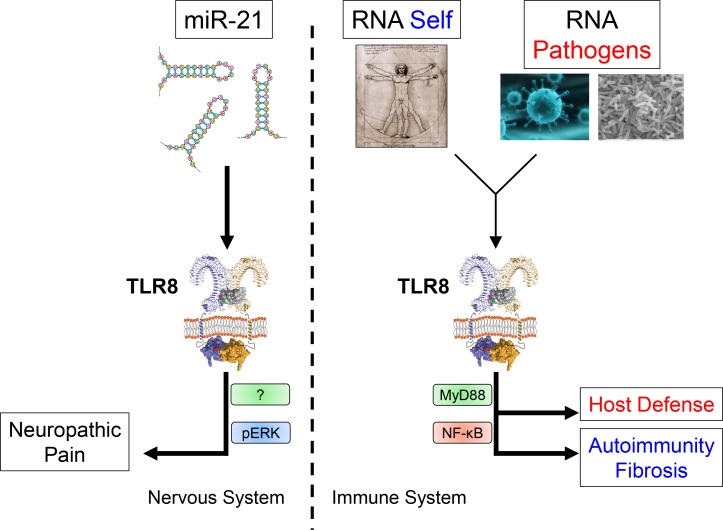
miR21-mediated activation of TLR8 controls neuropathic pain.
Abstract
TLRs have been well characterized in the context of immunity, although TLR8 is understudied due to its controversial function in mice. In this issue of JEM, the new work by Zhang et al. (https://doi.org/10.1084/jem.20180800) demonstrates that TLR8 activated by miR-21 controls neuropathic pain using a non-MyD88–dependent pathway.
In this issue of JEM, Zhang et al. describe the role played by TLR8 in controlling neuropathic pain in mice. The work demonstrates that the microRNA miR-21 can act as an endogenous ligand for TLR8 and identify MyD88-independent pathways as important for nonimmune-related TLR responses.

Insights from Franck J. Barrat.
The role and importance of the ten human TLRs in controlling immune responses to pathogens has been well characterized (Brubaker et al., 2015). The focus has been on multiple fronts, including determining (i) their respective agonists, both endogenous and derived from pathogens, and defining ligand–TLR recognition motifs; (ii) the nature of the response induced and the key signaling molecules involved in their signaling; and (iii) their role in vivo using series of genetic approaches targeting the TLRs themselves or adaptor molecules critical for their signaling. Although TLR8 recognizes RNA, and the importance of nucleic acid–sensing TLRs has been well acknowledged in physiological and disease situations, this TLR has been generally understudied mostly because it is thought to be either nonfunctional in mice or at best a regulator of the other RNA-sensing receptor TLR7. Difficulties in clearly establishing the respective contribution of the two RNA-sensing TLR7 and TLR8 during immune responses stems from both the controversial activity of TLR8 in the mouse and the presence of TLR7 in cells in mice that do not express it in human (Guiducci et al., 2013). In particular, the murine TLR7 is present on cells from the myeloid compartment, while it is restricted to B cells and plasmacytoid dendritic cells in humans, suggesting that TLR7 might compensate for the lack of a functional TLR8 in mice. Thus mice represent poor models for studying the normal and disease-associated functions of both TLR7 and TLR8. In addition, the majority of studies looking at the function of TLR8 in the mouse have been done either using transfected HEK293 cells activated by synthetic ligands with an NF-κB–dependent readout or using TLR7-deficient cells looking primarily at cytokine responses to various known agonists of the human TLR8 (Hemmi et al., 2002; Lund et al., 2004). The key conclusion from these studies should not be that the mouse TLR8 is not functional; rather, it is possible that most agonists for the human TLR8 are not recognized by the mouse counterpart or that ligand sensing is not associated with the NF-κB pathway.
In a new study published in the current issue of JEM, Zhang et al. (2018) have made a number of striking observations linking mTLR8 signaling induced by miR-21 in small dorsal root ganglion (DRG) neurons with the induction of pain hypersensitivity in mice. First, they observed that TLR8 is expressed in the spinal cord and, more specifically, in the ipsilateral dorsal horn and ventral horn where TLR8 accumulates after coming from DRG neurons. Interestingly, using a model of neuropathic pain induced by spinal nerve ligation (SNL), they show that TLR8 expression is actually induced in the neurons, and, by single cell expression analysis, they demonstrate that TLR7 and TLR8 can, in some situations, be coexpressed in DRG neurons. The role of TLR8 in controlling pain was then addressed using a series of complementary approaches. The authors generated a new strain of TLR8-deficient mice targeting the Tlr8 gene with transcription activator–like effector nucleases to avoid impacting the close by Tlr7 gene. In contrast to previously generated TLR8-deficient mice in which a larger portion of the gene was deleted by inserting a lacZ/neo cassette (Demaria et al., 2010), these mice have normal expression of TLR7 and no apparent sign of autoimmunity. Body weight, the weight and size of the spleen, thymus, and lymph node, and TLR7 expression in the spleen, DRG, and spinal cord are not significantly different as compared with WT littermates. Surprisingly, nerve injury–induced mechanical allodynia and heat hyperalgesia were significantly reduced in the TLR8-deficient mice following SNL. The conclusion from these data is that TLR8 has a critical role in the maintenance of neuropathic pain. This was supported by subsequent experiments in which the authors delivered siRNA specific for TLR8 directly to spinal nerves with similar observations. To determine whether TLR8 by itself is sufficient to induce neuropathic pain, the TLR8 agonist VTX-2337 was injected intrathecally, which resulted in mechanical allodynia in a dose-dependent manner.
