Abstract
The human microbiota maintains an enormous and diverse capacity to produce a diet-dependent metabolome that impacts both host tissue and microbial community homeostasis. Recent discoveries support a growing appreciation that microbial metabolites derived from bioactive foods are also important regulators of host immune and metabolic functions. To gain a better understanding of the current evidence for the roles of dietary and microbial metabolites in tumor immunity, the Division of Cancer Biology and the Division of Cancer Prevention, National Cancer Institute, cosponsored a workshop on August 31 and September 1, 2016, in Bethesda, Maryland. Workshop participants examined several lines of converging science that link nutrition, microbiology, and tumor immunology and identified key concepts and research opportunities that will accelerate our understanding of these interactions. In addition, the participants identified some of the critical gaps and research challenges that could be addressed through interdisciplinary collaborations, including future opportunities for translating new information into novel cancer prevention and treatment strategies based on targeting host immune functions that are altered by metabolite sensing pathways.
Although it is well established that dietary and microbial metabolites can modulate both innate and adaptive immune system responses to inflammatory stimuli, the molecular mechanisms and signal pathways by which these metabolites regulate immune responses, and their contribution to either tumor promotion or antitumor immunity, remain poorly defined (1,2). Accumulating evidence from preclinical and human studies suggests that the enormous and diverse metabolic output of gastrointestinal (GI) microbial communities contributes to the modulation of host immune function during carcinogenesis (3). This has given rise to an emerging appreciation that many of the immune-modulating effects of bioactive food components are mediated by microbial activities that can either directly yield immunomodulatory metabolites (eg, butyrate, indoles, bile acids) or that can alter host/microbe interactions that indirectly regulate innate and adoptive immune responses (4–7).
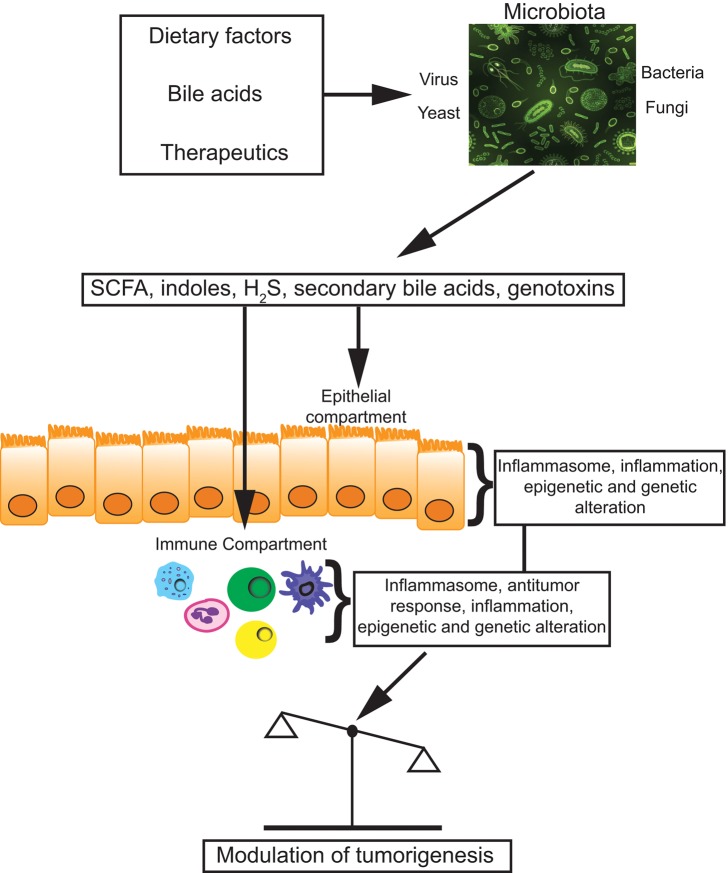
To assess the impact of microbial and dietary metabolites on tumor immune responses, the National Cancer Institute (NCI) convened a diverse panel of experts to examine the converging science that links nutrition, microbiology, and tumor immunology and to evaluate current evidence that dietary and/or microbial metabolites regulate immune responses that impact cancer development, progression, and treatment. The workshop, co-chaired by Christian Jobin (University of Florida, Gainesville, FL) and Giorgio Trinchieri (NCI, Bethesda, MD), explored several key concepts regarding metabolite regulation of host immunity and examined several critical molecular signaling pathways that regulate antitumor immune responses via interactions with metabolites from dietary and microbial sources (Figure 1). Panelists (see the Supplementary Materials, available online, for the complete list of panelists) provided a critical analysis of the current state of the science and identified knowledge gaps and research opportunities that would inform future collaborations and accelerate discoveries of metabolite signaling to the host immune system. Key points from the workshop are summarized below.
