Abstract
Objective
To explore whether plasma inflammatory mediators on postoperative day 3 (POD3) are associated with pain scores in older adults after hip fracture surgery.
Design
Cross-sectional study.
Setting
Mount Sinai Hospital, New York, New York.
Subjects
Forty patients age 60 years or older who presented with acute hip fracture at Mount Sinai Hospital between November 2011 and April 2013.
Methods
Plasma levels of six inflammatory mediators of the nuclear factor kappa B pathway were measured using blood collected on POD3. Self-reported pain scores (i.e., pain with resting, walking, and transferring) were assessed at baseline (prefracture) and on POD3. Linear regression models using log-transformed data were performed to determine associations between inflammatory mediators and postoperative pain.
Results
Interleukin 18 (IL-18) was positively associated with POD3 resting pain score in the unadjusted model (β = 0.66, P = 0.03). Tumor necrosis factor α (TNF-α) and soluble TNF receptor II (sTNF-RII) were positively associated with POD3 resting pain score in the adjusted model (β = 0.99, P = 0.03, and β = 0.86, P = 0.04, respectively). Moreover, TNF-α was positively associated with POD3 walking pain score in the adjusted model (β = 1.59, P = 0.05). Pain with transferring was not associated with these inflammatory mediators.
Conclusions
These findings suggest that TNF-α and its receptors may influence pain following hip fracture. Further study of the TNF-α pathway may inform future clinical applications that monitor and treat pain in the vulnerable elderly who are unable to accurately report pain.
Keywords: Biomarkers, Hip Fracture, Postoperative Pain, Inflammation
Introduction
Hip fracture, a common occurrence in older adults, is associated with mortality, delirium, functional decline, and pain. In 2010, there were 258,000 hospital admissions for hip fracture among adults age 65 years and older in the United States [1]. By 2030, this number is expected to rise to 289,000 [2]. The mortality rate in the Medicare population after hip fracture is 7% at one month, 13% at three months, and 24% at 12 months [3]. Delirium after hip fracture surgery is seen in nearly 30% of older adults, and the average duration of delirium is a predictor of six-month mortality [4]. Hip fracture has also been linked to substantial functional decline in older adults [5,6].
Pain is a less explored but significant consequence of hip fracture. Patients often experience significant pain following hip fracture, and the clinical impact of this pain can be substantial. Older adults with undertreated pain have been found to be nine times more likely to develop delirium after hip fracture [7]. Higher postoperative pain scores in older adults following hip fracture have been associated with increased hospital length of stay, decreased probability of ambulation by postoperative day 3 (POD3), and lower locomotion scores at six months [8]. Early mobilization, a key factor in recovery from hip fracture surgery [9], can be negatively impacted by high postoperative pain [10].
Despite the negative effects of pain on hip fracture recovery in older adults, the biological mechanisms contributing to this pain remain unclear. A potential mechanism mediating post–hip fracture pain is the continued activation of inflammatory cascades past the immediate postoperative period. Pro-inflammatory mediators regulated through the nuclear factor kappa B (NF-κB) pathway have been linked to pain [11,12]. Tumor necrosis factor α (TNF-α) and its soluble receptors (sTNF-RI and sTNF-RII) [11,13–19] can influence pain directly or by interaction with other inflammatory mediators that are part of the NF-κB-mediated pathway (i.e., interleukin [IL] 1 receptor antagonist/IL-1ra, IL-6, IL-18) [11,19–21] and are also associated with pain [12,20,22–25]. Studies have shown that the plasma concentrations of inflammatory mediators often change following surgery or stress [26–28]. Given that trauma and surgery induce higher levels of inflammatory mediators and that these same mediators are linked to pain in a variety of different settings, there is a clear rationale to investigate a potential link between inflammatory mediator levels in blood and pain after hip fracture surgery. Thus, we postulate that elevated inflammatory mediators post–hip fracture surgery may contribute to development of postoperative pain.
While a small number of studies have investigated the relationship between inflammatory mediators and functional outcomes following hip fracture in older adults [29], to our knowledge, no one has studied the relationship of inflammatory mediators with pain in the hip fracture setting. A better understanding of this relationship may have prognostic and therapeutic implications in post–hip fracture pain management. In this study, we hypothesized that higher levels of inflammatory mediators on POD3 after hip fracture surgery are associated with increased pain scores. We therefore attempted to determine the relationship between POD3 plasma concentrations of NF-κB pathway inflammatory mediators and pain scores (i.e., pain with resting, walking, and transferring) in a group of adults age 60 years or older hospitalized due to acute hip fracture.
