ABSTRACT
MDR1 is an ATP-dependent transmembrane transporter primarily studied for its role in the detoxification of tissues and for its implication in resistance of tumor cells to chemotherapy treatment. Several studies also report on its expression on immune cells where it plays a protective role from xenobiotics and toxins. This review provides an overview of what is known on MDR1 expression in immune cells in human, and its implications in different pathologies and their treatment options.
keywords: MDR1, Th1.17, chemo-resistance, drug efflux, anti-tumor immunity, autoimmunity, immune regulation, inhibitors, combination therapy
Introduction
The Multidrug Resistance Protein 1 (MDR1/P-gp) belongs to the ABC (ATP-binding cassette) transporters family which includes 48 members in human.1,2 This efflux pump can exclude several substances such as medical drugs from cells. Consequently, its role has been largely investigated in cancer cells owing to its contribution in chemotherapy resistances.3,4
Physiologically, MDR1 is expressed in several tissues (intestine, colon, placenta, liver, blood brain barrier) where it plays a protective role by reducing the accumulation of xenobiotic molecules in sensitive organs or cells. In addition, MDR1 is also involved in secretion processes as it allows the release of lipid molecules, such as steroid hormones and corticoids,5,6 but also peptides like amyloid-β.7
Over the last decade, several studies have shown the expression of MDR1 in immune cell subsets at different levels and the importance of its expression as well as its functionality to achieve different cellular processes. Nevertheless, MDR1 expression in specific immune cells can influence the treatment of autoimmune diseases and HIV patients. In the context of tumors, the expression of MDR1 on different immune populations could be an advantage as it will confer them resistance to chemotherapy as described for cancer cells, and thereby limit the corresponding immune suppression of these compounds.
In this review, we briefly describe the already well detailed structure of MDR1 and give and overview on current knowledge about its substrates and regulators, with a specific interest for immune modulating drugs. We then focus on the expression and functionality of MDR1 in the different human innate and adaptive immune cell subsets. Finally, we discuss the concerns of MDR1 expression on immune populations when considering treatment options in various pathologies with immune cell deregulations.
Part I: molecular biology of MDR1
Studies carried out on MDR1 during these last decades allowed to better understand the functionality and regulation of this transporter. This was achieved using structure modelling, substrate identification and the use of specific inhibitors.
1-. Gene and protein structure
MDR1 is encoded by the ABCB1 gene, consisting of a 210 kb region with 29 exons, located on chromosomal region 7q21 that contains also the ABCB4 gene, coding for MDR2/3 implicated in phosphatidylcholine transport. Its transcription gives rise to 4 mRNA variants, translated, for three of them, into a single protein of 1280 amino acids.8 At the protein level (Figure 1), MDR1 is formed by a single 170 kDa monomer of 12 transmembrane domains (TMs), glycosylated on its first extracellular loop (between TM1 and 2).1,2 Two distinct cytoplasmic ATP binding sites, respectively located on the loop between the TMs 6 and 7 and after the TM 12, work alternatively and cooperatively to efflux the substrate.9
Figure 1.
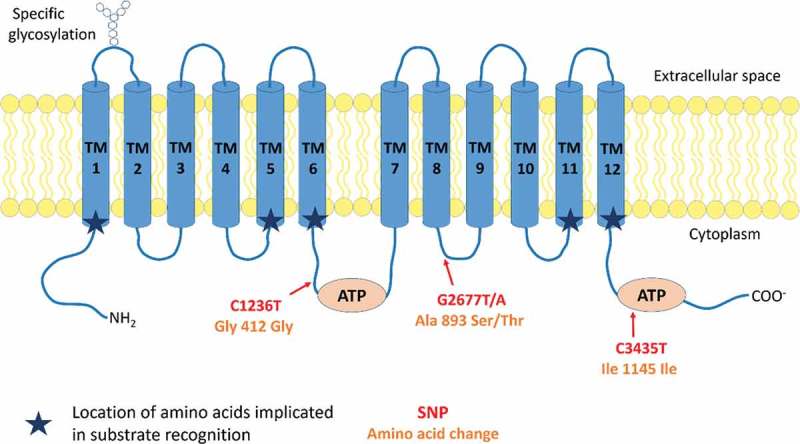
Protein structure and conformation of MDR1.
MDR1 is composed of 12 transmembrane domains (TMs) and two cytoplasmic ATP binding domains located on the loop between the TMs 6 and 7 and the loop after the TM 12. The first extracellular loop is glycosylated. The location of the 3 most frequent MDR1 SNPs (C1236T, G2677T/A and C3435T) are reported on the molecule according to the location of their corresponding amino acid. Changes implied in amino acid are also indicated. Two (C1236T and C3435T) out of the three SNPs are silent mutations. They improve however transcription processes.
Due to the conformational arrangement of its TMs in two pseudo-symmetric bundles of six helices, with the TMs 1–3,6,10,11 and TMs 4,5,7–9,12, having each one nucleotide binding domain, MDR1 forms an active pore.10 Directed mutagenesis performed on the different TMs of MDR1 revealed the implication of different residues on the TMs 1, 5, 6, 11 and 12 in substrates recognition (Figure 1). However, the interaction domains of MDR1 with its different substrates are variable and depend on both their nature and conformation. For instance, the F335A mutation on TM 6 induces a decreased efflux of actinomycin D and vinblastine without modulation of the transport of doxorubicin and colchicine.11–13
2-. Substrates and inhibitors
MDR1 effluxes a large variety of molecules differing in their chemical structure (cyclic, linear, charged or not, hydrophobic, aromatic) as well as their molecular weight (250 to 4000 Da).1 We find some endogenous compounds like amyloid-β, steroids and platelet-activating factor (PAF)5–7,14 but mainly a very large number of therapeutic drugs (Table 1).15 However, two other important ABC transporters, Breast Cancer Resistance Protein 1 (BCRP-1)16 and Multidrug Resistance Protein 1 (MRP-1),17 have also been implicated in chemotherapeutic resistance with a partially shared substrate specificity with MDR1 (Table 1). Polymorphisms of ABCB1 can also modify the efflux capacities of various substrates. Among the 1816 Single Nucleotide Polymorphisms (SNPs) described, only 566 give rise to missense mutations. In the literature, 3 SNPs (C1236T, G2677T/A and C3435T) well described due to their relative high frequency18 have been extensively studied in different pathological contexts. In kidney-transplanted patients, C3435T polymorphism has an impact on the efficacy of the immune suppressant tacrolimus due to a decrease of intestinal drug absorption19 but also immunosuppression resistance of CD4+ and CD8+ T cells, observed on patients with TT genotype.20 Other associations with specific pathologies have been established and are reviewed further in this review (cf. Part III- auto immunity and HIV).
Table 1.
Substrates of MDR1 classified according to their use and chemical origins. Family and mode of action is specified for anti-tumor drugs substrates of MDR1. The substrates known to act as MDR1 inhibitors are in bold, substrates shared with BCRP-1 are mentioned with (*) and substrates shared with MRP-1 are designated with (°).
