Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer death worldwide; its incidence is increasing in the United States. Depending on disease extent and underlying liver status, patients may be treated with local, locoregional, and/or systemic therapy. Recent data indicates that radiotherapy (RT) can play a meaningful role in the management of HCC. Here, we review published experiences using RT for HCC, including the use of radiosensitizers and stereotactic RT. We discuss methods for performing preclinical studies of RT for HCC and biomarkers of response. As a part of the HCC Working Group, an informal committee of the National Cancer Institute’s Radiation Research Program, we suggest how RT should be implemented in the management of HCC and identify future directions for the study of RT in HCC.
The Molecular Radiation Therapeutics Branch (MRTB) is a Radiation Research Program (RRP) in-house branch activity that serves as a focal point for collaborations with the Developmental Therapeutics Program (DTP) and the Cancer Therapy Evaluation Program (CTEP) in the Division of Cancer Treatment and Diagnosis (DCTD), investigators in the Radiation Biology and Radiation Oncology branches in the Center for Cancer Research (CCR), and academia and industry collaborators addressing research and development needs in combined modality therapy using radiation. Through these efforts, the MRTB stimulates discussion among various disease site/biology working groups that interact periodically to introduce new agents as radiation modifiers from either the CTEP portfolio or company interactions. The hepatocellular cancer (HCC) Working Group is one of eight disease site groups focused on the development and incorporation of novel modalities for the management of HCC. This review is a thorough summary of the discussions and recommendations that have resulted from the activities of this working group.
HCC is the third most common cause of cancer death worldwide ( 1 ). It ranks sixth worldwide in terms of incidence, and it is particularly common in developing countries ( 2 ). Rates of HCC diagnosis are increasing in many parts of the world, including the United States ( 3 ). The majority of patients diagnosed with HCC are not eligible for radical curative therapy, and median survival for such patients is less than one year ( 4 ). While the multitargeted small molecule tyrosine kinase inhibitor sorafenib has been shown to prolong overall survival (OS) in advanced HCC, it yields low radiographic response rates and transient stability, with no chance of tumor ablation or cure ( 5 ). Randomized trials have failed to identify a systemic therapy regimen that is superior to sorafenib ( 6–8 ).
Local therapeutic options for unresectable HCC include radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), other ablative procedures, and transarterial chemoembolization (TACE). Encouraging local control rates and cases of long-term survival following RFA or PEI have been reported, typically when small (<2–3 cm) lesions are treated ( 9–12 ). Lesions not suitable for local ablation are generally treated with TACE. While TACE provides a survival benefit over supportive care for unresectable HCC, objective responses are seen in only approximately one-third of patients ( 13 ), and long-term survival following TACE for HCC is rare ( 14 ).
Because of a lack of randomized trial data supporting its safety and efficacy, ionizing external beam radiotherapy (RT) has been used relatively rarely in the management of HCC ( 15 ). Because of recent advances, RT is now listed in the National Comprehensive Cancer Network Guidelines as a locoregional treatment option for inoperable HCC (Category 2B, http://www.nccn.org ). In this paper, we briefly review published experiences using various forms of external beam RT for HCC. We summarize available data regarding the combination of RT and radiosensitizing agents, and we suggest that the unique attributes of HCC necessitate novel approaches to studying multimodality treatment regimens, including RT.
Radiotherapy as Monotherapy for HCC
The widespread adoption of RT for HCC has been hindered by several challenges. RT has historically yielded suboptimal results in the treatment of HCC with regards to both treatment efficacy and toxicity. The prospects of using RT effectively for HCC, however, have improved with the development of improved treatment techniques.
Whole liver RT, which can be an effective palliative measure for patients with painful liver metastases, can only be delivered safely to doses of approximately 30 Gy using standard fractionation ( 16 ). When the whole liver is treated to doses above 30 Gy, the risk of radiation-induced liver damage (RILD) increases substantially. RILD typically occurs within three months after hepatic irradiation. Patients may present with fatigue, weight gain, hepatomegaly, anicteric ascites, and a relatively isolated elevation in alkaline phosphatase compared with other liver enzymes. Patients with liver cirrhosis are at increased risk for RILD compared with patients with healthy livers ( 17 , 18 ). Patients with chronic hepatic disease may also develop “nonclassic” RILD, which can present with jaundice and an elevation in all liver enzymes ( 19 ). Because of strong associations between liver disease, HCC formation, and intolerance to hepatic RT, the “therapeutic window” for effectively treating HCC with whole liver RT is essentially nonexistent in the absence of effective strategies to protect against or reverse RILD. Low-dose RT may be used to palliate symptoms from end-stage HCC ( 20 ).
The delivery of conformal partial liver RT can allow for safe dose escalation as the liver parenchyma is arranged with functionally parallel architecture. Single-institution experiences in which HCC patients received partial liver RT with median doses of 40 to 66 Gy using standard fractionation demonstrate response rates of 57% to 92% and severe (grade ≥ 3) late toxicity rates of less than 15%. Median overall survival in those series ranges from nine to 16 months ( 21–24 ).
Recent technological advances in target definition, treatment planning, and setup verification have allowed radiation oncologists to explore hypofractionated stereotactic body RT (SBRT) for a number of malignancies, including HCC. Potential benefits of SBRT include decreased normal tissue irradiation, delivery of increased biologically effective doses to target tissues, and exploitation of tumoricidal mechanisms that are not active when standard fractionation is used ( 25 ). Condensed treatment courses also mitigate concerns regarding accelerated tumor cell repopulation and are more convenient for patients.
Numerous experiences using SBRT for primary liver tumors have now been reported. A wide variety of dosing and fractionation schedules have been used, with total doses ranging from 24 to 60 Gy over three to 10 fractions ( 26–33 ). Review of the largest series describing SBRT for primary liver tumors leads to the following conclusions:
SBRT can be implemented safely in properly selected HCC patients. Reported severe toxicity rates following SBRT are generally less than 10% in Child-Pugh A patients (26-32,34,35). Over the past two decades, radiation oncologists have gained a greater understanding of the dose-volume effects of partial liver RT. Normal tissue complication probability (NTCP) models are now able to predict the risk of classic RILD associated with a given treatment plan ( 36 , 37 ). In many institutions, SBRT dosing is being individualized for each patient based on tumor size, predicted NTCP, and/or liver function ( 26 , 27 , 29 , 32 ).
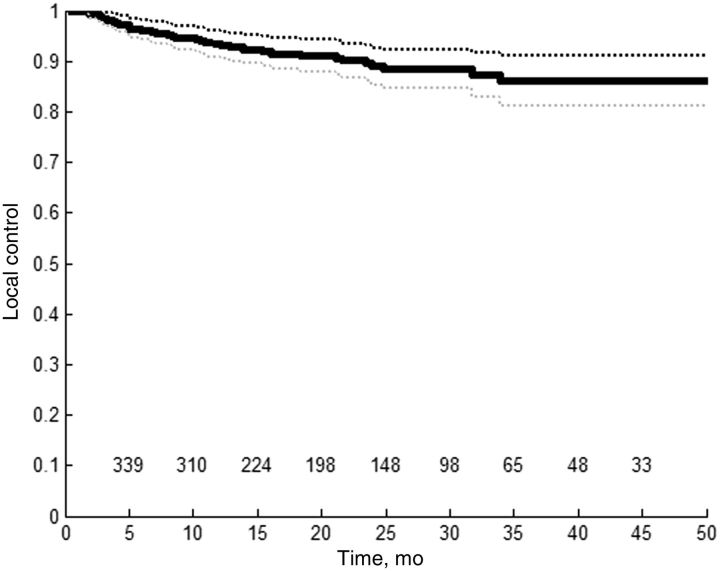
SBRT for HCC yields excellent local tumor control rates. Reported local control rates two to three years following SBRT for HCC range from 68% to 95% (26-28,31-33,35,38-40). These series are summarized in Table 1 . In a systematic review synthesizing data from the treatment of nearly 400 lesions, actuarial local control rates at one, two, and three years were 93%, 89%, and 86%, respectively ( 41 ) ( Figure 1 ). A recent retrospective study suggested that SBRT may achieve better local control rates than RFA for tumors larger than 2 cm ( 39 ). It is difficult to comment on control rates at time points beyond two to three years as follow-up is limited in published series and long-term survival for patients with inoperable HCC remains rare.