Methods
All study procedures including written informed consent were approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Study Design and Enrollment
Recruitment and enrollment of elderly hip fracture patients presenting to Mount Sinai Hospital (MSH), New York, New York, took place between November 2011 and April 2013. All 40 participants met the following inclusion criterion: a radiographically diagnosed acute hip fracture of the following types—articular, extra-articular, extracapsular, femoral head, femoral neck, intertrochanteric, intracapsular, peritrochanteric, subcapital, transcervical, or trochanteric. Exclusion criteria were applied as follows: patients who were/had 1) younger than age 60 years; 2) non-English speaking; 3) noncommunicating; 4) transferred from another hospital after hip fracture repair; 5) receiving immunosuppressive medications such as prednisone, tacrolimus, azathioprine, or mycophenolate mofetil; 6) scored lower than 4 on the Six Item Cognitive Screener [30]; 7) concurrent major internal injuries to the chest, abdomen, or pelvis; 8) limited fracture in the pelvis or acetabulum; 9) femoral shaft fracture; or 10) proximal femur fracture more than 2 cm below the lesser trochanter.
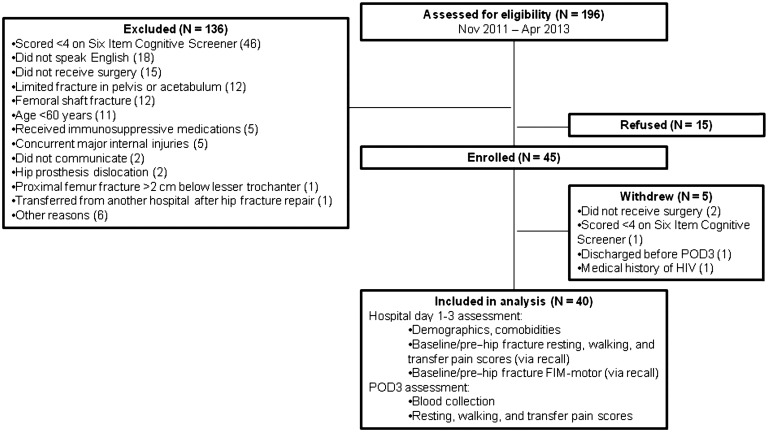
Research coordinators completed daily screens of all MSH adult emergency room patients using the hospital’s electronic medical records system to identify patients with potential acute hip fractures. A total of 196 patients were identified during the study’s enrollment period and assessed for eligibility (Figure 1). One hundred thirty-six of those patients were excluded for a variety of reasons, the most common of which was for scoring lower than 4 on the Six Item Cognitive Screener, indicating the presence of cognitive deficits (N = 46). Of the remaining patients, 15 declined to participate, leaving 45 enrolled. Five of the enrolled patients were subsequently removed from the study due to either not having received surgery (N = 2), scoring lower than 4 on the Six Item Cognitive Screener (N = 1), having a medical history of HIV (N = 1), or being discharged from the hospital prior to POD3 (N = 1). The remaining patients were assessed for baseline (pre–hip fracture) resting, walking, and transfer pain score via recall; baseline (pre–hip fracture) functional independence measure–motor score (baseline FIM-motor) via recall; demographics; and medical comorbidities. All participants received hip fracture surgery, anesthesia, and postsurgical care according to the standard of practice, and all care was directed by the attending surgeons and anesthesiologists, none of whom were directly involved in this study. On POD3, participants were asked to ambulate using a walker under the supervision of a physical therapist, were assessed for pain scores associated with three different activities (i.e., resting, walking, and transferring), and underwent blood collection. Participants were discharged on or after POD3 to rehabilitation facilities or home.
Figure 1.
Study design and participant enrollment.
Pain Assessment
Self-reported pain scores were utilized to assess pain severity. Participants were asked to rate their pain on a 0–10 scale, with 0 being no pain and 10 being the worst pain imaginable. Pain scores have been used in prior studies to assess pain following hip fractures [8,10] and have been shown to be reliable and valid for use across a number of different populations, including the elderly [31–34]. Pain scores were assessed in association with three different activities—at rest (resting pain), while walking (walking pain), and while transferring from bed to standing (transfer pain). After enrollment, participants provided baseline (pre–hip fracture) resting, walking, and transfer pain scores based on recall of their pain prior to hip fracture. In addition, the participants provided self-reported resting, walking, and transfer pain scores on POD3 after hip fracture surgery.