Although these data point to TLR8 as an important component of the pathway leading to neuropathic pain, they did not identify the physiological triggers of TLR8. Further, these observations do not fit with previous reports that small molecule agonists of the human TLR8, including VTX-2337, had no effect on the mouse TLR8 due to differences in the ligand recognition motifs on the TLR8 protein (Liu et al., 2010). Starting with the latter, the authors showed that TLR8 signaling in DRG in response to VTX-2337 occurs even in absence of MyD88 and involves the ERK pathway. Strikingly, they observed in a series of experiments that the entire TLR8-induced neuropathic pain response is independent of MyD88. By transfecting HEK293 with TLR8 and with either a NF-κB– or ERK-dependent readout, they confirmed these findings by showing that VTX-2337 does not activate the NF-κB pathway, as previously reported, but does indeed induce pERK, suggesting that TLR8 signaling may be more complex than originally envisioned. Next, Zhang et al. (2018) observed that miR-21 is dramatically induced in DRG neurons following SNL and colocalizes with TLR8-positive neurons. When injected intrathecally in WT but not in TLR8-deficient mice, miR-21 induced similar response as described with VTX-2337, including mechanical allodynia associated with increased pERK amount in the DRG neurons and subsequent inflammatory response. Blocking miR-21 also reduced SNL-induced mechanical allodynia, suggesting that miR-21 is a critical endogenous player in TLR8-induced neuropathic pain.
TLR8 can participate in key functions of the immune and nervous systems in both physiological and disease situations.
What can we learn from these studies?
Although some work has been done looking at the role played by TLRs in pain (Liu et al., 2012), and TLR8 has been shown to play a role in neurite outgrowth (Ma et al., 2006), the bulk of our understanding of the biology of TLRs comes from their contribution to immunity where the study of TLRs has greatly contributed to the rebirth of interest in innate immunity (Janeway and Medzhitov, 2002). The current study by Zhang et al. (2018) suggests a need for a better understanding of how TLRs, as well as other innate receptors, influence other key aspects of both mouse and human biology. The dependence on cell lines such as HEK293 cells to define TLR biology should be revisited at best. The existence of MyD88-independent pathways implies that strategies that better address the complexity of the signaling pathways involved in TLR responses should be used. Similar to what has been shown in immune cells, the involvement of TLRs, and in particular how TLR7 and TLR8 control pain, is very dependent on their expression pattern in cells of the nervous system. This pattern is very distinct in mice as compared with humans, and the role of both receptors in humans may need the use of engineered mice where the expression pattern is comparable (Guiducci et al., 2013).
One of the most surprising findings is that microRNAs (miRs) might act as endogenous agonists for TLRs. The mechanism by which miRs can silence genes and regulate gene expression at the posttranscriptional level has been well characterized, and their impact on immune function is an active field of research. These new data suggest that miRs can have other functions and may be recognized by nucleic acid sensors such as TLRs. The mechanism by which these may be in a situation to encounter such sensors is unclear and should be further studied. Importantly, as TLR8 has been associated with autoimmune and fibrotic diseases (Ah Kioon et al., 2018), the study by Zhang et al. (2018) points to TLR8 as a potential target for the treatment of neuropathic pain as well.
This work also raises a series of new questions. As we now uncover the critical role that evolutionary pressure has had on TLRs (Barreiro et al., 2009), one wonders why a nucleic acid–specific receptor like TLR8 would be associated with controlling pain. Even though Zhang et al. (2018) show that TLR8 appears to be expressed in DRG in human, the role of TLR8 in pain in humans should be explored. The impact of TLR8 on TLR7 expression and function should be clarified as well. Two different TLR8-deficient strains have been generated with conflicting data on how TLR8 regulates TLR7 expression levels (Demaria et al., 2010; Zhang et al., 2018). Defining selective and specific TLR7 and TLR8 agonists has always been a problem in particular, as their characterization often relies on TLR-transfected cells. The possibility that miRs may act as agonists will undoubtedly be an interesting area for future investigation. Finally, are there other non-MyD88–dependent responses by TLRs, especially ones previously regarded as completely MyD88 dependent? Moreover, whether the entire impact of TLR8 in pain is ERK dependent should be further defined.
Overall, the role of TLR8, both in mouse and human, outside the realm of the immune system has not been well studied, and the study by Zhang et al. (2018) identifies non–NF-κB signaling by TLR8 as part of the function of the nervous system. This work should spark new studies looking at the role of TLRs in nonimmune systems and supports targeting TLR8 for the treatment of neuropathic pain.
References
- Ah Kioon M.D., et al. 2018. Sci. Transl. Med. 10:eeam8458 10.1126/scitranslmed.aam8458 [DOI] [Google Scholar]
- Barreiro L.B., et al. 2009. PLoS Genet. 5:e1000562 10.1371/journal.pgen.1000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker S.W., et al. 2015. Annu. Rev. Immunol. 33:257–290. 10.1146/annurev-immunol-032414-112240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria O., et al. 2010. J. Clin. Invest. 120:3651–3662. 10.1172/JCI42081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C., et al. 2013. J. Exp. Med. 210:2903–2919. 10.1084/jem.20131044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H., et al. 2002. Nat. Immunol. 3:196–200. 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- Janeway C.A. Jr, and Medzhitov R.. 2002. Annu. Rev. Immunol. 20:197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Liu J., et al. 2010. Mol. Immunol. 47:1083–1090. 10.1016/j.molimm.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., et al. 2012. Neurosci. Bull. 28:131–144. 10.1007/s12264-012-1219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., et al. 2004. Proc. Natl. Acad. Sci. USA. 101:5598–5603. 10.1073/pnas.0400937101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., et al. 2006. J. Cell Biol. 175:209–215. 10.1083/jcb.200606016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-J., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20180800. [DOI] [Google Scholar]