ABSTRACT
Somatic gain-of-function mutations in isocitrate dehydrogenase (NADP(+)) 1, cytosolic (IDH1) or isocitrate dehydrogenase (NADP(+)) 2, mitochondrial (IDH2) are bona fide oncogenic drivers of acute myeloid leukemia and glioma because the neomorphic enzymes catalyze the synthesis of R-2-hydroxylutarate (R-2-HG), an oncometabolite with robust epigenetic effects. Recent data indicate that R-2-HG released by malignant cells can accumulate in the extracellular space and be taken up by T lymphocytes, ultimately compromising their capacity to mediate anticancer immune responses. Thus, R-2-HG drives oncogenesis and tumor progression not only as a cancer cell-autonomous epigenetic modifier, but also as an immunosuppressive metabolite. Chemical inhibitors of mutant IDH1 and IDH2, which currently are under clinical evaluation, may therefore mediate dual anticancer effects by targeting cancer cells and, at the same time, relieving R-2-HG-mediated immunosuppression.
KEYWORDS: cancer-associated fibroblasts, cytotoxic T lymphocytes, HIF-1α, immunosurveillance, immunotherapy, ivosidenib
R-2-hydroxyglutarate (R-2-HG) is a bona fide ‘oncometabolite’ because it accumulates in (pre-) malignant cells as a consequence of a somatic gain-of-function mutations and is causally involved in malignant transformation and tumor progression.1 In particular, gain-of-function mutations in isocitrate dehydrogenase (NADP(+)) 1, cytosolic (IDH1) or isocitrate dehydrogenase (NADP(+)) 2, mitochondrial (IDH2), which are particularly prevalent among acute myeloid leukemia (AML) and glioma patients,2,3 result in the acquisition of a neomorphic enzymatic function that catalyzes the direct conversion of alpha-ketoglutarate (α-KG, a key intermediate of the Krebs cycle) into R-2-HG.4–6 Intracellular R-2-HG accumulation coupled to α-KG depletion has oncogenic effects because it inhibits histone lysine demethylases (KDMs) and the TET family of DNA hydroxylases,7,8 resulting in histone hypermethylation, epigenetic programming and disruption of normal stem cell differentiation coupled to the acquisition of additional mutations.9 Until recently, R-2-HG has received considerable attention also because it can be detected in body fluids (including the plasma and cerebrospinal fluid) and organs (by nuclear magnetic resonance), hence constituting a biomarker that can be monitored non-invasively for diagnostic purposes as well as for measuring the therapeutic effects of clinically relevant IDH1 or IDH2 inhibitors.10,11 Recent clinical data from a Phase I trial demonstrate indeed that ivosidenib, a chemical inhibitor of mutant IDH1 can be safely administered to AML patients and is associated with an objective response rate of 40%.12 These findings constituted the ground for the approval of ivosidenib (commercialized under the name of Tbsovo®) by the US Food and Drug Administration for the treatment of relapsed or refractory AML.13
Although cancer has long been considered as a cellular disease driven by (epi-)genetic alterations, it has recently become clear that malignant cells emerge, progress and respond to therapy in the context of a complex and bidirectional crosstalk with the host immune system.14 In particular, tumors become clinically manifest only when immunosurveillance fails, which can occur for different reasons that include, but are not limited to: (i) primary immune defects rendering the host immune system unable to recognize (pre-)malignant cells, (ii) active secretion by (pre-)malignant cells of inhibitory factors that interfere with immune functions locally or systemically; or (iii) evolution of (pre-)malignant cells towards a state of reduced antigenicity of adjuvanticity.15–18 In this context, it appeared logical that R-2-HG would mediate some immunosubversive effects.