Biochemical Measurements
Participant blood samples were collected using EDTA or heparin-coated vacutainers on POD3 between 4 and 6 am. Blood samples were immediately centrifuged, and the supernatant (plasma) frozen and stored in aliquots in a –80°C freezer. Using the stored plasma samples, the concentrations of six inflammatory mediators—IL-1ra, IL-6, IL-18, TNF-α, sTNF-RI, sTNF-RII—were measured in duplicates using enzyme-linked immunosorbent assay (ELISA; Life Technologies Corporation, Carlsbad, CA, USA). The ELISAs were performed according to manufacturer protocols. The intra-assay coefficient of variation (CV%) and minimum detectable concentration for each ELISA target are as follows: 1) IL-1ra, 4.8%, 4 pg/mL; 2) IL-6, 6.3%, 0.25 pg/mL; 3) IL-18, 5.03%, 12.5 pg/mL; 4) TNF-α, 5.2%, 1.7 pg/mL; 5) sTNF-RI, 1.7%, 50 pg/mL; and 6) sTNF-RII, 2.1%, 0.1 ng/mL.
Statistical Analysis
In this study, the dependent variables were POD3 resting, walking, and transfer pain scores. The independent variables included POD3 concentrations of the six inflammatory mediators (IL-1ra, IL-6, IL-18, TNF-α, sTNF-RI, sTNF-RII). Simple linear regression (unadjusted model) and multiple linear regression (multivariable-adjusted model) were used to determine the associations of the independent variables with POD3 pain scores. The multivariable-adjusted model corrected for a prespecified set of baseline covariates (i.e., age, sex, baseline pain, baseline-FIM motor, and American Society of Anesthesiologists [ASA] physical status classification system). In these models, both the inflammatory mediator concentrations and pain scores were log-transformed due to non-normal distributions. The log-transformed concentrations of inflammatory mediators were compared between groups of participants who were administered general vs spinal anesthesia using t tests. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Both ASA and FIM-motor scores were included as covariates in the multivariable-adjusted model due to their potential confounding effects on pain. The ASA physical status classification allows anesthesiologists to note the overall health status of a patient before surgery so that uniform interpretation and patient stratification based on illness severity could be ascertained [35]. The presurgery ASA score has been associated with postoperative pain [36,37] and has been adjusted for in prior pain-related studies as a potential confounder [38,39]. The FIM-motor uses 13 tasks to assess functional ability in four domains (i.e., self-care, sphincter control, transfer ability, locomotion). Each task is scored on a 1–7 scale, with 1 being total assistance and 7 being complete independence. The individual responses to these 13 tasks can be summed to derive an FIM-motor score (maximum score of 91, or 13 × 7). Prior studies have shown the FIM-motor to be sensitive to changes in pain intensity [8,9].
Results
Characteristics of Study Participants
The study participants’ baseline characteristics are shown in Table 1. The average age was 79 ± 7.8 years. Sixty percent were women, 77.5% were white, and 12.5% were black or African American. The baseline cognition score was 5.1 ± 0.9 (out of a maximum of 6), and the baseline FIM-motor score was 85.7 ± 10.1 (out of a maximum of 91), suggesting relatively high pre–hip fracture surgery cognitive and functional status. The baseline mean pain scores (i.e., prior to fracture) were low, with resting pain of 1.9 ± 3.2, walking pain of 1.5 ± 2.7, and transfer pain of 1.9 ± 3.3, all scored on a 0–10 scale. Most participants were ASA class 3 (67.5%), and the most common comorbid medical condition was hypertension (57.5%).
Table 1.
