | 1.09 (0.96 to 1.23) | 33.9% (11.0% to 100.0+%) | 1.00 (0.86 to 1.17) | 94.5% (20.0% to 100.0+%) |
| Base + allo-isoleucine | – | – | 1.01 (0.87 to 1.18) | 79.0% (19.7% to 100.0+%) |
| Base + 2-methylbutyrylcarnitine | – | – | 1.04 (0.89 to 1.20) | 45.8% (4.5% to 100.0+%) |
| Base + 3-methylglutarylcarnitine | 1.10 (0.97 to 1.24) | 30.4% (10.6% to 100.0+%) | 1.01 (0.87 to 1.18) | 78.9% (14.4% to 100.0+%) |
| Base + BCAA-related metabolites† | – | – | 0.95 (0.82 to 1.12) | 100.0+% (41.5% to 100.0+%) |
| Base + combined metabolites‡ | 1.06 (0.93 to 1.20) | 57.6% (21.8% to 100.0+%) | 0.91 (0.78 to 1.07) | 100.0+% (60.5% to 100.0+%) |
Odds ratios were estimated with conditional logistic regression and adjusted for age at blood draw (years), age at menarche (≤12 years, 12–13 years or missing, ≥14 years), age at first live birth and number of live births (nulliparous, age ≤19 years and one or more live births, age 20–29 years with one or two live births, age 20–29 with three or more live births or missing, age 30+ with one or more live births), type of menopause and age at menopause (natural and <45 years, natural and 45–49 years, natural and 50–54 years, natural and ≥55 years, bilateral oophorectomy/surgery, drugs/radiation, hysterectomy or missing), menopausal hormone therapy use at blood draw (never, former, current), history of benign breast disease (no or missing, yes), first-degree family history of breast cancer (no or missing, yes), race/ethnicity (non-Hispanic white or missing, other). For models with adjustment for metabolites, metabolites are included on a continuous basis on the log-scale. BMI = body mass index; CI = confidence interval; ER = estrogen receptor; OR = odds ratio.
BCAA-related metabolites consist of allo-isoleucine, 2-methylbutyrylcarnitine, and 3-methylglutarylcarnitine.
For breast cancer, the combined metabolites consist of 16a-hydroxy DHEA 3-sulfate and 3-methylglutarylcarnitine. For ER+ breast cancer, the combined metabolites consist of all of 16a-hydroxy DHEA 3-sulfate, allo-isoleucine, 2-methylbutyrylcarnitine, and 3-methylglutarylcarnitine. The resulting odds ratio can be interpreted as the direct effect.
Table 4.
BMI-associated metabolites associated with postmenopausal breast cancer and estrogen receptor–positive breast cancer in forward selection models*
| Breast cancer |
ER+ breast cancer |
||||||
|---|---|---|---|---|---|---|---|
| Metabolite† | Chemical class | OR (95% CI)‡ | P | Q§ | OR | P | Q |
| 16a-hydroxy DHEA 3-sulfate | Lipid | 1.65 (1.22 to 2.22) | .001 | 0.07 | 1.84 (1.27 to 2.67) | .001 | 0.09 |
| Allo-isoleucine | Amino acid | – | – | – | 1.76 (1.23 to 2.51) | .002 | 0.09 |
| 2-methylbutyrylcarnitine | Amino acid | – | – | – | 1.89 (1.22 to 2.91) | .004 | 0.09 |
| 3-methylglutarylcarnitine | Amino acid | 1.67 (1.21 to 2.30) | .002 | 0.07 | 1.91 (1.23 to 2.96) | .004 | 0.09 |
Multivariable odds ratios and 95% confidence intervals are provided for a comparison of 90th and 10th percentile levels of BMI-associated metabolites. BMI = body mass index; CI = confidence interval; OR = odds ratio.