Figure 1.
Metabolites modulate antitumor immunity. The human commensal microbiota modulates important metabolic and immune responses during carcinogenesis. Various compounds (eg, diet, bile acids, therapeutics) either modify microbiota or influence metabolic activities (eg, SCFA, secondary bile acids), which then elicit a series of host-derived responses (eg, inflammation, inflammasome, genetic, and epigenetics) from epithelial and immune cells (eg, innate lymphocytes, dendritic cells, macrophages, and lymphocytes) that impact tumor progression.
Overview of Host/Microbe Interactions
Dr. Trinchieri introduced the concept of humans as metaorganisms, where cell and tissue functions are regulated by host-commensal interactions. The commensal microbiota are essential for optimal immune system development, inflammatory responses to pathogens, and the establishment of autoimmunity. A eubiotic gut microbiota also mediates important metabolic functions including nutrient absorption, vitamin synthesis, and fermentation of dietary carbohydrates, whose byproducts are important for regulating mucosal barrier homeostasis and tumor immune surveillance. The microbiota also modulate immune responses that affect tumor growth, and they are required for the effectiveness of anticancer immunotherapy and chemotherapy (8,9). These host-microbe interactions change over time and are affected by geography, lifestyle, nutrition, probiotics, hygiene, and infections. Even relatively small changes in microbiota composition and function can affect tumor immunity of the host. For example, changes in a single commensal such as S. epidermidis in the skin or Corynebacterium in the oral cavity have been shown to alter T cell response and could be expected to locally affect tumor growth (10).
Dr. Jobin focused on mechanisms of microbiota signaling to tumor and mucosal immune cells, noting that the entire output of the microbiome, including RNA, DNA, proteins, and metabolites, is involved in maintaining host immune and mucosal barrier homeostasis. It was stressed that polymicrobial interactions likely influence cancer development. Thus, even though specific microorganisms such as Salmonella and Fusobacteria spp have been linked to carcinogenesis, there are likely multiple contributions of microbial factors, such as adherence factors (eg, FadA, Fab2), virulence factors (eg, CagA, AvrA), secreted toxins (eg, B. fragilis toxin, colibactin), and metabolic byproducts (eg, hydrogen sulfide, radical oxygen, and ethanol) that are implicated in tumorigenesis (11). Using gnotobiotic technology, it was shown that some microorganisms (eg, E. coli NC101) could promote inflammation and colorectal cancer as a single community whereas others (Atopobium parvulum) require the presence of a complex community. Genetic and pharmacological approaches showed that blocking secondary metabolite production alleviated development of colitis and colorectal cancer.
In contrast to these tumor-promoting properties, Dr. Jobin stressed that numerous beneficial microbial-derived metabolic activities are implicated in the regulation of epithelial and immune cell responses and are necessary for maintaining a homeostatic (eubiotic) state. Perturbations of microbial eubiosis may lead to a dysbiotic state often reported in pathologies, including cancer. Dysbiosis can affect the metabolic output of the microbial community, leading to changes in, for example, secondary metabolites, bile acid pools, polyamine biosynthesis, hydrogen sulfide, and short chain fatty acid (SCFA) production, all of which may be targets for future therapeutic intervention (12–14).
The session also featured talks from National Institutes of Health program officers Lita Proctor (National Human Genome Research Institute) and Victor Kipnis (NCI), who spoke about cutting edge tools and resources developed by the Human Microbiome Project (https://commonfund.nih.gov/hmp/index) and the NCI biometry program (https://prevention.cancer.gov/research-groups/biometry). These resources include biospecimens, strains, and curated sequence data (15), as well as expert consultations on biostatistical and epidemiological methodologies and mathematical modeling that control for false discovery error rate and ensure appropriate power for the analyses of large data sets that are now commonly generated by metabolomic and metagenomic studies.
