Abstract
Background
The main goal of cervical screening programs is to detect and treat precancer before cancer develops. Human papillomavirus (HPV) testing is more sensitive than cytology for detecting precancer. However, reports of rare HPV-negative, cytology-positive cancers are motivating continued use of both tests (cotesting) despite increased testing costs.
Methods
We quantified the detection of cervical precancer and cancer by cotesting compared with HPV testing alone at Kaiser Permanente Northern California (KPNC), where 1 208 710 women age 30 years and older have undergone triennial cervical cotesting since 2003. Screening histories preceding cervical cancers (n = 623) and precancers (n = 5369) were examined to assess the relative contribution of the cytology and HPV test components in identifying cases. The performances of HPV testing and cytology were compared using contingency table methods, general estimating equation models, and nonparametric statistics; all statistical tests were two-sided.
Results
HPV testing identified more women subsequently diagnosed with cancer (P < .001) and precancer (P < .001) than cytology. HPV testing was statistically significantly more likely to be positive for cancer at any time point (P < .001), except within 12 months (P = .10). HPV-negative/cytology-positive results preceded only small fractions of cases of precancer (3.5%) and cancer (5.9%); these cancers were more likely to be regional or distant stage with squamous histopathology than other cases. Given the rarity of cancers among screened women, the contribution of cytology to screening translated to earlier detection of at most five cases per million women per year. Two-thirds (67.9%) of women found to have cancer during 10 years of follow-up at KPNC were detected by the first cotest performed.
Conclusions
The added sensitivity of cotesting vs HPV alone for detection of treatable cancer affected extremely few women.
Cervical cancer screening guidelines have changed profoundly over the last 10 to 15 years, following introduction of testing for the dozen high-risk human papillomavirus (HPV) types that cause virtually all cervical cancer and its precursors (“precancers”) (1). Despite increased etiologic understanding and the introduction of preventive HPV vaccines, screening will remain important and comprise many millions of tests annually for decades to come. Unfortunately, improved screening methods have introduced some confusion, even controversy.
An HPV test, in which a cervicovaginal specimen is tested for the presence of the nucleic acids of carcinogenic types of HPV, has superior sensitivity compared with cervical cytology (a microscopic examination of exfoliated cells) for detection of precancers (2–7). Thus, if a single screening method were chosen to complement HPV vaccination, primary HPV testing likely would gradually supplant cytology (8). In the United States, interim guidance issued by a committee of experts from several clinical societies recommended primary HPV testing every three years, the same as cytology (9). Alternatively, current guidelines recommend performing both HPV testing and cytology (“cotesting”), but, in recognition of the additional reassurance provided by this approach compared with cytology alone, the screening interval is extended to every five years (10). Draft guidelines from the US Preventive Services Task Force recently recommended either primary HPV testing every five years or cytology every three years for women age 30 to 64 years, and did not recommend cotesting.
Internationally, primary HPV testing at extended intervals (eg, every five or more years) has received more consideration than cotesting (11–14). In the United States, however, some screening authorities recommend cotesting at three-year intervals, even more often than the national guidelines, in order to match or exceed the safety achievable at considerable effort by yearly high-quality cytology (11). Also in advocating cotesting, recent well-publicized reports have elicited concern about the safety of HPV testing alone, suggesting in particular that cytology can detect already-invasive cancers missed by HPV tests (15–17). The existence of these cases, though very rare, is very influential in shaping screening policy.
Any confusion in an important public health practice like cervical screening is unhelpful and must be addressed. The accumulated evidence supports inclusion of HPV testing in screening; thus, the main choice moving forward is between cotesting and primary HPV testing alone. Realistic performance data are needed that quantify the additional benefit of the cytology component of cotesting as the costs of intensive screening of all women using two screening tests are substantial.
There is some urgency to deciding how to proceed. Far-reaching decisions regarding cervical screening policy are being made in the coming year or two in the United States and internationally. As mentioned, the US Preventive Services Task Force is re-evaluating cervical screening methods and strategies (18). Independently, planning for the next round of multi-organizational clinical guidelines is underway (19).
The most extensive source of data in the United States regarding cervical cotesting comes from Kaiser Permanente Northern California (KPNC). In January 2003, just prior to US Food and Drug Administration approval of HPV and cytology cotesting in mid-2003 (20) and interim guidelines (21) in 2004, KPNC, a large integrated health care organization, introduced three-year cotesting in women age 30 years and older. KPNC has now screened more than a million women by cotesting. To our knowledge, this remains the most extensive experience of HPV testing incorporated into routine screening in the world.
To inform guidelines development and support dissemination of an optimal strategy, we have analyzed cotesting data from the KPNC program. Specifically, we wished to quantify the relative contributions of the cytology and HPV test components of cotesting in the detection of cervical precancer and cancer to help guidelines groups judge whether cotesting, and the attendant cost of dual assays, should be recommended.