| Antitumor drugs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Molecules | Family | Mode of action | ||||||
| daunorubicin*°, epirubicin°, doxorubicin (Adriamycin)*° docetaxel°, paclitaxel° vincristine°, vinblastine° etoposide°, teniposide irinotecan*°, topotecan mitoxantrone, bisantrene melphalan° mitomycin C actinomycin D tamoxifen |
Anthracycline Taxane Vinca-alkaloïd Podophyllotoxin Camptothecin Anthracenedione Nitrogen mustard Antibiotic Antibiotic Anti-oestrogen |
DNA and RNA synthesis, topoisomerase II inhibition and chromatin damages induction Microtubules depolymerisation inhibition Microtubules polymerisation inhibition Topoisomerase II inhibition Topoisomerase I inhibition DNA Alkylation DNA Alkylation Topoisomerase II inhibition, DNA Alkylation Transcription Inhibition Oestrogen secretion reduction |
||||||
| HIV Protease inhibitors |
Antimicrobial agents |
Steroids |
Cardiac drugs |
|||||
| amprenavir indinavir° nelfinavir ritonavir*° saquinavir° lopenavir |
amoxicillin azithromycin erythromycin ciprofloxacin° clarythromycin doxycyclin ketoconazole itraconazole levofloxacin puromycin rifampicin tetracycline sparfloxacin |
aldosterone estradiol° progesterone glucocorticoids:
|
AHC-93 bunitrolol carvedilol celiprolol digitoxin digoxin diltiazem mibefradil nicardipine quinidine prazosin reserpine talinolol verapamil |
|||||
| Anti-histamines |
Fluorescent dye |
Opioids |
Immuno-suppressants |
Others |
||||
| cimetidine fexo fenadine phenothiazine terfenadine |
calcein° hoechst 33342* rhodamine 123° |
asimadoline domperidone fentanyl metadone morphine loperamide pentazocine |
cyclosporin A* sirolimus tacrolimus |
carbazepine clomipramine omeprazole* ondansetron Ranitidin atorvastatin* amyloid β PAF |
||||
Owing to MDR1 capacity to induce chemotherapeutic resistance on cells expressing it, many studies investigated MDR1 inhibitors. These have been classified into three generations according to their specificity and toxicity. The first generation of MDR1 inhibitors act as competitive inhibitors, such as the immunosuppressive cyclosporin-A and the calcium channel blocker verapamil. However, because of the high and potentially toxic serum concentration required for complete MDR1 inhibition, these poor specific inhibitors were quickly abandoned for this use. The second generation of inhibitors, such as valspodar (PSC833, cyclosporin-A analogue), possess high affinity for MDR1 and inhibit also other ABC transporters and the cytochrome P450-3A4 (CYPA4) involved in drug metabolism.
More recently, a third generation of inhibitors, with increased specificity to MDR1 and lower toxicity, has been developed in which we can quote elacridar (GG918) and tariquidar (XR9576).21 Nevertheless, new studies show that most of these recent inhibitors also inhibit BCRP-1 or MRP-1 to a certain extent. Zosuquidar (LY335979), that reached phase-III clinical trials to improve acute myeloid leukaemia (AML) treatment, possesses the highest specificity and efficiency.22,23 The development of a fourth generation of inhibitors, even more specific and with lower toxicity, based on the use of natural products is still ongoing.24,25 Other approaches to inhibit MDR1, like the use of the monoclonal antibodies (mAb) MRK-1626 or UIC2,27 have also been evaluated. However, UIC2 mAb inhibits only partially membrane expressed MDR1, due to its recognition of a conformational epitope only fully revealed in the presence of cyclosporin-A or valspodar.28 Moreover, the systemic use of such inhibitors should be cautious regarding their possible side effects, especially because of the important role played by MDR1 at the organism barrier sites to preserve sensitive organs from xenobiotics.
3-. Regulation
In cancer cell lines, MDR1 is regulated at different levels.4 Several studies have suggested the ability of PKC and PKA to increase the functionality of MDR1 or its membrane integration.29,30 Other studies showed that the preferential localization of MDR1 in the lipid rafts and caveolae, characterized as detergent resistant membranes (DRM), is required for its functionality.31,32 Indeed, cholesterol depletion that disrupts lipid rafts, abolishes membrane MDR1 localization and is associated with reduced efflux capacities.33,34 Hypoxia can also induce MDR1 expression through the Hypoxia Inducible Factor 1α (HIF-1α) that binds the promoter of ABCB1 and activates its transcription.35 The modulation of glucose levels and the oxidative stress also induces HIF-1α and MDR1 expression.36,37 MDR1 is also regulated at RNA level by micro RNAs (miR) such as miR-145, miR-27a and miR-331-5p that bind directly the 3ʹUTR of ABCB1 mRNA to decrease MDR1 expression.38,39 Some other miR (miR-137) indirectly decrease MDR1 expression by targeting the YB-1 transcription factor that upregulates MDR1 expression by fixation on ABCB1 promoter.40,41
Only few studies have been interested in MDR1 regulation on immune cells, and this will be developed in Part II.
4-. MDR1 in animal models
Even if MDR1 is highly conserved during evolution, animal models do not completely reflect human organization of the locus coding for this transporter. Indeed, in mouse, Mdr1 is encoded by 3 genes (Mdr1a (Mdr3), Mdr1b (Mdr1), Mdr1c (Mdr2)) that code for Mdr1a, Mdr1b and Mdr1c respectively. Only Mdr1a and Mdr1b confer multidrug resistance while Mdr1c displays the same function as the human MDR2/3.42 Even if the sequences of these two proteins are relatively similar to the human MDR1, Mdr1a and Mdr1b do not share the same substrates. Consequently, this should be taken into account when analyzing experiments evaluating drug effects or MDR1 inhibitors in mice.
Comparing of Mdr1a−/- and control mice enables also to evidence that Mdr1a modulates the functions of intraepithelial lymphocytes (IEL) in the small intestine. Indeed, lack of Mdr1a expression on these cells alter their proliferative capacity and their cytokine production (IL-2 and IFN-γ) in vitro.43 Mdr1a−/- mice also display a decreased regulatory T cells frequency (Treg) in the intestine, resulting from a reduction of induced Treg (iTreg) development from naïve CD4+ T cells upon exposure to TGF-β.44
Part II- MDR1 expression and function in immune cell populations
MDR1 is expressed by many immune cell types with functional consequences on their migration, differentiation, survival, or cytotoxic function. Molecular and cellular analyses of MDR1 expression in peripheral mononuclear cells show expression at high levels on NK cells and B cells. It is also present at the membrane of CD4+ and CD8+ T cells and recent studies highlight a tight association between MDR1 expression and the differentiation of specific T cell subsets. It is also found in some resident dendritic cell (DC) populations as well as in macrophages (Mϕ) but not in monocytes. Thus, MDR1 plays determinant roles in all these subsets (Figure 2).
Figure 2.
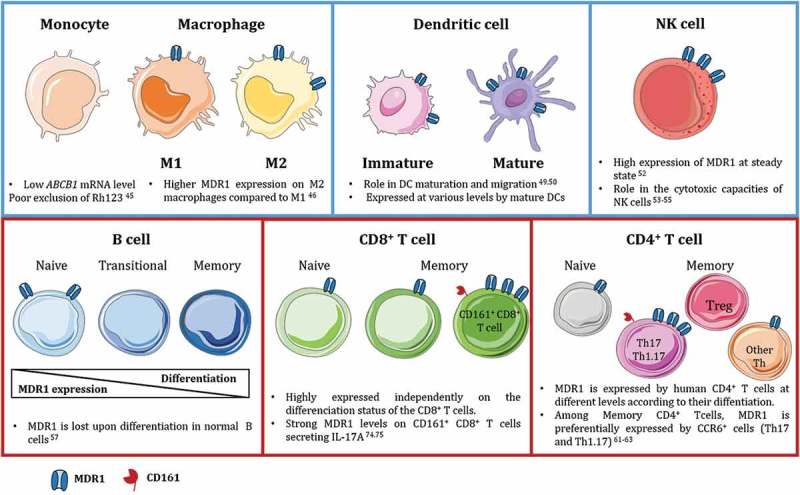
MDR1 expression in innate and adaptive immune cells in human.
MDR1 is widely expressed by immune cells from the innate (blue squares) and adaptive (red squares) compartments. MDR1 plays different role in maturation, migration and survival of the different subsets. Interestingly, it is tightly associated to specific CD4+ and CD8+ T lymphocytes subsets expressing CD161 and secreting IL-17A.