Recent data confirm that R-2-HG may mediate both direct and indirect immunosuppressive effects (Figure 1). First, R-2-HG limits the ability of cancer cells to secrete C-X-C motif chemokine ligand 10 (CXCL10), thus reducing the recruitment of T cells to the tumor bed.19,20 Second, R-2-HG can be taken up by non-malignant cells of the tumor microenvironment, including cancer-associated fibroblasts and myeloid cells, which respond to R-2-HG with increased proliferation rates and activation of the pro-inflammatory transcription factor NF-κB, respectively,21,22 ultimately generating a microenvironment that favors tumor progression.23 Third, R-2-HG and its enantiomer (S-2-HG) can be incorporated by immune effectors and mediate immunosuppressive effects. Reportedly, S-2-HG enters activated mouse CD8+ T cells to inhibit histone and DNA demethylation and activate hypoxia inducible factor 1 subunit alpha (HIF1A, best known as HIF-1α), resulting in suppressed T cell proliferation and effector functions.24 Conversely, R-2-HG has been suggested to destabilize HIF-1α in human naïve T cells to boost oxidative phosphorylation, culminating with increased differentiation towards CD4+CD25+FOXP3+ regulatory T (TREG) cells at the expenses of TH17 helper cells.25 Yet another recent paper indicates that R-2-HG can be taken up by human T cells through the plasma membrane transporter solute carrier family 13 member 3 (SLC13A3) irrespective of their activation status, hence interfering with nuclear factor of activated T cells 1 (NFATC1) signaling and limiting proliferative potential and effector functions.26
Figure 1.
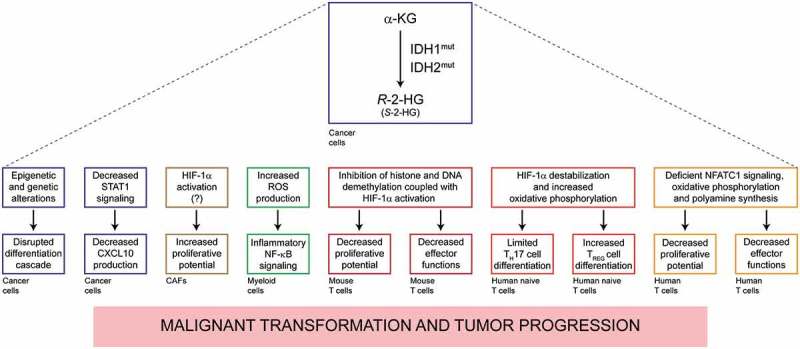
Dual action of R-2-HG in the pathogenesis of cancers with IDH1 or IDH2 mutations. Gain-of-function mutations in isocitrate dehydrogenase (NADP(+)) 1, cytosolic (IDH1) or isocitrate dehydrogenase (NADP(+)) 2, mitochondrial (IDH2) drive the synthesis of R-2-hydroxylutarate (R-2-HG). The accumulation of R-2-HG (and to some extent its enantiomer S-2-HG) supports oncogenesis and tumor progression not only by causing the epigenetic reprogramming of (pre-)malignant cells, but also by favoring the establishment of an immunosuppressive microenvironment. The therapeutic activity of IDH1 inhibitors may therefore involve a robust immunological component. α-KG, alpha-ketoglutarate; CXCL10, C-X-C motif chemokine ligand 10; HIF-1α (official name, HIF1A), hypoxia inducible factor 1 subunit alpha; NFATC1, nuclear factor of activated T cells 1; ROS, reactive oxygen species; STAT1, signal transducer and activator of transcription 1; TREG, regulatory T.
Intriguingly, these effects are at least partially mediated by some degree of ATP shortage resulting from inhibition of oxidative phosphorylation,27 because they can be reverted by supplementation of R-2-HG-treated T lymphocytes with a cell-permeable variant of ATP.26 Moreover, they are tied to a pathway in which R-2-HG inhibits the activity of ornithine decarboxylase (ODC), the rate-limiting enzyme for polyamine biosynthesis, either directly or indirectly upon ODC phosphorylation by 5ʹ-AMP-activated protein kinase (AMPK). Thus, the polyamine putrescine can interfere with the ability of R-2-HG to suppress T cell proliferation in vitro.26 Unfortunately, it has not been determined whether polyamines would negate the immunosuppressive effects of IDH1 or IDH2 mutations in vivo. This stands out as a feasible strategy because spermidine has potent immunostimulatory effects that can be harnessed for boosting natural and therapy-driven anticancer immunosurveillance.28,29
Based on these observations, it is tempting to speculate (pending mechanistic validation) that the clinically efficacy of IDH1 (and presumably also IDH2) inhibitors may be ascribed to a dual effect, namely (i) a cancer cell-autonomous action that interferes with R-2-HG-dependent epigenetic reprogramming, and (ii) the reinstatement of anticancer immunosurveillance. Thus, IDH1 inhibitors do not seem to escape the general rule that anticancer agents can only be successful if they favor anticancer immune responses.30,31 Future will tell whether IDH1 inhibitors can be combined with other immunotherapeutic agents to improve the clinical management of patients with AML or glioma.