Methods
Population
The KPNC population evaluated here has been described previously (22). From January 1, 2003, to December 31, 2015, 1 208 710 women age 30 years or older underwent cotesting. For each woman, we considered the first available cotest in this study period as the beginning of follow-up. Cervical histopathology outcomes were also collected through December 31, 2015. The KPNC institutional review board (IRB) approved use of the data, and the National Institutes of Health Office of Human Subjects Research and Albert Einstein College of Medicine IRB deemed this study exempt from review.
Screening and Clinical Management
Per KPNC clinical guidelines, women who cotested HPV negative and cytology negative (HPV-/cytology-) were offered screening again in three years (in contrast to five years in the national guidelines). Positive cotest results were managed according to internal Kaiser guidelines (23), which were broadly concordant with national standards at the time (21,24–26). Women with definite cytologic abnormalities were referred to colposcopy (25–27). Accelerated retesting at one year was performed for those women with HPV-positive/cytology-negative (HPV+/cytology-) or HPV-negative/cytology-equivocal (HPV-/ASC-US) results; the details evolved over time as previously described (22).
Statistical Analyses
Using the medical records, 907 cervical cancers were identified. We excluded 55 cases (6.1%) diagnosed in women younger than age 30 years because cotesting was not routinely performed in this age group, and 229 cases (25.2%) because they did not have a cotesting result prior to diagnosis and thereby could not answer the questions that we were asking. As result of these exclusions, there were 623 cervical cancers with at least one cotest up to the date of diagnosis (“prediagnostic” cotests) included in this analysis. Cancers were categorized as squamous cell carcinoma (SCC), adenocarcinoma (ADC; including adenosquamous carcinoma), microinvasive cancer regardless of histology type, and cancers of other or uncertain histology (other cancers). Stage data were available for 434 cases (69.7%). Microinvasive cancers were considered localized if their stage data were missing or unknown (n = 21). Thus, there were 455 (73.0%) with stage data: 333 (73.2%) localized, 93 (20.4%) regional, and 29 (6.4%) distant, a distribution that is more localized than the US distribution (https://seer.cancer.gov/statfacts/html/cervix.html).
Of the 623 cancers included in this analysis, there were 351 (56.3%) squamous cell carcinomas, 212 (34.0%) adenocarcinomas (including 19 adenosquamous carcinomas, three adenocarcinomas that favored endocervical [vs endometrial] tissue, and nine cases in which there was uncertainty as to whether the cancer tissue was endocervical or endometrial), 41 (6.6%) microinvasive cancers, and 19 (3.0%) other cancers. This represents a larger proportion of adenocarcinoma than is typically seen in the United States (28). There was no difference in the distribution of diagnostic categories between cancers included in and excluded from these analyses (P = .65).
We included for comparison all 5369 precancers, including 4929 cervical intraepithelial neoplasia grade 3 (CIN3) and 440 adenocarcinoma in situ (AIS), diagnosed in women age 30 years and older during this same period when the cancers were diagnosed. We compared HPV, cytology, and cotesting performance of all prediagnostic cotests for cancer, overall and by cancer diagnostic category (ie, SCC, ADC, microinvasive, or other cancers) and stage. The positivity of a screening test prior to diagnosis can be thought of as a kind of prognostic sensitivity. We performed a similar analysis for precancer diagnoses, overall and by diagnostic category (ie, CIN3 or AIS).
We repeated these analyses stratified for cotests obtained less than 12 vs 12 or more months prior to diagnosis; the examination of cotests taken less than 12 months prior to cancer diagnosis was undertaken in response to recent reports regarding suboptimal HPV test performance in detecting already prevalent invasive cancers (15–17). We compared HPV and cytology cotesting results by time intervals for all cancers and for SCC and ADC separately, and for all precancers and for CIN3 and AIS separately. We also compared the cotesting results by whether the last round of cotesting prior to diagnosis was the first, second, or third (or subsequent) cotest performed at KPNC prior to diagnosis.
Women could contribute multiple cotests during any period of time. Raw numbers and percentages are presented. General estimating equation (GEE) P values were calculated using Z scores from GEE parameter estimates and empirical standard error estimates. Results using GEE were largely similar to raw values except for cells with small numbers of women (data not shown); when cells contained zero counts, McNemar P values were used. Trend tests for HPV+, Cyto+, and HPV+/Cyto- vs HPV-/Cyto+ by increasing cancer stage were calculated.
In the Supplementary Tables (available online), the P values were derived from the exact Pearson chi-square test for the frequencies (stage and race) and Kruskal-Wallis for the summary statistics (number of cotests, time from cotests, and ages). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Characteristics of Cases
Characteristics of the 623 women diagnosed with cancer are shown in Supplementary Table 1 (available online) and 5369 women diagnosed with precancer are shown in Supplementary Table 2 (available online). Among those with cancer, women diagnosed with other cancers were older (median age = 57 years) and those diagnosed with microinvasive cancer were younger (median age = 40 years) than those diagnosed with SCC (median age = 47 years) and ADC (median age = 47 years; P = .001, Kruskal-Wallis). By comparison, women diagnosed with precancer had a median age of 38 years (of note, this analysis excluded women younger than 30 years of age, elevating the average age of precancer).
