Abstract
Objective
Opioid-based analgesics are a major component of the lengthy pain management of burn patients, including military service members, but are problematic due to central nervous system–mediated side effects. Peripheral analgesia via targeted ablation of nociceptive nerve endings that express the transient receptor potential vanilloid channel 1 (TRPV1) may provide an improved approach. We hypothesized that local injection of the TRPV1 agonist resiniferatoxin (RTX) would produce long-lasting analgesia in a rat model of pain associated with burn injury.
Methods
Baseline sensitivities to thermal and mechanical stimuli were measured in male and female Sprague-Dawley rats. Under anesthesia, a 100 °C metal probe was placed on the right hind paw for 30 seconds, and sensitivity was reassessed 72 hours following injury. Rats received RTX (0.25 μg/100 μL; ipl) into the injured hind paw, and sensitivity was reassessed across three weeks. Tissues were collected from a separate group of rats at 24 hours and/or one week post-RTX for pathological analyses of the injured hind paw, dorsal spinal cord c-Fos, and primary afferent neuropeptide immunoreactivity.
Results
Local RTX reversed burn pain behaviors within 24 hours, which lasted through recovery at three weeks. At one week following RTX, decreased c-Fos and primary afferent neuropeptide immunoreactivities were observed in the dorsal horn, while plantar burn pathology was unaltered.
Conclusions
These results indicate that local RTX induces long-lasting analgesia in a rat model of pain associated with burn. While opioids are undesirable in trauma patients due to side effects, RTX may provide valuable long-term, nonopioid analgesia for burn patients.
Keywords: Resiniferatoxin, Burn, Peripheral Analgesia, c-Fos, TRPV1, Opioid Sparing, Spinal Cord, Substance P, CGRP, Opioids
Introduction
Burn patients typically undergo hospital stays in order to continuously debride and change wound dressings for successful healing. Pain management is challenging in this setting as burn patients require complex and long-term treatment with numerous therapeutics. Furthermore, military service members represent a unique subpopulation of burn patients whose condition is often further complicated by polytrauma, resuscitation/evacuation from the battlefield, and lengthy rehabilitation and recovery. With improvements in both military armor [1] and medical procedures [2], there is now an increased survival rate for military service members with severe burn and blast injuries [3]. Unfortunately, burn patients must contend with ongoing pain associated with burn recovery and rehabilitation. The uniquely stressful environment and wound care may worsen and prolong the pain associated with burn injuries [4], further opposing optimal pain control.
Blocking the pain signal at the periphery, presents an opportunity for analgesia that avoids drug-induced CNS-mediated side effects [5–7]. Locally administered opioid-based therapeutics can also act at the periphery; however, their ability to provide optimal analgesia remains unclear [8], with clinical trials reporting little to no efficacy [9], with the exception of intra-articular administration sites [10]. One promising nonopioid pain therapeutic that targets the periphery is the novel compound resiniferatoxin (RTX), extracted from the Moroccan plant Euphorbia resinifera [11–14]. RTX is an ultrapotent agonist of the transient receptor potential vanilloid 1 (TRPV1) channel protein and is an analog of capsaicin, another naturally occurring TRPV1 agonist found in chili peppers. TRPV1 ion conductance is the main neural sensor of elevated temperature (>42 °C) and thus pain associated with heat [15]. Furthermore, TRPV1 can be activated or sensitized by prolonged exposure to additional stimuli, including protons, nerve growth factor (NGF), bradykinin (BK), serotonin (5HT), and other inflammatory mediators [16–18]. Importantly, overstimulation of the channel can lead to desensitization, or diminished pain signaling, and therefore can ameliorate pain [5,14,19,20]. For example, a topical patch containing high concentrations of capsaicin has been reported to be effective for the relief of arthritis and joint pain [21,22]; however, the acutely evoked pain due to capsaicin’s agonist activity can be hard to control, while the analgesic effects are quite transient [23].
Alternatively, RTX has a higher affinity for TRPV1 than capsaicin [20] and is a candidate for long-lasting, nonopioid peripheral analgesia [24,25]. RTX temporarily ablates TRPV1-expressing nociceptive nerve fibers for up to two weeks without damaging sensory neurons in rodent models of inflammatory hyperalgesia [11]. RTX is currently in clinical trials for cancer pain management [12] using intrathecal [26,27] and periganglionic [28,29] routes of administration (NCT00804154 and NCT02522611, respectively). Due to its long duration of action and targeting of peripheral nerve endings, local administration of RTX may provide improved pain control at the onset of injury and throughout long-duration burn wound treatments and rehabilitation.
The objective of the present study was to determine if RTX could produce long-lasting analgesia in a rat model of pain associated with full thickness thermal injury. We hypothesized that local injection of RTX at the burn site would attenuate thermal hyperalgesia and mechanical allodynia with a corresponding reduction in peptidergic activity in the spinal dorsal horn. Impairment of wound healing has not been observed following RTX treatment in Rhesus monkeys [30]; thus we also hypothesize that local RTX treatment will not have detrimental effects on the burn wound healing time course. The results from this study aim to provide evidence that reversible ablation of the TRPV1 population of peripheral nociceptors by local treatment with RTX provides long-lasting peripheral analgesia for burn pain. This intervention could be incorporated into an optimized pain management strategy for burn patients, including burn-injured military service members on the battlefield immediately following injury and during wound debridement, recovery, and rehabilitation, all while avoiding CNS-mediated negative side effects.
Methods
Subjects
A total of 32 adult (250–350 g) intact male and 12 cycling female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were used in these experiments. Rats were separated by sex and pair-housed in a 12:12 hour light: dark cycle with ad libitum access to food and water. All studies were approved by the US Army Institute of Surgical Research Institutional Animal Care and Use Committee and conform to federal guidelines and guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. This study was conducted in strict compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
Drugs
Resiniferatoxin powder (LC Laboratories, Woburn, MA) was dissolved in 95% ethanol. RTX was then further diluted to 100 μg per 1 mL in vehicle consisting of 7.5% Tween 80 and 0.05% ascorbic acid in 1X PBS at pH 7.2, and stored at −80 °C [14]. RTX for injections was freshly diluted to 0.25 μg in 100 μL in vehicle on the day of use and brought to room temperature prior to injection. Control rats received injections of 100 μL of the vehicle alone.
Full Thickness Thermal Injury
A rat model of thermal injury was induced as previously characterized [31]. Briefly, rats were anesthetized by 4% isofluorane inhalation and laid ventrally. A 100°C slanted soldering tip connected to a temperature-controlled super soldering station (RX-80HRT-5.4D; Goot, Hiroshima, Japan) was steadily applied to the midplantar surface of the right hind paw for 30 seconds to induce a full thickness thermal injury of <1% of the total body surface area. The area of the chisel of the soldering tip applied was 5.4 mm x 6 mm. Five minutes following injury and once daily for the next four days, 1% silver sulfadiazine cream (Watson Laboratories, Corona, CA, USA) was applied to the injured hind paw to prevent infection.