Out-of-field disease progression following SBRT for HCC is common. In contrast to the encouraging local control rates quoted above, two- to three-year progression-free survival (PFS) rates following SBRT range from 21% to 48% (26-28,30,31,33,35,40). Following SBRT, disease progression most often occurs in the untreated liver ( 28 , 31 ). This, in combination with underlying patient characteristics (poor functional status, chronic liver disease), produces disappointing two- to three-year overall survival rates following SBRT of 21% to 69% (26-31,40,42).
Response assessment following SBRT for HCC is difficult. Following SBRT, 37% to 85% of HCC lesions demonstrate a partial or complete response using RECIST criteria or similar tools (26-29,31,32,34,42). RECIST and other conventional response assessment algorithms, however, may not be predictive of clinical outcomes following localized therapy for HCC ( 43 ). Other radiographic response criteria, based on visualization of tumor necrosis and/or intratumoral arterial enhancement, have been developed and examined as predictors of clinical outcomes following TACE or RFA ( 42 , 44–46 ). Few reports have examined the relationship between treatment response and eventual clinical outcomes following SBRT for HCC ( 26 , 28 ). Functional imaging such as FDG-PET may also have a role in this setting and is being explored by some groups.
Table 1.
Results from selected series of stereotactic radiotherapy for hepatocellular carcinoma reporting local control rates at two to three years
| First author (country) | Sample size | SBRT schedule | Prescription point/volume | Median follow-up (range) | Local control |
|---|---|---|---|---|---|
| Andolino (United States) | 60 patients | 24–48 Gy, 3–5 fx | PTV (80% IDL) | 27 mo | 90% at 2 y |
| Dewas (France) | 42 patients * , 48 lesions * | Median 45 Gy, 3 fx | PTV (80% IDL) | 15 mo | 91% at 2 y |
| Honda (Japan) | 30 patients * | Median 48 Gy, 4 fx | Isocenter | 12 mo ( 6–38 ) | 94% at 2 y |
| Jang (Korea) | 82 patients, 95 lesions | <45 Gy, 3 fx (n = 11) 45–54 Gy, 3 fx (n = 47) >54 Gy, 3 fx (n = 57) | PTV (70%–80% IDL) | 30 mo ( 4–81 ) | 87% at 2 y |
| Kang (Korea) | 47 patients | Risk-adapted, 3 fx | PTV (70%–80% IDL) | 17 mo ( 6–38 ) | 95% at 2 y |
| Kwon (Korea) | 42 patients | Median 33 Gy, 3 fx | PTV (70%–85% IDL) | 29 mo ( 8–49 ) | 68% at 3 y |
| Sanuki (Japan) | 185 patients | 35 Gy, 5 fx (n = 48) 40 Gy, 5 fx (n = 137) | PTV (70%–80% IDL) | 25 mo† ( 3–80 ) | 91% at 3 y |
| Wahl (United States) | 63 patients, 83 lesions | 27–60 Gy, 3–5 fx | PTV (75%–85% IDL) | 13 mo | 84% at 2 y |
*Subset of larger cohort with hepatocellular carcinoma. fx = fractions; IDL = isodose line; PTV = planning target volume; SBRT = stereotactic body radiotherapy.
†Estimate.
Figure 1.
Kaplan-Meier curve for local control following stereotactic body radiotherapy for hepatocellular carcinoma, based on a recent systematic review ( 41 ). Sample size = 394 lesions. Numbers remaining at risk are listed above the x-axis.
Radiosensitization for HCC
For many solid tumors, concurrent radiosensitizing chemotherapy is routinely added to definitive radiotherapy based on randomized trials demonstrating improvements in local control and/or overall survival. A typical example is cervical squamous cell carcinoma. Cisplatin was established as the cornerstone of therapy for metastatic disease over 30 years ago ( 47 ). Subsequent preclinical reports suggested that cisplatin affects the radiosensitivity of cervical carcinoma cell lines ( 48 ). RT was already an option for the definitive treatment of cervical cancer, so clinical trials were performed to test the combination of cisplatin and RT in the curative setting ( 49 , 50 ). Based on the success of those trials, cisplatin-based chemoradiotherapy is now the standard of care for locally advanced cervical cancer.
While classical radiosensitizers have been studied in HCC as well, clinical results thus far have been less encouraging. In a prospective trial performed by the RTOG in the 1980s, nearly 200 HCC patients were treated with whole liver RT using either conventional fractionation (21.0 Gy in 3.0 Gy daily fractions) or a hyperfractionated schedule (24.0 Gy in 1.2 Gy fractions given twice daily) with concurrent doxorubicin and 5-FU ( 51 ). Increased toxicity rates were seen in patients treated with hyperfractionated RT, yet response rates were only approximately 20% in both groups. Median survival was approximately five months in each arm.
Partial liver RT, using a variety of dosing and fractionation schedules, has also been tested with numerous radiosensitizers for HCC. In a phase II study performed at the University of Michigan, radiotherapy was delivered concurrently with hepatic arterial floxuridine for patients with unresectable intrahepatic tumors ( 52 ). This chemotherapy was administered as a continuous infusion, typically through a percutaneous hepatic arterial catheter placed through the brachial artery or with a hepatic artery catheter and pump placed during a previous laparotomy. Cumulative radiotherapy doses were chosen to provide a 10% to 15% estimated maximum risk of RILD for each patient. The median RT dose delivered was 60.75 Gy delivered in 1.5 Gy fractions given twice daily (interquartile range = 51 to 75 Gy), and RILD actually occurred in only 4% of patients. Other serious adverse events were also rare. Among the 35 of 108 subjects with a diagnosis of HCC, objective responses were seen in 40% of patients, and median survival was an encouraging 15 months. In a Korean study of 40 HCC patients with portal vein thrombosis who were treated with partial liver RT and hepatic arterial 5-FU given continuously during weeks 1 and 5, similar results (45% response rate, 13 month median survival [95% confidence interval {CI} = 2 to 37 months]) were obtained ( 53 ).
Systemic administration of chemotherapy has also been used concurrently with conformal RT for HCC. A Korean group has published several reports describing their experience using partial liver RT in combination with chemotherapy ( 54–56 ). Patients were treated with conventionally fractionated RT to a dose of 45 Gy over five weeks, with infusional 5-FU administered during weeks 1 and 5. Patients then went on to receive TACE. Approximately one-half of patients displayed endoscopic evidence of radiation-induced gastroduodenal complications, and 15% had serious gastroduodenal complications ( 54 ). Thirty-four percent of patients demonstrated a radiographic response following chemoradiotherapy. Median PFS was 6.5 months (95% = 5.5 to 7.5 months), and median OS was 11.3 months (95% = 10.2 to 12.5 months). Among patients who underwent FDG-PET imaging prior to RT, high maximal tumor SUV was associated with decreased PFS and OS ( 56 ). In another analysis, AFP response, defined as a 50% reduction from pretreatment baseline, was reported in 68% of patients. AFP response correlated with radiographic response and an approximate doubling in median PFS and OS when compared with AFP nonresponders. Both radiographic response and AFP response were independent predictors of prolonged PFS and OS on multivariable analysis ( 55 ).
Smaller series in which conformal RT doses of 40-50 Gy have been combined with other agents such as capecitabine ( 57 ) and thalidomide ( 58 ) have demonstrated encouraging toxicity profiles, with median survival ranging from nine to 12 months.
Numerous groups are exploring the combination of multitargeted small molecule tyrosine kinase inhibitor sorafenib with RT. Two randomized trials demonstrated that sorafenib prolongs overall survival for patients with inoperable HCC and established sorafenib monotherapy as the first-line systemic treatment for advanced HCC around the world ( 5 , 59 , 60 ). Preclinical data also demonstrates that sorafenib may act as a potent radiosensitizer in HCC cell lines ( 61 ). Several case reports describe impressive radiographic responses when HCC has been treated with the combination of sorafenib and RT ( 62 , 63 ). A similar agent, sunitinib, has yielded impressive results when added to conformal RT in a single-institution experience ( 64 ). A search of ClinicalTrials.gov reveals approximately 10 ongoing trials testing the combination of sorafenib and RT, all of which were initiated after the 2007 FDA approval of sorafenib for HCC. RT techniques employed in these studies include conventionally fractionated partial liver RT, SBRT, proton beam RT, and selective internal RT (SIRT). In RTOG 1112, nearly 400 patients with inoperable HCC unsuitable for TACE are being randomly assigned to receive sorafenib monotherapy vs SBRT followed by sorafenib. This sequential approach has been chosen to minimize toxicity risks. There are a few ongoing trials testing other agents (eg, bevacizumab, thalidomide) with RT.