Cotesting History Prior to Cancer Diagnoses, Evaluating the Contribution of Cytology vs HPV Testing
Paired HPV and cytology testing results (n = 1137) are shown for all cotests preceding the diagnosis of cancer in 623 women (Table 1). Overall, prediagnostic HPV testing (76.7%) was more likely to be positive (ie, was more clinically sensitive) than cytology (59.1%, P < .001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; 5.9% of the cotests were positive by cytology alone (HPV negative). Stratified on histopathology, the differences in positivity between HPV and cytology antecedent to SCC were less pronounced (75.0% vs 69.6%, respectively, P = .03) than for ADC (79.0% vs 45.4%, respectively, P < .001).
Table 1.
Comparison of all high-risk human papillomavirus DNA and cytology cotest results prior to cancer diagnosis, overall and by specific histology*
| Cancer | Total No. (%) | HPV+No. (%) | Cyto+No. (%) | Any+No. (%) | HPV+Cyto+No. (%) | HPV+Cyto-No. (%) | HPV-Cyto+No. (%) | HPV-Cyto-No. (%) | P† |
|---|---|---|---|---|---|---|---|---|---|
| All cancers | 1137 (100.0) | 872 (76.7) | 672 (59.1) | 939 (82.6) | 605 (53.2) | 267 (23.5) | 67 (5.9) | 198 (17.4) | <.001 |
| SCC | 576 (100.0) | 432 (75.0) | 401 (69.6) | 478 (83.0) | 355 (61.6) | 77 (13.4) | 46 (8.0) | 98 (17.0) | .03 |
| ADC | 443 (100.0) | 350 (79.0) | 201 (45.4) | 361 (81.5) | 190 (42.9) | 160 (36.1) | 11 (2.5) | 82 (18.5) | <.001 |
| Micro | 73 (100.0) | 64 (87.7) | 54 (74.0) | 69 (94.5) | 49 (67.1) | 15 (20.5) | 5 (6.8) | 4 (5.5) | .08 |
| Other | 45 (100.0) | 26 (57.8) | 16 (35.6) | 31 (68.9) | 11 (24.4) | 15 (33.3) | 5 (11.1) | 14 (31.1) | .10 |
Some women contributed more than one cotesting result prior to diagnosis. “+” indicates a positive result, and “-” indicates a negative result; “Any+” means that HPV and/or Pap were positive. ADC = adenocarcinoma; Cyto = cytology; HPV = human papillomavirus; Micro = microinvasive; Other = other cancers; SCC = squamous cell carcinoma.
Two-sided P values comparing HPV tests with cytology were based on Z scores calculated from general estimating equation parameter estimates and empirical standard error estimates.
Paired HPV and cytology testing results (n = 10 999) are shown for all cotests preceding the diagnosis of precancer in 5369 women (Table 2). Overall, prediagnostic HPV testing (83.8%) was more likely to be positive than cytology (61.9%, P < .001); 87.3% of prediagnostic cotests were positive by HPV and/or cytology; 3.5% of the cotests were positive by cytology alone (HPV negative). HPV testing was more likely to be positive than cytology prior to both CIN3 (83.9% vs 62.8%, P < .001) and AIS (82.2% vs 53.2%, P < .001).
Table 2.
Comparison of all high-risk human papillomavirus DNA and cytology cotest results prior to precancer diagnosis, overall and by specific histology*
| Precancer | TotalNo. (%) | HPV+No. (%) | Pap+No. (%) | Any+No. (%) | HPV+Cyto+No. (%) | HPV+Cyto-No. (%) | HPV-Cyto+No. (%) | HPV-Cyto-No. (%) | P† |
|---|---|---|---|---|---|---|---|---|---|
| All precancers | 10 999 (100.0) | 9215 (83.8) | 6805 (61.9) | 9604 (87.3) | 6416 (58.3) | 2799 (25.4) | 389 (3.5) | 1395 (12.7) | <.001 |
| CIN 3 | 9975 (100.0) | 8373 (83.9) | 6260 (62.8) | 8731 (87.5) | 5902 (59.2) | 2471 (24.8) | 358 (3.6) | 1244 (12.5) | <.001 |
| AIS | 1024 (100.0) | 842 (82.2) | 545 (53.2) | 873 (85.3) | 514 (50.2) | 328 (32.0) | 31 (3.0) | 151 (14.7) | <.001 |
Some women contributed more than one cotesting result prior to diagnosis. “+” indicates a positive result, and “-” indicates a negative result; “Any+” means that HPV and/or Pap were positive. AIS = adenocarcinoma in situ; CIN 3 = cervical intraepithelial neoplasia grade 3; Cyto = cytology; HPV = human papillomavirus.
Two-sided P values comparing HPV tests with cytology were based on Z scores calculated from general estimating equation parameter estimates and empirical standard error estimates.