Behavioral Testing
Rats were acclimated to the testing apparatuses and baseline measurements were recorded prior to thermal injury. Basal thermal hyperalgesia was assessed using a Paw Thermal Stimulator (University of California, San Diego, CA, USA), as previously described [32]. To test their withdrawal latency to a noxious stimulus, rats were placed in a clear plexiglass box resting on an elevated glass plate maintained at 30°C. Subsequent to acclimation, a beam of light was positioned under the right hind paw, and the average time for the rat to withdrawal the paw from the thermal stimulus over three trials, performed at an intertrial interval of two to three minutes, was recorded in seconds and averaged as the paw withdrawal latency (PWL). The light beam’s intensity was set to produce basal PWLs of approximately 15 to 18 seconds. A maximal PWL of 20 seconds was used to prevent tissue damage from repeated application of the thermal stimulus.
A Dynamic Plantar Aesthesiometer (Ugo Basile; Collegeville, PA, USA) was used to elicit a paw withdrawal from a non-noxious stimulus to test for mechanical allodynia as previously described [33]. Rats were placed in a plexiglass box on an elevated grid platform, and a blunt mechanical stimulus was applied to the plantar surface of the right hind paw. The force (in grams) of the mechanical stimulus was increased with a ramp of 3 grams per second over 10 seconds, with a cutoff of 30 seconds to avoid mechanical lifting of the paw by the device. All behavioral testing was conducted by an observer blinded to the experimental condition.
Following collection of basal measures, 12 male rats received a thermal injury. Stimuli were applied approximately 5 mm proximal to the wound bed, as previously characterized [31], and PWLs and force to withdrawal were recorded at 72 hours post–thermal injury, a time point when burn-evoked thermal hyperalgesia and mechanical allodynia are peaking [31]. Awake rats then received one intraplantar injection of either RTX (0.25 μg/100 μL; 30 gauge; N = 6) or vehicle control (N = 6) adjacent to the thermal injury between the heel and the wound bed at a 20° angle from the right, and thermal hyperalgesia was assessed at 2.5 hours postinjection. Both thermal hyperalgesia and mechanical allodynia were then measured in the same rats 24 hours, seven days, 14 days, and 21 days following RTX injection. A period of four hours was retained between the two sensory testing modalities to avoid potential confounders of behavioral sensitization. The 2.5-hour time point postinjection was not measured for mechanical allodynia as thermal hyperalgesia was tested 2.5 hours and potential behavioral sensitization would confound testing mechanical allodynia at the same time point. The time points were chosen based on previous reports of RTX effects in rats [11,13] and the time course of full thickness thermal injury [31]. A group of eight female rats also received one intraplantar injection of either RTX (0.25 μg/100 μL; 30 gauge; N = 4) or vehicle control (N = 4) adjacent to the thermal injury, and thermal hyperalgesia was assessed at 2.5 hours postinjection, then at 24 hours, seven days, 14 days, and 21 days following RTX injection. A control group of four male and four female rats received a thermal injury only to detect a potential confounder of an underlying sex difference in burn-evoked thermal hyperalgesia.
Skin Histology
Ten male rats received a thermal injury and were euthanized by lethal injection of sodium pentobarbital (160 mg/kg; i.p.; Lundbeck Inc., Deerfield, IL, USA) at 24 hours and one week postinjury (N = 2–3 per time point) to visualize burn pathology. The collected paws were fixed in formalin and decalcified, and the plantar tissue was paraffin-embedded. The tissue was then sectioned sagittally and cross-sectioned at the center of injury at 5 µM onto glass slides and stained with hematoxylin and eosin for visualization. Images were captured at 40x magnification with a Nikon Eclipse 80i microscope equipped with a DS-Fi1 camera head. A board-certified veterinary pathologist characterized the degree and time course of burn based on tissue morphology.
Perfusion Fixation
Six thermally injured rats were euthanized (sodium pentobarbital; 160mg/kg; i.p.) at one week post-RTX or postvehicle injection (N = 3 rats per treatment) and perfusion-fixed. For perfusion fixation, heparin sodium (0.1 mL; 1,000 USP Units/mL; APP Pharmaceuticals, Schaumburg, IL, USA) was injected into the apex of the heart and rats were perfused transcardially with 250 mL of 0.9% sodium chloride containing 2% sodium nitrite as a vasodilator, followed by 250 mL 4% paraformaldehyde (pH 7.0) in 0.1 M phosphate buffer. A final rinse with 250 mL sodium chloride/sodium nitrite was used to remove residual paraformaldehyde. The lumbar segment of the spinal cord was extracted and placed into 30% sucrose solution and stored at 4°C for at least 24 hours prior to sectioning.
Immunohistochemistry
Perfusion-fixed lumbar spinal cords were sectioned at 30 microns under −20°C directly onto slides with a Microm HM 560 cryostat (ThermoFisher Scientific; Rockford, IL, USA) and stored at −20°C until use. Tissue was fixed to the slide with 4% paraformaldehyde during a 10-minute incubation. A 1:4 series through the rostrocaudal axis of the lumbar L3, L4, and L5 spinal cord was processed for CGRP and substance P as previously described [31], or Fos immunoreactivity using standard immunohistochemical techniques. Briefly, sections were serially rinsed with potassium phosphate buffered saline (KPBS) and incubated in primary antibody solution rabbit anti-CGRP (1:10,000; Immunostar; cat # 24112; Hudson, WI, USA), rabbit anti-substance-P (1:50,000; Immunostar; cat # 20064), or rabbit anti-c-Fos (1:10,000; AbCam; cat # ab7963; Cambridge, MA, USA) in KPBS containing 1% Triton-X100 at room temperature for one hour followed by 48-hour incubation at 4°C. A separate series of sections received the Fos antibody incubated with Fos-blocking peptide (100 µg at 0.2 mg/mL; AbCam) for four hours at room temperature prior to applying to the tissue to verify antibody specificity. Tissue was then serially rinsed (eight times in KPBS for six minutes each) and incubated for one hour in biotinylated goat anti-rabbit IgG (1:600; Jackson Immunoresearch; West Grove, PA, USA). After, secondary incubation tissue was serially rinsed (six times in KPBS for five minutes each) and incubated for one hour in avidin-biotin peroxidase complex (1:10; ABC Elite Kit; Vector Laboratories; Burlingame, CA, USA). Tissue was rinsed, and staining was visualized using nickel sulfate (250 mg/10 mL) intensified 3,3’-diaminobenzidine solution (2 mg/10 mL) containing 0.8% hydrogen peroxide in 0.175 M sodium acetate buffer, pH 7.2. A final serial rinse was conducted with 0.175 M sodium acetate (pH 6.8), followed by KPBS. Slides were then dehydrated in a series of graded alcohols, cleared with HistoChoice (Sigma), and glass-coverslipped using Permount (Fisher Scientific; Fair Lawn, NJ, USA).
Anatomical Quantification
Bilateral images of the dorsal horn quadrant, as the region of interest that included superficial lamina I-III, were captured with a Nikon Eclipse 80i microscope equipped with a DS-Fi1 camera head at 10x magnification. Three representative sections each from caudal L3, mid L4, and rostral L5, based on known innervation of the spinal cord from the plantar surface of the hind paw [34,35], were chosen for quantification. Peptide immunohistochemistry was quantified by densitometry. Images were imported into NIH Image J-64 (http://rsb.info.nih.gov/ij/), converted to 16-bit gray scale to be sampled for average gray scale pixel value (sum gray values/number pixels) following background correction (set a ∼150 pixels) [36–38]. For Fos immunohistochemistry, Fos-immunoreactive nuclei were directly counted bilaterally. The tissue was sectioned at 30 μm so that 150 μm separates each analyzed level, thus avoiding counting the same cell twice. All quantification was performed by an experimenter blinded to the condition. All tissue was processed in parallel and for the same time duration to ensure stain homogeneity. Following quantification, images for illustration were adjusted for brightness/contrast to optimize visualization for publication.