Regulation of Innate Immune Circuits
Recent studies have indicated that immune responses are determined, at least in part, by the local milieu of endogenous metabolites, as well as by exogenous metabolites derived from the diet or microbiome. The latter are thought to operate both locally at mucosal barrier tissues and systemically to regulate both immune cell development and function. Presentations in this session addressed metabolite signaling to various components of the innate immune system and provided examples of how innate immune responses may be perturbed by metabolite signaling during carcinogenesis.
Dmitry Gabrilovich (Wistar Institute, Philadelphia, PA) discussed how dendritic cell (DC) cross-presentation of tumor antigens to CD8+ T lymphocytes is profoundly inhibited by the accumulation of excess oxidized lipids from the tumor microenvironment (16). Cross-presentation is a critical DC activity required for stimulating antitumor immunity, and many tumors disable this pathway to evade immune surveillance. Thus, a major goal for improving cancer immunotherapy is to understand the mechanistic basis for this defect in order to reprogram DCs to restore cross-presentation capability.
Dr. Gabrilovich described experiments in which DCs incubated in tumor explant supernatants were unable to stimulate T cell responses. This inability to cross-present antigens was associated with uptake of oxidized lipids and their accumulation in lipid bodies (LBs). These lipids form a large class of chemically similar structures derived from both tumor cells and bacteria and that have been shown to trigger a CD1d-dependent inflammatory response within intestinal tissues and that may also affect iNKT cell function (17). Electrophilic oxidized lipids were shown to localize to the surface of the LBs and covalently bind protein chaperones, which is necessary for trafficking peptide-MHC (pMHC) complexes to the cell surface and to lysosomes/late endosomes. These data demonstrate a novel mechanism regulating cross-presentation in tumors and suggest potential therapeutic avenues to improve cancer immunotherapy.
Gregory Sonnenberg (Cornell University, Ithaca, NY) discussed the role of innate lymphoid cells (ILCs) in regulating host-commensal bacteria interactions in human health and disease. The GI tract comprises the largest immune organ system and contains 60% to 80% of total immune cells. The IL-22 receptor is abundant on the intestinal epithelium and stimulates secretion of mucins and antimicrobial peptides and promotes tissue homeostasis and regeneration. Recent discoveries indicate that ILC3 (CD3-, NK1.1−, c-kit+, CD127+, CD90+, RORγt+) cells are the dominant source of IL-22 in the lamina propria. ILC3 cells are highly enriched in the gut, are expanded by the cytokines IL-1β, IL-23, IL-6, and retinoic acid, and play an essential role in IL-22 and IL-17 dependent innate immune responses and in balancing CD4-dependent inflammatory immune responses to commensal bacteria (18). Precisely how ILC3 cells restrict microbiota-dependent CD4 inflammatory response in the GI tract is not clear but could involve one or more peripheral deletion mechanisms (19).
Consistent with their role in regulating inflammation, ILC3 cells were found to be reduced in pediatric inflammatory bowel disease (IBD) biopsies, and this was inversely correlated with increased IL-17 expression. Although the role of ILCs in human cancers is an emerging area of study, it is appreciated that immunotherapy, such as checkpoint blockade, increases susceptibility to colitis. Novel interventions targeting ILCs to reduce inflammation could ameliorate these effects.
Mihai Netea (Radbound University, Nijmegen, the Netherlands) discussed the role of adaptive responses to pathogens, such as Candida albicans, by innate immune cells as evidenced by a protective inflammatory response upon re-exposure. Vaccinated T cell–deficient Rag1-/- mice demonstrated increased survival upon rechallenge with Candida albicans, whereas myeloid-deficient CCR2-/- mice showed no such protection, indicating that components of the innate immune system possess the ability to develop a protective memory immune response (20). This memory response, referred to as “trained immunity,” is largely mediated by epigenetic reprogramming that maintains critical genes in an open chromatin conformation that allows rapid gene upregulation when rechallenged.
Inflammatory cytokines IL-6 and TNF were associated with an increase in H3K4me3 at their promoters, and ChIP analysis revealed similar chromatin modifications at toll-like receptor (TLR) and G-protein coupled receptor (GPCR) genes (21). Dr. Netea outlined how microbiota exposure both reduced oxidative phosphorylation and increased fermentation and glycolysis, indicating an extensive reprogramming of metabolic and epigenetic cell activity (22). Further studies are needed to assess the extent and contribution of trained innate immunity in tumor progression.