Table 3 shows the timing of the cotest and follow-up screening intervals relative to time of cancer (Table 3) and precancer diagnoses (Table 4). Cotests done within 12 months of diagnosis were more likely to be HPV and/or cytology positive than cotests done 12 months or longer before the diagnosis. HPV was statistically significantly more likely to be positive than cytology less than 12 months prior to a precancer diagnosis (96.2% vs 89.8%, respectively, P < .001) but not immediately prior to a cancer diagnosis (89.2% vs 86.3%, P = .1). By comparison, HPV was statistically significantly more likely to be positive than cytology 12 or more months before both a precancer diagnosis (70.6% vs 32.4%, respectively, P < .001) and a cancer diagnosis (62.8% vs 28.7%, P < .001).
Table 3.
Comparison of high-risk human papillomavirus DNA and cytology cotest results less than 12 months vs 12 or more months prior to diagnosis of cancer, to compare with previously published data by Blatt et al., 2015 (15), and precancer*
| Cotesting results in time period | TotalNo (%) | HPV+No (%) | Cyto+No (%) | Any+No (%) | HPV+Cyto+No (%) | HPV+Cyto-No (%) | HPV-Cyto+No (%) | HPV-Cyto-No (%) | P† |
|---|---|---|---|---|---|---|---|---|---|
| Cancer | |||||||||
| <12 mo | 600 (100.0) | 535 (89.2) | 518 (86.3) | 577 (96.2) | 476 (79.3) | 59 (9.8) | 42 (7.0) | 23 (3.8) | .10 |
| ≥12 mo | 537 (100.0) | 337 (62.8) | 154 (28.7) | 362 (67.4) | 129 (24.0) | 208 (38.7) | 25 (4.7) | 175 (32.6) | <.001 |
| Precancer | |||||||||
| <12 mo | 5646 (100.0) | 5434 (96.2) | 5068 (89.8) | 5627 (99.7) | 4875 (86.3) | 559 (9.9) | 193 (3.4) | 19 (0.3) | <.001 |
| ≥12 mo | 5353 (100.0) | 3781 (70.6) | 1737 (32.4) | 3977 (74.3) | 1541 (28.8) | 2240 (41.8) | 196 (3.7) | 1376 (25.7) | <.001 |
“+” indicates a positive result, and “-” indicates a negative result; “Any+” means that HPV and/or cytology were positive. Cyto = cytology; HPV = human papillomavirus.
Two-sided P values comparing HPV tests with cytology were based on Z scores calculated from general estimating equation parameter estimates and empirical standard error estimates.
Table 4.
Comparison of last high-risk human papillomavirus DNA and cytology cotest result prior to cancer diagnosis, by stage*
| Stage | TotalNo (%) | HPV+No (%) | Cyto+No (%) | Any+No (%) | HPV+Cyto+No (%) | HPV+Cyto-No (%) | HPV-Cyto+No (%) | HPV-Cyto-No (%) | P† |
|---|---|---|---|---|---|---|---|---|---|
| Local | 333 (100.0) | 302 (90.7) | 285 (85.6) | 319 (95.8) | 268 (80.5) | 34 (10.2) | 17 (5.1) | 14 (4.2) | .02 |
| Regional | 93 (100.0) | 67 (72.0) | 72 (77.4) | 78 (83.9) | 61 (65.6) | 6 (6.5) | 11 (11.8) | 15 (16.1) | .33 |
| Distant | 29 (100.0) | 15 (51.7) | 20 (69.0) | 20 (69.0) | 15 (51.7) | 0 (0.0) | 5 (17.2) | 9 (31.0) | .06 |
| All | 455 (100.0) | 384 (84.4) | 377 (82.9) | 417 (91.6) | 344 (75.6) | 40 (8.8) | 33 (7.3) | 38 (8.4) | .48 |
“+” indicates a positive result, and “-” indicates a negative result; “Any+” means that HPV and/or cytology were positive. Cyto = cytology; HPV = human papillomavirus.
The P values listed on this table are two-sided exact McNemar chi-square P values. With regard to trends by stage, the Ptrend values are the following: HPV+ column Ptrend < .001, Cyto+ column Ptrend = .007, and both the HPV+/Cyto- and HPV-/Cyto+ columns Ptrend = .002.