Statistical Analysis
Behavioral data are expressed as mean ± standard error of the mean paw withdrawal latencies in seconds or the force to withdrawal in grams. Behavioral data were analyzed by one-way or two-way repeated measures analysis of variance (ANOVA). Individual groups were compared using Bonferroni post hoc analysis. Anatomical data are expressed as mean ± standard error of the mean number of counted immunoreactive cells or the mean gray value following densitometry. Anatomical data were analyzed by unpaired Student’s t tests. Statistical significance was tested at P < 0.05. All data were analyzed using GraphPad software version 5 (GraphPad, San Diego, CA, USA).
Results
Peripheral RTX Significantly Attenuates Thermal Injury-Evoked Pain Behaviors in Male Rats
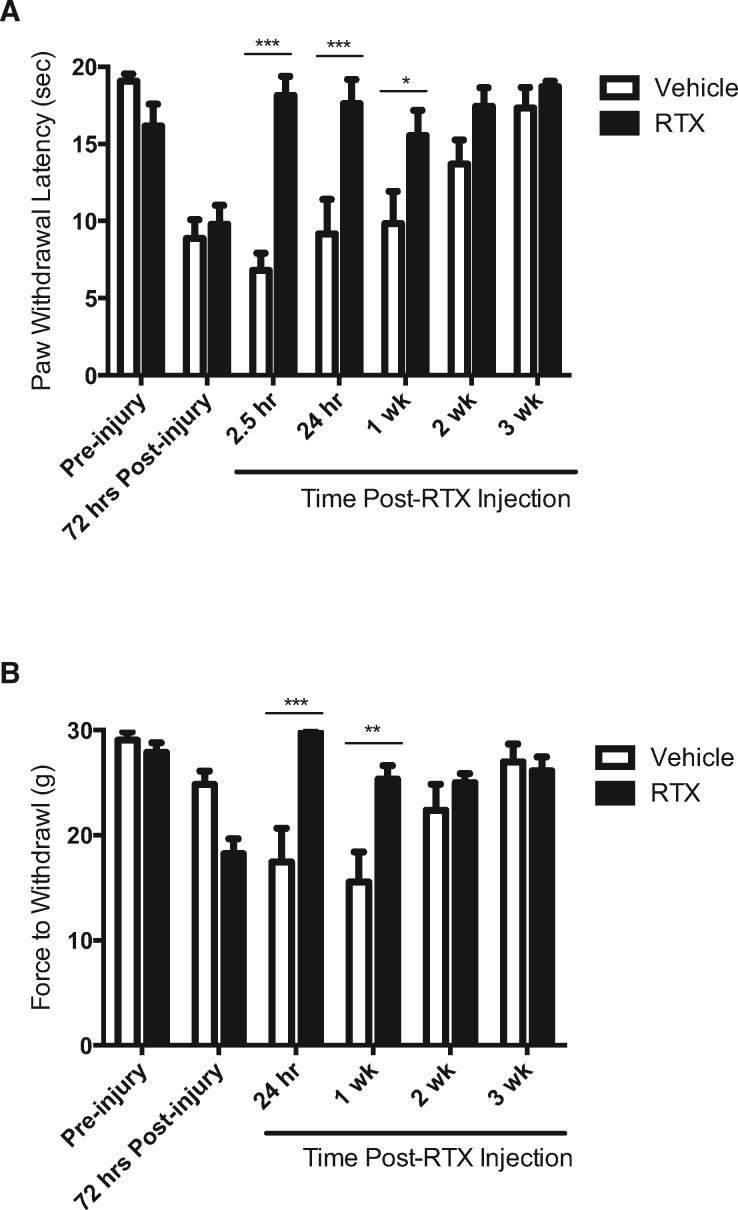
Full thickness thermal injury in male rats evoked significant thermal hyperalgesia within 72 hours that lasted up to two weeks (F(6,41) = 17.32, P = 0.0004) (Figure 1A, open bars), similar to our previous reports [4,31]. At 72 hours following thermal injury, rats received an intraplantar injection of either RTX or vehicle and thermal hyperalgesia was reexamined. Brief nocifensive behaviors, including licking and shaking, were observed for approximately five to 15 minutes immediately following injection, a time course similar to earlier observations [39]. RTX significantly reversed thermal hyperalgesia within 2.5 hours of injection that lasted through recovery at three weeks postinjury (Figure 1A) (F(6,60) = 8.396, P < 0.0001). At 2.5 hours, 24 hours, and one week following RTX injection, thermal hyperalgesia was significantly reduced compared with vehicle controls (P < 0.05).
Figure 1.
Resiniferatoxin (RTX) reverses thermal hyperalgesia and mechanical allodynia in male rats following full thickness thermal injury. (A) Full thickness thermal injury evoked significant thermal hyperalgesia within 72 hours in male rats. Thermal hyperalgesia was reversed at 2.5 hours, 24 hours, and one week following RTX injection (closed bars) compared with vehicle controls (open bars). (B) Full thickness thermal injury also evoked significant mechanical allodynia, which was reversed at 24 hours and one week following RTX injection (closed bars) compared with vehicle controls (open bars). Asterisks denote significant differences between RTX and vehicle treatment groups. * denotes significance at P < 0.05; ** denotes significance at P < 0.01; *** denotes significance at P < 0.001; **** denotes significance at P < 0.0001.
Full thickness thermal injury in male rats also evoked significant mechanical allodynia lasting up to two weeks (F(5,35) = 7.233, P = 0.0045) (Figure 1B, open bars), similar to our previous reports [4,31]. RTX significantly reversed mechanical allodynia within 24 hours following injection that lasted through recovery at two weeks post-thermal injury (F(5,50) = 9.999, P < 0.0001) (Figure 1B). At 24 hours and one week following RTX injection, mechanical allodynia was significantly reduced compared with vehicle controls (P < 0.05). Examination of the full time course showed that both thermal hyperalgesia and mechanical allodynia following RTX treatment were not significantly different from pre-injury sensitivity at all time points measured (P > 0.05).