1-. Innate immunity
a. Monocytes/Mf
Monocytes express low ABCB1 mRNA levels correlated with a limited capacity to excrete Rhodamine 123 (Rh123) substrate.45 This expression is increased in monocyte-derived Mϕ but not homogenously between Mϕ subtypes with higher expression by M2-Mϕ than M1-Mϕ.46 Cytokines content in the inflammatory environment can also influence MDR1 expression in Mϕ. Indeed, using THP-1 cell line, Liu et al. demonstrated the upregulation of ABCB1 by soluble factors including IL-6, TNF-α, IL-17, IL-1β and LPS.47 IFNγ has also been shown to increase MDR1 expression in monocyte-derived Mϕ, the mechanism underlying this upregulation remains however still unknown.48
b. DC
Many DC subtypes including Langerhans cells and monocyte-derived DC (MoDC) express varying MDR1 levels. Functionally, MDR1 is especially required for efficient DC maturation. Indeed, MDR1 blockade with valspodar during DC differentiation inhibits their maturation, impairing their acquisition of activation markers like CD80 or CD40 as well as their IL-12p70 secretion capacity.49 Furthermore, under hypoxia, MDR1 is expressed at higher level on mature DCs (DC-Lamp+) compared to immature ones, also suggesting a role in the maturation of iDCs and modulation of its function by hypoxia. MDR1 is also required for DC migration as it mediates the production of an unidentified substrate triggering a migration signaling.50 The MDR1 inhibitor verapamil was shown to specifically upregulate MDR1 expression in both MoDC and Mϕ51 whereas no such effect has been described on immortalized hematopoietic cell lines or T cells.
c. NK cells
Several studies conducted in the late 90’s reported high expression of MDR1 on NK cells. It was also shown to be highly functional on these cells based on Rh123 assay.52 Inhibition with verapamil decreases their cytotoxic functions in a dose dependent manner.53 MDR1 might indeed influence the intracellular pH in NK cells and thereby their cytotoxic function.54 Using cardiac drugs (AHC-93 and nicardipine) that are also MDR1 inhibitors, Takahashi et al. reported that in NK cells, MDR1 controls secretion of cytotoxic granules without modifying their Fas-mediated cytotoxicity.55 These studies, however, rely on the use of unspecific first generation MDR1 inhibitors, and further studies with more specific inhibitors could help decipher its roles in NK cell biology.
2-. Adaptive immunity
a. B lymphocytes
In the early 90’s, Chaudhary et al demonstrated the expression of functional MDR1 in B cells.56 However, recent works evidenced that MDR1 expression remains limited to naive (CD27neg) B cells and undeniably discriminates them from transitional and memory B cells.57 Moreover, the double MDR1/CD27 staining allows to delineate the switched and transitional B cells, the latter being CD27negMDR1neg. The role of MDR1 on naïve B cells is not yet established but could play a role in B cell migration like in DCs or in the lipid composition of their membrane, which is decisive for B cell activation.58
b. T lymphocytes
i. CD4+ T lymphocytes
Pattern of MDR1 expression on CD4+ T cells. Pioneer studies on MDR1 in T lymphocytes have shown its expression on only half of CD4+ T cells.56 In-depth MDR1 functional analysis based on CD45RA/CCR7 expression with Mitotracker green (MTG) dye,59 showed that naive cells (CD45RA+CCR7+) exclude less MTG from their cytoplasm than CD4+ effector (CD45RAnegCCR7neg, TEM) and central (CD45RAnegCCR7+, TCM) memory cells.60 The majority of CD4+ T cells expressing MDR1 are therefore memory cells with the exception of Treg (FoxP3+ or CD25highCD127low/neg) lacking MDR1 expression.60 However, their described hyper-sensitivity to cyclophosphamide does not rely on that since this drug is not a MDR1 substrate.
MDR1 association with Th17/Th1.17 features. In healthy subjects, among CD4+ memory T cells, MDR1 delineates a subset of effector T cells (Teff) mainly composed of Th1.17 also called pathogenic Th17.61 Moreover, our team recently characterized, in human, the CD73+ Teff population that is particularly enriched in Th1.17 and expressed higher MDR1 levels than their CD73neg counterpart.62 A recent study also suggests that MDR1 is also selectively expressed by Teff bearing the lectin-like receptor CD161.63 When analyzed in detail, it appears that this CD161+Rh123low (as a surrogate marker of MDR1) population has strong Th1.17 characteristics and that its high MDR1 expression could contribute to their long-term life span among virus-specific antigen memory T cell pool. This polyfunctional Teff population co-producing IFN-γ and IL-17A and co-expressing MDR1, CD161 and CD73 might therefore be an interesting therapeutic target to modulate their pro-inflammatory features in auto-immune and tumor context.
Functional impact of MDR1 expression on CD4+ T cells. In CD4+ T cells, MDR1 seems to endorse several functions. It notably plays a role in their survival as shown in in vitro models of leukemic T cells made sensitive to caspase-dependent apoptosis after exposure to verapamil or anti-MDR1 mAb.64,65 An alleged role for MDR1 in cytokine secretion by T lymphocytes was also suggested although it is not yet well-defined. Indeed, a study evidenced that this transporter is possibly involved in the export of several cytokines including IL-2, IL-4 and IFNγ66 whereas the use of MDR1-deficient mice didn’t display IL-2 production defects, nor did human T cell lines KO for MDR1 expression.67 Two major components of a chronically inflamed environment, detected in autoimmune disorders (IL-2 and fragmented hyaluronan), have been characterized as efficient activators of MDR1 expression by CD4+ T cells.68,69 Considering this, recent works have proposed MDR1 blockade as a therapeutic strategy in autoimmune disorders or to limit allograft rejection.
ii. CD8+ T lymphocytes
Contrary to CD4+ T cells, most CD8+ T cells exhibit a functional MDR1 identified by Rh123 assay, independently from their differentiation stage but with a significant association to their CD73 expression (unpublished data). However, several studies have shed light on specific CD8+ subsets expressing particularly high MDR1 levels in line with their functions. Recent work suggests that MDR1 is expressed in the bone marrow by a quiescent population of early memory CD8+ T cells that proliferates efficiently in vitro in presence of viral peptides.70 Turtle et al. also reported a memory CD8+ T cell subset (IL18Rα+CD161+CD62L±) with high MDR1 expression endowed with stem cell properties and able to withstand high doses chemotherapy.71 Recently, the expression of MDR1 has also been reported on circulating “young memory” cells (TYM) distinct from TCM and TEM, expressing CD73, CXCR3 and with a marked ALDH1 activity that could relate to cells with self-renewal properties able to survive high dose chemotherapy.72
Like NK cells, it has been shown that high MDR1 expression on CD8+ T cells can contribute to their cytotoxic function73 although no validating data have been published with recent tools available for MDR1 function study. Furthermore, there are no report of MDR1 inhibition affecting the pattern of cytokines secreted by CD8+ T cells in contrast to that described by some teams on CD4+ T cells.
iii. Mucosal tissue associated T cells
A CD161int MDR1hi memory CD8+ T cell population was described as enriched in the gut, and caught up interest in the case of autoimmune Crohn disease (CD) and ulcerative colitis (UC) treatment strategy.74 In addition, xenobiotic resistance of mucosal-associated invariant T cells (MAIT) CD8+ T cells (TCR Vα7.2+) has been associated to their MDR1 expression. These MAIT also express high levels of CD161 and secrete IL-17A or both IFN-γ and IL-17A, displaying Tc17 and Tc1.17-like phenotypes.75 Together with data discussed on CD4+ Teff, these results suggest a common regulation of CD161 and MDR1 in T cells and a common pathway of development and related functions. CD4+ and CD8+ T cells expressing CD161 might indeed share closer similarities in their respective ontogenic pathways than CD161neg counterparts.76 CD161 expression by T lymphocytes has also been linked to their capacity to secrete IL-17A.77 MDR1 expression might therefore be required for their proper function and to preserve them from toxins and xenobiotics particularly abundant in the intestine. In this matter, a recent work from Cao et al. using Mdr1a−/- mice shows that MDR1 initially plays a protective role in the gut since tissue resident MDR1 expressing CD4+ Teff could resist better to bile acids and not induce inflammation while MDR1neg T cells would favor it.78 Indeed, CD4+ Teff from the ileum upregulate MDR1 expression in presence of bile acids to maintain homeostasis. First required as a protective mechanism for mucosal-associated T cells, MDR1 expression is therefore two-sided since it contributes to homeostasis but can become an obstacle to treatment in pathological contexts.
Part III- MDR1 expression on immune cells and its impacts in pathologies and therapeutic strategies
Since MDR1 is widely expressed throughout the immune system, multiple evidences suggest it contributes to modulate the action of some drugs as well as the response or resistance of patients to treatments in various pathologies. We review the known consequences of MDR1 expression on immune cells in several human systemic autoimmune disorders, as well as in the context of viral infection (HIV) and anti-tumor immune response.