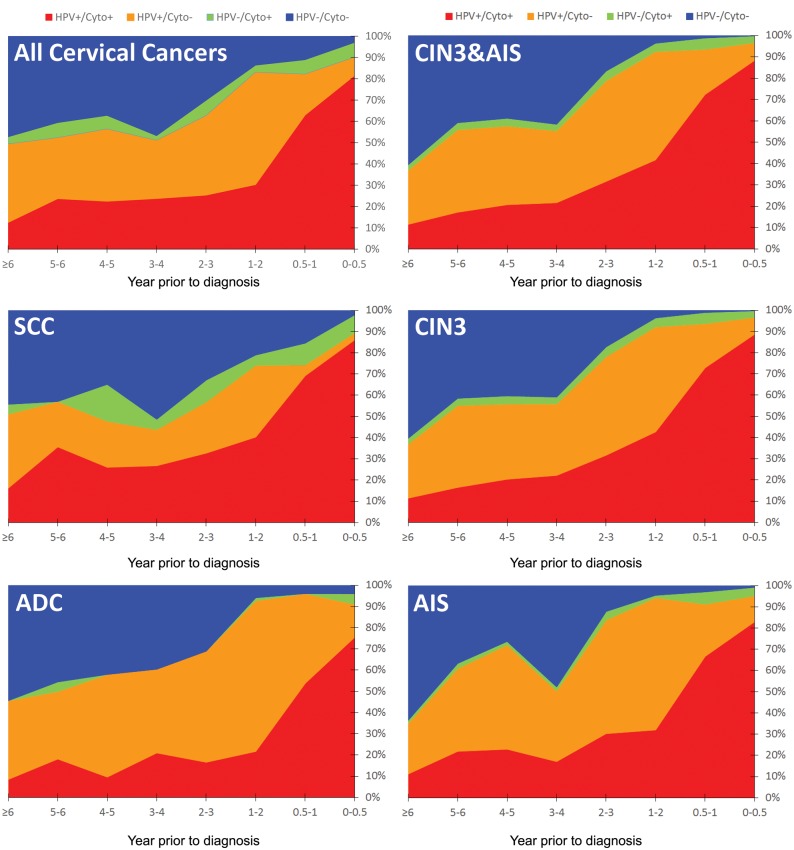
The percentages of the results for paired HPV and cytology cotesting by time interval prior to diagnosis for both precancers and cancers, overall and by main histology types, are shown in Figure 1. Women diagnosed with precancer or cancer were more likely to test HPV positive than cytology positive at any time interval prior to diagnosis. A positive cotest was only very slightly more likely (absolute, not relative, increase) for precancers (as a percentage of the total cotested precancer population) and for cancers than testing HPV positive alone at any time interval prior to diagnosis.
Figure 1.
Distribution of cotesting results prior to cervical precancer and cancer diagnoses by time period. Shown are all cervical cancers, squamous cell carcinomas (SCC), and adenocarcinomas (ADC) and all precancers (cervical intraepithelial neoplasia grade 3 [CIN3] and adenocarcinoma in situ [AIS]), CIN3, and AIS. From top to bottom the order of the test results is as follows: HPV and cytology negative (HPV−/Cyto−), HPV negative and cytology positive (HPV−/Cyto+), HPV positive and cytology negative (HPV+/Cyto−), and HPV and cytology positive (HPV+/Cyto+).
Cotesting Screening History Prior to Precancer vs Cancer Diagnoses
As shown in Table 4, the last cotest prior to diagnosis (median = 0.15 years, mean = 0.42 years, interquartile range = 0.32–0.06 years, and range = 7.25–0.00 years prior to diagnosis) was less likely to be HPV positive (Ptrend < .001) or cytology positive (Ptrend = .007) with more severe stage of cancer. However, an increasing percentage was positive only by cytology (HPV negative) with more severe stage (5.1% for localized, 11.8% for regional, and 17.2% for distant spread) while the reverse was true for HPV testing (cytology negative; 10.2% for localized, 6.5% for regional, and 0.0% for distant) (Ptrend = .002). Thus, for the last cotest prior to diagnosis, HPV testing was more sensitive for localized cancer (90.7% vs 85.6%, P = .02) but was marginally less sensitive for distant cancer (51.7% vs 69.0%, P = .06) compared with cytology.
We estimated by review of all cancer cases with at least one preceding HPV-negative test (n = 55 women) that 14 were tested at the time of colposcopy as part of the diagnostic work-up, not as part of screening. At maximum, there were 23 women whose cancers were missed by HPV in a prior screen but caught by cytology (at the definite threshold of LSIL or greater). We included in this count even those instances in which the positive cytology result would have advanced the diagnostic date by a very small amount of time, and also included those cases for which the cancer stage was distant spread at diagnosis. The same result expressed as a rate indicates that, in 2010, there were 23 additional true-positive screens per 1 618 394 women, or per 4 917 774 woman-years (3.04 years of follow-up on average per woman), that is, 4.7 per million woman-years.
Sequential Cotests Prior to Diagnosis
Table 5 addresses the relative performances of cotests when more than one was taken for a woman prior to diagnosis; most precancers (58.3%) and cancers (67.9%) diagnosed at KPNC were found after the first cotest was taken. HPV was more likely than cytology to test positive on the first, second, and third or subsequent cotests prior to a precancer diagnosis (P < .001 for all comparisons) and on the first (P < .001), second (P = .02), and third or subsequent cotests (P < .001) prior to a cancer diagnosis. The sensitivity of cotesting for diagnosis of cancer declined from the first cotest to the second and third or subsequent cotests prior to the cancer diagnosis (5.7%, 13.0%, and 12.9% negative cotests, respectively). Moreover, the cotesting results were less likely to be positive prior to a cancer diagnosis compared with a precancer diagnosis (P < .001 for all comparisons).
Table 5.