Peripheral RTX Also Significantly Attenuates Thermal Injury-Evoked Pain Behaviors in Female Rats
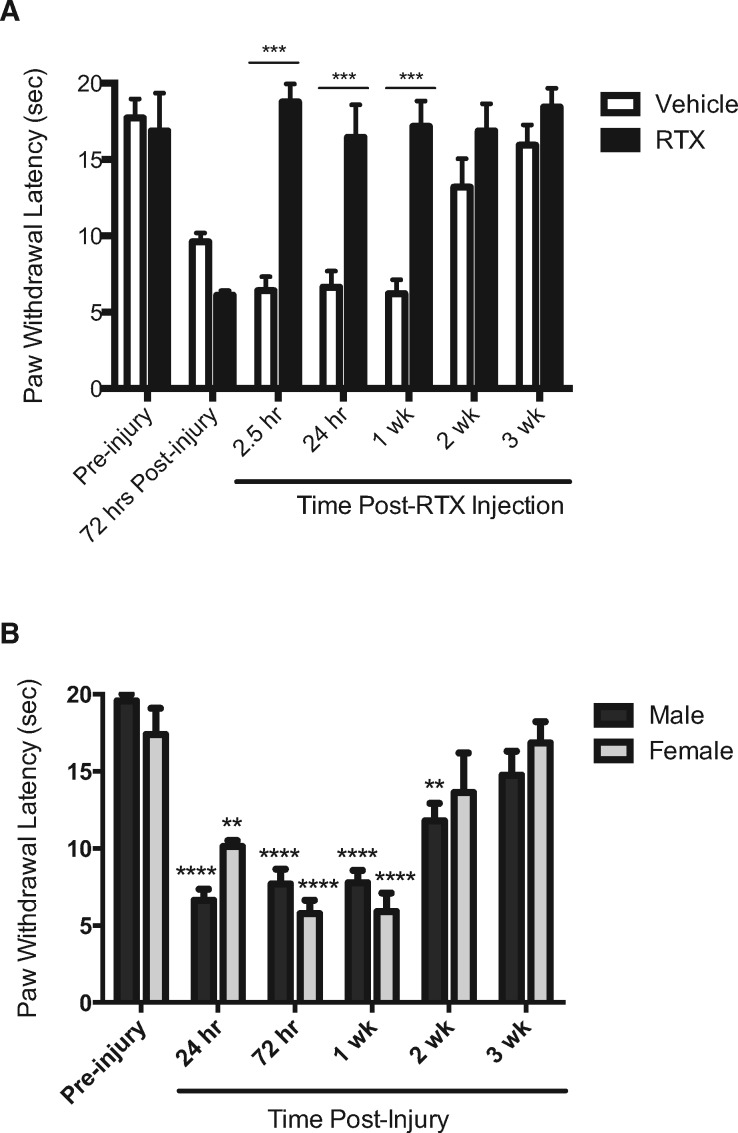
Full thickness thermal injury in female rats evoked significant thermal hyperalgesia within 72 hours and lasting up to two weeks (F(6,27) = 15.27, P = 0.0043) (Figure 2A, open bars). Similar to males, RTX significantly reversed thermal hyperalgesia within 2.5 hours of injection that lasted through recovery at three weeks postinjury (F(6,36) = 9.131, P < 0.0001) (Figure 2A). At 2.5 hours, 24 hours, and one week following RTX injection, thermal hyperalgesia was significantly different compared with vehicle controls (P < 0.05). Thermal hyperalgesia following RTX treatment was not significantly different from pre-injury sensitivity at all time points measured (P > 0.05). There were no significant sex differences in the degree and time course of thermal hyperalgesia following burn injury (F(1,20) = 0.1704, P = 0.7009) (Figure 2B), which was equally reversed by RTX in both sexes.
Figure 2.
Resiniferatoxin (RTX) reverses thermal hyperalgesia in female rats following full thickness thermal injury. (A) Full thickness thermal injury evoked significant thermal hyperalgesia within 72 hours in female rats. Thermal hyperalgesia was reversed at 2.5 hours, 24 hours, and one week following RTX injection (closed bars) compared with vehicle controls (open bars). (B) No sex differences in degree or time course were observed following full thickness thermal injury. Asterisks denote significant change from baseline within sexes. * denotes significance at P < 0.05; ** denotes significance at P < 0.01; *** denotes significance at P < 0.001; **** denotes significance at P < 0.0001.
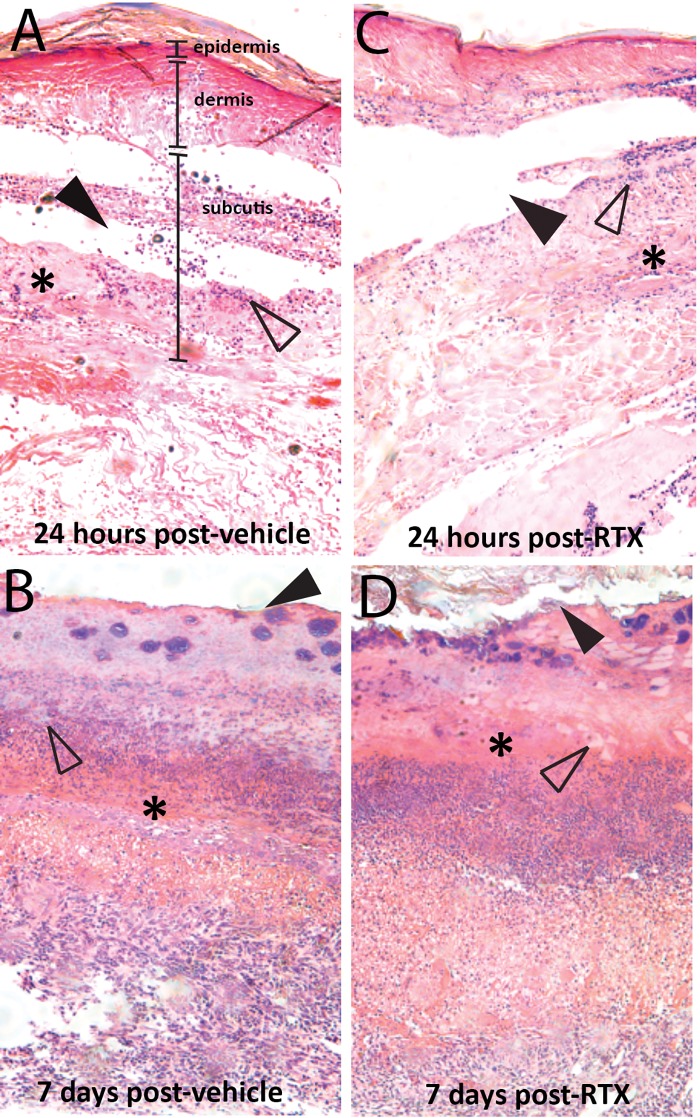
Local injection of RTX at the site of thermal injury does not alter burn pathology during wound healing
In vehicle-treated rats, 24 hours following injections (96 hours following injury), there was significant damage to the epidermis and dermis, extending into the subcutis and underlying muscle, as previously reported following full thickness thermal injury in this model (Figure 3A) [31]. Plantar epithelial necrosis and denatured collagen (asterisks) were focally extensive, with evidence of moderate neutrophilic infiltration (open arrowheads) and multifocal detachment (solid arrowheads) of dermal layers. At seven days following vehicle injections (10 days following injury), the necrotic tissue was sloughed, with evidence of a serocellular crust (solid arrowheads), multifocal neovascularization (open arrowheads), and chronic inflammation observed as granulation tissue (asterisks) extending to the deep flexor tendon (Figure 3B). There were no significant morphological differences between tissue receiving an intraplantar injection of vehicle compared with tissue injected with RTX at either 24 hours (Figure 3C) or seven days (Figure 3D) postinjection.
Figure 3.
No effect of local resiniferatoxin (RTX) injection on burn pathology observed at 24 hours and seven days following injection. Photomicrographs of the plantar hind paw epidermis, dermis, and subcutis layers at the site of burn injury at (A) 24 hours following vehicle injection, (B) seven days following vehicle injection, (C) 24 hours following RTX injection, and (D) seven days following RTX injection. Asterisks denote plantar epithelial necrosis and denatured collagen at 24 hours followed by replacement with granulation tissue at seven days; open arrowheads denote neutrophil infiltration at 24 hours followed by multifocal neovascularization at seven days; solid arrowheads denote multifocal detachment at 24 hours followed by replacement with a serocellular crust at seven days.