Funding Statement
The authors are supported by the Dept. of Radiation Oncology at Weill Cornell Medicine (New York, US); Luke Heller TECPR2 Foundation (Boston, US); Phosplatin (New York, US); Sotio a.s. (Prague, Czech Republic); Lytix (Oslo, Norway); Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2; ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix); Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); SIRIC Cancer Research and Personalized Medicine (CARPEM).
Author disclosure
LG provides remunerated consulting to AstraZeneca (Gathersburg, MD, US), VL47 (New York, NY, US) and OmniSEQ (Buffalo, NY, US), and is a member of the Scientific Advisory Committee of OmniSEQ (Buffalo, NY, US).
References
- 1.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G.. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 2.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, Spath D, Kayser S, Zucknick M, Gotze K, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 4.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G.. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21:805–821. doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Ye D, Guan KL, Xiong Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin Cancer Res. 2012;18:5562–5571. doi: 10.1158/1078-0432.CCR-12-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang L, Su SM. Isocitrate dehydrogenase mutation and (R)-2-Hydroxyglutarate: from basic discovery to therapeutics development. Annu Rev Biochem. 2017;86:305–331. doi: 10.1146/annurev-biochem-061516-044732. [DOI] [PubMed] [Google Scholar]
- 10.Choi C, Raisanen JM, Ganji SK, Zhang S, McNeil SS, An Z, Madan A, Hatanpaa KJ, Vemireddy V, Sheppard CA, et al. Prospective longitudinal analysis of 2-Hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol. 2016;34:4030–4039. doi: 10.1200/JCO.2016.67.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: a systemic review and meta-analysis. Eur Radiol. 2018. doi: 10.1007/s00330-018-5608-7. [DOI] [PubMed] [Google Scholar]
- 12.DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, Swords R, Collins RH, Mannis GN, Pollyea DA, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon S. Ivosidenib: first global approval. Drugs. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–1138. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 15.Zitvogel L, Pitt JM, Daillere R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16:759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 16.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 17.Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, O’Brien T, Lopez JI, Watkins TBK, Nicol D, et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx renal. Cell. 2018;173:595–610 e11. doi: 10.1016/j.cell.2018.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA, Veeriah S, Rosenthal R, et al. Corrigendum: phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2018;554:264. doi: 10.1038/nature25161. [DOI] [PubMed] [Google Scholar]
- 19.Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, Chheda ZS, Downey KM, Watchmaker PB, Beppler C, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127:1425–1437. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucca LE, Hafler DA. Resisting fatal attraction: a glioma oncometabolite prevents CD8+ T cell recruitment. J Clin Invest. 2017;127:1218–1220. doi: 10.1172/JCI93565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvorak A, Zelenka J, Smolkova K, Vitek L, JeZek P. Background levels of neomorphic 2-hydroxyglutarate facilitate proliferation of primary fibroblasts. Physiol Res. 2017;66:293–304. [DOI] [PubMed] [Google Scholar]
- 22.Chen JY, Lai YS, Tsai HJ, Kuo CC, Yen BL, Yeh SP, Sun HS, Hung WC. The oncometabolite R-2-hydroxyglutarate activates NF-kappaB-dependent tumor-promoting stromal niche for acute myeloid leukemia cells. Sci Rep. 2016;6:32428. doi: 10.1038/srep32428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018: in press. doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 24.Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8(+) T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bottcher M, Renner K, Berger R, Mentz K, Thomas S, Cardenas-Conejo ZE, Dettmer K, Oefner PJ, Mackensen A, Kreutz M, et al. D-2-hydroxyglutarate interferes with HIF-1alpha stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. Oncoimmunology. 2018;7:e1445454. doi: 10.1080/2162402X.2018.1445454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, Alansary D, Sonner JK, Green E, Deumelandt K, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018;24:1192–1203. doi: 10.1038/s41591-018-0095-6. [DOI] [PubMed] [Google Scholar]
- 27.Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, Pai MY, Li S, Ta L, Fazlollahi F, et al. 2-Hydroxyglutarate inhibits ATP synthase and mTOR signaling. Cell Metab. 2015;22:508–515. doi: 10.1016/j.cmet.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30:147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:eaan2788. [DOI] [PubMed] [Google Scholar]
- 30.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 31.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]