Odds ratios were estimated with conditional logistic regression and adjusted for age at blood draw (years), age at menarche (≤12 years, 12–13 years or missing, ≥14 years), age at first live birth and number of live births (nulliparous, age ≤19 years and one or more live births, age 20–29 years with one or two live births, age 20–29 with three or more live births or missing, age 30+ with one or more live births), type of menopause and age at menopause (natural and <45 years, natural and 45–49 years, natural and 50–54 years, natural and ≥55 years, bilateral oophorectomy/surgery, drugs/radiation, hysterectomy or missing), menopausal hormone therapy use at blood draw (never, former, current), history of benign breast disease (no or missing, yes), first-degree family history of breast cancer (no or missing, yes), race/ethnicity (non-Hispanic white or missing, other), education (high school or less, post–high school training besides college, some college or missing, completed college, postgraduate), smoking history (never, former, current), diabetes history (no or missing, yes), leisure time physical activity (none, less than one hour/week, one hour/week, two hours/week, three hours/week, four or more hours/week, missing), and body mass index (<25 kg/m2, 25.0–<30 kg/m2 or missing, >30 kg/m2).
The models include all metabolites statistically significantly associated with breast cancer or ER+ breast cancer (false discovery rate <0.2). Odds ratios and P values are based on mutually adjusted models. For breast cancer, 16a-hydroxy DHEA 3-sulfate and 3-methylglutarylcarnitine were included in the model. For ER+ breast cancer, 16a-Hydroxy DHEA 3-sulfate, Allo-isoleucine, 2-methylbutyrylcarnitine, and 3-methylglutarylcarnitine were included in the model. Forward selection models were used to identify a parsimonious set of metabolites statistically significantly and independently associated with risk. Specifically, models were constructed by adding the metabolite with the lowest P value to the model, retesting all remaining metabolites for statistical significance, and then adding the metabolite with the lowest P value from this new set. We did this until the false discovery rate threshold of 0.20 was reached.
The Q value is the estimated probability of a false discovery.
Mediation of Obesity and Breast Cancer Associations
In a model without metabolites and that excluded potential mediators of BMI-cancer associations (eg, diabetes), the odds ratio per 5 kg/m2 increase in BMI was 1.14 (95% CI = 1.01 to 1.28). After adding 16a-hydroxy-DHEA-3-sulfate and 3-methylglutarylcarnitine to the model, the odds ratio per 5 kg/m2 BMI dropped to 1.06 (95% CI = 0.93 to 1.20). The effect of BMI was attenuated by 57.6% (95% CI = 21.8% to 100.0+%) in this model, which can be attributed to BMI’s indirect effect as mediated by the two metabolites (OR = 1.08, 95% CI = 1.05 to 1.16). Conversely, adding BMI to a model with metabolites had little effect on metabolite–breast cancer associations (Supplementary Table 7, available online).
For ER+ breast cancer, in a model without metabolites, the odds ratio per 5 kg/m2 BMI was 1.07 (95% CI = 0.92 to 1.23), which, contrary to expectations, was weaker than the association for overall breast cancer. Adjusting for allo-isoleucine, 2-methylbutyrylcarnitine, and 3-methylglutarylcarnitine together—grouped because of their common role in branched-chain amino acid (BCAA) catabolism (Figure 2)—decreased the odds ratio substantially (OR = 0.95) and attenuated the logOR by 100.0+% (95% CI = 41.5% to 100.0+%). Adding 16a-hydroxy-DHEA-3-sulfate decreased the odds ratio further (OR = 0.91, 95% CI = 0.78 to 1.07), and the final indirect effect was OR = 1.18 (95% CI = 1.08 to 1.27). These estimates for ER+ cancer, however, should be interpreted with caution owing to the weaker-than-expected association with BMI.