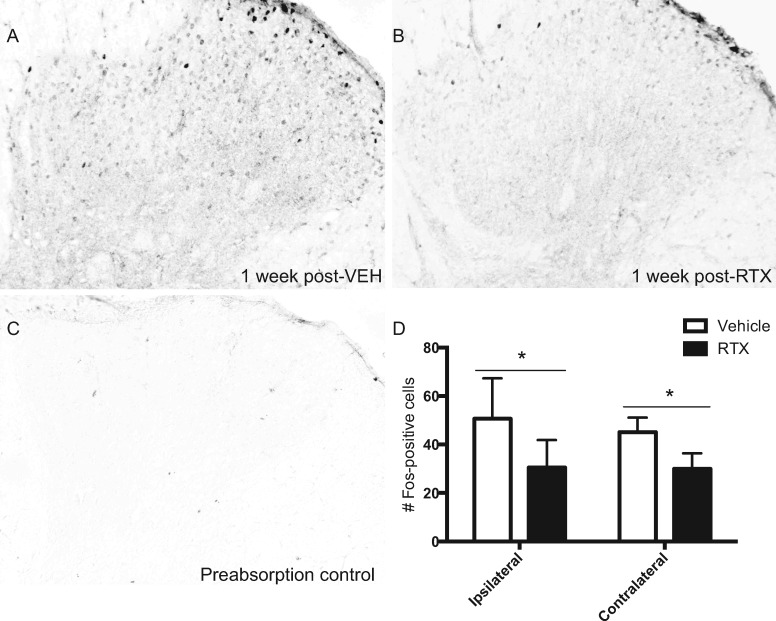
Fos Immunoreactivity in the Spinal Dorsal Horn Is Significantly Attenuated in RTX-Treated Rats
In vehicle-treated rats, burn injury–induced Fos expression was observed in the ipsilateral and contralateral lumbar dorsal horn across superficial lamina I-III at one week following injection (Figure 4A). As there was no significant difference in Fos expression between the L3, L4, and L5 lumbar sections (P > 0.05), data were combined across levels for analysis and presentation. Fos immunoreactivity was markedly reduced in RTX-treated rats one week following treatment (Figure 4B). Antibody specificity was confirmed by preabsorption of the antibody with the antigenic Fos peptide (Figure 4C). Quantification of Fos-positive cells corresponding to one week postinjection of vehicle compared with RTX revealed a significant reduction in Fos immunoreactivity in RTX-treated rats vs vehicle controls (ipsilateral t = 2.21, df = 14, P = 0.044; contralateral t = 2.51, df = 14, P = 0.025) (Figure 4D).
Figure 4.
Resiniferatoxin (RTX) attenuates burn-evoked Fos immunoreactivity in the lumbar spinal dorsal horn one week following treatment. Photomicrographs of Fos-positive nuclei in the lumbar spinal dorsal horn at one week following a local injection of either (A) vehicle or (B) RTX in male rats at 72 hours following burn injury. (C) Photomicrograph illustrating the preabsorption control. (D) Quantification of Fos-positive cells in both vehicle-treated (open bar) and RTX-treated (solid bar) groups. * denotes significance at P < 0.05.
Proinflammatory Peptide Expression in the Spinal Dorsal Horn Is Significantly Attenuated in RTX-Treated Rats
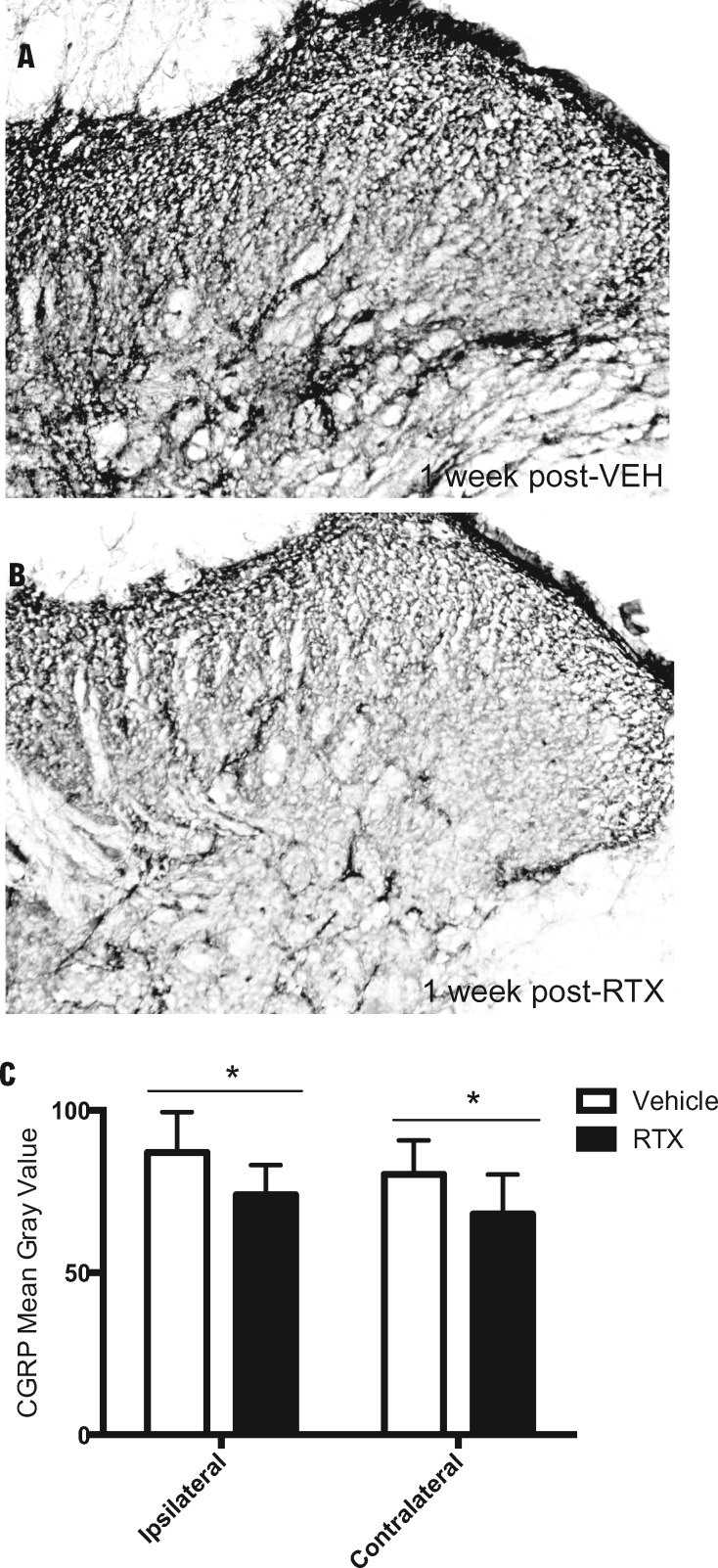
In vehicle-treated rats, a burn injury–induced increase in CGRP immunoreactivity was observed in the lumbar dorsal horn across superficial lamina I-III at one week following injection (Figure 5A), similar to our previous report using this model [31]. In comparison, the elevated CGRP immunoreactivity was reduced in RTX-treated rats at one week following injection (Figure 5B). Densitometry of CGRP immunoreactivity corresponding to one week postinjection of vehicle compared with RTX treatment revealed a significant reduction in CGRP immunoreactivity in RTX-treated rats vs vehicle controls (ipsilateral t = 2.48, df = 15, P = 0.025; contralateral t = 2.241, df = 15, P = 0.041) (Figure 5C).