1-. Auto-immune diseases
a. Rheumatoid autoimmune disorders: rheumatoid arthritis (RA) and psoriatic arthritis (PsA)
T cell hyper activation and resistance to Treg inhibition play a central role in rheumatoid autoimmune diseases including RA and PsA. In blood of RA patients with highly active disease, high MDR1 expression has been evidenced on memory B cells, involved in the physiopathology of the disease.79 This strongly contrasts with the MDR1 expression restricted on naïve B cells observed in healthy donors.57 The most commonly used treatments in RA and PsA rely on synthetic disease modifying anti rheumatic drugs (DMARDs) like methotrexate (MTX), sulfasalazine (SSZ), leflunomide (LEF) or glucocorticoids (GC). GC are substrates of MDR1 and have a direct influence on patient response over time. Moreover, increased MDR1 functionality on T cells from RA patients treated with prednisolone was reported in 1996.80 It highlighted the role that MDR1 could play in resistance of T cells to DMARDs. Despite contradictory data in the literature, MTX is not a substrate of MDR1 but of MRP1.81 Under MTX treatment, MDR1 expression might therefore not be a resistance factor but rather reflect disease activity.82 Strategies combining drugs that are not substrates of MDR1 (e.g., MTX, infliximab) with MDR1 inhibition by tacrolimus partially restored response to treatment in refractory RA patients and decrease MDR1 expression on both T and B lymphocytes.79
Recently, ABCB1 SNPs (G2677A/T, and less significantly C1236T and C3435T) have been associated with good prognosis in RA patients treated with GC since they reduce MDR1 function on B cells and to a lesser extent on other PBMCs, thus lowering GC resistance.83
b. Crohn’s disease (CD) and ulcerative colitis (UC)
Gut tissues are naturally enriched with Th17 and Th1.17, due to chronic exposure of T cells to many pathogens as well as to a pro-inflammatory cytokine-rich environment (IL-17A, IL-1β, TNF-α), stimulating their differentiation. A tolerance break induced by uncontrolled chronic stimulation of these immune cells is the starting point to UC and CD pathogenesis. Th17 and Th1.17 are particularly active in the pathogenesis of these affections and Ramesh et al. demonstrated, in CD patients, a MDR1 enrichment within Th1.17 that resist to GC treatment.61 On the contrary, MDR1 loss of function was identified in a subset of patients with an aggressive form of ileal CD.78 Therefore, MDR1 role is dual in this pathology. It plays a protective role at first, but its loss can lead to a breach in homeostasis and favor the disease progression. In other cases, where loss of homeostasis involves other mechanisms, MDR1 expression can favor the selection of treatment-resistant T cells thereafter active in the pathophysiology of the diseases. In addition, ABCB1 SNPs (G2677T/A and C3435T) are associated with refractory UC and CD and might play a role in the resistance of T cells to GC treatment.84 Their good or poor prognosis could depend on the cases depicted above indicating that either MDR1 loss of function or over-expression can lead to the same phenotype depending on the pathophysiological mechanisms primarily involved.
Altogether, these data related to MDR1 impact on disease course and treatment are strong arguments to personalized therapy for patients with autoimmune disorders. Efforts should also be done on the evaluation of MDR1 expression and function in the different immune subsets involved in autoimmune diseases to better understand the roles it can play in disease course and response to treatment.
2-. HIV treatment and resistance
As described in Table 1, MDR1 can exclude protease inhibitor (PI) used to treat HIV infected patients, thus conferring treatment resistances.85 MDR1 polymorphisms, especially the T variant of the C1236T SNP, increase affinity for some PI resulting in a low intestinal absorption and an increased PI efflux by CD4+ T cells.86 Moreover, PI concentrations in PBMC from HIV infected patients inversely correlate with mRNA levels of ABCB1 in the cells85, suggesting the implication of MDR1 in HIV treatment resistances.
The proportions of CD4+ and CD8+ T cells expressing MDR1 are higher in HIV infected patients than in healthy controls and seem to increase during the progression of the infection. Nonetheless, MDR1 functionality is reduced in HIV patients as illustrated by a higher intracellular accumulation of Rh123 compared to controls that is not altered by cyclosporin-A.87 Furthermore, this altered MDR1 functionality is also observed on patients’ NK cells as it increases with the progression of the infection and correlates with a decrease of their cytotoxic functions.88 However, this impairment seems to be transient and shaped by the environment as the in vitro stimulation of these NK cells with IL-15 restores MDR1 functionality as well as NK cell cytotoxic functions.89 In contrast, the increased MDR1 expression at transcriptomic and protein levels detected on CD8+ T cells in treated patients is associated with a better efflux capacity.90
Concerning Mϕ, M2-Mϕ express higher MDR1 levels than M1-Mϕ and their increased efflux of lopenavir favor the accumulation of latent virus.46
Altogether, these data suggest that the evaluation of MDR1 expression on some immune subsets might be relevant to assess the state of disease development or guide the therapeutic option in HIV infected patients.
3-. Anti-cancer drugs and impact on immune cells
In the cancer field, two scenarios can be distinguished when studying MDR1 expression and function on immune cells. Either the malignant cells are immune cells, originally expressing or not MDR1, or the immune cells infiltrate solid tumors from other tissue origin. We consider both cases showing the alternatives of treatment and the challenges it raises.
a. MDR1 expression in T leukemic cells, lymphomas and myelomas
Many immune cell types giving rise to hematological malignancies naturally express MDR1 as described previously (B cells, T cells, myeloid cells). Therefore, emerging hematological tumors are often resistant to chemotherapies that are MDR1 substrates. AML or multiple myelomas (MM) patients displaying high MDR1 expression on malignant cells indeed show poorer response to many treatments compared to patients with lower MDR1 expression.91–93 This natural expression by myeloid and lymphoid cells is also strengthened by oncogenic mechanisms inducing aberrant MDR1 re-expression on cell types normally MDR1neg (e.g., memory B cells) such as Diffuse Large B Cell lymphoma (DLBCL) and Follicular Lymphoma (FL) or enhancing preexisting MDR1 expression (e.g., naïve and transitory B cells, monocytes). Indeed, upregulation of ABCB1 is related to hyper activation of the MAPK/ERK signal transduction pathway that activates YB-1 nuclear translocation and this amplification loop is increased in B-cell lymphomas.94 To improve treatment efficacy, combination of MDR1 inhibitors with specific drugs have been tested, showing notably encouraging results in term of overall survival in patients with AML.22,95–97 Most of these studies used first or second generation inhibitors. The use of much more specific third or upcoming fourth generation inhibitors could allow to improve these results and decrease toxicities associated with combination treatments. Interestingly, MDR1-induced chemo-resistance in lymphoma cells can be overcome with anti-CD20 as well as anti-CD19 mAbs used in lymphoma therapy. In addition to their therapeutic effect based on complement-mediated and antibody-dependent cell-mediated cytotoxicity, both mAbs, could increase chemotherapy efficacy, by limiting MDR1 activity as fixation to their target could favor MDR1 re-localization outside of the lipid rafts where it is much less efficient.98,99 More recently, PI substrates of MDR1 have also been used as unconventional competitive inhibitors in MM to improve efficacy of carfilzomib (proteasome inhibitor).93 Understanding MDR1 regulation processes in normal and neoplastic immune cells therefore appears as an interesting lead to improve combination therapy options in hematological tumors and discover new molecules efficiently blocking MDR1 activity with limited side effects.
b. MDR1 expression on immune infiltrate in tumors
MDR1 has been extensively studied on tumor cells along with the phenomenon of multidrug resistance. However, little is known on the role of MDR1 on the tumor immune infiltrate. Could MDR1 expression by specific lymphocytes or NK cells represent an interesting mechanism of shaping of the immune infiltrate in solid tumors? The data we report here suggest that all immune cells do not respond the same way when exposed to MDR1-sensitive drugs. It is therefore highly likely that the infiltrate will be shaped upon such treatment regimen.
Presence of MDR1 positive T cells in solid tumors infiltrates
Very few data are so far available on the evaluation of MDR1 expression by the immune infiltrate and its possible impact on immune cells survival in solid tumors. A recent study, conducted on advanced human colorectal cancer (CRC) tissues showed, a significant higher frequency of MDR1+CD161+ T cells, considered mostly as MAIT cells by the authors, in the tumor tissue compared to adjacent healthy tissues.100 In this matter, evaluation of MDR1 expression by immune subtypes should be more extensively investigated. The hostile tumor micro-environment and its chemical composition must indeed shape the immune infiltrate, and MDR1 expression by anti-tumor immune cells may favor their preservation despite harsh environmental conditions. The immune infiltrate is also modulated by MDR1-associated anti-neoplastic agents,90,101 and evaluation of MDR1 level on immune cells could be an indicator of these treatments’ effects.