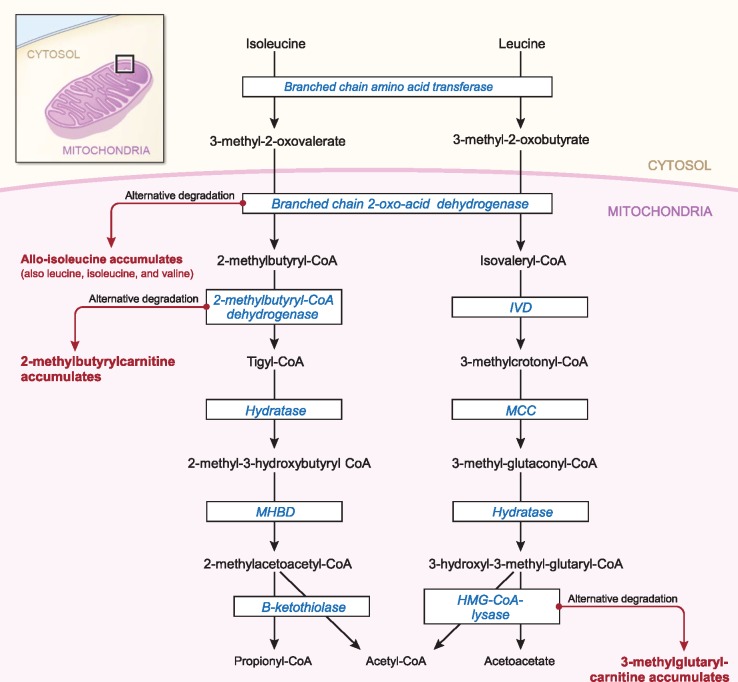
Figure 2.
Pathways of isoleucine and leucine catabolism. Isoleucine and leucine catabolism is shown by arrows, with the enzymes catalyzing the reactions shown inside boxes. Straight arrows indicate normal catabolism, whereas side (bent) arrows indicate the byproducts that accumulate when enzyme activity is insufficient to convert available substrates. Each metabolite at the end of side (bent) arrows was associated with breast cancer risk (either overall or ER+). Figure is adapted from Knerr, Vockley, and Gibson (28). IVD = Isovaleryl-CoA dehydrogenase; MCC = Methylcrotonyl-CoA carboxylase; MHBD = 2-methyl-3-hydroxybutyryl-CoA dehydrogenase.
Other Circulating Biomarkers
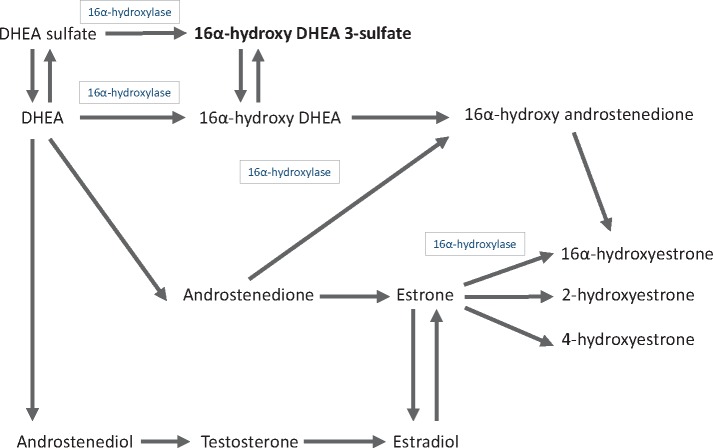
16a-hydroxy-DHEA-3-sulfate is biochemically related to DHEA, estrone, and estradiol (Figure 3), steroid hormones previously implicated in breast carcinogenesis (25–27). When we added DHEA-sulfate to models, the association of 16a-hydroxy-DHEA-3-sulfate with breast cancer risk attenuated by 8.7% (OR = 1.65, 95% CI = 1.22 to 2.22, before and 1.58, 95% CI = 1.06 to 2.33, after) (see Supplementary Table 8, available online). In contrast, adding 16a-hydroxy-DHEA-3-sulfate to the model attenuated the association of DHEA-sulfate with breast cancer risk by 80.0% (OR = 1.47, 95% CI = 1.08 to 2.01, before and 1.08, 95% CI = 0.71 to 1.62, after). Results for ER+ breast cancer were more equivocal, however, with neither metabolite predominating when modeled together (Supplementary Table 8, available online), possibly reflecting their collinearity (Pearson correlation = 0.68). Adjusting for estrone-sulfate had no effect on the 16a-hydroxy-DHEA-3-sulfate association (OR changed by less than 0.02), and estradiol had no correlation with 16a-hydroxy-DHEA-3-sulfate (r = –0.04).
Figure 3.
Pathways of steroid hormone metabolism. DHEA-sulfate metabolism is shown by arrows, with 16a-hydoxylase activity shown inside boxes. 16a-Hydroxy-DHEA 3-sulfate, the steroid metabolite we found to be associated with breast cancer risk, is shown in boldface.