Figure 5.
Resiniferatoxin (RTX) attenuates burn-evoked elevation in CGRP immunoreactivity in the lumbar spinal dorsal horn one week following treatment. Photomicrographs of CGRP immunoreactivity in the lumbar spinal dorsal horn at one week following a local injection of either (A) vehicle or (B) RTX in male rats at 72 hours following burn injury. (C) Densitometry values of CGRP immunoreactivity in both vehicle-treated (open bar) and RTX-treated (solid bar) groups. * denotes significance at P < 0.05.
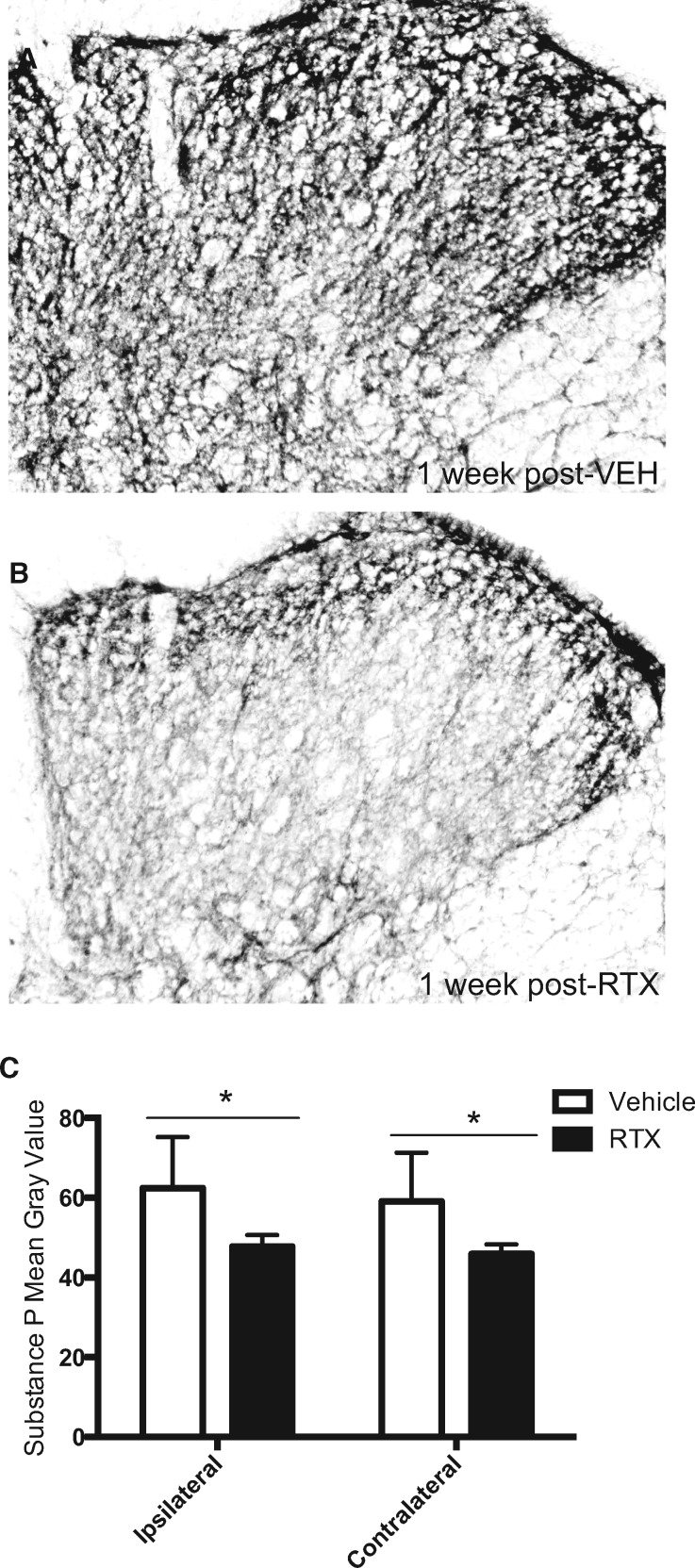
In vehicle-treated rats, a burn injury–induced elevation in substance P immunoreactivity was also observed in the lumbar dorsal horn across superficial lamina I-III at one week following injection (Figure 6A), similar to our previous report using this model [31]. In comparison, the elevated substance P immunoreactivity was reduced in RTX-treated rats at one week following injection (Figure 6B). Densitometry of substance P immunoreactivity corresponding to one-week postinjection of vehicle compared with RTX treatment revealed a significant reduction in substance P immunoreactivity in RTX-treated rats vs vehicle controls (ipsilateral t = 2.78, df = 16, P = 0.013; contralateral t = 2.64, df = 16, P = 0.018) (Figure 6C). As there was no significant difference in CGRP or substance P expression between the L3, L4, and L5 lumber sections (P > 0.05), data were combined across levels for analysis and presentation. Both CGRP and substance P immunoreactivities were comparable between the ipsilateral and contralateral dorsal horns in both treatment groups (P > 0.05).
Figure 6.
Resiniferatoxin (RTX) attenuates burn-evoked elevation in substance P immunoreactivity in the lumbar spinal dorsal horn one week following treatment. Photomicrographs of substance P immunoreactivity in the lumbar spinal dorsal horn at one week following a local injection of either (A) vehicle or (B) RTX in male rats at 72 hours following burn injury. (C) Densitometry values of CGRP immunoreactivity in both vehicle-treated (open bar) and RTX-treated (solid bar) groups. * denotes significance at P < 0.05.
Discussion
Burn patients, including military service members injured on the battlefield, are a unique patient population that experience severe injuries requiring prolonged treatment with expensive, suboptimal opioid-based analgesics. This pain management protocol is a substantial problem for trauma patients due to the multitude of CNS-mediated side effects elicited by opioids. Side effects that represent a significant barrier to pain management in burn patients include decreased motor coordination and cognition (especially for service members in a battlefield situation), further depression of burn-induced reductions in respiratory and cardiac output, and the development of tolerance and addiction during lengthy wound care and rehabilitation [40]. Novel nonopioid peripheral analgesics that avoid the CNS are desired.
As the TRPV1 agonist RTX has been reported to produce long-lasting peripheral analgesia in various pain models [11,13,14,28,30,41–43], we hypothesized that local injection of RTX would produce long-lasting analgesia in a rat model of pain associated with full thickness thermal injury. Our results indicate that intraplantar injection of RTX at the site of a full thickness thermal injury in the rat hind paw 1) reversed burn-evoked thermal hyperalgesia and mechanical allodynia as early as 2.5 hours following administration and lasting through recovery, 2) produced comparable peripheral analgesia in burn injured female rats, 3) did not have a detrimental effect on healing of the wound bed as observed by histopathological analysis, and 4) significantly reduced nociceptive activity in the dorsal horn of the lumbar spinal cord as observed by reduced Fos and proinflammatory peptide immunoreactivity one week following RTX treatment.