Host Metabolic Sensing Pathways Regulate Tumor Immune Response
Host cell metabolite sensing pathways can signal changes in immune responses that are important to pathogen response, tumor surveillance, and aging. Marc Veldhoen (Institute of Molecular Medicine, Lisbon, Portugal) examined signaling through the aryl hydrocarbon receptor (AhR) and its role in intestinal tissue homeostasis and gut barrier integrity via interactions with the mucosal immune system. Earlier studies had defined a role for AhR in the regulation of hepatic P450 enzyme levels and their activation of protocarcinogenic xenobiotics such as dioxins, heterocyclic amines, and PAHs. However, newly described functions for AhR reveal that it is essential for the maintenance of intraepithelial lymphocytes and is affected by dietary metabolites.
In Th-17 lymphocytes, TGF-β receptor signals through TIF1γ and Smad4 mediators to regulate AhR expression, which is critical for the production of IL-22. Bioactive dietary factors such an indole-3-carbinol can serve as weak AhR ligands, leading to production of protective levels of IL-22 and normal barrier function (23). Knocking out components of the TGFβ receptor signaling pathway resulted in decreased AhR function and decreased IL-22 production and damaged mucosal barrier function. This damage leads to bacterial translocation from the gut lumen and stimulates a pro-inflammatory TH17 responses and development of colorectal cancer in mouse models (24).
Scott Bultman (University of North Carolina, Chapel Hill, NC) discussed the role of butyrate and other short-chain fatty acids derived from soluble dietary fiber, and their anti-inflammatory and tumor-suppressive mechanisms. Colorectal cancer is associated with a diminished butyrate-producing microbiota, but prior human studies have examined end-stage disease, making it difficult to establish whether this association is a cause or a consequence of an altered microbiota. Butyrate is an important energy source for normal colonocytes and has tumor-suppressive epigenetic functions via HDAC inhibitory activity when it accumulates in glycolytic tumor cells (25). AOM/DSS-treated gnotobiotic mice colonized with altered Schaedler flora (ASF) and fed a high-fiber diet plus butyrate-producing microbes (B. fibrisolvens) showed decreased tumor formation compared with mice given non-butyrate-producing mutant strains. There was synergism between butyrate-producing microbes and a butyrate-rich diet, and these were associated with increased histone acetylation and expression of apoptosis-related genes.
Vishwa Deep Dixit (Yale University School of Medicine, New Haven, CT) focused on the impact of negative energy balance on the immune system by studying caloric restriction (CR), which has been shown to increase lifespan, health span, and reduce cancer incidence in mice. This effect is due in part to changes in cell metabolism from glycolysis to fatty acid oxidation, which reduces pro-inflammatory neuroendocrine and hormone signaling, although the mechanism remains unclear. One of the most striking observations from the CALERIE clinical trial (NCT00099138) has been that CR in humans induces immunometabolic changes that suggest upregulation of anti-inflammatory and anticancer pathways.
Similar responses were seen in mice, where calorie restriction leads to reduced glycolysis and increased lipid utilization primarily through ketogenesis and ketolysis (26). The primary metabolic product of fatty acid oxidation, beta-hydroxybutyrate, suppresses the NLRP3 inflammasome activity and attenuates activation and secretion of pro-inflammatory IL-1b, suggesting that the anti-inflammatory effects of CR or ketogenic diets are linked to the production of beta-hydroxybutyrate (27).
Immune-Modulating Metabolites
This session focused on specific examples of metabolites that affect immune function and how they may contribute to pathogenesis of specific cancers. Benjamin Marsland (University of Lausanne, Lausanne, Switzerland) discussed how SCFAs produced in the colon from insoluble fibers can influence peripheral immune responses in the lung (the gut-lung axis) (28) or in utero (29). For example, antigen-challenged mice fed low-fiber diets display a Th2-dominated immune response and statistically significantly higher levels of eosinophils, lymphocyte infiltration, mucus production, and levels of IL-4 and Il-5 in the lung when compared with mice fed regular and high-fiber (pectin) diets. An abundance of circulating SCFAs was associated with decreased activity of tissue-resident dendritic cells and decreased allergic airway inflammation. This raises the interesting possibility that high-fiber diets (HFDs) could be immunosuppressive and increase the risk of tumorigenesis and infection. However, in mice fed an HFD and challenged with a lethal dose of influenza, the severity of infection was reduced, possibly due to a reduced neutrophil response in the lung (30).