Combined Modality Therapy for HCC – Special Considerations
It is logical that combined modality approaches including RT are not utilized by most centers in the treatment of HCC as both RT and systemic therapy have traditionally played a relatively minor role in this disease. As the aforementioned experiences with partial liver RT for HCC demonstrate, local tumor control does not necessarily translate to long-term patient survival because it does not address HCC patients’ underlying liver dysfunction and the risk of disease progression in the untreated liver or elsewhere. If, based on tumor location and patient characteristics, ablative RT doses can be delivered safely to conformal target volumes using SBRT, the focus of combined modality therapy should shift away from classical radiosensitizers, which act synergistically with RT to augment local cytotoxic effects. Ideal drug candidates for combination with RT would instead address the risk of out-of-field disease progression, either by controlling micrometastatic disease, attenuating the field cancerization effect seen in diseased livers, or promoting host antitumor immunity. Identification of such agents, which might not demonstrate activity against HCC as monotherapy in preclinical tests, poses a substantial challenge. Hence, future preclinical screenings of novel agents should be tested using standard chemo-radiotherapy approaches.
There is no shortage of systemic agents being studied for the treatment of HCC. Advances in cancer biology have elucidated numerous cellular signaling mechanisms that are critical to HCC development, progression, and metastasis. Signaling cascades implicated in the pathophysiology of HCC include the MAPK/ERK pathway, the PI3Kinase/AKT/mTOR pathway, the Wnt/β-Catenin pathway, and angiogenic pathways ( 65 ). In a search of ClinicalTrials.gov, we identified over 100 distinct targeted agents that are in various phases of clinical study for advanced HCC ( Table 2 ). Many more agents are being evaluated in preclinical experiments. Our challenge is therefore to design an efficient platform for identifying which of these targeted agents has potential for working as a complement to RT. We will briefly describe several forms of preclinical tests that are used in the study of HCC and suggest which might be most suitable for this purpose.
Table 2.
Targeted agents in clinical hepatocellular carcinoma studies, grouped by biologic compartment
| Compartment | Target | Agents |
|---|---|---|
| Tumor growth factors | ||
| EGF | Cetuximab * , erlotinib * , vandetanib * | |
| PDGF | Axitinib, BIBF 1120, linifanib, MEDI-575, orantinib, preretinoin, pazopanib * , sorafenib * , sunitinib * | |
| IGF | AVE 1642, BIIB 022, cixutumumab, linsitinib, MEDI-573 | |
| TGF | LY2157299 | |
| HGF | TAC-101 | |
| Cell signaling pathways | ||
| PI3K/mTOR | AZD 8055, CC-223, NVP-BEZ 235, salirasib, evorlimus * , sirolimus * , tacrolimus * | |
| MEK/ERK | BAY 86-9766, isomalto oligosaccharide sulfate, PD 0325901, selumetinib | |
| JAK/STAT | AZD 1480, OPB-31121 | |
| “Pro-apoptotic” | Artemisinin, cantharidin analogues, fenretinide, genistein, melatonin, xanthohumol, XIAP antisense AEG 35156, fluvastatin * , simvastatin * | |
| HDAC | Belinostat, panobinostat, resminostat, vorinostat * | |
| Tumor microenvironment | ||
| VEGF | Apatinib, axitinib, brivanib, cediranib, cabozantinib, foretinib, linifanib, nintedanib, orantinib, ramucirumab, vatalanib, bevacizumab * , pazopanib * , sorafenib * , sunitinib * , vandetanib * | |
| PDGF | Axitinib, linifanib, nitedanib, orantinib, preretinoin, pazopanib * , sorafenib * , sunitinib * | |
| Other antiangiogenics | AMG 386, bavituximab, PI-88, tetrathiomolybdate, lenalidomide * , thalidomide * | |
| Hypoxia | Darinaparsin, TH-302 |
*US Food and Drug Administration–approved. EGF = epidermal growth factor; ERK = extracellular signal-regulated kinase; HDAC = histone deacetylase; HGF = hepatocyte growth factor; IGF = insulin-like growth factor; JAK = Janus kinase; MEK = mitogen-activated protein kinase; mTOR = mammalian target of rapamycin; PDGF = platelet-derived growth factor; PI3K = phosphoinositide 3-kinase; STAT = signal transducer and activator of transcription; TGF = transforming growth factor; VEGF = vascular endothelial growth factor.
In vitro Testing. The standard technique for determining the effectiveness of one or several antineoplastic agents is the clonogenic cell survival assay ( 66 ). Classical radiosensitizers, which act synergistically with RT to reduce cell survival, can be identified using this technique. While this test is relatively straightforward and can be performed rapidly, it has several limitations. Its results reflect activity against one or several cell lines, which may be poor representations of actual human tumors. An important drawback is the inability to model therapeutic effects on tumor stroma. This is particularly relevant in the case of targeted biologic agents, many of which act by altering interactions between a tumor and its microenvironment. In the aforementioned preclinical report testing the combination of sorafenib and RT against a colorectal cancer cell line, for example, there was little evidence of radiosensitization in vitro, yet substantial synergy between RT and sorafenib was demonstrated in vivo ( 67 ). Other tests that can be performed in vitro include assays of DNA damage (eg, γ-H2AX probes) and tests for activation of radiation-induced signaling pathways (eg, PI3K/mTOR).
In vivo Testing Rodents are commonly used for cancer research because of their high breeding capacity, short lifespan, and physiologic and genetic similarities to humans. Mice have been used extensively for in vivo HCC experiments, and we believe that mouse models will play a large role in the discovery of effective agents for combination with RT. The most relevant methodology for this purpose is probably the tumor growth delay assay, in which treatments are evaluated based on retardation of disease progression in tumor-bearing mice. Several forms of mouse HCC models have been developed.
In xenograft cancer models, HCC tumors are formed by injecting human cancer cells into immunodeficient mice. Both the source and the target of the xenograft cells may vary. Tumors can be established by direct implantation of material from biopsy or resection of a human HCC. Injections can be ectopic (typically in the subcutaneous tissue of the mouse flank) or orthotopic (into the mouse liver). In the case of orthotopic models, sophisticated animal imaging platforms may be required for accurate tumor measurement ( 68 ). The use of established cell lines facilitates comparison of results from different experiments. Tumor phenotypes can vary greatly between cell lines, however, so it important to use several cell lines when using the xenograft model. The primary advantage of xenograft models is the short time span required for tumor formation. A key drawback is that the important interplay between tumors, the immune system, and cancer therapeutics is lost when experiments are performed using immunocompromised mice.
The relevance of murine models to the human HCC population may be heightened in nonxenograft models, where chronic chemical exposure is used induce liver disease and HCC formation in mice. Agents that have been used for this purpose include N-nitrosodiethylamine, aflatoxins, peroxisome proliferators, carbon tetrachloride, and thioacetamide ( 69 ). The incidence of chemically induced HCC varies between agents but is typically greater than 70%. The timing of tumor formation also varies between compounds and dose levels and is generally between 20 and 100 weeks. The aggressiveness, metastatic potential, and molecular characteristics of the HCC tumors also depend on the carcinogenic agent. One advantage of chemically induced models is that they mimic the injury-fibrosis-malignancy cycle seen in humans. The hepatic tumor environment may therefore resemble that of human HCC patients, and the tolerance of the injured murine liver to aggressive treatments may be comparable with that of a cirrhotic human liver.
Combining the two approaches described above, some investigators have studied HCC by stimulating murine liver damage using carbon tetrachloride or alcohol and injecting HCC cells directly into the fibrotic livers ( 70 ). Tumor growth and metastasis is accelerated in these models, but the use of cell lines necessitates repetition with several HCC variants.
Finally, genetically modified models (GMMs) can be engineered to mimic the behavioral and molecular features of human HCC in mice. Transgenic mouse genomes can be constructed to include fragments of viral DNA (eg, hepatitis B virus, hepatitis C virus), to overexpress oncogenes (eg, c-myc, β-catenin), to overexpress growth factors (eg, TGF-α, EGF), or to have deficient protein transport mechanisms (eg, alpha-1 antitrypsin). Each of these alteratiofns leads to HCC formation and/or hepatic fibrosis in mice ( 69 ).