Participant baseline characteristics, fracture descriptions, and anesthesia and surgical modalities received (N = 40)
| Characteristics | Value |
|---|---|
| Age, mean (SD) | 79 (7.8) |
| Female, No. (%) | 24 (60) |
| Race, No. (%) | |
| White | 31 (77.5) |
| Black or African American | 5 (12.5) |
| More than one race | 2 (5) |
| Unknown or not reported | 2 (5) |
| Baseline cognition score [30], mean (SD) | 5.1 (0.9) |
| Baseline FIM-motor score, mean (SD) | 85.7 (10.1) |
| Baseline pain score | |
| Baseline resting pain | 1.9 (3.2) |
| Baseline walking pain | 1.5 (2.7) |
| Baseline transfer pain | 1.9 (3.3) |
| ASA classification* | |
| ASA 2, No. (%) | 7 (17.5) |
| ASA 3, No. (%) | 27 (67.5) |
| ASA 4, No. (%) | 6 (15) |
| Medical comorbidities, No. (%) | |
| Hypertension | 23 (57.5) |
| Myocardial infarction | 2 (5) |
| Alzheimer's disease | 6 (15) |
| Cerebrovascular accident | 2 (5) |
| Prior fracture on opposite hip | 6 (15) |
| Cancer | 7 (17.5) |
| Past disease | 5 (12.5) |
| Current disease | 2 (5) |
| Fracture side, No. (%) | |
| Left | 21 (52.5) |
| Right | 19 (47.5) |
| Fracture type, No. (%) | |
| Intertrochanteric | 21 (52.5) |
| Peritrochanteric | 1 (2.5) |
| Femoral neck | 17 (42.5) |
| Subcapital | 1 (2.5) |
| Anesthesia type, No. (%) | |
| Spinal (subarachnoid block) | 19 (47.5) |
| General | 21 (52.5) |
| Surgery type, No. (%) | |
| Hemi-arthroplasty | 6 (15) |
| Total arthroplasty | 6 (15) |
| Intramedullary nail | 18 (45) |
| Dynamic hip screw | 6 (15) |
| Internal fixation, cannulated screw | 1 (2.5) |
| Percutaneous fixation, cannulated screw | 3 (7.5) |
ASA = American Society of Anesthesiologists; FIM = functional independence measure.
ASA status as determined by anesthesiologist during pre-op evaluation.
Left-sided hip fractures occurred in 52.5% of the participants. The most common types were intertrochanteric (52.5%) and femoral neck (42.5%) fractures. General anesthesia and spinal block were administered to 52.5% and 47.5% of the participants, respectively. The most common surgical repair procedure was intramedullary nail fixation (45%). All participants underwent hip fracture surgery within two to four days of presenting to the MSH emergency room (i.e., 23 on hospital day 2; 16 on hospital day 3; and 1 on hospital day 4). All participants received patient-controlled analgesia immediately after surgery, and none had received postoperative Pain Service consult for pain management.
Associations between POD3 Inflammatory Mediator Concentrations and POD3 Pain Scores
The plasma concentrations of inflammatory mediators measured on POD3 after surgery in all participants were as follows: IL-1ra, 499.1 ± 418.4 pg/mL; IL-6, 61.9 ± 53.6 pg/mL; IL-18, 68.4 ± 37.0 pg/mL; TNF-α, 23.3 ± 7.3 pg/mL; sTNF-RI, 5.9 ± 2.2 ng/mL; and sTNF-RII, 8.6 ± 3.4 ng/mL. In comparing groups of participants who were administered general vs spinal anesthesia, the plasma concentrations of each of these six inflammatory mediators were not significantly different between the two groups (IL-1ra, P = 0.84; IL-6, P = 0.33; IL-18, P = 0.74; TNF-α, P = 0.23; sTNF-RI, P = 0.99; and sTNF-RII, P = 0.57). The pain scores on POD3 were as follows: resting pain, 3.0 ± 3.2; walking pain, 4.1 ± 3.8; and transfer pain 4.5 ± 3.7. Forty participants provided a resting pain score, 29 provided a walking pain score, and 35 provided a transfer pain score. Those participants who were unable to walk or transfer on POD3 did not provide a score, thus accounting for the missing values.
The associations between the log values of plasma inflammatory mediator concentrations (IL-1ra, IL-6, IL-18, TNF-α, sTNF-RI, sTNF-RII) and the log values of POD3 resting, walking, and transfer pain scores are shown in Table 2. In the simple linear regression model (unadjusted), IL-18 was positively associated with POD3 resting pain (β = 0.66, P = 0.03). This association was not statistically significant in the multiple linear regression model (adjusted) after correcting for age, sex, baseline pain, baseline FIM-motor and ASA scores (β = 0.60, P = 0.08).
Table 2.