The importance of NOD-like receptor (NLR) ligands (eg, dsRNA, unmethylated CpG motifs, peptidoglycan, and other microbial associated molecular patterns) in modulating inflammasome activity and innate immune responses was examined by Thirumala-Devi Kanneganti (St. Jude Children’s Research Hospital, Memphis TN). Genetic variation in and low expression of NLRs (ie, NLRX1, NLRC3, NLRP3, NLRP1, NLRC4, AIM2) are highly associated with susceptibility to inflammatory diseases (eg, osteomyelitis and colon adenocarcinoma), although the mechanism is currently unknown.
Dr. Kanneganti showed that in the AOM/DSS model of colitis Aim2 deficiency resulted in increased tumorigenesis and mortality, as well as an altered microbiome that included a statistically significant increase in Akkermansia muciniphila—demonstrating that both genetic and environmental factors may act in concert during development of colorectal cancer. It was also shown that AIM2 has inflammasome-independent functions that regulate colonic stem cell cycling and proliferation via the PI3K-AKT signal pathway (31). Bile acid metabolites are important regulators of immune responses and mediate microbiota-driven inflammation relevant to liver cancer.
Dr. Yu-Jui Yvonne Wan (UC Davis, Davis, CA) examined how diet may influence microbial production of secondary bile acids (DCA, LCA) that regulate the activity of dendritic cells, T cells, APC, CD4+, CD8+ T cells, macrophages, and monocytes that effect liver inflammation, epithelial cell integrity, and insulin sensitivity (12). Diets including a high-fat diet, Western diet (high fat and high sweet), alcohol intake, hepatitis viral infection, and aflatoxin β1 exposure can disrupt bile acid homeostasis and impact hepatic metabolism, cell proliferation, autoimmune disorders, and carcinogenesis process. The physiological effects of altered bile acid composition appear sex dependent as liver cancer is a male-dominant disease.
In an farnesoid X receptor (FXR) knockout mouse model of liver cancer, male mice fed a Western diet had more tumors, leukocyte infiltration, and inflammation when compared with female FXR knockout mice on the same diet, indicating an FXR-dependent pathway influencing gender difference in liver carcinogenesis (32). Moreover, during pregnancy, estrogen and progesterone can reduce bile acid transport and induce cholestasis of pregnancy (33). Elevated bile acid levels have a profound effect in CD4+ T cell–mediated pro-inflammatory responses (TH1 and TH17).
Benoit Chassaing (Georgia State University, Atlanta, GA) described how processed food additives such as emulsifiers, which are detergent-like molecules commonly used to improve texture and homogeneity, may alter the intestinal microbiota in a detrimental way, allowing bacteria to penetrate the normally sterile mucus layer and inducing chronic, microbiota-dependent low-grade GI inflammation that associated with colorectal cancer (34). Mice fed polysorbate 80 (P80) or carboxymethylcellulose (CMC) had a five-fold increase of fecal lipocalin 2, a marker of low-grade GI inflammation, and demonstrated an altered epithelial cell layer homeostasis that resulted in increased colorectal cancer susceptibility. Mice fed P80 or CMC followed by AOM-DSS treatment developed more tumors than the AOM-DSS-only treated animals, suggesting a detrimental effect of those agents in tumorigenesis (35). Information on additional immune-modulating metabolites can be found in Supplementary Table 1 (available online).
Translational Studies of Metabolites on Immune Response
The final session provided discussion of how metabolite-immune cell interactions may be targeted to modulate immune responses to reduce cancer risk or increase antitumor therapeutic efficacy. Rex Gaskins (University of Illinois, Urbana-Champaign, IL) discussed the effects of diet on the composition and abundance of sulfidogenic bacteria and the risk of colorectal cancer. Bacterial-derived hydrogen sulfide has been linked with human colorectal cancer (CRC) risk because of its putative genotoxic and pro-inflammatory effects on intestinal epithelial cells (14,36). Several species of sulfidogenic bacteria colonize human gut mucosa and produce hydrogen sulfide from either inorganic sulfate/sulfite or organic sulphur (cysteine or taurine). Taurine is generated by microbial hydrolysis of the conjugated bile acid taurocholate, which is abundant in people with high-fat and animal meat diets and is preferentially metabolized by the sulfidogenic bacteria Bilophila wadsworthia (37).