We believe that the best models for studying combined modality treatment approaches involving RT are those in which HCC tumors develop within diseased liver tissue. Additionally, small animal irradiation platforms should be used to deliver targeted RT to the mouse tumors, just as RT would be implemented in HCC patients. This will allow for concurrent evaluation of radiographic and histopathologic responses, damage to uninvolved hepatic tissue, and out-of-field disease progression rates. Agents that yield promising results with regards to these endpoints should then be studied in clinical HCC trials.
A relatively new consideration in experimental oncology is the cancer stem cell (CSC) theory. CSCs are thought to possess unique survival mechanisms and have the ability to self-renew, differentiate, and proliferate, even after a prolonged period of quiescence ( 71 ). CSCs may also be resistant to conventional chemotherapy and RT ( 72 ). Several recent reports strongly support the CSC hypothesis ( 73-75 ), highlighting CSCs as appealing therapeutic targets. Numerous markers (eg, CD133, CD44, EpCAM) for HCC CSCs have been identified, and several pathways (eg, Wnt/β-catenin, AKT, IL-6) that are central to hepatic CSC signaling have been described ( 76 ). Agents that target HCC CSCs by inhibiting these pathways may be ideal candidates for combination with local treatments such as RT.
Defining the Current Role of RT in HCC
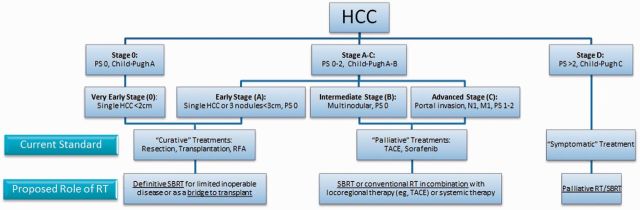
Based on the clinical data summarized above, we believe that RT can be incorporated into the management of HCC in several situations, depending on disease extent and patient characteristics. Figure 2 depicts how we suggest RT might be incorporated into the BCLC staging system ( 77 , 78 ). Of note, there are many factors (eg, tumor location, specific tumor size beyond 3 cm, prior treatments received) that are not included in the BCLC algorithm that are critical in selecting the optimal treatment pathway for a specific patient. We therefore advocate careful multidisciplinary evaluation for every HCC patient. We believe that the evidence supporting our recommendations falls into NCCN Category 2B or USPSTF Level II.
Figure 2.
Current Barcelona Clinic Liver Classification system and proposed roles for radiotherapy in the management of hepatocellular carcinoma. HCC = hepatocellular carcinoma; PS = performance status; RFA = radiofrequency ablation; RT = radiotherapy; SBRT = stereotactic body radiotherapy; TACE = transarterial chemoembolization.
For early-stage disease (including “very early-stage”), SBRT may be added to our armamentarium of ablative therapies that are likely to achieve long-term local control. With other modalities, such as RFA, it has already been established that treatment efficacy decreases as target size increases ( 39 , 79 ). This may be the case for SBRT as well, as large lesions are paradoxically generally treated with less aggressive dosing schedules based on toxicity risks ( 80 ). One important exception to this generalization might be in the use of particle therapy, whose highly conformal treatment delivery might allow large lesions to be treated safely with aggressive SBRT schedules ( 81–83 ). In any case, the selection of the best treatment modality should be based on the perceived chances of achieving tumor control without causing toxicity for each individual patient. For lesions located near the diaphragm, liver capsule, or large vessels, where RFA may not be optimal, SBRT may be the preferred ablative therapy.
For intermediate-stage disease (defined in the BCLC classification as “multinodular, PS 0” but perhaps also including patients with large solitary tumors and/or somewhat impaired performance status), it is likely that the best outcomes will be achieved using a combined-modality approach. Randomized trials suggest that RFA combined with TACE yields better outcomes than either modality alone ( 84–86 ), particularly for large lesions ( 85 ). Similarly, the combination of TACE and RT seems to yield better outcomes than TACE alone ( 35 , 87 ). Combined-modality strategies that can be considered include the combination of SBRT with another locoregional treatment or the combination of RT with a classical radiosensitizer in cases where SBRT might be unsafe because of nearby radiosensitive organs at risk.
For advanced disease (eg, diffuse liver involvement, extrahepatic disease, disease refractory to locoregional therapy), RT may be used in a palliative role ( 88 ). SBRT may even be considered in selected patients with advanced cirrhosis ( 89 ).
Future Directions for RT in HCC
RT for Downstaging Prior to Liver Transplantation
Liver transplantation is an established treatment for early-stage HCC that eliminates the liver tumor(s), removes the major organ at risk for disease progression, and allows recovery of liver function. The Milan criteria were established to select patients with limited disease burden who are likely to have favorable oncologic outcomes following transplantation ( 90 ). For patients who are initially outside of transplant criteria, liver-directed treatments such as TACE, RFA, and/or radioembolization have “downstaged” patients to be within criteria in 24% to 69% of cases ( 91 ). Outcomes for such patients who undergo transplantation are comparable with outcomes for patients who are eligible for upfront transplantation ( 91 ).
Reports on the use of RT as a means to downstage HCC patients prior to liver transplantation are extremely limited. In one case report, conventionally fractionated RT (54 Gy in 27 fractions) was used to treat a 7.6 cm lesion that had progressed after TACE ( 92 ). The patient had a complete radiographic response and underwent transplantation, and explant pathology revealed a complete pathologic response. Given that excellent local control rates that have been observed when SBRT has been used for inoperable HCC patients, we believe that SBRT should be compared with other liver-directed treatments as a means for downstaging patients who are outside of transplant criteria. Downstaging may serve as a valuable clinical endpoint because patients who undergo transplant can be expected to have a favorable prognosis.
Combining RT With Immunotherapy for HCC
Immunotherapy, which is an emerging tool in oncology, has not yet been established in the treatment of HCC. Unlike some other malignancies, HCC cells do not appear to be inherently immunogenic. Furthermore, viral hepatitis infection and liver cirrhosis, which are exceedingly common in HCC patients, may generate an immunosuppressed state. Recent reports from early-phase clinical trials testing checkpoint inhibitors for HCC, however, demonstrate promising results with regards to treatment efficacy and tolerability ( 93 ). A recent randomized trial demonstrated that adjuvant treatment with activated killer T-cells may prolong survival following resection or tumor ablation ( 94 ).
There is a growing body of evidence indicating that RT may enhance the antitumor effects of immunotherapeutic agents through dissemination of tumor-associated antigens, activation of cellular danger signals, and modifications of the host microenvironment. A number of preclinical studies have demonstrated synergy between RT and agents targeting immune checkpoint proteins, such as CTLA-4 ( 95 , 96 ) and PD-1 ( 97 , 98 ). Clinical trials are now testing combinations of RT and various forms of immunotherapy ( 99 , 100 ).
Preclinical data using human HCC tumor cultures and murine models demonstrates that RT increases cell surface expression of immunogenicity markers and increases sensitivity to dendritic cell therapy ( 101–103 ). This strategy is particularly appealing, as conforming RT to a small target volume may enhance the efficacy of immunotherapy, leading to eradication of untreated macroscopic lesions and/or occult microscopic disease.
Preclinical and clinical studies demonstrate particular promise for the combination of RT and C-X-C chemokine receptor type 4 (CXCR4) blockade in the management of HCC. CXCR4 inhibition mobilizes hematopoietic stem cells into the bloodstream. CXCR4 signaling plays a role in HCC progression, and high CXCR4 expression in HCC patient tumor specimens has been correlated with advanced disease stage and inferior clinical outcomes ( 104 ). In several tumor models, CXCR4 silencing has been shown to increase tumor responsiveness to RT and chemotherapy ( 105 , 106 ). CXCR4 inhibition has recently been shown to increase sensitivity to anti-PD1 immunotherapy in a murine HCC model ( 107 ). Importantly, CXCR4 is implicated in liver fibrosis ( 108 ), and CXCR4 inhibition is being explored as a treatment for cirrhosis. The addition of CXCR4 inhibition may widen the therapeutic window for RT in HCC both by enhancing treatment efficacy and preventing progressive liver dysfunction.
Epigenetic Agents and Radiotherapy
Epigenetic modifications are heritable changes that affect gene expression without altering the genes sequences. Several agents with epigenetic mechanisms have gained approval for cancer therapy. Histone deacetylase (HDAC) inhibitors, in particular, have shown promise for the treatment of HCC ( 109 , 110 ). These agents have been found to have radiosensitizing properties in a variety of tumor models ( 111 , 112 ), including HCC ( 113 ). Early-phase clinical trials combining RT and HDAC inhibitors have been performed for a variety of malignancies ( 114 , 115 ). There is rationale for performing similar studies in HCC patients.