Human papillomavirus and cytology cotesting results directly prior to diagnosis of cervical cancers and precancers, for first, second, and third or later cotest performed*
| Diagnosis | Total | HPV+/Cyto+ | HPV+/Cyto- | HPV-/Cyto+ | HPV-/Cyto- | P† |
|---|---|---|---|---|---|---|
| Cancer | ||||||
| 1st cotest | ||||||
| No. | 423 | 296 | 77 | 26 | 24 | <.001 |
| % of 1st cotests | 100.0 | 70.0 | 18.2 | 6.1 | 5.7 | |
| % of total cancers | 67.9 | 47.5 | 12.4 | 4.2 | 3.9 | |
| 2nd cotests | ||||||
| No. | 115 | 70 | 22 | 8 | 15 | .02 |
| % of 2nd cotests | 100.0 | 60.9 | 19.1 | 7.0 | 13.0 | |
| % of total cancers | 18.5 | 11.2 | 3.5 | 1.3 | 2.4 | |
| ≥3rd cotests | ||||||
| No. | 85 | 49 | 21 | 4 | 11 | <.001 |
| % of > 3rd cotests | 100.0 | 57.6 | 24.7 | 4.7 | 12.9 | |
| % of total cancers | 13.6 | 7.9 | 3.4 | 0.6 | 1.8 | |
| Total | ||||||
| No. | 623 | 415 | 120 | 38 | 50 | <.001 |
| % of total tests | 100.0 | 66.6 | 19.3 | 6.1 | 8.0 | |
| % of total cancers | 100.0 | 66.6 | 19.3 | 6.1 | 8.0 | |
| Precancer | ||||||
| 1st cotest | ||||||
| No. | 3131 | 2338 | 720 | 65 | 8 | <.001 |
| % of 1st cotests | 100.0 | 74.7 | 23.0 | 2.1 | 0.3 | |
| % of total precancers | 58.3 | 43.5 | 13.4 | 1.2 | 0.1 | |
| 2nd cotest | ||||||
| No. | 1309 | 938 | 313 | 51 | 7 | <.001 |
| % of 2nd cotests | 100.0 | 71.7 | 23.9 | 3.9 | 0.5 | |
| % of total precancers | 24.4 | 17.5 | 5.8 | 0.9 | 0.1 | |
| ≥3rd cotests | ||||||
| No. | 929 | 695 | 183 | 47 | 4 | <.001 |
| % of >3rd cotests | 100.0 | 74.8 | 19.7 | 5.1 | 0.4 | |
| % of total precancers | 17.3 | 12.9 | 3.4 | 0.9 | 0.1 | |
| Total | ||||||
| No. | 5369 | 3971 | 1216 | 163 | 19 | <.001 |
| % of total tests | 100.0 | 74.0 | 22.6 | 3.0 | 0.4 | |
| % of total precancers | 100.0 | 74.0 | 22.6 | 3.0 | 0.4 |
“+” indicates a positive result, and “-” indicates a negative result. Cyto = cytology; HPV = human papillomavirus.
A two-sided McNemar chi-square test was used to test for statistical significance between HPV and cytology positivity.
Discussion
This systematic evaluation of the very few cancers diagnosed within the first and most extensive major HPV/cytology cotesting program in the United States confirms the sensitivity advantage of HPV tests. The evaluation also demonstrates that recent reports questioning the sensitivity of HPV testing for detection of cancer were based on partial analyses with short follow-up that accentuated the role of cytology (15,16,29). Viewed more comprehensively, the contribution of the cytology component to cotesting performance is quite limited for detection of treatable precancers and early curable cancers. Warnings that reliance on primary HPV testing would encourage cervical cancer mortality are overstated (15).
To discuss in more depth the critical point of the analysis, as shown here and previously reported (30,31), limiting analyses of cotesting results to the year before diagnosis reveals increased test positivity for HPV but even more so for cytology (perhaps due to recognition of tumor diathesis or debris devoid of viable cells). The clinical value is uncertain; testing positive at a time in which the cancer is already present (even advanced) is not prevention and thus is not consistent with the fundamental goals of cervical cancer screening. In any case, the percentage of all cancers combined that was detected by cytology alone was about 5% of an extremely small absolute number, as previously reported from a number of trials and epidemiologic studies (23,32,33).
Given that cytologic screening of the approximate 90+% of women who are HPV negative, or repeating screening at shorter intervals, would virtually automatically produce a small increase in diagnosis of precancers and treatable cancers (23), the question becomes what is the threshold of acceptable cancer risk, given that no reasonable approach will achieve perfect protection against cervical cancer mortality. In other words, what screening method and interval achieve the best benefits-to-harms ratio and cost-effectiveness over a “lifetime” of screening. There are potential harms associated with excessive screening, including the identification and overtreatment of potentially regressive CIN2 lesions (34,35) and possible increased risk of negative reproductive outcomes such as preterm delivery (36–39). The choice of acceptable level of safety compared with disadvantages of frequent intensive screening is not a scientific question. Rather it requires societal consensus/compromise beyond the scope of this presentation.