Profiling of the sulfidogenic microbiome in populations consuming a diet high in fat and meat revealed a correlation with their abundance in the colonic mucosa. Furthermore, populations with CRC had statistically significantly higher levels of sulfidogenic bacteria (Bilophila, Lactococcus, Odoribacter, Porphyomonas, and Pyramidobacter) than controls. Controlled diet studies revealed that switching to a high-fiber, low-meat diet reduced levels of sulfidogenic bacteria and decreased several indicators of colonic cell proliferation and inflammation (38). Importantly, hydrogen sulfide also has been proposed to play a protective role in CRC by sensitizing many anaerobic microbes to immune control in the colonic mucosa. Thus, managing hydrogen sulfide production to maintain protective levels could be an important strategy for CRC prevention.
Pavan Reddy (University of Michigan, Ann Arbor, MI) discussed approaches to mitigate graft vs host disease (GVHD) following allogenic bone marrow transplantation (allo-BMT). In GVHD, 80% to 90% of mortality derives from intestinal epithelial cell (IEC) damage. Changes in intestinal microbiota have been documented during GVHD (39), but whether this results in an altered metabolome that effects IEC damage has not been explored.
Dr. Reddy has identified statistically significantly reduced levels of the microbiota-derived short-chain fatty acid (SCFA) butyrate within intestinal tissues after allo-BMT, resulting in decreased histone acetylation in IECs (40). This decrease correlated with lowered expression of key proteins of anti-apoptotic pathways and junctional complexes (Bcl2l10 and occluding/claudins). Restoration of butyrate levels either by exogenous oral administration or introduction of selected strains of butyrate-producing Clostridia (40) into the intestinal lumen enhanced epithelial cell integrity and function and reduced severity and mortality of GVHD (41).
An alternate approach to increasing IEC butyrate levels was tested by adding resistant starch (RS) in the diet. Adding RS to the diet caused a shift in the gut microbiota toward the RS-degrading organisms Bifidobacterium adolescentis/Ruminococcus bromii and the bytyrogenic microbe Eubacterium rectale. Ongoing studies will reveal if host tissue response to injury modulated by diet and microbiota can be used as adjuvants to immunosuppression/immune tolerance regimens to reduce the GVDH severity.
Thomas Gajewski (University of Chicago, Chicago, IL) discussed two issues: 1) immune response escape by tumor cells and 2) impact of the commensal microbiota on antitumor immunity. Dr. Gajewski has identified different gene signatures in human melanomas that indicate the presence or absence of T cell infiltration. Patients with T cell–infiltrated tumors expressed high levels of IDO, PD-L1, and FoxP3+ Tregs, which mark immune inhibitory pathways (42). Treating these patients alone or in combination with anti-PD1/PD-L1 antibody and an IDO inhibitor resulted in a statistically significantly better response than in patients with low T cell infiltration. Gene expression analysis revealed activation of WNT/β-catenin target genes in 48% of tumor samples, which correlated with low CD8+T cell counts within the tumor (43). One target gene, ATF3, is a negative regulator of CCL4, a major cytokine responsible for recruiting dendritic cells (DCs). Reduced CCL4 results in exclusion of immune cells from the tumor tissue and resistance to immunotherapy.
Other studies have clearly indicated an influence of commensal microbiota on endogenous antitumor responses. Genetically identical C57BL/6 mice from Jackson laboratory (JAX) and Taconic Farms (TAC) exhibited statistically significant differences in tumor growth and anti-PD-L1 response. Cohousing or fecal transplant from JAX mice to TAC almost eliminated this difference, revealing the role of microbiota in controlling the antitumor immune response. Microbiome analysis of TAC mice that received JAX fecal transplant identified higher abundance of 11 bacterial species. Among them, the Bifidobacterium spp. activated host dendritic cells and correlated with positive T cell response in tumors. Furthermore, administration of Bifidobacterium with anti-PD-L1 antibody TAC mice statistically significantly improved tumor-specific immunity and response to therapy (44). Additionally, Bifidobacterium abundance is statistically significantly lower in colon cancer and metastatic melanoma patients. Ongoing and future studies will examine if pharmacological approaches to inhibit the β-catenin pathway or the manipulation of commensal microbiota could be used to improve checkpoint inhibitor efficacy.