Tumor-associated immune infiltrate could be skewed by the use of chemotherapies substrates of MDR1
It is expected that MDR1-expressing immune cells in the tumor microenvironment will resist better to MDR1-associated chemotherapies. The Th1.17 subset expressing high MDR1 level and also secreting pro-inflammatory cytokines,61,62 appears as an asset in the anti-tumor immune response in contrast to its deleterious impact in autoimmune diseases. In long term chemotherapy treated AML patients, the non-malignant CD4+CD161+MDR1+ T cells fraction is increased, and they conserve their anti-viral properties and display self-renewal capacities.63 In addition, we showed an enrichment in MDR1-positive cells within CD4+CD73+ Teff infiltrating breast and ovarian carcinomas (unpublished data) that secrete anti-tumor cytokines (IFNγ, IL-17A, TNFα) ex vivo in the absence of adenosine or ATP/AMP.62 In addition, we evidenced that this specific subset expresses very low level of inhibitory immune checkpoints like PD-1 and CTLA-4. This highly polyfunctional CD73+MDR1+ Teff population might therefore be of interest in therapeutic approaches combining MDR1-associated chemotherapy and anti-CD73 targeting therapy to avoid adenosine production and potentiate pro-inflammatory response of this specific subset. Moreover, determining which IL-17A secreting immune cells express MDR1 in the tumor microenvironment could help solve the controversial role played by IL-17A in solid tumors. Enrichment of the specific Th1.17 subset at the tumor site subsequently to the use of MDR1-associated chemotherapy may indeed favor anti-tumor properties of IL-17A. We can also predict curtailment of the Treg frequency since their lack of MDR1 will increased their sensitivity to high doses of these treatments. Considering the Mϕ, we can on the contrary expect a skewing towards pro-tumoral M2-Mϕ upon high doses chemotherapy since they express higher levels of MDR1. NK cells are also key players in the anti-tumor immune response, and quantitative but also functional alteration of NK cells in primary and metastatic tumors have been evidenced.102 Since NK cells were shown to express high levels of MDR1 in physiological context it would be worthwhile evaluating MDR1 expression on the NK infiltrate in tumor environment and determine if it correlates with tumor destruction or if therapeutic choices influence positively or negatively NK cell cytotoxicity.
In the case of poorly chemo-resistant tumors displaying low MDR1 activity like breast tumors,103 the use of high doses of MDR1-associated chemotherapeutic agents, could therefore have a double advantage: it should be effective against tumor cells to a certain extent favoring the release of tumor antigens and could also induce the selection of immune subsets with interesting anti-tumor features (Th1.17, CD161+CD8+ T cells), while limiting MDR1neg Treg expansion. However, one should keep in mind that this selection could also favor pro-tumor M2-Mϕ expansion in the tumor since they express MDR1 at high level.
On the contrary, the use of MDR1 inhibitors in combination treatment of chemo-resistant solid tumors may help restore chemo-sensitivity of tumor cells but could in the same time impair critical anti-tumor T cell populations. Changes induced by such treatment on immune infiltrate must especially be considered in the therapeutic strategy to anticipate variations of the immune players at the tumor site to try to achieve the most efficient anti-tumor response.
Funding Statement
This work has been financially supported by the Breast Cancer Research Fundation (BCRF), grants from the Puy de Dôme comity of the “Ligue Contre le Cancer”, the SIRIC project (LyriCAN, grant n°INCa-DGOS-INSERM_12563), the FP7 European TumAdoR project (grant n° 602200) and the LABEX DEVweCAN (ANR-10-LABX-0061) of the University of Lyon, within the program “Investissements d’Avenir” organized by the French National Research Agency (ANR). We also acknowledge the "Ligue Nationale contre le Cancer" for A Di-Roio PhD fellowship.
Acknowledgments
We would like to thank Dr. L.P. Jordheim for critical reading of the manuscript.
References
- 1.Borst P, Elferink RO.. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 2.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/S0169-409X(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 3.Nakai E, Park K, Yawata T, Chihara T, Kumazawa A, Nakabayashi H, Shimizu K. Enhanced MDR1 expression and chemoresistance of cancer stem cells derived from glioblastoma. Cancer Invest. 2009. 27(9);901–908. doi: 10.3109/07357900801946679. [DOI] [PubMed] [Google Scholar]
- 4.Katayama K, Noguchi K, Sugimoto Y. Regulations of P-Glycoprotein/ABCB1/MDR1 in human cancer cells. New J Sci. 2014. accessed 2018 March23. doi: 10.1155/2014/476974. [DOI] [Google Scholar]
- 5.Ueda K, Okamura N, Hirai M, Tanigawara Y, Saeki T, Kioka N, Komano T, Hori R. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267(34):24248–24252. [PubMed] [Google Scholar]
- 6.Bello-Reuss E, Ernest S, Holland OB, Hellmich MR. Role of multidrug resistance P-glycoprotein in the secretion of aldosterone by human adrenal NCI-H295 cells. Am J Physiol Cell Physiol. 2000. 278(6);C1256–1265. doi: 10.1152/ajpcell.2000.278.6.C1256. [DOI] [PubMed] [Google Scholar]
- 7.Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, Cascorbi I, Walker LC, Kroemer HK, Warzok RW, et al. MDR1-P-Glycoprotein (ABCB1) mediates transport of Alzheimer’s amyloid-beta peptides–implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17(4):347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodor M, Kelly EJ, Ho RJ. Characterization of the human MDR1 gene. AAPS J. 2005. 7(1);E1–5. doi: 10.1208/aapsj070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrycyna CA, Ramachandra M, Ambudkar SV, Ko YH, Pedersen PL, Pastan I, Gottesman MM. Mechanism of action of human P-glycoprotein ATPase activity. Photochemical cleavage during a catalytic transition state using orthovanadate reveals cross-talk between the two ATP sites. J Biol Chem. 1998;273(27):16631–16634. doi: 10.1074/jbc.273.27.16631. [DOI] [PubMed] [Google Scholar]
- 10.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323(5922):1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajiji S, Talbot F, Grizzuti K, Van Dyke-Phillips V, Agresti M, Safa AR, Gros P. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry. 1993;32(16):4185–4194. doi: 10.1021/bi00067a005. [DOI] [PubMed] [Google Scholar]
- 12.Hafkemeyer P, Dey S, Ambudkar SV, Hrycyna CA, Pastan I, Gottesman MM. Contribution to substrate specificity and transport of nonconserved residues in transmembrane domain 12 of human P-glycoprotein. Biochemistry. 1998. 37(46);16400–16409. doi: 10.1021/bi980871+. [DOI] [PubMed] [Google Scholar]
- 13.Loo TW, Clarke DM. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J Membr Biol. 2005. 206(3);173–185. doi: 10.1007/s00232-005-0792-1. [DOI] [PubMed] [Google Scholar]