Associations between POD3 plasma concentrations of inflammatory mediators and POD3 pain scores
| Inflammatory mediator | Log (POD3 resting pain) |
Log (POD3 walking pain) |
Log (POD3 transfer pain) |
||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* | ||
| N = 40 | N = 40 | N = 29 | N = 29 | N = 35 | N = 35 | ||
| Log (TNF-α) | β | 0.60 | 0.99** | 0.67 | 10.59** | 0.38 | 0.22 |
| P | 0.17 | 0.03 | 0.12 | 0.05 | 0.34 | 0.68 | |
| Log (sTNF-RI) | β | 0.41 | 0.83 | 0.06 | −0.33 | −0.36 | −0.64 |
| P | 0.32 | 0.07 | 0.88 | 0.62 | 0.32 | 0.19 | |
| Log (sTNF-RII) | β | 0.65 | 0.86** | 0.28 | −0.03 | −0.05 | −0.52 |
| P | 0.09 | 0.04 | 0.46 | 0.96 | 0.89 | 0.28 | |
| Log (IL-1ra) | β | 0.04 | 0.11 | −0.12 | −0.29 | −0.09 | −0.30 |
| P | 0.85 | 0.58 | 0.50 | 0.35 | 0.17 | 0.14 | |
| Log (IL-18) | β | 0.66** | 0.60 | 0.27 | −0.11 | 0.17 | −0.38 |
| P | 0.03 | 0.08 | 0.36 | 0.83 | 0.55 | 0.33 | |
| Log (IL-6) | β | 0.22 | 0.26 | 0.24 | 0.25 | 0.04 | 0.07 |
| P | 0.14 | 0.10 | 0.11 | 0.24 | 0.79 | 0.69 | |
IL-1ra = interleukin 1 receptor antagonist; IL-18 = interleukin 18; IL-6 = interleukin 6; POD3 = postoperative day 3; sTNF-RI = soluble tumor necrosis factor receptor I; sTNF-RII = soluble tumor necrosis factor receptor II; TNF-α = tumor necrosis factor α.
Adjusted for age, sex, baseline pain, baseline functional independence measure–motor, and American Society of Anesthesiologists status.
Association is significant at the 0.05 level.
In the adjusted models, TNF-α (β = 0.99, P = 0.03) and sTNF-RII (β = 0.86, P = 0.04) were positively associated with POD3 resting pain. Moreover, TNF-α was positively associated with POD3 walking pain (β = 1.59, P = 0.05). In addition, the association of sTNF-RI with POD3 resting pain was not statistically significant (β = 0.83, P = 0.07). In contrast, POD3 transfer pain was not associated with any of the inflammatory mediators measured.
Discussion
In this study of adults age 60 years or older with acute hip fracture, we found that on POD3 after hip fracture surgery: 1) elevated TNF-α, sTNF-RII, and IL-18 plasma levels were associated with increased resting pain and 2) elevated TNF-α plasma level was associated with increased walking pain. These novel results suggest that there may be a link between inflammatory mediators of the NF-κB pathway and pain after hip fracture surgery in older adults, specifically with respect to TNF-α and its receptors.
Nuclear factor kappa B is a protein complex that regulates gene expression, cytokine production, and is a key mediator of the cellular stress response [40]. The NF-κB pathway is activated by, or regulates production of, IL-1ra, IL-6, IL-18, TNF-α, sTNF-RI, and sTNF-RII. Several of these inflammatory mediators have been investigated in the context of pain in a variety of studies. For instance, TNF-α and its receptors (sTNF-RI and sTNF-RII) have been linked to diabetic or nondiabetic neuropathic pain, complex regional pain syndrome, hyperalgesia, and osteoarthritis joint pain [11,13–19]. TNF-α can either act directly on nociceptors or interact with other NF-κB inflammatory mediators (IL-1ra, IL-6, IL-18) [11,19–21] that have been associated with pain [12,20,22–25]. Moreover, the levels of inflammatory mediators often change in response to surgery [26–28], and observational studies have shown that IL-1ra, IL-6, and TNF-α levels increase within the first three days after acute hip fracture in older adults [41–43]. With this background, we hypothesized that an elevation of inflammatory mediators of the NF-κB pathway after hip fracture surgery may influence postoperative pain. Our findings that TNF-α and sTNF-RII plasma levels are associated with some measures of pain three days after hip fracture surgery provided new evidence to support this hypothesis. These associations are consistent with what is known in the literature, but novel in the context of hip fracture pain in older adults. Interestingly, the associations between TNF-α, sTNF-RII, and postoperative pain were limited to pain with resting and walking, but not with transferring. This observation supports the notion that pain is a complex sensory experience and that multiple factors (e.g., tissue damage, physical exertion), in addition to inflammatory mediators, may precipitate pain after hip fracture surgery.