A final concept that bears mentioning is that the role of RT in HCC may expand if effective treatments for liver disease become available. Hepatocyte transplantation (HT) has already been proposed as an alternative to liver transplantation for the treatment of metabolic and end-stage liver diseases ( 116 ). Mouse models have demonstrated the potential of using HT to ameliorate RILD ( 117 ). Preclinical studies have also shown that multipotent bone marrow–derived cells have therapeutic potential in liver cirrhosis ( 118 ). Further development of strategies to improve the hepatic function of HCC patients prior to therapy would minimize the risk of treatment-related toxicity, and/or reverse treatment sequelae would expand the therapeutic window for RT in HCC.
Biomarkers
The presence of HCC and therapeutic interventions can lead to critical modifications in several components of both the tumor microenvironment and the surrounding normal tissue compartment. Measuring these changes could provide valuable predictive information regarding treatment efficacy and toxicity.
Alpha-fetoprotein is well-established as a blood-based tumor marker in HCC ( 119 ). AFP-L3, an isoform of AFP, is currently being tested as a more specific tumor marker for HCC ( 120 ). It may be particularly useful for patients who have indeterminate levels of AFP. Levels of the prothrombin precursor des-gamma-carboxyprotrombin (DCP) are also elevated in many HCC patients while it is not detectable in most other liver diseases. DCP and AFP-L3 are being studied as biomarkers in phase II clinical trials ( 121 ). Other potential tumor biomarkers of interest include hepatocyte growth factor (HGF) ( 122 ) and alpha-L-fucosidase (AFU) ( 123 ) . There has been an attempt to discover biomarkers using the proximity ligation assay of multiplex protein analysis in serum. Through this assay, four biomarkers were identified and tested in clinical settings ( 124 ).
Biomarkers of liver injury might aid with therapeutic decisions and/or prompt initiation of measures to mitigate treatment-related toxicities ( 125 ). While several biomarkers for liver injury have been studied ( 126 ), radiation-specific toxicity biomarkers in HCC have not been identified. This area warrants additional attention.
Clinical Research Priorities
Technological advancements have improved the therapeutic index of RT for HCC, such that SBRT appears to be at least comparable with other locoregional treatments for appropriately selected patients. Prospective clinical trials are needed to solidify the role of RT in the management of HCC.
Several randomized trials have demonstrated comparable outcomes when small HCC lesions are treated with RFA or resection ( 127 , 128 ). Similar trials will be needed in order to conclusively establish the role of SBRT in this setting.
RTOG 1112 is a pivotal trial seeking to incorporate SBRT into the multimodality treatment of patients with more advanced disease. Successful completion of this study will demonstrate that SBRT for HCC can be studied in the cooperative group setting and pave the way for future trials.
The possibility of using SBRT for downstaging has received relatively little attention to date. We believe that prospective trials testing this concept and comparing RT with other locoregional treatments as a means to achieve downstaging should be a priority. The ability to study explant pathology following neoadjuvant therapy may yield novel insights into the mechanisms by which RT for HCC can be optimized.
Translational Research Priorities
Preclinical and translational discoveries will be needed to unlock the full potential of RT and meaningfully improve outcomes in the HCC patient population. Clinical trials have thus far failed to establish a role for immunotherapy in HCC, but future studies will focus on combination immunotherapies and the detection of biomarkers to guide treatment selection ( 129 ). Epigenetic biomarkers and epigenetic inhibitors have shown promise in HCC models and may one day serve valuable roles in both the detection and treatment of HCC ( 130 ).
Conclusions
Recent technological advances have generated renewed interest for incorporating RT in the management of unresectable HCC. Depending on disease extent, current evidence supports the use of RT as a curative local therapy, in combination with regional or systemic therapy, and as a palliative measure. Available data suggest that RT may play a role in downstaging patients who are initially ineligible for liver transplant or as a means to enhance the efficacy of novel systemic treatments. Well-designed clinical trials are needed to establish how RT should be aligned with other therapies with specific biomarker monitoring for the optimal management of HCC.
References
- 1. Ferenci P, Fried M, Labrecque D , et al. . World Gastroenterology Organisation Guideline. Hepatocellular carcinoma (HCC): a global perspective . J Gastrointestin Liver Dis. 2010. ; 19 ( 3 ): 311 – 317 . [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM , et al. . Global cancer statistics . CA Cancer J Clin. 2011. ; 61 ( 2 ): 69 – 90 . [DOI] [PubMed] [Google Scholar]
- 3. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005 . J Clin Oncol. 2009. ; 27 ( 9 ): 1485 – 1491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davila JA, Duan Z, McGlynn KA , et al. . Utilization and outcomes of palliative therapy for hepatocellular carcinoma: a population-based study in the United States . J Clin Gastroenterol. 2012. ; 46 ( 1 ): 71 – 77 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llovet JM, Ricci S, Mazzaferro V , et al. . Sorafenib in advanced hepatocellular carcinoma . N Engl J Med. 2008. ; 359 ( 4 ): 378 – 390 . [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX, Rosmorduc O, Evans TR , et al. . SEARCH: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Sorafenib Plus Erlotinib in Patients With Advanced Hepatocellular Carcinoma . J Clin Oncol. 2015. ; 33 ( 6 ): 559 – 566 . [DOI] [PubMed] [Google Scholar]
- 7. Cainap C, Qin S, Huang WT , et al. . Linifanib Versus Sorafenib in Patients With Advanced Hepatocellular Carcinoma: Results of a Randomized Phase III Trial . J Clin Oncol . 2014. ; in press. [DOI] [PMC free article] [PubMed]
- 8. Johnson PJ, Qin S, Park JW , et al. . Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study . J Clin Oncol . 2013. ; 31 ( 28 ): 3517 – 3524 . [DOI] [PubMed] [Google Scholar]
- 9. Kuang M, Xie XY, Huang C , et al. . Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma . J Gastrointest Surg. 2011. ; 15 ( 12 ): 2165 – 2171 . [DOI] [PubMed] [Google Scholar]
- 10. Shiina S, Tateishi R, Arano T , et al. . Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors . Am J Gastroenterol. 2012. ; 107 ( 4 ): 569-577 ; quiz 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010 . Oncology. 2010. ; 78(Suppl 1) : 113 – 124 . [DOI] [PubMed] [Google Scholar]
- 12. Arii S, Yamaoka Y, Futagawa S , et al. . Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan . Hepatology. 2000. ; 32 ( 6 ): 1224 – 1229 . [DOI] [PubMed] [Google Scholar]
- 13. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival . Hepatology. 2003. ; 37 ( 2 ): 429 – 442 . [DOI] [PubMed] [Google Scholar]
- 14. El-Serag HB, Siegel AB, Davila JA , et al. . Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study . J Hepatol. 2006. ; 44 ( 1 ): 158 – 166 . [DOI] [PubMed] [Google Scholar]
- 15. Schwarz RE, Smith DD. Trends in local therapy for hepatocellular carcinoma and survival outcomes in the US population . Am J Surg. 2008. ; 195 ( 6 ): 829 – 836 . [DOI] [PubMed] [Google Scholar]
- 16. Russell AH, Clyde C, Wasserman TH , et al. . Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol . Int J Radiat Oncol Biol Phys. 1993. ; 27 ( 1 ): 117 – 123 . [DOI] [PubMed] [Google Scholar]