Conclusions
We are still in the very early stages of connecting microbiome function to tumor development, progression, and response to therapy. Although technological advances have increased our capacity to sequence, identify, and culture microbial flora and detect their metabolites, their functional impact on tumor immunity remains unclear. Properly designed mechanistic studies that define and validate critical host-microbe interactions are needed to improve our understanding of how metabolites signal the immune system and to identify potential molecular targets for preventive and therapeutic interventions.
A current challenge in studying the effects of microbial metabolites on immune function is our reliance on metagenomic (16S) data. While metagenomic data provide information on the range of bacteria present, it is difficult to infer dynamic relationships between diet, metabolic outputs of microbial origin, and the intermediate metabolism of the host. This problem parallels the experience with human genomic data, which showed that identifying somatic or germline mutations from tumors often does not inform about mechanism or offer clear therapeutic targets.
The integration of functional studies that validate host-microbial interactions is difficult to perform. New technologies such as “crypts-on-a-chip” are needed to enable coculturing of human tissue mimetics and prokaryotic communities. This will afford the opportunity to interrogate specific niches in a biologically relevant context and identify and annotate metabolites from both host and microbial sources. Gas chromatography–mass spectroscopy platforms combined with open-source metabolome databases, such as HMDB, METLIN, and NIST, are currently the primary method for detecting and quantifying microbial metabolites. However, improved technology is needed for a more accessible, high-throughput means of defining putative immune-modulatory metabolites and their modified forms (oxidation, phosphorylation). A clinically significant metabolite may be the culmination of intermediate metabolism by several distinct microbial species as well as the host.
Experimental animal models can provide valuable mechanistic insights and a means to validate hypotheses on microbial-host metabolism and immunity in cancer. Syngeneic or genetically engineered animal models have the flexibility of studying microbial composition and immunological functions over time as a cancer is induced and progresses, in conjunction with analysis of metabolic profiles. Correlative studies can be further distilled through specific intervention approaches to demonstrate proof of concept. A limitation of these studies is an appreciation for the species-specific differences between experimental animals and humans. Beyond these inherent differences are factors that can be systematically controlled in experimental design, such as housing conditions, diet, sex, and circadian rhythms (Supplementary Table 2, available online).
Several key concepts and gaps in the field that emerged from the workshop include:
Both diet and unique host traits (eg, genetic polymorphisms, age, gender) modulate human microbiota composition and metabolic output; how they combine to regulate host immune functions is a key area for study in cancer prevention and treatment.
Biomarker analysis using bacterial 16S rRNA sequencing must be done with appropriate statistical power and validated with independent experiments to minimize false discovery rates. A combination of preclinical and clinical approaches is needed to access mechanism and establish the rationale for microbial contributions to cancer prevention, progression, or treatment.
Careful analysis of both the lymphoid and myeloid populations is needed to reveal connections between the microbiome, metabolism, and immune dysbiosis that occur in cancer. In particular, discriminating the myeloid population subsets may clarify important ROS, RNS, cell death, cytokine, or chemokine pathways and determine their contribution to antitumor responses.
Microbiota in different body niches (gut, skin, lung) may confer distinct impacts on host immunity. The specific metabolic intermediates and relative effect on immunity will likely vary in accord with the individual, type, and stage of cancer.
Little information exists on the bidirectionality of signals between microbes and host that occurs with cancers. Microbial-based metabolites have different effects dependent on the cell type (cancer, immune, stromal, stem) and tissue compartment context. Further studies that determine the durability of these effects over time and how malleable they may be for cancer treatment and prevention are needed.
Open questions remain on how to best leverage human cohorts to study microbial-host metabolism and tumor immunity. The Human Microbiome Project may provide a key pre-disease-onset resource for investigators who aim to develop prevention strategies. While the use of antibiotics is well entrenched in medicine, the community has not adopted intervention approaches that propose to harness diet and microbial metabolism proactively as part of a cancer treatment strategy, whether it be conventional (chemotherapy, irradiation), immunotherapy, or a combination. The addition of microbiome- and metabolomic discovery–based analysis to clinical trials may in time provide a rationale for inclusion and refinement of microbe-based adjuvant therapies. Questions and controversy may persist in the field, but workshops such as the one detailed here will hopefully spark collaborations and deep conversations about the next steps for the field.