Interleukin 18 modulates activities of other pro-inflammatory mediators such as TNF-α, IL-6, and IL-1β and plays a role in neuropathic pain [44,45]. It has been observed that IL-18 level increases in microglia following peripheral nerve injury, suggesting that IL-18 influences development of neuropathic pain [24]. In addition, intrathecal injection of IL-18 enhances neuropathic pain processing similarly to those seen after nerve injury [25]. Taken together, we anticipated elevated postoperative IL-18 level to be associated with increased pain after hip fracture surgery. This positive association was observed between POD3 plasma level of IL-18 and resting pain in the simple linear regression model and approached statistical significance in the multivariable-adjusted model. While the analysis was limited by the small sample size, this finding provided preliminary evidence that IL-18 may have a role in resting pain in older adults after hip fracture surgery.
Pain following hip fracture surgery in older adults has a variety of effects, including higher incidence of delirium, increased length of hospitalization, and worsened long-term locomotion and rehabilitation [7,8,10]. Properly assessing and controlling pain following hip fracture surgery can have a positive effect on recovery and rehabilitation. However, the assessment of pain in older patients is complex, and thus pain in older adults is often underrecognized and undertreated [31]. The literature has shown that older patients are asked about pain less often and are administered both less frequent and lower doses of analgesia than younger patients [46]. Diminished cognitive function in some elderly patients, especially postsurgery, further exacerbates these barriers and limitations in pain management. Moreover, studies have shown that both health care providers and the patient’s caregivers often provide inaccurate pain estimates, specifically among the cognitively impaired [47,48]. Morrison and colleagues have found that hospitalized hip fracture patients with severe cognitive impairment receive less analgesia than cognitively intact older patients with similar fractures [7]. Additional barriers to accurate assessment and treatment of pain in the elderly include the belief that pain is persistent and cannot be helped and a fear of addiction and dependence [49]. Thus, the results of this study may be of particular benefit to older adults given the high frequency of hip fracture, high prevalence of pain following surgery, and the challenges in obtaining adequate pain assessment in this vulnerable population. Further validation of the role of TNF-α and its receptors and IL-18 in the pathogenesis of post–hip fracture surgery pain may identify novel targets for the assessment, monitoring, and treatment of pain in this clinical context. Specifically, TNF-α and other inflammatory biomarkers may provide an additional method to approximate pain in older adults who struggle to communicate their pain level. Furthermore, these inflammatory mediators may provide novel nonopioid targets for pain management after hip fracture. This may be particularly important given the current national concerns related to misuse of opioid analgesics. Finally, results from this study raise the question as to whether postoperative inflammatory mediator levels are associated with chronic postoperative pain, a condition that is common in a variety of surgeries. Future research addressing this topic may add to the growing rationale of using multimodal analgesia (e.g., use of opioids and gabapentinoids) in the peri-operative setting to optimize pain control after surgery.
This study had several limitations. Because of a small sample size of 40 and the high heterogeneity of human biological samples, the analyses used may lack sufficient statistical power to detect associations between some inflammatory mediators and POD3 pain scores. This may explain the near statistically significant associations between POD3 resting pain and sTNF-RI and IL-18. The subjectivity of self-reported pain score is another potential limitation. Pain is a complex sensory experience known only to the person subjected to it. The perception of pain could be influenced by factors such as pain medication, mood, and, in the case of baseline pain scores, recall bias, which may impact the reporting of pain. However, the pain rating scale used in this study is valid and reliable, and self-reporting remains the most accurate and appropriate tool for pain assessment [31]. Because narcotic requirement may provide a less subjective measure of pain severity and better approximate pain management, accurate quantification of this parameter (i.e., daily morphine equivalent) needs to be incorporated in future studies in this area of research. In addition, because the effect of peri-operative changes in inflammatory mediators on postoperative pain after hip fracture is unknown, additional time points of blood sampling (e.g., POD0, POD1, POD2) need to be incorporated in future study design to allow an expanded assessment in the influence of trajectory of these mediators on progression of postoperative pain. Although there is evidence suggesting that anesthesia type could affect levels of inflammatory mediators [50,51], this did not appear to be the case in our study. Despite our observation, anesthesia type and modality should be considered in the analysis of future postoperative pain biomarker investigations. Finally, findings from this study are correlational and not causal, nor are they mechanistic. However, these novel associations provide the rationale for future studies that investigate the mechanistic and pathological role of NF-κB pathway mediators in impacting pain sensation in older adults after hip fracture surgery.
Conclusions
In conclusion, this study provides novel evidence suggesting that the postoperative levels of some NF-κB pathway inflammatory mediators may influence resting and walking pain in older adults three days after hip fracture surgery. These findings support the hypothesis that post–hip fracture surgery inflammation may have a biological role in the development of postoperative pain. Further exploration and research in this area may lead to findings that help to improve assessment, monitoring, and management of pain after hip fracture surgery in older adults.