- 17. Tai A, Erickson B, Li XA. Extrapolation of normal tissue complication probability for different fractionations in liver irradiation . Int J Radiat Oncol Biol Phys. 2009. ; 74 ( 1 ): 283 – 289 . [DOI] [PubMed] [Google Scholar]
- 18. Liang SX, Zhu XD, Xu ZY , et al. . Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance . Int J Radiat Oncol Biol Phys. 2006. ; 65 ( 2 ): 426 – 434 . [DOI] [PubMed] [Google Scholar]
- 19. Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration . Semin Radiat Oncol. 2011. ; 21 ( 4 ): 256 – 263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soliman H, Ringash J, Jiang H , et al. . Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases . J Clin Oncol. 2013. ; 31 ( 31 ): 3980 – 3986 . [DOI] [PubMed] [Google Scholar]
- 21. Seo YS, Kim JN, Keum B , et al. . Radiotherapy for 65 patients with advanced unresectable hepatocellular carcinoma . World J Gastroenterol. 2008. ; 14 ( 15 ): 2394 – 2400 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park W, Lim DH, Paik SW , et al. . Local radiotherapy for patients with unresectable hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2005. ; 61 ( 4 ): 1143 – 1150 . [DOI] [PubMed] [Google Scholar]
- 23. Seong J, Park HC, Han KH , et al. . Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients . Int J Radiat Oncol Biol Phys. 2003. ; 55 ( 2 ): 329 – 336 . [DOI] [PubMed] [Google Scholar]
- 24. Mornex F, Girard N, Beziat C , et al. . Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies--mature results of the French Phase II RTF-1 trial . Int J Radiat Oncol Biol Phys. 2006. ; 66 ( 4 ): 1152 – 1158 . [DOI] [PubMed] [Google Scholar]
- 25. Park HJ, Griffin RJ, Hui S , et al. . Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) . Radiat Res. 2012. ; 177 ( 3 ): 311 – 327 . [DOI] [PubMed] [Google Scholar]
- 26. Kang JK, Kim MS, Cho CK , et al. . Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization . Cancer. 2012. ; 118 ( 21 ): 5424 – 5431 . [DOI] [PubMed] [Google Scholar]
- 27. Andolino DL, Johnson CS, Maluccio M , et al. . Stereotactic body radiotherapy for primary hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2011. ; 81 ( 4 ): e447 – e453 . [DOI] [PubMed] [Google Scholar]
- 28. Kwon JH, Bae SH, Kim JY , et al. . Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer . BMC Cancer. 2010. ; 10 : 475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tse RV, Hawkins M, Lockwood G , et al. . Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma . J Clin Oncol. 2008. ; 26 ( 4 ): 657 – 664 . [DOI] [PubMed] [Google Scholar]
- 30. Choi BO, Jang HS, Kang KM , et al. . Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma . Jpn J Clin Oncol. 2006. ; 36 ( 3 ): 154 – 158 . [DOI] [PubMed] [Google Scholar]
- 31. Huang WY, Jen YM, Lee MS , et al. . Stereotactic body radiation therapy in recurrent hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2012. ; 84 ( 2 ): 355 – 361 . [DOI] [PubMed] [Google Scholar]
- 32. Bujold A, Massey CA, Kim JJ , et al. . Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma . J Clin Oncol. 2013. ; 31 ( 13 ): 1631 – 1639 . [DOI] [PubMed] [Google Scholar]
- 33. Dewas S, Bibault JE, Mirabel X , et al. . Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy . Radiat Oncol. 2012. ; 7 : 166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Facciuto ME, Singh MK, Rochon C , et al. . Stereotactic body radiation therapy in hepatocellular carcinoma and cirrhosis: evaluation of radiological and pathological response . J Surg Oncol. 2012. ; 105 ( 7 ): 692 – 698 . [DOI] [PubMed] [Google Scholar]
- 35. Honda Y, Kimura T, Aikata H , et al. . Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma . J Gastroenterol Hepatol. 2012. ; 28 ( 3 ): 530 – 536 . [DOI] [PubMed] [Google Scholar]
- 36. Dawson LA, Normolle D, Balter JM , et al. . Analysis of radiation-induced liver disease using the Lyman NTCP model . Int J Radiat Oncol Biol Phys. 2002. ; 53 ( 4 ): 810 – 821 . [DOI] [PubMed] [Google Scholar]
- 37. Cheng JC, Liu HS, Wu JK , et al. . Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease . Int J Radiat Oncol Biol Phys. 2005. ; 62 ( 4 ): 1150 – 1156 . [DOI] [PubMed] [Google Scholar]
- 38. Sanuki N, Takeda A, Oku Y , et al. . Stereotactic body radiotherapy for small hepatocellular carcinoma: A retrospective outcome analysis in 185 patients . Acta Oncol. 2014. ; 53 ( 3 ): 399 – 404 . [DOI] [PubMed] [Google Scholar]
- 39. Wahl DR, Stenmark MH, Tao Y , et al. . Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma . J Clin Oncol. 2015. ; 34 ( 5 ): 452 – 459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jang WI, Kim MS, Bae SH , et al. . High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma . Radiat Oncol. 2013. ; 8 : 250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohri N, Jackson A, Mendez Romero A , et al. . Local Control Following Stereotactic Body Radiotherapy for Liver Tumors: A Preliminary Report of the AAPM Working Group for SBRT . Int J Radiat Oncol Biol Phys. 2014. ; 90(1, Supplement) : S52 . [Google Scholar]
- 42. Price TR, Perkins SM, Sandrasegaran K , et al. . Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma . Cancer. 2012. ; 118 ( 12 ): 3191 – 3198 . [DOI] [PubMed] [Google Scholar]
- 43. Sanuki-Fujimoto N, Takeda A, Ohashi T , et al. . CT evaluations of focal liver reactions following stereotactic body radiotherapy for small hepatocellular carcinoma with cirrhosis: relationship between imaging appearance and baseline liver function . Br J Radiol. 2010. ; 83 ( 996 ): 1063 – 1071 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruix J, Sherman M, Llovet JM , et al. . Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver . J Hepatol. 2001. ; 35 ( 3 ): 421 – 430 . [DOI] [PubMed] [Google Scholar]
- 45. Forner A, Ayuso C, Varela M , et al. . Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009. ; 115 ( 3 ): 616 – 623 . [DOI] [PubMed] [Google Scholar]
- 46. Shim JH, Lee HC, Kim SO , et al. . Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models . Radiology. 2012. ; 262 ( 2 ): 708 – 718 . [DOI] [PubMed] [Google Scholar]
- 47. Bonomi P, Blessing JA, Stehman FB , et al. . Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study . J Clin Oncol. 1985. ; 3 ( 8 ): 1079 – 1085 . [DOI] [PubMed] [Google Scholar]
- 48. Britten RA, Evans AJ, Allalunis-Turner MJ , et al. . Effect of cisplatin on the clinically relevant radiosensitivity of human cervical carcinoma cell lines . Int J Radiat Oncol Biol Phys. 1996. ; 34 ( 2 ): 367 – 374 . [DOI] [PubMed] [Google Scholar]
- 49. Keys HM, Bundy BN, Stehman FB , et al. . Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma . N Engl J Med. 1999. ; 340 ( 15 ): 1154 – 1161 . [DOI] [PubMed] [Google Scholar]
- 50. Morris M, Eifel PJ, Lu J , et al. . Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer . N Engl J Med. 1999. ; 340 ( 15 ): 1137 – 1143 . [DOI] [PubMed] [Google Scholar]
- 51. Stillwagon GB, Order SE, Guse C , et al. . 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study . Int J Radiat Oncol Biol Phys. 1989. ; 17 ( 6 ): 1223 – 1229 . [DOI] [PubMed] [Google Scholar]
- 52. Ben-Josef E, Normolle D, Ensminger WD , et al. . Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies . J Clin Oncol. 2005. ; 23 ( 34 ): 8739 – 8747 . [DOI] [PubMed] [Google Scholar]
- 53. Han KH, Seong J, Kim JK , et al. . Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis . Cancer. 2008. ; 113 ( 5 ): 995 – 1003 . [DOI] [PubMed] [Google Scholar]
- 54. Chon YE, Seong J, Kim BK , et al. . Gastroduodenal complications after concurrent chemoradiation therapy in patients with hepatocellular carcinoma: endoscopic findings and risk factors . Int J Radiat Oncol Biol Phys. 2011. ; 81 ( 5 ): 1343 – 1351 . [DOI] [PubMed] [Google Scholar]