- 14.Raggers RJ, Vogels I, van Meer G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem J. 2001;357(Pt 3):859–865. doi: 10.1042/bj3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics. 2011. 21(3);152–161. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003. 22(47);7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z-S, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in Cancer chemotherapy and genetic diseases. FEBS J. 2011. 278(18);3226–3245. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gow JM, Hodges LM, Chinn LW, Kroetz DL. Substrate-dependent effects of human ABCB1 coding polymorphisms. J Pharmacol Exp Ther. 2008. 325(2);435–442. doi: 10.1124/jpet.107.135194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbas SH, Bilgen T, Keser I, Tuncer M, Yucetin L, Tosun O, Gultekin M, Luleci G. The effect of MDR1 (ABCB1) polymorphism on the pharmacokinetic of tacrolimus in Turkish renal transplant recipients. Transplant Proc. 2006. 38(5);1290–1292. doi: 10.1016/j.transproceed.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 20.Vafadari R, Bouamar R, Hesselink DA, Kraaijeveld R, van Schaik RHN, Weimar W, Baan CC, van Gelder T. Genetic polymorphisms in ABCB1 influence the pharmacodynamics of tacrolimus. Ther Drug Monit. 2013. 35(4);459–465. doi: 10.1097/FTD.0b013e31828c1581. [DOI] [PubMed] [Google Scholar]
- 21.Amin ML. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights. 2013;7:27–34. doi: 10.4137/DTI.S12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang R, Faussat A-M, Perrot J-Y, Marjanovic Z, Cohen S, Storme T, Morjani H, Legrand O, Marie J-P. Zosuquidar restores drug sensitivity in P-glycoprotein expressing acute myeloid leukemia (AML). BMC cancer. 2008;8:51. doi: 10.1186/1471-2407-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rl S, Cao J, Jj S, Ah D. Modulation of P-glycoprotein but not MRP1- or BCRP-mediated drug resistance by LY335979. Int J Cancer. 2003. 103(1);121–125. doi: 10.1002/ijc.10792. [DOI] [PubMed] [Google Scholar]
- 24.Wu C-P, Ohnuma S, Ambudkar SV. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12(4):609–620. doi: 10.2174/138920111795163887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karthikeyan S, Hoti SL. Development of fourth generation ABC inhibitors from natural products: a novel approach to overcome cancer multidrug resistance. Anticancer Agents Med Chem. 2015;15(5):605–615. doi: 10.2174/1871520615666150113103439. [DOI] [PubMed] [Google Scholar]
- 26.Mickisch GH, Pai LH, Gottesman MM, Pastan I. Monoclonal antibody MRK16 Reverses the multidrug resistance of multidrug-resistant transgenic mice. Cancer Res. 1992;52(16):4427–4432. [PubMed] [Google Scholar]
- 27.Mechetner EB, Roninson IB. Efficient inhibition of P-glycoprotein-mediated multidrug resistance with a monoclonal antibody. Proc Natl Acad Sci. 1992. 89(13);5824–5828. doi: 10.1073/pnas.89.13.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goda K, Fenyvesi F, Bacsó Z, Nagy H, Márián T, Megyeri A, Krasznai Z, Juhász I, Vecsernyés M, Szabó G. Complete inhibition of P-glycoprotein by simultaneous treatment with a distinct class of modulators and the UIC2 monoclonal antibody. J Pharmacol Exp Ther. 2007. 320(1);81–88. doi: 10.1124/jpet.106.110155. [DOI] [PubMed] [Google Scholar]
- 29.Chambers TC, Pohl J, Glass DB, Kuo JF. Phosphorylation by protein kinase C and cyclic AMP-dependent protein kinase of synthetic peptides derived from the linker region of human P-glycoprotein. Biochem J. 1994;299(Pt 1):309–315. doi: 10.1042/bj2990309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojtal KA, De Vries E, Hoekstra D, van Ijzendoorn SCD. Efficient trafficking of MDR1/P-glycoprotein to apical canalicular plasma membranes in HepG2 cells requires PKA-RIIalpha anchoring and glucosylceramide. Mol Biol Cell. 2006. 17(8);3638–3650. doi: 10.1091/mbc.E06-03-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci U S A. 2002. 99(16);10347–10352. doi: 10.1073/pnas.162366399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothnie A, Theron D, Soceneantu L, Martin C, Traikia M, Berridge G, Higgins CF, Devaux PF, Callaghan R. The importance of cholesterol in maintenance of P-glycoprotein activity and its membrane perturbing influence. European biophysics journal: EBJ. 2001;30(6):430–442. [DOI] [PubMed] [Google Scholar]
- 33.Troost J, Lindenmaier H, Haefeli WE, Weiss J. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol Pharmacol. 2004. 66(5);1332–1339. doi: 10.1124/mol.104.002329. [DOI] [PubMed] [Google Scholar]
- 34.Luker GD, Pica CM, Kumar AS, Covey DF, Piwnica-Worms D. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry. 2000;39(26):7651–7661. doi: 10.1021/bi9928593. [DOI] [PubMed] [Google Scholar]
- 35.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible Factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–3394. [PubMed] [Google Scholar]
- 36.Seebacher NA, Richardson DR, Jansson PJ. Glucose modulation induces reactive oxygen species and increases P-glycoprotein-mediated multidrug resistance to chemotherapeutics. Br J Pharmacol. 2015. 172(10);2557–2572. doi: 10.1111/bph.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzón-Daza ML, Cuellar-Saenz Y, Nualart F, Ondo-Mendez A, Del Riesgo L, Castillo-Rivera F, Garzón R. Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions. J Cell Biochem. 2017. 118(7);1868–1878. doi: 10.1002/jcb.25890. [DOI] [PubMed] [Google Scholar]
- 38.Feng -D-D, Zhang H, Zhang P, Zheng Y-S, Zhang X-J, Han B-W, Luo X-Q, Xu L, Zhou H, Qu L-H, et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15(10):2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikemura K, Yamamoto M, Miyazaki S, Mizutani H, Iwamoto T, Okuda M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol Pharmacol. 2013. 83(2);399–405. doi: 10.1124/mol.112.081844. [DOI] [PubMed] [Google Scholar]
- 40.Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W. miR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim Biophys Sin (Shanghai). 2013. 45(2);80–86. doi: 10.1093/abbs/gms099. [DOI] [PubMed] [Google Scholar]
- 41.Dolfini D, Mantovani R. Targeting the Y/CCAAT box in cancer: YB-1 (YBX1) or NF-Y? Cell Death Differ. 2013. 20(5);676–685. doi: 10.1038/cdd.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devault A, Gros P. Two members of the mouse mdr gene family confer multidrug resistance with overlapping but distinct drug specificities. Mol Cell Biol. 1990;10(4):1652–1663. doi: 10.1128/MCB.10.4.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eisenbraun MD, Mosley RL, Teitelbaum DH, Miller RA. Altered development of intestinal intraepithelial lymphocytes in P-glycoprotein-deficient mice. Dev Comp Immunol. 2000. 24(8);783–795. doi: 10.1016/S0145-305X(00)00029-X. [DOI] [PubMed] [Google Scholar]
- 44.Tanner SM, Staley EM, Lorenz RG. Altered generation of induced regulatory T cells in the FVB.mdr1a-/- mouse model of colitis. Mucosal Immunol. 2013. 6(2);309–323. doi: 10.1038/mi.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype [see comments]. Blood. 1992;80(11):2729–2734. [PubMed] [Google Scholar]
- 46.Cory TJ, He H, Winchester LC, Kumar S, Fletcher CV. Alterations in P-glycoprotein expression and function between macrophage subsets. Pharm Res. 2016. 33(11);2713–2721. doi: 10.1007/s11095-016-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Zhou F, Chen Q, Kang A, Lu M, Liu W, Zang X, Wang G, Zhang J. Chronic inflammation up-regulates P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice. Sci Rep. 2015;5:13558. doi: 10.1038/srep13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puddu P, Fais S, Luciani F, Gherardi G, Dupuis ML, Romagnoli G, Ramoni C, Cianfriglia M, Gessani S. Interferon-gamma up-regulates expression and activity of P-glycoprotein in human peripheral blood monocyte-derived macrophages. Lab Invest. 1999;79(10):1299–1309. [PubMed] [Google Scholar]
- 49.Pendse SS, Behjati S, Schatton T, Izawa A, Sayegh MH, Frank MH. P‐glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am J Transplant. 2006. 6(12);2884–2893. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 50.Laupèze B, Amiot L, Bertho N, Grosset JM, Lehne G, Fauchet R, Fardel O. Differential expression of the efflux pumps P-glycoprotein and multidrug resistance-associated protein in human monocyte-derived dendritic cells. Hum Immunol. 2001;62(10):1073–1080. [DOI] [PubMed] [Google Scholar]
