Jameson discusses the generation of a genetic mouse model for the conditional depletion of γδ T cells, confirming the fetal origin and persistence of γδ Th17 cells.
Abstract
In this issue of JEM, Sandrock et al. (https://doi.org/10.1084/jem.20181439) compare the origin of IL-17–producing γδ T cells (Tγδ17) with other γδ T cell populations and demonstrate the role Tγδ17 cells play in skin pathology. Using two genetically modified mouse models, one with inducible γδ T cell–specific labeling and the other with conditional γδ T cell depletion, the authors find that Tγδ17 are mostly long-lived lymphocytes and that depleting γδ T cells protects mice from psoriasis.
The link between γδ T cells and inflammatory skin disease has been an area of intense research over the last decade. IL-17A and F are required for the protection of epithelial surfaces from fungal and bacterial infection; however, a fine balance is required because IL-17 is a key driver of inflammation and autoimmunity (Hawkes et al., 2018). Vγ4+ T cells were originally identified as a cellular source of IL-17 by Roark et al. (2007) in experiments using intradermal CFA injection with collagen to induce arthritis. In 2011, several groups characterized the dermal IL-17–secreting γδ T cells as a motile, TCR-intermediate, CCR6hi population expressing either the Vγ4 or Vγ6 chain (Cai et al., 2011; Gray et al., 2011; Sumaria et al., 2011).

Insights from Julie M. Jameson.
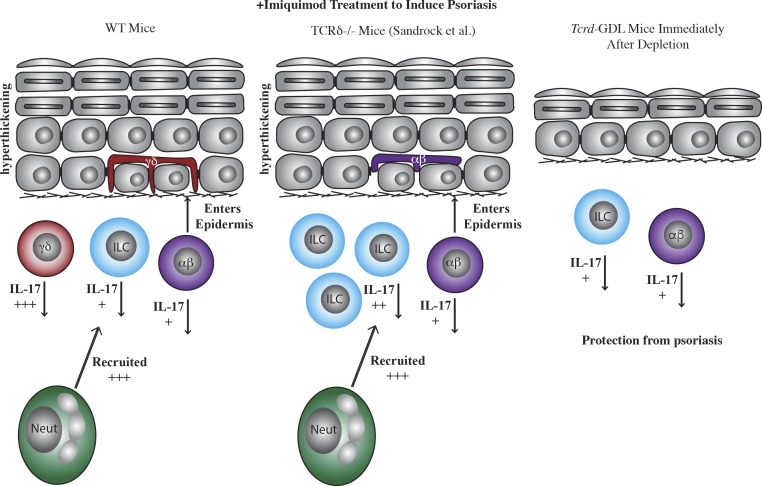
Dermal Tγδ17 cells play roles in inflammatory diseases such as psoriasis (Cai et al., 2011; Pantelyushin et al., 2012; Ramírez-Valle et al., 2015). One of the confounding issues up to now has been that the roles played by γδ T cells in disease pathogenesis have been based on studies using mice with a genetic disruption of the delta chain (TCRδ−/−) or in mice treated with γδ TCR blocking antibodies. The caveat of TCRδ−/− mice is that other cellular populations take up residence in the space left by absent γδ T cells. For example, in the epidermis, Vγ5+ T cells are replaced by a smaller number of αβ T cells (Jameson et al., 2004). In addition, γδ TCR–depleting antibodies do not actually deplete γδ T cells, but instead down-regulate the TCR, rendering them “functionally inactive” (Koenecke et al., 2009). These caveats complicate the conclusions that can be drawn from the data on murine γδ T cells as the role of Tγδ17 cells may have redundancy with Th17 cells and innate lymphoid cells (ILCs).
Sandrock et al. have overcome these problems by developing a novel mouse model for acute γδ T cell depletion. The novel Tcrd-GDL mice have GFP, human diphtheria toxin receptor, and luciferase knocked-in under internal ribosome entry site control in the 3′UTR of Tcrd. Together, these genes allow for visualization of γδ T cells and the ability to deplete γδ T cells with the addition of diphtheria toxin. Luciferase and GFP show great utility in visualizing γδ T cell number and morphology throughout the tissues of the animal, including the intestine and skin. Upon γδ T cell depletion, γδ T cell subsets reemerge at different kinetics, showing both that the depletion is reversible and that the development of different γδ T cell populations varies.
In the lymph node and spleen, IFN-γ–producing CD27+ γδ T cells return to pre-deletion numbers within 7 wk, while IL-17–producing CD27− γδ T cells remain low in number after 7 wk. This finding supports earlier published work by the authors that Tγδ17 cells develop before birth and are self-renewing (Haas et al., 2012), similar to γδ T cell populations with specific TCR rearrangements that seed the epidermis, reproductive tract, and intestine (Asarnow et al., 1988; Havran and Allison, 1988; Itohara et al., 1990). Intestinal Vγ7+ and lymph node Vγ1+ T cells recover their numbers within 7 wk of depletion, but Vγ6 and Vγ4 T cells from the spleen and lymph node do not. Interestingly, the TCR repertoire is similar between the rare “recovering” Vγ17 T cells and those examined before depletion, suggesting that some cells survive the diphtheria toxin treatment and expand subsequently.