- 55. Kim BK, Ahn SH, Seong JS , et al. . Early alpha-fetoprotein response as a predictor for clinical outcome after localized concurrent chemoradiotherapy for advanced hepatocellular carcinoma . Liver Int. 2011. ; 31 ( 3 ): 369 – 376 . [DOI] [PubMed] [Google Scholar]
- 56. Kim BK, Kang WJ, Kim JK , et al. . ( 18) F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma . Cancer. 2011. ; 117 ( 20 ): 4779 – 4787 . [DOI] [PubMed] [Google Scholar]
- 57. McIntosh A, Hagspiel KD, Al-Osaimi AM , et al. . Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma . Cancer. 2009. ; 115 ( 21 ): 5117 – 5125 . [DOI] [PubMed] [Google Scholar]
- 58. Ch'ang HJ, Hsu C, Chen CH , et al. . Phase II study of concomitant thalidomide during radiotherapy for hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2012. ; 82 ( 2 ): 817 – 825 . [DOI] [PubMed] [Google Scholar]
- 59. Cheng AL, Kang YK, Chen Z , et al. . Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial . Lancet Oncol. 2009. ; 10 ( 1 ): 25 – 34 . [DOI] [PubMed] [Google Scholar]
- 60. Song P, Tobe RG, Inagaki Y , et al. . The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011 . Liver Int. 2012. ; 32 ( 7 ): 1053 – 1063 . [DOI] [PubMed] [Google Scholar]
- 61. Yu W, Gu K, Yu Z , et al. . Sorafenib potentiates irradiation effect in hepatocellular carcinoma in vitro and in vivo . Cancer Lett. 2013. ; 329 ( 1 ): 109 – 117 . [DOI] [PubMed] [Google Scholar]
- 62. Horgan AM, Dawson LA, Swaminath A , et al. . Sorafenib and radiation therapy for the treatment of advanced hepatocellular carcinoma . J Gastrointest Cancer. 2012. ; 43 ( 2 ): 344 – 348 . [DOI] [PubMed] [Google Scholar]
- 63. Hsieh CH, Jeng KS, Lin CC , et al. . Combination of sorafenib and intensity modulated radiotherapy for unresectable hepatocellular carcinoma . Clin Drug Investig. 2009. ; 29 ( 1 ): 65 – 71 . [DOI] [PubMed] [Google Scholar]
- 64. Chi KH, Liao CS, Chang CC , et al. . Angiogenic blockade and radiotherapy in hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2010. ; 78 ( 1 ): 188 – 193 . [DOI] [PubMed] [Google Scholar]
- 65. Frenette C, Gish R. Targeted systemic therapies for hepatocellular carcinoma: clinical perspectives, challenges and implications . World J Gastroenterol. 2012. ; 18 ( 6 ): 498 – 506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoffman RM. In vitro sensitivity assays in cancer: a review, analysis, and prognosis . J Clin Lab Anal. 1991. ; 5 ( 2 ): 133 – 143 . [DOI] [PubMed] [Google Scholar]
- 67. Suen AW, Galoforo S, Marples B , et al. . Sorafenib and radiation: a promising combination in colorectal cancer . Int J Radiat Oncol Biol Phys. 2010. ; 78 ( 1 ): 213 – 220 . [DOI] [PubMed] [Google Scholar]
- 68. Ma X, Liu Z, Yang X , et al. . Dual-modality monitoring of tumor response to cyclophosphamide therapy in mice with bioluminescence imaging and small-animal positron emission tomography . Mol Imaging . 2011. ; 10 ( 4 ): 278 – 283 . [DOI] [PubMed] [Google Scholar]
- 69. Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research . Int J Exp Pathol. 2009. ; 90 ( 4 ): 367 – 386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kornek M, Raskopf E, Tolba R , et al. . Accelerated orthotopic hepatocellular carcinomas growth is linked to increased expression of pro-angiogenic and prometastatic factors in murine liver fibrosis . Liver Int. 2008. ; 28 ( 4 ): 509 – 518 . [DOI] [PubMed] [Google Scholar]
- 71. Reya T, Morrison SJ, Clarke MF , et al. . Stem cells, cancer, and cancer stem cells . Nature. 2001. ; 414 ( 6859 ): 105 – 111 . [DOI] [PubMed] [Google Scholar]
- 72. Rich JN. Cancer stem cells in radiation resistance . Cancer Res. 2007. ; 67 ( 19 ): 8980 – 8984 . [DOI] [PubMed] [Google Scholar]
- 73. Schepers AG, Snippert HJ, Stange DE , et al. . Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas . Science. 2012. ; 337 ( 6095 ): 730 – 735 . [DOI] [PubMed] [Google Scholar]
- 74. Mascre G, Dekoninck S, Drogat B , et al. . Distinct contribution of stem and progenitor cells to epidermal maintenance . Nature. 2012. ; 489 ( 7415 ): 257 – 262 . [DOI] [PubMed] [Google Scholar]
- 75. Chen J, Li Y, Yu TS , et al. . A restricted cell population propagates glioblastoma growth after chemotherapy . Nature. 2012. ; 488 ( 7412 ): 522 – 526 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma . Semin Oncol. 2012. ; 39 ( 4 ): 461 – 472 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification . Semin Liver Dis. 1999. ; 19 ( 3 ): 329 – 338 . [DOI] [PubMed] [Google Scholar]
- 78. Llovet JM. Updated treatment approach to hepatocellular carcinoma . J Gastroenterol. 2005. ; 40 ( 3 ): 225 – 235 . [DOI] [PubMed] [Google Scholar]
- 79. Tateishi R, Shiina S, Teratani T , et al. . Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases . Cancer. 2005. ; 103 ( 6 ): 1201 – 1209 . [DOI] [PubMed] [Google Scholar]
- 80. Dawson LA, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT . Acta Oncol. 2006. ; 45 ( 7 ): 856 – 864 . [DOI] [PubMed] [Google Scholar]
- 81. Chiba T, Tokuuye K, Matsuzaki Y , et al. . Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients . Clin Cancer Res. 2005. ; 11 ( 10 ): 3799 – 3805 . [DOI] [PubMed] [Google Scholar]
- 82. Petersen JB, Lassen Y, Hansen AT , et al. . Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours . Acta Oncologica. 2011. ; 50 ( 6 ): 823 – 828 . [DOI] [PubMed] [Google Scholar]
- 83. Wang X, Krishnan S, Zhang X , et al. . Proton radiotherapy for liver tumors: dosimetric advantages over photon plans . Med Dosim. 2009. ; 33 ( 4 ): 259 – 267 . [DOI] [PubMed] [Google Scholar]
- 84. Peng ZW, Zhang YJ, Chen MS , et al. . Radiofrequency Ablation With or Without Transcatheter Arterial Chemoembolization in the Treatment of Hepatocellular Carcinoma: A Prospective Randomized Trial . J Clin Oncol. 2013. ; 31 ( 4 ): 426 – 432 . [DOI] [PubMed] [Google Scholar]
- 85. Lu Z, Wen F, Guo Q , et al. . Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials . Eur J Gastroenterol Hepatol. 2013. ; 25 ( 2 ): 187 – 194 . [DOI] [PubMed] [Google Scholar]
- 86. Ni JY, Liu SS, Xu LF , et al. . Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis . J Cancer Res Clin Oncol. 2013. ; 139 ( 4 ): 653 – 659 . [DOI] [PubMed] [Google Scholar]
- 87. Meng MB, Cui YL, Lu Y , et al. . Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis . Radiother Oncol. 2009. ; 92 ( 2 ): 184 – 194 . [DOI] [PubMed] [Google Scholar]
- 88. Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: from palliation to cure . Cancer. 2006. ; 106 ( 8 ): 1653 – 1663 . [DOI] [PubMed] [Google Scholar]
- 89. Culleton S, Jiang H, Haddad CR , et al. . Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma . Radiother Oncol. 2014. ; 111 ( 3 ): 412 – 417 . [DOI] [PubMed] [Google Scholar]
- 90. Mazzaferro V, Regalia E, Doci R , et al. . Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis . N Engl J Med. 1996. ; 334 ( 11 ): 693 – 699 . [DOI] [PubMed] [Google Scholar]
- 91. Gordon-Weeks AN, Snaith A, Petrinic T , et al. . Systematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteria . Br J Surg. 2011. ; 98 ( 9 ): 1201 – 1208 . [DOI] [PubMed] [Google Scholar]
- 92. Wigg A, Hon K, Mosel L , et al. . Down-staging of hepatocellular carcinoma via external-beam radiotherapy with subsequent liver transplantation: a case report . Liver Transpl . 2013. ; 19 ( 10 ): 1119 – 1124 . [DOI] [PubMed] [Google Scholar]
- 93. Sangro B, Gomez-Martin C, de la Mata M , et al. . A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C . J Hepatol. 2013. ; 59 ( 1 ): 81 – 88 . [DOI] [PubMed] [Google Scholar]