The remaining breast cancer–associated metabolites are involved in BCAA metabolism, which recent studies implicate in insulin resistance (7,29,30). Study participants lacked fasting insulin measures, and so, on an exploratory basis, we adjusted instead for levels of the insulin resistance–related metabolites of 2-hydroxybutyrate, urate, and mannose. In comparison with fasting insulin, which has a correlation of –0.5 with insulin sensitivity as measured by the gold standard euglycemic clamp method, 2-hydroxybutyrate and urate have more modest correlations of approximately –0.3 (29,30). Mannose is a glucose epimer that outperforms fasting glucose for prediction of future diabetes (31–34) and is insensitive to recent food intake (35). When adjusting associations for these metabolites, the odds ratios for BCAA-related metabolites attenuated by at most 11.8% (Supplementary Table 9, available online). In contrast, after adjusting for BCAA-related metabolites, the associations of insulin resistance–related metabolites with breast cancer risk attenuated by 17.7% to 73.9% (Supplementary Table 10, available online).
Discussion
In this metabolomics analysis, we found that 67 of 617 identified metabolites were associated with BMI and that two of these metabolites—16a-hydroxy DHEA 3-sulfate and 3-methylglutarylycarnitine—were strongly associated with breast cancer risk. These metabolites and allo-isoleucine and 2-methylbutyrylcarnitine were also associated with ER+ breast cancer. The addition of these metabolites to models caused the BMI–breast cancer association to attenuate. Taken together, our findings point toward metabolic pathways that may contribute to breast carcinogenesis and that may underlie the association of BMI with increased risk of postmenopausal breast cancer.
Biologically, the four breast cancer–associated metabolites cluster into two distinct pathways, namely steroid hormone metabolism and BCAA metabolism. With respect to steroid hormones, our results specifically implicate changes in the metabolism of DHEA, a precursor to androstenedione, testosterone, and, ultimately, estrone and estradiol (Figure 3). High DHEA levels are associated with increased postmenopausal breast cancer risk (25–27), putatively due to its role as an estradiol precursor and/or androgen receptor agonist.
Our results further suggest that metabolism of DHEA into 16a-hydroxy DHEA 3-sulfate, a rarely studied DHEA metabolite formed after 16α-hydroxylation of DHEA, may independently contribute to breast cancer risk. 16a-hydroxy DHEA 3-sulfate was positively associated with breast cancer risk independent of levels of DHEA-sulfate and estrone-sulfate, and this metabolite was uncorrelated with estradiol levels. In laboratory studies, 16a-hydroxy DHEA 3-sulfate binds and activates the β estrogen receptor (36) and, perhaps more importantly, is metabolized into 16a-hydroxyestrone and other 16-hydroxylation pathway estrogen metabolites (37). Increased formation of these metabolites, relative to 2-hydroxylation pathway metabolites, has been consistently associated with increased risk of postmenopausal breast cancer (19,23,38,39). We caution, however, that further studies are needed to differentiate findings for 16a-hydroxy DHEA 3-sulfate from those of other intercorrelated sex steroid hormones, especially DHEA.
The other metabolic pathway that our results suggest may be important is BCAA metabolism. BCAAs comprise 33% of amino acids in the body (40) and are catabolized according to well-regulated and well-characterized enzymatic processes (41). In some instances, however, catabolism of BCAAs can become dysregulated, leading to higher levels of BCAAs and/or metabolic byproducts indicative of alternative degradation pathways. Elevated circulating concentrations of BCAAs, for example, are a known sequala of excess body weight (6–9,42,43), and levels of isoleucine and leucine, two of the three BCAAs, have been prospectively associated with pancreatic cancer risk (44).
Strikingly, our study found that three metabolites known to indicate flux through alternative BCAA degradation pathways (41,45–48) were positively and independently associated with breast cancer risk (Figure 2). Specifically, elevated levels of allo-isoleucine, 2-methylbutyrylcarnitine, and 3-methylglutarylcarnitine are formed as byproducts when BCAA metabolites are not fully degraded by the respective enzymes of branched-chain 2-oxo-acid dehydrogenase (45,46), 2-methylbutyryl-coenzyme A dehydrogenase (41,47), and 3-hydroxy-3-methylglutaryl-coenzyme A lyase (48). To our knowledge, no prior studies have examined these BCAA-related metabolites in relation to breast cancer risk.