Supplementary Material
References
- 1. Shapiro H, Thaiss CA, Levy M, Elinav E.. The cross talk between microbiota and the immune system: Metabolites take center stage. Curr Opin Immunol. 2014;30(11):54–62. [DOI] [PubMed] [Google Scholar]
- 2. Holmes E, Li JV, Marchesi JR, et al. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;165:559–564. [DOI] [PubMed] [Google Scholar]
- 3. Louis P, Hold GL, Flint HJ, et al. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Micro. 2014;1210:661–672. [DOI] [PubMed] [Google Scholar]
- 4. Davis CD, Milner JA.. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;2010:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elinav E, Nowarksi R, Christoph A, et al. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. [DOI] [PubMed] [Google Scholar]
- 6. Fung TC, Artis D, Sonnenberg G.. Anatomical localization of commensal bacteria in immune cell homeostasis and disease. Immunol Rev. 2014;2601:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rooks MG, Garrett WS.. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;166:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;3426161:967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vetizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naik S, Bouladoux N, Linehan JL, et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520(7545):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwabe RF, Jobin C.. The microbiome and cancer. Nat Rev Cancer 2013;1311:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ridlon JM, Bajaj JS.. The human gut sterolbiome: Bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm Sin B. 2015;52:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chaturvedi R, de Sablet T, Coburn LA, et al. Arginine and polyamines in Helicobacter pylori–induced immune dysregulation and gastric carcinogenesis. Amino Acids. 2012;42(2–3):627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Attene-Ramos M S, Nava GM, Muellner MG, et al. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen. 2010;51(4):304–314. [DOI] [PubMed] [Google Scholar]
- 15. The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: Dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;163:276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao W, Ramakrishnan R, Tuyrin VA, et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer—oxidized lipids and DCs in cancer. J Immunol. 2014;1926:2920–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dingding AD, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156(1–2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;4987452:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hepworth MR, Fung TC, Masur SH, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;3486238:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Netea MG, Brown GD, Kullberg BJ, Gow NA.. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;61:67–78. [DOI] [PubMed] [Google Scholar]
- 21. Saeed S, Quintin J, Kerstens HH, Rao NA, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;3456204:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ostuni R., Kratochvill F, Murray PJ, Natoli G.. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2015;364:229–239. [DOI] [PubMed] [Google Scholar]
- 23. Zhou L. AHR function in lymphocytes: Emerging concepts. Trends Immunol. 2016;371:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. [DOI] [PubMed] [Google Scholar]
- 25. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ.. The Warburg Effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;484:612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Youm YH, Horvatha TL, Mangelsdoret DJ, et al. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc Natl Acad Sci U S A. 2016;113(4):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. [DOI] [PubMed] [Google Scholar]
- 29. Thornburn AN, McKenzie CI, Shen SJ, Stanley D, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Comms. 2015;6:1–13. [DOI] [PubMed] [Google Scholar]
- 30. Balmer ML, Ma EH, Bantug GR, et al. Memory CD8(+) T cells require increased concentrations of acetate induced by stress for optimal function. Immunity. 2016;446:1312–1324. [DOI] [PubMed] [Google Scholar]
- 31. Man SM, Zhu Q, Zhu L, et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;1621:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu HX, Keane R, Sheng L, Wan YJ.. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;636:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larson SP, Kovilam O, Agrawal DK.. Immunological basis in the pathogenesis of intrahepatic cholestasis of pregnancy. Expert Rev Clin Immunol. 2016;121:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viennois E, Merlin D, Gewirtz AT, Chassaing B.. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2016;771:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deplancke B, Gaskins HR.. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB. 2003;17(10):1310–1312. [DOI] [PubMed] [Google Scholar]
- 37. Ridlon JM, Wolf PG, Gaskins HR.. Taurocholic acid metabolism by gut microbes and colon cancer. Gut microbes. 2016;73:201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Keefe SJ, Li JV, Lahti L, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Comm. 2015;6:6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eriguchi Y, Takashima S, Oka H, Shimoji S, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120(1):223–231. [DOI] [PubMed] [Google Scholar]
- 40. Atarashi K, Tanoue T, Oshima K, Suda W, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. [DOI] [PubMed] [Google Scholar]
- 41. Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gajewski TF, Schreiber H, Fu YX.. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Spranger S, Bao R, Gajewski TF.. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. [DOI] [PubMed] [Google Scholar]
- 44. Sivan A, Corrales L, Hubert N, Williams JB, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.