Acknowledgments
We wish to thank Taja Ferguson for her contribution to participant recruitment and data collection.
References
- 1. National Center for Health Statistics. National Hospital Discharge Survey. Available at: http://205.207.175.93/hdi/ReportFolders/ReportFolders.aspx?IF_ActivePath=P,18External (accessed August 2014).
- 2. Stevens JA, Rudd RA.. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int 2013;24:2725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu-Yao GL, Baron JA, Barrett JA, Fisher ES.. Treatment and survival among elderly Americans with hip fractures: A population-based study. Am J Public Health 1994;84:1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellelli G, Mazzola P, Morandi A, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc 2014;62:1335–40. [DOI] [PubMed] [Google Scholar]
- 5. Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM.. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med 2014;174:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci 2000;55:M498–507. [DOI] [PubMed] [Google Scholar]
- 7. Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci 2003;58:76–81. [DOI] [PubMed] [Google Scholar]
- 8. Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain 2003;103:303–311. [DOI] [PubMed] [Google Scholar]
- 9. Siu AL, Penrod JD, Boockvar KS, Koval K, Strauss E, Morrison RS.. Early ambulation after hip fracture: Effects on function and mortality. Arch Intern Med 2006;166:766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chin RP, Ho CH, Cheung LP.. Scheduled analgesic regimen improves rehabilitation after hip fracture surgery. Clin Orthop Relat Res 2013;471:2349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watkins LR, Maier SF, Goehler LE.. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995;63:289–302. [DOI] [PubMed] [Google Scholar]
- 12. Haddad JJ. On the enigma of pain and hyperalgesia: A molecular perspective. Biochem Biophys Res Commun 2007;353:217–24. [DOI] [PubMed] [Google Scholar]
- 13. Ortmann KLM, Chattopadhyay M.. Decrease in neuroimmune activation by HSV-mediated gene transfer of TNFα soluble receptor alleviates pain in rats with diabetic neuropathy. Brain Behav Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez-Clemente JM, Mauricio D, Richart C, et al. Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2005;63: 525–9. [DOI] [PubMed] [Google Scholar]
- 15. Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M.. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol 2006;59:843–51. [DOI] [PubMed] [Google Scholar]
- 16. Birklein F, Drummond PD, Li W, et al. Activation of cutaneous immune responses in complex regional pain syndrome. J Pain 2014;15:485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schafers M, Sorkin LS, Geis C, Shubayev VI.. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett 2003;347:179–82. [DOI] [PubMed] [Google Scholar]
- 18. Sommer C, Schmidt C, George A.. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol 1998;151:138–42. [DOI] [PubMed] [Google Scholar]
- 19. Miller RE, Miller RJ, Malfait AM.. Osteoarthritis joint pain: The cytokine connection. Cytokine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommer C, Kress M.. Recent findings on how proinflammatory cytokines cause pain: Peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neuroscience Letters. 2004;361:184–87. [DOI] [PubMed] [Google Scholar]
- 21. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bao Y, Fang J, Peng L, et al. Comparison of preincisional and postincisional parecoxib administration on postoperative pain control and cytokine response after total hip replacement. J Int Med Res 2012;40:1804–11. [DOI] [PubMed] [Google Scholar]
- 23. Cunha JM, Cunha FQ, Poole S, Ferreira SH.. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol 2000;130:1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daigo E, Sakuma Y, Miyoshi K, Noguchi K, Kotani J.. Increased expression of interleukin-18 in the trigeminal spinal subnucleus caudalis after inferior alveolar nerve injury in the rat. Neurosci Lett 2012;529:39–44. [DOI] [PubMed] [Google Scholar]
- 25. Miyoshi K, Obata K, Kondo T, Okamura H, Noguchi K.. Interleukin-18-mediated microglia/astrocyte interaction in the spinal cord enhances neuropathic pain processing after nerve injury. J Neurosci 2008;28:12775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kudoh A, Katagai H, Takazawa T, Matsuki A.. Plasma proinflammatory cytokine response to surgical stress in elderly patients. Cytokine 2001;15: 270–3. [DOI] [PubMed] [Google Scholar]