There were limitations of this analysis. First, we had screening data only from KPNC, a single integrated health system whose members and practices are not representative of all populations. Second, why certain tests or procedures were done at any given time was not always clear. Third, at KPNC the cotest is collected as two separate specimens, with the HPV tests being collected right after the cytologic specimen, which may have reduced the HPV testing performance compared with primary HPV testing or cospecimen collection of both specimens in a single cell collection.
With regard to strengths of this work, we were able to examine the long-term relative history of HPV tests and cytology prior to cancer diagnosis. We could show that the results of previous analyses are at least in part the result of design decisions leading to bias. Restriction to cases occurring within one year of screening tests biases against the use of HPV testing because HPV-positive, cytology-negative results lead to recommendations of return in one year. Any cancer cases diagnosed among women who return even slightly late are missed in the analysis, making HPV testing appear to be less sensitive than it is. The goal of screening is to diagnose precancers and treatable cancers as early as possible (40,41), certainly more than a year before diagnosis of regional or distant stage invasive disease. When properly examined over the long term, the contribution of cytology to screening is shown to be very small, and the need for continued rounds of cotesting is worth questioning. Others have suggested that a hybrid strategy might be to start with cotesting for women with unknown screening histories and then switch to primary HPV testing after one or two negative cotests (42,43). The trade-offs in benefits and harms as well as the cost-effectiveness of such a complex strategy will need to be evaluated, and its implementation may pose some practical challenges.
It is possible that screening intrinsically worked less well for those few women who developed cancer vs those who were diagnosed with precancer. Women diagnosed with cancer were less likely to test HPV positive and/or cytology positive than those diagnosed with precancer, overall, at any time point, or for any round of cotesting. Indeed, comparing cotesting results prior to a precancer diagnosis with results five years prior to a cancer diagnosis (when the latter might still have been precancer), raise the question of inherently less effective screening efficacy in women developing cancer.
The introduction of HPV testing alone as a cervical cancer screening option would perform nearly the same as HPV and cytology cotesting (44). The choice between the two strategies and the screening interval chosen, whether three years or five years or more, depends on societal judgments (eg, cancer prevention benefits vs resource allocation) and not scientific facts. Nevertheless, even using cotesting at three-year intervals (the most aggressive strategy in common use), cervical cancer continues to occur rarely (albeit typically at a curable stage). Excessive screening in an attempt to prevent every case could have minimal cancer prevention benefits while increasing the harms of screening.
Funding
Dr. Castle received partial support by the Intergovernmental Personnel Act mobility program with the National Cancer Institute (NCI). The Intramural Research Program of NCI supported the analysis.
Notes
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The NCI has received cervical cytology and HPV testing results for independent NCI-directed studies at reduced or no cost from Roche and Becton Dickinson. Dr. Castle has received HPV tests and biomarker assays for research at a reduced or no cost from Roche, Becton Dickinson, Cepheid, and Arbor Vita Corporation.
Supplementary Material
References
- 1. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S.. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 2. Cuzick J, Clavel C, Petry KU et al. , Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 2006;119(5):1095–1101. [DOI] [PubMed] [Google Scholar]
- 3. Mayrand MH, Duarte-Franco E, Rodrigues I et al. , Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. [DOI] [PubMed] [Google Scholar]
- 4. Naucler P, Ryd W, Tornberg S et al. , Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. [DOI] [PubMed] [Google Scholar]
- 5. Rijkaart DC, Berkhof J, Rozendaal L et al. , Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: Final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13(1):78–88. [DOI] [PubMed] [Google Scholar]
- 6. Ronco G, Giorgi-Rossi P, Carozzi F et al. , Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. [DOI] [PubMed] [Google Scholar]
- 7. Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM.. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: A subanalysis of the ATHENA study. Lancet Oncol. 2011;12(9):880–890. [DOI] [PubMed] [Google Scholar]
- 8. Kim JJ, Burger EA, Sy S, Campos NG.. Optimal cervical cancer screening in women vaccinated against human papillomavirus. J Natl Cancer Inst. 2017;109(2):djw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huh WK, Ault KA, Chelmow D et al. , Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182. [DOI] [PubMed] [Google Scholar]
- 10. Saslow D, Solomon D, Lawson HW et al. , American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W.. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315. [DOI] [PubMed] [Google Scholar]
- 12. Davis M, Feldman S.. Making sense of cervical cancer screening guidelines and recommendations. Curr Treat Options Oncol. 2015;16(12):55. [DOI] [PubMed] [Google Scholar]
- 13. Naber SK, de Kok IM, Matthijsse SM, van Ballegooijen M.. The potential harms of primary human papillomavirus screening in over-screened women: A microsimulation study. Cancer Causes Control. 2016;27(4):569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felix JC, Lacey MJ, Miller JD, Lenhart GM, Spitzer M, Kulkarni R.. The clinical and economic benefits of co-testing versus primary hpv testing for cervical cancer screening: A modeling analysis. J Womens Health (Larchmt). 2016;25(6):606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS.. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123(5):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng B, Li Z, Griffith CC et al. , Prior high-risk HPV testing and Pap test results for 427 invasive cervical cancers in China's largest CAP-certified laboratory. Cancer Cytopathol. 2015;123(7):428–434. [DOI] [PubMed] [Google Scholar]
- 17. Tao X, Griffith CC, Zhou X et al. , History of high-risk HPV and Pap test results in a large cohort of patients with invasive cervical carcinoma: Experience from the largest women's hospital in China. Cancer Cytopathol. 2015;123(7):421–427. [DOI] [PubMed] [Google Scholar]
- 18. US Preventive Services Task Force. 2015. http://www.uspreventiveservicestaskforce.org/Page/Document/draft-research-plan179/cervical-cancer-screening2. Accessed May 28, 2015.