WT mice subjected to IMQ treatment exhibit the clinical signs of psoriasis due to IL-17 production by dermal γδ T cells, which leads to neutrophil infiltration and epidermal hyperproliferation. Sandrock et al. (2018) show that TCRδ–/– mice also exhibit hyperproliferation of the epidermis and neutrophil infiltration; however, this appears to be due to an abundance of IL-17–producing ILCs that makes up for the absent γδ T cells. The Tcrd-GDL mice, on the other hand, do not have prominent IL-17–producing cells and are resistent to the clinical signs of psoriasis upon acute depletion.
As expected, fetal thymus-derived Vγ5+ dendritic epidermal T cells (DETCs) remain fully depleted at 7 wk in Tcrd-GDL mice, with only sporadic surviving cells that expand as clonal colonies. Interestingly, dermal γδ T cells also remain depleted with only a few mice that begin to recover low numbers of γδ T cells. The lack of αβ T cell infiltration in the epidermis suggests that αβ T cell migration may happen during the developmental waves of T cell seeding of the epithelia in TCRδ−/− mice.
Sandrock et al. (2018) use a fate-mapping system to label and quantify turnover of γδ T cell populations. IFN-γ–producing CD27+ γδ T cells exhibit full cellular replenishment within 7 wk, while IL-17–producing CD27− γδ T cells remain labeled in the lymph node, spleen, and liver. Interestingly, in the skin, dermal Tγδ17 cells exhibit a higher rate of turnover than DETCs, suggesting a potential for dermal Tγδ17 migration to and from the lymph nodes as previously reported (Ramírez-Valle et al., 2015). Dermal Tγδ17 cells are highly motile as compared with their DETC neighbors, further supporting exchange with the lymph nodes (Gray et al., 2011).
Previous reports have identified detrimental roles played by Tγδ17 cells in psoriasis (Cai et al., 2011; Pantelyushin et al., 2012), and here the acute γδ T cell depletion in Tcrd-GDL mice results in a similar finding. Psoriasis induction with imiquimod (IMQ) in Tcrd-GDL mice leads to less severe neutrophil infiltration, reduced thickening of the ear, and reduced disease score as compared with control mice. Tγδ17 cells are highly motile in response to IMQ and exhibit migration into the hyperthickened epidermis. The current study does deviate from the previously published findings in that Sandrock et al. (2018) do not observe exacerbated IMQ-induced disease in TCRδ−/− mice, but instead find that TCRδ−/− mice are similar to wild-type mice.
This leads the authors to a set of experiments to address how the cellular milieu in the dermis changes in the absence of γδ T cells (Sandrock et al., 2018). Within 2 mo after Tγδ17 cells are depleted in Tcrd-GDL mice, ILC3s increase in number and become the major IL-17–producing cells in the dermis. By this time, the mice have also regained their susceptibility to IMQ-induced pathology. In a final culminating experiment, the authors deplete the γδ T cells to determine once and for all whether the newly increased ILCs can compensate for the Tγδ17 cells and exacerbate disease. However, mice that are depleted once again have less severe disease, suggesting Tγδ17 cells play a nonredundant role in psoriasis.
The generation of a conditional γδ T cell–depleted mouse model is a considerable leap forward, as this model allows for normal γδ T cell seeding of the tissues to occur during development before specific γδ T cell depletion. Thus, autoimmune disease, infection, or tissue damage can be initiated immediately upon depletion before any reseeding of αβ T cells or ILC to elucidate specific roles for γδ T cells in disease.
References
- Asarnow D.M., et al. . 1988. Cell. 55:837–847. 10.1016/0092-8674(88)90139-0 [DOI] [PubMed] [Google Scholar]
- Cai Y., et al. . 2011. Immunity. 35:596–610. 10.1016/j.immuni.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E.E., et al. . 2011. J. Immunol. 186:6091–6095. 10.4049/jimmunol.1100427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J.D., et al. . 2012. Immunity. 37:48–59. 10.1016/j.immuni.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Havran W.L., and Allison J.P.. 1988. Nature. 335:443–445. 10.1038/335443a0 [DOI] [PubMed] [Google Scholar]
- Hawkes J.E., et al. . 2018. J. Immunol. 201:1605–1613. 10.4049/jimmunol.1800013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S., et al. . 1990. Nature. 343:754–757. 10.1038/343754a0 [DOI] [PubMed] [Google Scholar]
- Jameson J.M., et al. . 2004. J. Immunol. 172:3573–3579. 10.4049/jimmunol.172.6.3573 [DOI] [PubMed] [Google Scholar]
- Koenecke C., et al. . 2009. Eur. J. Immunol. 39:372–379. 10.1002/eji.200838741 [DOI] [PubMed] [Google Scholar]
- Pantelyushin S., et al. . 2012. J. Clin. Invest. 122:2252–2256. 10.1172/JCI61862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Valle F., et al. . 2015. Proc. Natl. Acad. Sci. USA. 112:8046–8051. 10.1073/pnas.1508990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark C.L., et al. . 2007. J. Immunol. 179:5576–5583. 10.4049/jimmunol.179.8.5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock I.,, et al. J. Exp. Med. 2018 doi: 10.1084/jem.20181439. [DOI] [Google Scholar]
- Sumaria N., et al. . 2011. J. Exp. Med. 208:505–518. 10.1084/jem.20101824 [DOI] [PMC free article] [PubMed] [Google Scholar]