Biologically, degradation of BCAAs through alternative pathways has been postulated to promote tumor growth by providing nutritive building blocks needed for mitosis (49,50), and by anaplerotically replenishing the TCA cycle (50), thus helping cancer cells to meet energetic demands. BCAA byproducts also may trigger cell signaling cascades related to unchecked growth of cancer, including PI3K/AKT/mTOR (7,51,52). In several types of tumors, including non–small cell lung carcinomas (53), myeloid leukemias (54), and glioblastomas (55), dysregulated catabolism of BCAAs is a key metabolic feature that underlies their unchecked growth.
Historically, obesity’s link with postmenopausal breast cancer risk has been attributed to its role in increasing levels of steroid hormones, insulin resistance, and inflammation (4,5,56–61). Our finding for 16a-hydroxy DHEA 3-sulfate extends upon the steroid hormone hypothesis by suggesting that a high BMI, besides increasing levels of estradiol and/or DHEA, may shift hormonal metabolism toward 16a-hydroxylation and separately influence breast cancer risk through this pathway. Our finding for BCAA-related metabolism suggests a new hypothesis, namely that higher levels of circulating BCAAs among heavier individuals may overload BCAA catabolic pathways, leading to metabolic byproducts that nutritively enhance breast cancer cell growth. Alternately, elevated levels of BCAAs may be a marker of insulin resistance (31,32,62), which has been previously associated with breast cancer risk (55). Although our exploratory analyses suggest that these BCAA-related associations are independent from insulin resistance, further studies with measures of fasting insulin are needed to more definitely ascertain this.
Our study identified numerous metabolites to be positively or inversely associated with BMI that were not related to breast cancer risk, including gamma-tocopherol, methyl glucopyranoside, threonate, and catechol sulfate—metabolites reflective of intake of fried foods, fruits, supplements, and coffee, respectively (63–66). A high BMI was also associated with presence of hydrochlorothiazide, a diuretic used to lower blood pressure. Like prior studies (6,8,42,67), we found that disproportionate numbers of amino acids and lipids were associated with BMI, and the magnitudes of association observed were highly similar to those of our prior report (Supplementary Figure 2, available online). While most BMI-associated metabolites were not breast cancer associated, several may yet prove relevant for other disease outcomes, such as the association of mannose with diabetes risk (34).
Our study has several strengths, including many cases and controls, detailed information on breast cancer risk factors, hormone receptor status data, prediagnostically collected serum, many identified metabolites, and a highly reliable metabolomics platform. Additionally, our focus on BMI-associated metabolites allowed us to thoughtfully interrogate the biology underlying the association of BMI with breast cancer, including a detailed evaluation of whether our candidate mechanistic mediators were distinct from those implicated by prevailing hypotheses (eg, estrone/estradiol).
A key limitation is that we cannot fully rule out confounding by unmeasured lifestyle or biological factors. We did, however, control for many known breast cancer risk factors. Other limitations include that participants were not required to fast and that an individual’s metabolite levels may vary over time; these factors can affect metabolite levels (17,68,69) and cause attenuation of odds ratios. Of the seven breast cancer–associated metabolites, we previously showed that levels of three of them (3-methyl-2-oxobutyrate, 2-methylbutyrylcarnitine, 4-androsten-3beta-17beta-diol disulfate 2) were unrelated to fasting status (P > .05) and were stable over a study year (one-year ICCs = 0.50–0.81) (17). Additionally, our study consisted primarily of non-Hispanic white women; thus generalizability of results to other women is unknown. Finally, our findings need to be validated through replication using other metabolomics platforms, with different analytical approaches, and in other cohorts or a consortia of cohorts, such as the Consortium of Metabolomics Studies (70).
In summary, our metabolomics analysis suggests that steroid hormone and BCAA metabolism may be important pathways related to breast cancer risk, and that these pathways may help explain why excess body weight increases postmenopausal breast cancer risk. Future studies should aim to replicate these findings with different platforms, analytical approaches, and cohorts and, if findings replicate, determine which interventions—pharmacologic (71,72) and/or behavioral—might modulate these pathways and reduce breast cancer risk.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and by the Breast Cancer Research Stamp Fund, awarded through competitive review.