- 19. Schiffman M, Wentzensen N, Khan MJ et al. , Preparing for the next round of ASCCP-sponsored cervical screening and management guidelines. J Low Genit Tract Dis. 2017;21(2):87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castle PE, de Sanjose S, Qiao YL, Belinson JL, Lazcano-Ponce E, Kinney W.. Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine. 2012;30(suppl 5):F117–F122. [DOI] [PubMed] [Google Scholar]
- 21. Wright TC Jr, Schiffman M, Solomon D et al. , Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103(2):304–309. [DOI] [PubMed] [Google Scholar]
- 22. Katki HA, Kinney WK, Fetterman B et al. , Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: A population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gage JC, Schiffman M, Katki HA et al. , Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106(8):dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright TC Jr, Massad LS, Dunton CJ et al. , 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. J Low Genit Tract Dis. 2007;11(4):223–239. [DOI] [PubMed] [Google Scholar]
- 25. Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D.. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11(4):201–222. [DOI] [PubMed] [Google Scholar]
- 26. Wright TC Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ.. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287(16):2120–2129. [DOI] [PubMed] [Google Scholar]
- 27. Massad LS, Einstein MH, Huh WK et al. , 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. [DOI] [PubMed] [Google Scholar]
- 28. Adegoke O, Kulasingam S, Virnig B.. Cervical cancer trends in the United States: A 35-year population-based analysis. J Womens Health (Larchmt). 2012;21(10):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou H, Mody RR, Luna E et al. , Clinical performance of the Food and Drug Administration-approved high-risk HPV test for the detection of high-grade cervicovaginal lesions. Cancer Cytopathol. 2016;124(5):317–323. [DOI] [PubMed] [Google Scholar]
- 30. Miller RA, Waters LL, Mody DR, Tams KC.. Squamous cell carcinoma of the cervix: A cytology-histology-human papillomavirus correlation in clinical practice. Arch Pathol Lab Med. 2015;139(6):776–781. [DOI] [PubMed] [Google Scholar]
- 31. Miller RA, Mody DR, Tams KC, Thrall MJ.. Glandular lesions of the cervix in clinical practice: A cytology, histology, and human papillomavirus correlation study from 2 institutions. Arch Pathol Lab Med. 2015;139(11):1431–1436. [DOI] [PubMed] [Google Scholar]
- 32. Dillner J, Rebolj M, Birembaut P et al. , Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European cohort study. BMJ. 2008;337:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arbyn M, Ronco G, Meijer CJ, Naucler P.. Trials comparing cytology with human papillomavirus screening. Lancet Oncol. 2009;10(10):935–936. [DOI] [PubMed] [Google Scholar]
- 34. Trimble CL, Piantadosi S, Gravitt P et al. , Spontaneous regression of high-grade cervical dysplasia: Effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11(13):4717–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castle PE, Schiffman M, Wheeler CM, Solomon D.. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009;113(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E.. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: Systematic review and meta-analysis. Lancet. 2006;367(9509):489–498. [DOI] [PubMed] [Google Scholar]
- 37. Castanon A, Landy R, Brocklehurst P et al. , Is the increased risk of preterm birth following excision for cervical intraepithelial neoplasia restricted to the first birth post treatment? BJOG. 2015;122(9):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sasieni P, Castanon A, Landy R et al. , Risk of preterm birth following surgical treatment for cervical disease: Executive summary of a recent symposium. BJOG. 2016;123(9):1426–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wuntakal R, Castanon A, Landy R, Sasieni P.. How many preterm births in England are due to excision of the cervical transformation zone? Nested case control study. BMC Pregnancy Childbirth. 2015;15:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saslow D, Solomon D, Lawson HW et al. , American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeronimo J, Castle PE, Temin S, Shastri SS.. Secondary prevention of cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline summary. J Oncol Pract. 2017;13(2):129–133. [DOI] [PubMed] [Google Scholar]
- 42. Kitchener HC. HPV primary cervical screening: Time for a change. Cytopathology. 2015;26(1):4–6. [DOI] [PubMed] [Google Scholar]
- 43. Herbert A. Primary HPV testing: A proposal for co-testing in initial rounds of screening to optimise sensitivity of cervical cancer screening. Cytopathology. 2017;28(1):9–15. [DOI] [PubMed] [Google Scholar]
- 44. Silver MI, Schiffman M, Fetterman B et al. , The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: Reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.