- 94. Lee JH, Lee J-H, Lim Y-S , et al. . Adjuvant Immunotherapy with Autologous Cytokine-induced Killer Cells for Hepatocellular Carcinoma . Gastroenterology. 2015. ; 148 ( 7 ): 1383 – 1391, e6 . [DOI] [PubMed] [Google Scholar]
- 95. Demaria S, Kawashima N, Yang AM , et al. . Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer . Clin Cancer Res. 2005. ; 11 ( 2 Pt 1 ): 728 – 734 . [PubMed] [Google Scholar]
- 96. Yoshimoto Y, Suzuki Y, Mimura K , et al. . Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model . PLoS ONE. 2014. ; 9 ( 3 ): e92572 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Verbrugge I, Hagekyriakou J, Sharp LL , et al. . Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies . Cancer Res. 2012. ; 72 ( 13 ): 3163 – 3174 . [DOI] [PubMed] [Google Scholar]
- 98. Zeng J, See AP, Phallen J , et al. . Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas . Int J Radiat Oncol Biol Phys. 2013. ; 86 ( 2 ): 343 – 349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Seung SK, Curti BD, Crittenden M , et al. . Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses . Sci Transl Med. 2012. ; 4 ( 137 ): 137ra74 . [DOI] [PubMed] [Google Scholar]
- 100. Brody JD, Ai WZ, Czerwinski DK , et al. . In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study . J Clin Oncol. 2010. ; 28 ( 28 ): 4324 – 4332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin CC, Wang TE, Liu CY , et al. . Potentiation of the immunotherapeutic effect of autologous dendritic cells by pretreating hepatocellular carcinoma with low-dose radiation . Clin Invest Med . 2008. ; 31 ( 3 ): E150 – E159 . [DOI] [PubMed] [Google Scholar]
- 102. Kawashita Y, Deb NJ, Garg M , et al. . An autologous in situ tumor vaccination approach for hepatocellular carcinoma. 1. Flt3 ligand gene transfer increases antitumor effects of a radio-inducible suicide gene therapy in an ectopic tumor model . Radiat Res. 2014. ; 182 ( 2 ): 191 – 200 . [DOI] [PubMed] [Google Scholar]
- 103. Kawashita Y, Deb NJ, Garg MK , et al. . An Autologous In Situ Tumor Vaccination Approach for Hepatocellular Carcinoma. 2. Tumor-Specific Immunity and Cure after Radio-Inducible Suicide Gene Therapy and Systemic CD40-Ligand and Flt3-Ligand Gene Therapy in an Orthotopic Tumor Model . Radiat Res. 2014. ; 182 ( 2 ): 201 – 210 . [DOI] [PubMed] [Google Scholar]
- 104. Neve Polimeno M, Ierano C, D'Alterio C , et al. . CXCR4 expression affects overall survival of HCC patients whereas CXCR7 expression does not . Cell Mol Immunol. 2015. ; 12 ( 4 ): 474 – 482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liang S, Peng X, Li X , et al. . Silencing of CXCR4 sensitizes triple-negative breast cancer cells to cisplatin . Oncotarget. 2015. ; 6 ( 2 ): 1020 – 1030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Trautmann F, Cojoc M, Kurth I , et al. . CXCR4 as biomarker for radioresistant cancer stem cells . Int J Radiat Biol. 2014. ; 90 ( 8 ): 687 – 699 . [DOI] [PubMed] [Google Scholar]
- 107. Chen Y, Ramjiawan RR, Reiberger T , et al. . CXCR4 inhibition in tumor microenvironment facilitates anti-PD-1 immunotherapy in sorafenib-treated HCC in mice . Hepatology. 2015. ; 61 ( 5 ): 1591 – 1602 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ding BS, Cao Z, Lis R , et al. . Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis . Nature. 2014. ; 505 ( 7481 ): 97 – 102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee YH, Seo D, Choi KJ , et al. . Antitumor effects in hepatocarcinoma of isoform-selective inhibition of HDAC2 . Cancer Res. 2014. ; 74 ( 17 ): 4752 – 4761 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Venturelli S, Berger A, Bocker A , et al. . Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells . PLoS ONE. 2013. ; 8 ( 8 ): e73097 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. De Schutter H, Kimpe M, Isebaert S , et al. . A systematic assessment of radiation dose enhancement by 5-Aza-2'-deoxycytidine and histone deacetylase inhibitors in head-and-neck squamous cell carcinoma . Int J Radiat Oncol Biol Phys. 2009. ; 73 ( 3 ): 904 – 912 . [DOI] [PubMed] [Google Scholar]
- 112. Munshi A, Kurland JF, Nishikawa T , et al. . Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity . Clin Cancer Res. 2005. ; 11 ( 13 ): 4912 – 4922 . [DOI] [PubMed] [Google Scholar]
- 113. Lu YS, Chou CH, Tzen KY , et al. . Radiosensitizing effect of a phenylbutyrate-derived histone deacetylase inhibitor in hepatocellular carcinoma . Int J Radiat Oncol Biol Phys. 2012. ; 83 ( 2 ): e181 – e189 . [DOI] [PubMed] [Google Scholar]
- 114. Ree AH, Dueland S, Folkvord S , et al. . Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study . Lancet Oncol. 2010. ; 11 ( 5 ): 459 – 464 . [DOI] [PubMed] [Google Scholar]
- 115. Shi W, Lawrence YR, Choy H , et al. . Vorinostat as a radiosensitizer for brain metastasis: a phase I clinical trial . J Neurooncol. 2014. ; 118 ( 2 ): 313 – 319 . [DOI] [PubMed] [Google Scholar]
- 116. Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease . Semin Liver Dis. 1999. ; 19 ( 1 ): 39 – 48 . [DOI] [PubMed] [Google Scholar]
- 117. Guha C, Sharma A, Gupta S , et al. . Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation . Cancer Res . 1999. ; 59 ( 23 ): 5871 – 5874 . [PubMed] [Google Scholar]
- 118. Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease . Gut. 2007. ; 56 ( 5 ): 716 – 724 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Marrero JA. Screening tests for hepatocellular carcinoma . Clin Liver Dis. 2005. ; 9 ( 2 ): 235 – 251 , vi. [DOI] [PubMed] [Google Scholar]
- 120. Yamagata Y, Shimizu K, Nakamura K , et al. . Simultaneous determination of percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein and alpha-fetoprotein concentration using the LiBASys clinical auto-analyzer . Clin Chim Acta. 2003. ; 327 ( 1-2 ): 59 – 67 . [DOI] [PubMed] [Google Scholar]
- 121. Marrero JA, Su GL, Wei W , et al. . Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients . Hepatology. 2003. ; 37 ( 5 ): 1114 – 1121 . [DOI] [PubMed] [Google Scholar]
- 122. Yamazaki H, Oi H, Matsumoto K , et al. . Biphasic changes in serum hepatocyte growth factor after transarterial chemoembolization therapy for hepato-cellular carcinoma . Cytokine. 1996. ; 8 ( 2 ): 178 – 182 . [DOI] [PubMed] [Google Scholar]
- 123. Ishizuka H, Nakayama T, Matsuoka S , et al. . Prediction of the development of hepato-cellular-carcinoma in patients with liver cirrhosis by the serial determinations of serum alpha-L-fucosidase activity . Intern Med. 1999. ; 38 ( 12 ): 927 – 931 . [DOI] [PubMed] [Google Scholar]
- 124. Tsai C-L, Koong AC, Hsu F-M , et al. . Biomarker studies on radiotherapy to hepatocellular carcinoma . Oncology . 2012. ; 84 : 64 – 68 . [DOI] [PubMed] [Google Scholar]
- 125. Seidensticker M, Seidensticker R, Damm R , et al. . Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity . PLoS One. 2014. ; 9 ( 11 ): e112731 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Flores A, Marrero JA. Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics . Clin Med Insights Oncol. 2014. ; 8 : 71 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen MS, Li JQ, Zheng Y , et al. . A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma . Ann Surg. 2006. ; 243 ( 3 ): 321 – 328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Feng K, Yan J, Li X , et al. . A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma . J Hepatol. 2012. ; 57 ( 4 ): 794 – 802 . [DOI] [PubMed] [Google Scholar]
- 129. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG , et al. . The yin and yang of evasion and immune activation in HCC . J Hepatol. 2015. ; 62 ( 6 ): 1420 – 1429 . [DOI] [PubMed] [Google Scholar]
- 130. Banaudha KK, Verma M. Epigenetic biomarkers in liver cancer . Methods Mol Biol. 2015. ; 1238 : 65 – 76 . [DOI] [PubMed] [Google Scholar]