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors disclose no potential conflicts of interest related to this study.
Supplementary Material
References
- 1. Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lauby-Secretan B, Scoccianti C, Loomis D, et al. . Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold M, Leitzmann M, Freisling H, et al. . Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016;41:8–15.http://dx.doi.org/10.1016/j.canep.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 4. Calle EE, Kaaks R.. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591.http://dx.doi.org/10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- 5. Renehan AG, Zwahlen M, Egger M.. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498.http://dx.doi.org/10.1038/nrc3967 [DOI] [PubMed] [Google Scholar]
- 6. Moore SC, Matthews CE, Sampson JN, et al. . Human metabolic correlates of body mass index. Metabolomics. 2014;10(2):259–269.http://dx.doi.org/10.1007/s11306-013-0574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newgard CB, An J, Bain JR, et al. . A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326.http://dx.doi.org/10.1016/j.cmet.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho JE, Larson MG, Ghorbani A, et al. . Metabolomic profiles of body mass index in the Framingham Heart Study reveal distinct cardiometabolic phenotypes. PLoS One. 2016;11(2):e0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wurtz P, Wang Q, Kangas AJ, et al. . Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park S, Sadanala KC, Kim EK.. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol Cell. 2015;38(7):587–596.http://dx.doi.org/10.14348/molcells.2015.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin AC, Fasshauer M, Filatova N, et al. . Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20(5):898–909.http://dx.doi.org/10.1016/j.cmet.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanahan D, Weinberg RA.. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674.http://dx.doi.org/10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 13. Vander Heiden MG, Cantley LC, Thompson CB.. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033.http://dx.doi.org/10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prorok PC, Andriole GL, Bresalier RS, et al. . Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 suppl):273S–309S. [DOI] [PubMed] [Google Scholar]
- 15. Andriole GL, Crawford ED, Grubb RL 3rd, et al. . Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian cancer screening trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132.http://dx.doi.org/10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans AM, Bridgewater BR, Liu Q, et al. . High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4(2):132. [Google Scholar]
- 17. Sampson JN, Boca SM, Shu XO, et al. . Metabolomics in epidemiology: Sources of variability in metabolite measurements and implications. Cancer Epidemiol Biomarkers Prev. 2013;22(4):631–640.http://dx.doi.org/10.1158/1055-9965.EPI-12-1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munsell MF, Sprague BL, Berry DA, et al. . Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136.http://dx.doi.org/10.1093/epirev/mxt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore SC, Matthews CE, Ou Shu X, et al. . Endogenous estrogens, estrogen metabolites, and breast cancer risk in postmenopausal Chinese women. J Natl Cancer Inst. 2016;108(10):djw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanderweele TJ, Vansteelandt S.. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348.http://dx.doi.org/10.1093/aje/kwq332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benjamini Y HY. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc B. 1995;57:289–300. [Google Scholar]
- 22. Ganna A, Lee D, Ingelsson E, et al. . Rediscovery rate estimation for assessing the validation of significant findings in high-throughput studies. Brief Bioinform. 2015;16(4):563–575.http://dx.doi.org/10.1093/bib/bbu033 [DOI] [PubMed] [Google Scholar]
- 23. Fuhrman BJ, Schairer C, Gail MH, et al. . Estrogen metabolism and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2012;104(4):326–339.http://dx.doi.org/10.1093/jnci/djr531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones ME, Schoemaker MJ, Rae M, et al. . Reproducibility of estradiol and testosterone levels in postmenopausal women over 5 years: Results from the breakthrough generations study. Am J Epidemiol. 2014;179(9):1128–1133.http://dx.doi.org/10.1093/aje/kwu027 [DOI] [PubMed] [Google Scholar]
- 25. Key T, Appleby P, Barnes I, et al. . Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616.http://dx.doi.org/10.1093/jnci/94.8.606 [DOI] [PubMed] [Google Scholar]