- 51.Ishri RK, Menzies S, Halliday GM. Verapamil induces upregulation of P-glycoprotein expression on human monocyte derived dendritic cells. Immunol Invest. 2006. 35(1);1–18. doi: 10.1080/08820130500496746. [DOI] [PubMed] [Google Scholar]
- 52.Vasquez EM, Petrenko Y, Jacobssen V, Sifontis NM, Testa G, Sankary H, Benedetti E. An assessment of P-glycoprotein expression and activity in peripheral blood lymphocytes of transplant candidates. Transplant Proc. 2005. 37(1);175–177. doi: 10.1016/j.transproceed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Ludescher C, Pall G, Irschick EU, Gastl G. Differential activity of P-glycoprotein in normal blood lymphocyte subsets. Br J Haematol. 1998;101(4):722–727. [DOI] [PubMed] [Google Scholar]
- 54.Yamashiro T, Watanabe N, Kobayashi Y. Reduction of intracellular pH by inhibitors of natural killer cell activity, nicardipine, methyl 2-(N-benzyl-N-methylamino)ethyl-2,6-dimethyl-4-(2-isopropyl-pyrazolo[1, 5-a]pyridine-3-yl)-1,4-dihydro-pyridine-3,5-dicarboxylate (AHC-52), and 4,4ʹ-diisothiocyano-2,2ʹ-disulfonic acid stilbene (DIDS). Biochem Pharmacol. 1997;54(1):143–148. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Misawa Y, Watanabe N, Kawanishi T, Tanaka H, Shigenobu K, Kobayashi Y. Role of P-glycoprotein in human natural killer-like cell line-mediated cytotoxicity. Exp Cell Res. 1999. 253(2);396–402. doi: 10.1006/excr.1999.4696. [DOI] [PubMed] [Google Scholar]
- 56.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991. 66(1);85–94. doi: 10.1016/0092-8674(91)90141-K. [DOI] [PubMed] [Google Scholar]
- 57.Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol. 2005. 35(12);3433–3441. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- 58.Yagi K, Yamamoto K, Umeda S, Abe S, Suzuki S, Onishi I, Kirimura S, Fukayama M, Arai A, Kitagawa M, et al. Expression of multidrug resistance 1 gene in B-cell lymphomas: association with follicular dendritic cells. Histopathology. 2013;62(3):414–420. doi: 10.1111/his.12035. [DOI] [PubMed] [Google Scholar]
- 59.Marques-Santos LF, Oliveira JGP, Maia RC, Rumjanek VM. Mitotracker green is a emphasis Type=“Italic” P emphasis-glycoprotein substrate. Biosci Rep. 2003. 23(4);199–212. doi: 10.1023/B:BIRE.0000007693.33521.18. [DOI] [PubMed] [Google Scholar]
- 60.Sarah D, Corina F, Marco F, Gubser Patrick M, Leyla R, Bantug Glenn R, Morgane R, Anja L, Christoph H. Human regulatory T cells lack the cyclophosphamide‐extruding transporter ABCB1 and are more susceptible to cyclophosphamide‐induced apoptosis. Eur J Immunol. 2014. 44(12);3614–3620. doi: 10.1002/eji.201444879. [DOI] [PubMed] [Google Scholar]
- 61.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014. 211(1);89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gourdin N, Bossennec M, Rodriguez C, Vigano S, Machon C, Jandus C, Bauché D, Faget J, Durand I, Chopin N, et al. Autocrine Adenosine regulates tumor polyfunctional CD73+CD4+ effector T cells devoid of immune checkpoints. Cancer Res. 2018March20 doi: 10.1158/0008-5472.CAN-17-2405. [DOI] [PubMed] [Google Scholar]
- 63.Alsuliman A, Muftuoglu M, Khoder A, Ahn Y-O, Basar R, Verneris MR, Muranski P, Barrett AJ, Liu E, Li L, et al. A subset of virus-specific CD161+ T cells selectively express the multidrug transporter MDR1 and are resistant to chemotherapy in AML. Blood. 2017;129(6):740–758. doi: 10.1182/blood-2016-05-713347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnstone RW, Cretney E, Smyth MJ. P-Glycoprotein Protects Leukemia Cells Against Caspase-Dependent, but not Caspase-Independent, Cell Death. Blood. 1999;93(3):1075–1085. [PubMed] [Google Scholar]
- 65.Gollapud S, Gupta S. Anti-P-glycoprotein antibody-induced apoptosis of activated peripheral blood lymphocytes: a possible role of P-glycoprotein in lymphocyte survival. J Clin Immunol. 2001;21(6):420–430. doi: 10.1023/A:1013177710941. [DOI] [PubMed] [Google Scholar]
- 66.Drach J, Gsur A, Hamilton G, Zhao S, Angerler J, Fiegl M, Zojer N, Raderer M, Haberl I, Andreeff M, et al. Involvement of P-glycoprotein in the transmembrane transport of interleukin-2 (IL-2), IL-4, and interferon-gamma in normal human T lymphocytes. Blood. 1996;88(5):1747–1754. [PubMed] [Google Scholar]
- 67.Gollapudi S, Kim C, Gupta S. P-glycoprotein (encoded by multidrug resistance genes) is not required for interleukin-2 secretion in mice and humans. Genes Immun. 2000. 1(6);371–379. doi: 10.1038/sj.gene.6363693. [DOI] [PubMed] [Google Scholar]
- 68.Tsujimura S, Saito K, Nakayamada S, Nakano K, Tsukada J, Kohno K, Tanaka Y. Transcriptional regulation of multidrug resistance-1 gene by interleukin-2 in lymphocytes. Genes Cells: Devoted Mol Cell Mech. 2004. 9(12);1265–1273. doi: 10.1111/j.1365-2443.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 69.Tsujimura S, Saito K, Kohno K, Tanaka Y. Fragmented hyaluronan induces transcriptional up-regulation of the multidrug resistance-1 gene in CD4+ T cells. J Biol Chem. 2006. 281(49);38089–38097. doi: 10.1074/jbc.M601030200. [DOI] [PubMed] [Google Scholar]
- 70.Kudernatsch RF, Letsch A, Guerreiro M, Löbel M, Bauer S, Volk H-D, Scheibenbogen C. Human bone marrow contains a subset of quiescent early memory CD8+ T cells characterized by high CD127 expression and efflux capacity. Eur J Immunol. 2014;44(12):3532–3542. doi: 10.1002/eji.201344180. [DOI] [PubMed] [Google Scholar]
- 71.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR, Distinct A. Subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009. 31(5);834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murata K, Tsukahara T, Emori M, Shibayama Y, Mizushima E, Matsumiya H, Yamashita K, Kaya M, Hirohashi Y, Kanaseki T, et al. Identification of a novel human memory T-cell population with the characteristics of stem-like chemo-resistance. OncoImmunology. 2016;5(6):e1165376. doi: 10.1080/2162402X.2016.1165376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta S, Kim CH, Tsuruo T, Gollapudi S. Preferential expression and activity of multidrug resistance gene 1 product (P-glycoprotein), a functionally active efflux pump, in human CD8 + T cells: A role in cytotoxic effector function. J Clin Immunol. 1992. 12(6);451–458. doi: 10.1007/BF00918857. [DOI] [PubMed] [Google Scholar]
- 74.Fergusson JR, Hühn MH, Swadling L, Walker LJ, Kurioka A, Llibre A, Bertoletti A, Holländer G, Newell EW, Davis MM, et al. CD161(int)CD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol. 2016;9(2):401–413. doi: 10.1038/mi.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Bourhis LL, Soudais C, Treiner E, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17–secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 76.Fergusson JR, Fleming VM, Klenerman P. CD161-expressing human T cells. Front Immunol. 2011;2:36. doi: 10.3389/fimmu.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao W, Kayama H, Chen ML, Delmas A, Sun A, Kim SY, Rangarajan ES, McKevitt K, Beck AP, Jackson CB, et al. The xenobiotic transporter mdr1 enforces T Cell homeostasis in the presence of intestinal bile acids. Immunity. 2017;47(6):1182–1196.e10. doi: 10.1016/j.immuni.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsujimura S, Saito K, Nawata M, Nakayamada S, Tanaka Y. Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann Rheum Dis. 2008. 67(3);380–388. doi: 10.1136/ard.2007.070821. [DOI] [PubMed] [Google Scholar]