We previously characterized a rat model of full thickness thermal injury to model the burn wound severity and pain experienced by burn patients requiring treatment over extended periods of time [31]. We used this model to analyze the potential for RTX to abate burn pain behaviors and found that RTX significantly reduced thermal hyperalgesia and mechanical allodynia within 2.5 hours following injection. Importantly, RTX analgesia lasted through the duration of the burn injury. While burn pain behaviors lasted approximately three weeks in this model [31], a single administration of RTX fully blocked pain behaviors throughout this duration. Studies of peripheral RTX administration in inflammatory pain models report a similar long-duration time course for robust analgesia [11,13]. Furthermore, comparable analgesia was observed in both male and female burn-injured rats. These data provide the first account of the beneficial effects of peripherally induced resiniferatoxin analgesia on burn pain. A peripheral injection of RTX is likely the easiest route of administration in the austere environments of the battlefield. Intrathecal and periganglionic injections of RTX are also highly effective in many pain models; however, these routes yield a permanent analgesia because of loss of the neuronal perikarya and/or the centrally projecting dorsal root and are probably not appropriate routes for this patient population [5,12,13].
While the wound healing time course will be significantly different in burn patients, these data provide evidence of long-lasting peripheral analgesia produced by one injection of RTX into the wound bed. The dose of RTX used in this study has been previously shown to preserve motor function, preserve somato- and mechanosensations other than pain, and be reversible between two weeks and six months [13]. One potential side effect of local RTX injection is a transient burning sensation upon administration that may be experienced via RTX’s agonism of TRPV1 prior to nociceptor inactivation. This side effect can be overcome in clinical application by general anesthesia, nerve block, or topical, epidural, or intrathecal administration of short-acting anesthetics immediately prior to RTX administration [5,11,43]. Importantly, preemptive treatment or co-administration with local anesthetic does not interfere with the lesion effect of RTX on local nerve endings [44]. Additionally, the use of RTX in a pain control regimen can be tailored to the patient’s condition through regional, site-specific administration without concomitant CNS side effects.
Local administration of RTX into the burn wound bed does not appear to change the course of wound healing as observed by rat hind paw histopathology. In the present study, the burn injury site of hind paws was analyzed at 24 hours and seven days following RTX injection, which corresponds to four days and 10 days following burn injury, respectively. Within four days (24 hours following RTX or vehicle treatment), all injured rat hind paws displayed similar evidence of significant damage to the epithelium, dermis, and subcutis indicative of a third-degree burn, in concurrence with our previous research using this model [31]. At seven days following either RTX or vehicle treatment (10 days following burn), wound beds displayed evidence of tissue replacement and new vascularization consistent with wound healing, also comparable with our previous report [31]. Together, these data indicate that the extent of damage and healing was comparable and independent of whether the rat received vehicle or RTX injection into the wound bed, indicating that RTX neither exacerbated nor improved wound bed healing in this model. This is important clinically as it indicates that administration of RTX at the site of burn will likely not have detrimental effects on the wound healing time course. This is an improvement compared with other peripheral analgesics, such as opioids and nonsteroidal anti-inflammatory drugs, which have been reported to impair wound healing in rodent punch biopsy and surgical wound closure models [45–49].
It was previously reported that Fos expression, as a measure of cellular activity in the lumbar spinal dorsal horn, is decreased following RTX injection in models of inflammatory pain [11]. In the present study, we observed a significant increase in Fos immunoreactivity across the rostrocaudal axis of the L3-L5 spinal dorsal horn at seven days following vehicle treatment in burn-injured rats (10 days following burn injury). Fos expression was significantly reduced in RTX-treated rats, providing evidence of the ability of RTX to reduce pain processing at the spinal level in our rat model of pain associated with full thickness thermal injury. While a potential central action of RTX is not excluded in the present studies, RNA-Seq analyses of multiple human brain regions, organs, and peripheral tissues shows very low to no expression outside the DRG (http://www.gtexportal.org/home/gene/TRPV1 2016 Oct 07), although expression in specific subpopulations of cells has been mapped in the mouse using transgenic reporters [50]. Future immunohistochemical analysis of TRPV1 expression in the DRG following RTX treatment compared with vehicle-treated controls is warranted.
Full thickness thermal injury in the rat hind paw produces a significant increase in the release of the proinflammatory peptides CGRP and substance P in the lumbar dorsal horn of the spinal cord [31]. The time course of increased CGRP and substance P immunoreactivity mirrors the pain behavior time course, with increased expression observed during the first two weeks following thermal injury. In the present study, we compared the density of CGRP and substance P immunoreactivity in the spinal dorsal horn in vehicle-treated and RTX-treated rats one week following injection (10 days following burn injury). In vehicle-treated rats, a similar peptide expression was observed, while this expression was significantly decreased in the rats that received RTX. The reduction was observed across the rostrocaudal axis of the L3-L5 dorsal horn of the spinal cord. Tissues were observed at one week following injections to avoid potential confounders of measuring a potential transient increase in peptide expression due to the transient RTX-evoked hyperalgesia. These observations demonstrate a strong attenuation of burn-induced molecular manifestations of persistent nociceptive input from the injury site.
Conclusions
Overall, the present data provide the first evidence that the TRPV1 agonist RTX produces potent peripheral analgesia when injected into the burn wound bed of male and female rats with a full thickness thermal injury. The use of RTX as a peripheral analgesic, and possibly variants like TRPV1 positive allosteric modulators [51], has the potential to optimize pain management in burn patients, including the military service member population, who have severe burns and require a lengthy pain management regimen during hospitalization and rehabilitation. RTX could also be useful as a battlefield analgesic due to its lack of effect on motor function, cognition, and respiratory and cardiac output. Reducing opioid reliance through increasing the use of peripheral analgesics such as RTX that may provide comparable, if not superior, analgesia has significant implications for pain management in trauma patients, especially the burn patient population.
Acknowledgments
The authors would like to acknowledge the technical assistance of Matthew K. McIntyre, Terry S. Bakewell, and Thomas Garza. The authors thank Dr. Joseph Novak for expert burn pathology analysis and Drs. Marcie Fowler and Lawrence Petz for helpful comments on study design.