- 27. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109–17. [DOI] [PubMed] [Google Scholar]
- 28. Menger MD, Vollmar B.. Surgical trauma: Hyperinflammation versus immunosuppression? Langenbecks Arch Surg 2004;389:475–84. [DOI] [PubMed] [Google Scholar]
- 29. Miller RR, Cappola AR, Shardell MD, et al. Persistent changes in interleukin-6 and lower extremity function following hip fracture. J Gerontol A Biol Sci Med Sci 2006;61:1053–8. [DOI] [PubMed] [Google Scholar]
- 30. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC.. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–81. [DOI] [PubMed] [Google Scholar]
- 31. Catananti C, Gambassi G.. Pain assessment in the elderly. Surg Oncol 2010;19:140–8. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP.. Validity of four pain intensity rating scales. Pain 2011;152:2399–404. [DOI] [PubMed] [Google Scholar]
- 33. Hjermstad MJ, Fayers PM, Haugen DF, et al. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J Pain Symptom Manage 2011;41:1073–93. [DOI] [PubMed] [Google Scholar]
- 34. Collett B, O'Mahoney S, Schofield P, Closs SJ, Potter J.. The assessment of pain in older people. Clin Med 2007;7:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 2011;55:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinjo S, Sands LP, Lim E, Paul S, Leung JM.. Prediction of postoperative pain using path analysis in older patients. J Anesth 2012;26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caumo W, Schmidt AP, Schneider CN, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand 2002;46:1265–71. [DOI] [PubMed] [Google Scholar]
- 38. Goebel S, Steinert A, Vierheilig C, Faller H.. Correlation between depressive symptoms and perioperative pain: A prospective cohort study of patients undergoing orthopedic surgeries. Clin J Pain 2013;29:392–9. [DOI] [PubMed] [Google Scholar]
- 39. Baker PN, Petheram T, Avery PJ, Gregg PJ, Deehan DJ.. Revision for unexplained pain following unicompartmental and total knee replacement. J Bone Joint Surg Am 2012;94:e126. [DOI] [PubMed] [Google Scholar]
- 40. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007;8:49–62. [DOI] [PubMed] [Google Scholar]
- 41. Beloosesky Y, Hendel D, Weiss A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci 2007;62:420–6. [DOI] [PubMed] [Google Scholar]
- 42. Sedlar M, Kudrnova Z, Erhart D, et al. Older age and type of surgery predict the early inflammatory response to hip trauma mediated by interleukin-6 (IL-6). Arch Gerontol Geriatr 2010;51:e1–6. [DOI] [PubMed] [Google Scholar]
- 43. Knesek MJ, Litinas E, Adiguzel C, et al. Inflammatory biomarker profiling in elderly patients with acute hip fracture treated with heparins. Clin Appl Thromb Hemost 2010;16:42–50. [DOI] [PubMed] [Google Scholar]
- 44. Ten Hove T, Corbaz A, Amitai H, et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology 2001;121:1372–9. [DOI] [PubMed] [Google Scholar]
- 45. Plater-Zyberk C, Joosten LA, Helsen MM, et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest 2001;108:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Rond ME, de Wit R, van Dam FS, Muller MJ.. A pain monitoring program for nurses: Effects on communication, assessment and documentation of patients' pain. J Pain Symptom Manage 2000;20: 424–39. [DOI] [PubMed] [Google Scholar]
- 47. Cohen-Mansfield J, Lipson S.. Pain in cognitively impaired nursing home residents: How well are physicians diagnosing it? J Am Geriatr Soc 2002;50:1039–44. [DOI] [PubMed] [Google Scholar]
- 48. Shega JW, Hougham GW, Stocking CB, Cox-Hayley D, Sachs GA.. Factors associated with self- and caregiver report of pain among community-dwelling persons with dementia. J Palliat Med 2005;8:567–75. [DOI] [PubMed] [Google Scholar]
- 49. Weiner DK, Rudy TE.. Attitudinal barriers to effective treatment of persistent pain in nursing home residents. J Am Geriatr Soc 2002;50:2035–40. [DOI] [PubMed] [Google Scholar]
- 50. Ozcan S, Ozer AB, Yasar MA, Erhan OL.. Effects of combined general anesthesia and thoracic epidural analgesia on cytokine response in patients undergoing laparoscopic cholecystectomy. Niger J Clin Pract 2016;19:436–42. [DOI] [PubMed] [Google Scholar]
- 51. Xing ZM, Zhang ZQ, Zhang WS, Liu YF.. Effects of analgesia methods on serum IL-6 and IL-10 levels after cesarean delivery. Genet Mol Res 2015;14: 4778–83. [DOI] [PubMed] [Google Scholar]