- 26. Kaaks R, Rinaldi S, Key TJ, et al. . Postmenopausal serum androgens, oestrogens and breast cancer risk: The European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082.http://dx.doi.org/10.1677/erc.1.01038 [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Tworoger SS, Eliassen AH, et al. . Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 2013;137(3):883–892.http://dx.doi.org/10.1007/s10549-012-2391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knerr J IV, Gibson KM. Disorders of Leucine, Isoleucine, and Valine Metabolism. 2014. [Google Scholar]
- 29. Cobb J, Gall W, Adam KP, et al. . A novel fasting blood test for insulin resistance and prediabetes. J Diabetes Sci Technol. 2013;7(1):100–110.http://dx.doi.org/10.1177/193229681300700112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gall WE, Beebe K, Lawton KA, et al. . alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Menni C, Fauman E, Erte I, et al. . Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276.http://dx.doi.org/10.2337/db13-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Floegel A, Stefan N, Yu Z, et al. . Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–648.http://dx.doi.org/10.2337/db12-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S, Zhang C, Kilicarslan M, et al. . Integrated network analysis reveals an association between plasma mannose levels and insulin resistance. Cell Metab. 2016;24(1):172–184.http://dx.doi.org/10.1016/j.cmet.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu D, Moore SC, Matthews CE, et al. . Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sone H, Shimano H, Ebinuma H, et al. . Physiological changes in circulating mannose levels in normal, glucose-intolerant, and diabetic subjects. Metabolism. 2003;52(8):1019–1027.http://dx.doi.org/10.1016/S0026-0495(03)00153-7 [DOI] [PubMed] [Google Scholar]
- 36. Miller KK, Al-Rayyan N, Ivanova MM, et al. . DHEA metabolites activate estrogen receptors alpha and beta. Steroids. 2013;78(1):15–25.http://dx.doi.org/10.1016/j.steroids.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hampl R, Starka L.. Minireview: 16alpha-hydroxylated metabolites of dehydroepiandrosterone and their biological significance. Endocr Regul. 2000;34(3):161–163. [PubMed] [Google Scholar]
- 38. Sampson JN, Falk RT, Schairer C, et al. . Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res. 2017;77(4):918–925.http://dx.doi.org/10.1158/0008-5472.CAN-16-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziegler RG, Fuhrman BJ, Moore SC, et al. . Epidemiologic studies of estrogen metabolism and breast cancer. Steroids. 2015;99(pt A):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rajendram R, Preedy VR, Patel VB.. Branched Chain Amino Acids in Clinical Nutrition. New York: Humana Press; 2015. [Google Scholar]
- 41. Korman SH. Inborn errors of isoleucine degradation: A review. Mol Genet Metab. 2006;89(4):289–299.http://dx.doi.org/10.1016/j.ymgme.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 42. Rauschert S, Uhl O, Koletzko B, et al. . Metabolomic biomarkers for obesity in humans: A short review. Ann Nutr Metab. 2014;64(3-4):314–324. [DOI] [PubMed] [Google Scholar]
- 43. Felig P, Marliss E, Cahill GF Jr.. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–816.http://dx.doi.org/10.1056/NEJM196910092811503 [DOI] [PubMed] [Google Scholar]
- 44. Mayers JR, Wu C, Clish CB, et al. . Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–1198.http://dx.doi.org/10.1038/nm.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mamer OA, Reimer ML.. On the mechanisms of the formation of L-alloisoleucine and the 2-hydroxy-3-methylvaleric acid stereoisomers from L-isoleucine in maple syrup urine disease patients and in normal humans. J Biol Chem. 1992;267(31):22141–22147. [PubMed] [Google Scholar]
- 46. Olson KC, Chen G, Xu Y, et al. . Alloisoleucine differentiates the branched-chain aminoacidemia of Zucker and dietary obese rats. Obesity (Silver Spring). 2014;22(5):1212–1215.http://dx.doi.org/10.1002/oby.20691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Calcar SC, Baker MW, Williams P, et al. . Prevalence and mutation analysis of short/branched chain acyl-CoA dehydrogenase deficiency (SBCADD) detected on newborn screening in Wisconsin. Mol Genet Metab. 2013;110(1-2):111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roe CR, Millington DS, Maltby DA.. Identification of 3-methylglutarylcarnitine. A new diagnostic metabolite of 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency. J Clin Invest. 1986;77(4):1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hosios AM, Hecht VC, Danai LV, et al. . Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell. 2016;36(5):540–549.http://dx.doi.org/10.1016/j.devcel.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeBerardinis RJ, Chandel NS.. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cota D, Proulx K, Smith KA, et al. . Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930.http://dx.doi.org/10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- 52. Grabiner BC, Nardi V, Birsoy K, et al. . A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov. 2014;4(5):554–563.http://dx.doi.org/10.1158/2159-8290.CD-13-0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mayers JR, Torrence ME, Danai LV, et al. . Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353(6304):1161–1165.http://dx.doi.org/10.1126/science.aaf5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hattori A, Tsunoda M, Konuma T, et al. . Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 2017;545(7655):500–504.http://dx.doi.org/10.1038/nature22314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tonjes M, Barbus S, Park YJ, et al. . BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med. 2013;19(7):901–908.http://dx.doi.org/10.1038/nm.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gunter MJ, Hoover DR, Yu H, et al. . Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101(1):48–60.http://dx.doi.org/10.1093/jnci/djn415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gunter MJ, Wang T, Cushman M, et al. . Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst. 2015;107(9):djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Key TJ, Appleby PN, Reeves GK, et al. . Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95(16):1218–1226.http://dx.doi.org/10.1093/jnci/djg022 [DOI] [PubMed] [Google Scholar]
- 59. Key TJ, Appleby PN, Reeves GK, et al. . Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: Reanalysis of eighteen prospective studies. Steroids. 2015;99(pt A):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6(2):164–179.http://dx.doi.org/10.1007/BF00052777 [DOI] [PubMed] [Google Scholar]
- 61. Autier P, Koechlin A, Boniol M, et al. . Serum insulin and C-peptide concentration and breast cancer: A meta-analysis. Cancer Causes Control. 2013;24(5):873–883.http://dx.doi.org/10.1007/s10552-013-0164-6 [DOI] [PubMed] [Google Scholar]
- 62. Wang TJ, Larson MG, Vasan RS, et al. . Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453.http://dx.doi.org/10.1038/nm.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guertin KA, Moore SC, Sampson JN, et al. . Metabolomics in nutritional epidemiology: Identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100(1):208–217.http://dx.doi.org/10.3945/ajcn.113.078758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Playdon MC, Sampson JN, Cross AJ, et al. . Comparing metabolite profiles of habitual diet in serum and urine. Am J Clin Nutr. 2016;104(3):776–789.http://dx.doi.org/10.3945/ajcn.116.135301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Playdon MC, Ziegler RG, Sampson JN, et al. . Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr. 2017;106(2):637–649.http://dx.doi.org/10.3945/ajcn.116.150912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guertin KA, Loftfield E, Boca SM, et al. . Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am J Clin Nutr. 2015;101(5):1000–1011.http://dx.doi.org/10.3945/ajcn.114.096099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murphy RA, Moore SC, Playdon M, et al. . Metabolites associated with lean mass and adiposity in older black men. J Gerontol A Biol Sci Med Sci. 2017;72(10):1352–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Townsend MK, Bao Y, Poole EM, et al. . Impact of pre-analytic blood sample collection factors on metabolomics. Cancer Epidemiol Biomarkers Prev. 2016;25(5):823–829.http://dx.doi.org/10.1158/1055-9965.EPI-15-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fages A, Ferrari P, Monni S, et al. . Investigating sources of variability in metabolomic data in the EPIC study: The Principal Component Partial R-square (PC-PR2) method. Metabolomics. 2014;10(6):1074–1083.http://dx.doi.org/10.1007/s11306-014-0647-9 [Google Scholar]
- 70.COnsortium of METabolomics Studies. https://epi.grants.cancer.gov/comets/. Accessed November 3, 2017.
- 71. Xiao C, Giacca A, Lewis GF.. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60(3):918–924.http://dx.doi.org/10.2337/db10-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tso SC, Qi X, Gui WJ, et al. . Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain alpha-ketoacid dehydrogenase kinase. Proc Natl Acad Sci U S A. 2013;110(24):9728–9733.http://dx.doi.org/10.1073/pnas.1303220110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.