References
- 1. Patel TH, Wenner KA, Price SA, et al. A U.S. Army Forward Surgical Team's experience in Operation Iraqi Freedom. J Trauma 2004;572:201–7. [DOI] [PubMed] [Google Scholar]
- 2. Grissom TE, Farmer JC.. The provision of sophisticated critical care beyond the hospital: Lessons from physiology and military experiences that apply to civil disaster medical response. Crit Care Med 2005;33(1 suppl):S13–21. [DOI] [PubMed] [Google Scholar]
- 3. Gawande A. Casualties of war–military care for the wounded from Iraq and Afghanistan. N Engl J Med 2004;35124:2471–5. [DOI] [PubMed] [Google Scholar]
- 4. Nyland JE, McLean SA, Averitt DL.. Prior stress exposure increases pain behaviors in a rat model of full thickness thermal injury. Burns 2015;418:1796–804. [DOI] [PubMed] [Google Scholar]
- 5. Iadarola MJ, Gonnella GL.. Resiniferatoxin for pain treatment: An interventional approach to personalized pain medicine. Open Pain J 2013;6:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ness TJ. Pharmacology of peripheral analgesia. Pain Pract 2001;13:243–54. [DOI] [PubMed] [Google Scholar]
- 7. Brederson JD, Kym PR, Szallasi A.. Targeting TRP channels for pain relief. Eur J Pharmacol 2013;716(1–3):61–76. [DOI] [PubMed] [Google Scholar]
- 8. Nielsen BN, Henneberg SW, Schmiegelow K, Friis SM, Romsing J.. Peripherally applied opioids for postoperative pain: Evidence of an analgesic effect? A systematic review and meta-analysis. Acta Anaesthesiol Scand 2015;597:830–45. [DOI] [PubMed] [Google Scholar]
- 9. Picard PR, Tramer MR, McQuay HJ, Moore RA.. Analgesic efficacy of peripheral opioids (all except intra-articular): A qualitative systematic review of randomised controlled trials. Pain 1997;723:309–18. [DOI] [PubMed] [Google Scholar]
- 10. Stein C. Targeting pain and inflammation by peripherally acting opioids. Front Pharmacol 2013;4:123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neubert JK, Karai L, Jun JH, et al. Peripherally induced resiniferatoxin analgesia. Pain 2003;104(1–2):219–28. [DOI] [PubMed] [Google Scholar]
- 12. Iadarola MJ, Mannes AJ.. The vanilloid agonist resiniferatoxin for interventional-based pain control. Curr Top Med Chem 2011;1117:2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neubert JK, Mannes AJ, Karai LJ, et al. Perineural resiniferatoxin selectively inhibits inflammatory hyperalgesia. Mol Pain 2008;4:3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 2004;1139:1344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997;3896653:816–24. [DOI] [PubMed] [Google Scholar]
- 16. Chuang HH, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001;4116840:957–62. [DOI] [PubMed] [Google Scholar]
- 17. Loyd DR, Weiss G, Henry MA, Hargreaves KM.. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 2011;15210:2267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loyd DR, Henry MA, Hargreaves KM.. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol 2013;24(1):54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veldhuis NA, Lew MJ, Abogadie FC, et al. N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J Biol Chem 2012;28726:21765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karai LJ, Russell JT, Iadarola MJ, Olah Z.. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem 2004;27916:16377–87. [DOI] [PubMed] [Google Scholar]
- 21. Anand P, Bley K.. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 2011;1074:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim Y, Kim EH, Lee KS, et al. The effects of intra-articular resiniferatoxin on monosodium iodoacetate-induced osteoarthritic pain in rats. Korean J Physiol Pharmacol 2016;201:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheinfeld N. Topical treatments of skin pain: A general review with a focus on hidradenitis suppurativa with topical agents. Dermatol Online J 2014;207. [PubMed] [Google Scholar]
- 24. Lee JH, Lee Y, Ryu H, et al. Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J Comput Aided Mol Des 2011;254:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elokely K, Velisetty P, Delemotte L, et al. Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin. Proc Natl Acad Sci U S A 2016;1132:E137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heiss J, Iadarola M, Cantor F, et al. A Phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer (Abstract). J Pain 2014;154:S67. [Google Scholar]
- 27.National Institute of Dental and Craniofacial Research. Resiniferatoxin to treat severe pain associated with advanced cancer. In: ClinicalTrials.gov[Internet]. Bethesda (MD): National Library of Medicine (US). 2000-[2016 OCT 07]. Available from: https://clinicaltrials.gov/ct2/show/NCT00804154 NLM Identifier: NCT00804154.
- 28. Brown JD, Saeed M, Do L, et al. CT-guided injection of a TRPV1 agonist around dorsal root ganglia decreases pain transmission in swine. Sci Transl Med 2015;7305:305ra145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institute of Neurological Disorders and Stroke. Periganglionic resiniferatoxin for the treatment of intractable pain due to cancer-induced bone pain. In: ClinicalTrials.gov[Internet]. Bethesda (MD): National Library of Medicine (US). 2000-[2016 OCT 07]. Available from: https://clinicaltrials.gov/ct2/show/NCT02522611 NLM Identifier: NCT02522611.
- 30. Tender GC, Walbridge S, Olah Z, et al. Selective ablation of nociceptive neurons for elimination of hyperalgesia and neurogenic inflammation. J Neurosurg 2005;1023:522–5. [DOI] [PubMed] [Google Scholar]
- 31. Fowler M, Clifford JL, Garza TH, et al. A rat model of full thickness thermal injury characterized by thermal hyperalgesia, mechanical allodynia, pronociceptive peptide release and tramadol analgesia. Burns 2014;40(4):759-71. [DOI] [PubMed] [Google Scholar]
- 32. Hargreaves K, Dubner R, Brown F, Flores C, Joris J.. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;321:77–88. [DOI] [PubMed] [Google Scholar]
- 33. Gibbs JL, Flores CM, Hargreaves KM.. Attenuation of capsaicin-evoked mechanical allodynia by peripheral neuropeptide Y Y1 receptors. Pain 2006;124(1–2):167–74. [DOI] [PubMed] [Google Scholar]
- 34. Molander C, Grant G.. Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience 1986;191:297–312. [DOI] [PubMed] [Google Scholar]
- 35. Swett JE, Woolf CJ.. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol 1985;2311:66–77. [DOI] [PubMed] [Google Scholar]
- 36. Gassmann M, Grenacher B, Rohde B, Vogel J.. Quantifying Western blots: Pitfalls of densitometry. Electrophoresis 2009;3011:1845–55. [DOI] [PubMed] [Google Scholar]
- 37. Loyd DR, Wang X, Murphy AZ.. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci 2008;2852:14007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothman SM, Nicholson KJ, Winkelstein BA.. Time-dependent mechanics and measures of glial activation and behavioral sensitivity in a rodent model of radiculopathy. J Neurotrauma 2010;275:803–14. [DOI] [PubMed] [Google Scholar]
- 39. Yang HY, Mitchell K, Keller JM, Iadarola MJ.. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. J Neurochem 2007;1034:1628–43. [DOI] [PubMed] [Google Scholar]
- 40. Clifford JL, Fowler M, Hansen JJ, et al. State of the science review: Advances in pain management in wounded service members over a decade at war. J Trauma Acute Care Surg 2014;77(3 suppl 2):S228–36. [DOI] [PubMed] [Google Scholar]
- 41. Kissin I, Freitas CF, Bradley EL Jr.. Perineural resiniferatoxin prevents the development of hyperalgesia produced by loose ligation of the sciatic nerve in rats. Anesth Analg 2007;1045:1210–6. [DOI] [PubMed] [Google Scholar]
- 42. Tender GC, Li YY, Cui JG.. Vanilloid receptor 1-positive neurons mediate thermal hyperalgesia and tactile allodynia. Spine J 2008;82:351–8. [DOI] [PubMed] [Google Scholar]
- 43. Brown DC, Iadarola MJ, Perkowski SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005;1035:1052–9. [DOI] [PubMed] [Google Scholar]
- 44. Bates BD, Mitchell K, Keller JM, et al. Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain 2010;1493:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rook JM, McCarson KE.. Delay of cutaneous wound closure by morphine via local blockade of peripheral tachykinin release. Biochem Pharmacol 2007;745:752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rook JM, Hasan W, McCarson KE.. Temporal effects of topical morphine application on cutaneous wound healing. Anesthesiology 2008;1091:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rook JM, Hasan W, McCarson KE.. Morphine-induced early delays in wound closure: Involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochem Pharmacol 2009;7711:1747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin JL, Charboneau R, Barke RA, Roy S.. Chronic morphine treatment inhibits LPS-induced angiogenesis: Implications in wound healing. Cell Immunol 2010;2652:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martin JL, Koodie L, Krishnan AG, et al. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. Am J Pathol 2010;1762:786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cavanaugh DJ, Chesler AT, Jackson AC, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 2011;3113:5067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lebovitz EE, Keller JM, Kominsky H, et al. Positive allosteric modulation of TRPV1 as a novel analgesic mechanism. Mol Pain 2012;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]