- 80.Maillefert JF, Maynadie M, Tebib JG, Aho S, Walker P, Chatard C, Dulieu V, Bouvier M, Carli PM, Tavernier C. Expression of the multidrug resistance glycoprotein 170 in the peripheral blood lymphocytes of rheumatoid arthritis patients. The percentage of lymphocytes expressing glycoprotein 170 is increased in patients treated with prednisolone. Br J Rheumatol. 1996;35(5):430–435. doi: 10.1093/rheumatology/35.5.430. [DOI] [PubMed] [Google Scholar]
- 81.Maillefert J-F, Puéchal X, Falgarone G, Lizard G, Ornetti P, Solau E, Legré V, Lioté F, Sibilia J, Morel J, et al. Prediction of response to disease modifying antirheumatic drugs in rheumatoid arthritis. Joint Bone Spine. 2010;77(6):558–563. doi: 10.1016/j.jbspin.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal V, Mittal SK, Misra R. Expression of multidrug resistance-1 protein correlates with disease activity rather than the refractoriness to methotrexate therapy in rheumatoid arthritis. Clin Rheumatol. 2009. 28(4);427–433. doi: 10.1007/s10067-008-1071-1. [DOI] [PubMed] [Google Scholar]
- 83.Cuppen BVJ, Pardali K, Kraan MC, Marijnissen ACA, Yrlid L, Olsson M, Bijlsma JWJ, Lafeber FPJG, Fritsch-Stork RDE. Polymorphisms in the multidrug-resistance 1 gene related to glucocorticoid response in rheumatoid arthritis treatment. Rheumatol Int. 2017. 37(4);531–536. doi: 10.1007/s00296-017-3653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinar M, Cukovic-Cavka S, Bozina N, Ravic KG, Markos P, Ladic A, Cota M, Krznaric Z, Vucelic B. MDR1 polymorphisms are associated with inflammatory bowel disease in a cohort of Croatian IBD patients. BMC Gastroenterol. 2013;13:57. doi: 10.1186/1471-230X-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaillou S, Durant J, Garraffo R, Georgenthum E, Roptin C, Clevenbergh P, Dunais B, Mondain V, Roger PM, Dellamonica P. Intracellular concentration of protease inhibitors in HIV-1-infected patients: correlation with MDR-1 gene expression and low dose of ritonavir. HIV Clin Trials. 2002. 3(6);493–501. doi: 10.1310/hct.2002.3.6.007. [DOI] [PubMed] [Google Scholar]
- 86.Bellusci CP, Rocco C, Aulicino P, Mecikovsky D, Curras V, Hegoburu S, Bramuglia GF, Bologna R, Sen L, Mangano A. Influence of MDR1 C1236T polymorphism on lopinavir plasma concentration and virological response in HIV-1-infected children. Gene. 2013. 522(1);96–101. doi: 10.1016/j.gene.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 87.Andreana A, Aggarwal S, Gollapudi S, Wien D, Tsuruo T, Gupta S. Abnormal expression of a 170-kilodalton P-glycoprotein encoded by MDR1 gene, a metabolically active efflux pump, in CD4+ and CD8+ T cells from patients with human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1996. 12(15);1457–1462. doi: 10.1089/aid.1996.12.1457. [DOI] [PubMed] [Google Scholar]
- 88.Lucia MB, Cauda R, Malorni W, Rainaldi G, Tumbarello M, Tacconelli E, Rumi C, Donelli G, Ortona L. P-170 glycoprotein (P-170) is involved in the impairment of natural killer cell-mediated cytotoxicity in HIV+ patients. Immunol Lett. 1995;47(3):223–226. [DOI] [PubMed] [Google Scholar]
- 89.Chang KH, Kim JM, Yoo NC, Kim WH, Park JH, Choi IH, Kim HS, Lee KW, Song YG, Hong SK, et al. Restoration of P-glycoprotein function is involved in the increase of natural killer activity with exogenous interleukin-15 in human immunodeficiency virus-infected individuals. Yonsei Med J. 2000;41(5):600–606. doi: 10.3349/ymj.2000.41.5.600. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J-C, Xie F, Yu X-H, Deng Z-Y, Wang Y, Liang P, Sun L, Zhang F-X. Expression levels of P-glycoprotein in peripheral blood CD8+ T lymphocytes from HIV-1-infected patients on antiretroviral therapy. Int J Mol Med. 2014. 33(2);431–440. doi: 10.3892/ijmm.2013.1584. [DOI] [PubMed] [Google Scholar]
- 91.Brophy NA, Marie JP, Rojas VA, Warnke RA, McFall PJ, Smith SD, Sikic BI. Mdr1 gene expression in childhood acute lymphoblastic leukemias and lymphomas: a critical evaluation by four techniques. Leukemia. 1994;8(2):327–335. [PubMed] [Google Scholar]
- 92.Zenkov AN, Scvortsova NV, Chernolovskaya EL, Pospelova TI, Vlassov VV. Expression of the MDR1 and MRP genes in patients with lymphoma with primary bone marrow involvement. Nucleosides Nucleotides Nucleic Acids. 2004. 23(6–7);843–847. doi: 10.1081/NCN-200026029. [DOI] [PubMed] [Google Scholar]
- 93.Besse A, Stolze SC, Rasche L, Weinhold N, Morgan GJ, Kraus M, Bader J, Overkleeft HS, Besse L, Driessen C. Carfilzomib resistance due to ABCB1/MDR1 overexpression is overcome by nelfinavir and lopinavir in multiple myeloma. Leukemia. 2018. 32(2);391–401. doi: 10.1038/leu.2017.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen H, Xu W, Luo W, Zhou L, Yong W, Chen F, Wu C, Chen Q, Han X. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp Hematol. 2011. 39(5);558–569. doi: 10.1016/j.exphem.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 95.List AF, Spier C, Greer J, Wolff S, Hutter J, Dorr R, Salmon S, Futscher B, Baier M, Dalton W. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J Clin Oncol. 1993. 11(9);1652–1660. doi: 10.1200/JCO.1993.11.9.1652. [DOI] [PubMed] [Google Scholar]
- 96.List AF, Kopecky KJ, Willman CL, Head DR, Persons DL, Slovak ML, Dorr R, Karanes C, Hynes HE, Doroshow JH, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood. 2001;98(12):3212–3220. doi: 10.1182/blood.V98.12.3212. [DOI] [PubMed] [Google Scholar]
- 97.Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, Dugan K, Lum B, Chin DL, Dewald G, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III Trial (E2995). J Clin Oncol. 2004;22(6):1078–1086. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghetie M-A, Marches R, Kufert S, Vitetta ES. An anti-CD19 antibody inhibits the interaction between P-glycoprotein (P-gp) and CD19, causes P-gp to translocate out of lipid rafts, and chemosensitizes a multidrug-resistant (MDR) lymphoma cell line. Blood. 2004. 104(1);178–183. doi: 10.1182/blood-2003-12-4255. [DOI] [PubMed] [Google Scholar]
- 99.Ghetie M, Crank M, Kufert S, Pop I, Vitetta E. Rituximab but not Other anti-cd20 Antibodies Reverses Multidrug Resistance in 2 B lymphoma Cell Lines, Blocks the Activity of P-glycoprotein (p-gp), and Induces P-gp to Translocate out of Lipid Rafts. J Immunotherapy. 2006. 29(5);536–544. doi: 10.1097/01.cji.0000211307.05869.6c. [DOI] [PubMed] [Google Scholar]
- 100.Ling L, Lin Y, Zheng W, Hong S, Tang X, Zhao P, Li M, Ni J, Li C, Wang L, et al Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci Rep. 2016;6:20358. doi: 10.1038/srep20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panis C, Lemos LGT, Victorino VJ, Herrera ACSA, Campos FC, Colado Simão AN, Pinge-Filho P, Cecchini AL, Cecchini R. Immunological effects of taxol and adryamicin in breast cancer patients. Cancer immunology, immunotherapy: CII. 2012. 61(4);481–488. doi: 10.1007/s00262-011-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verronèse E, Delgado A, Valladeau-Guilemond J, Garin G, Guillemaut S, Tredan O, Ray-Coquard I, Bachelot T, N’Kodia A, Bardin-Dit-Courageot C, et al. Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay. Oncoimmunology. 2015;5:3. doi: 10.1080/2162402X.2015.1100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hegewisch-Becker S, Staib F, Löning T, Pichlmeier U, Kröger N, Reymann A, Hossfeld DK. No evidence of significant activity of the multidrug resistance gene product in primary human breast cancer. Ann Oncology: Off J Eur Soc Med Oncol. 1998;9(1):85–93. doi: 10.1023/A:1008255725515. [DOI] [PubMed] [Google Scholar]


