ABSTRACT
Purpose: To investigate the impact of programmed death-ligand 1 (PD-L1) expression, oncogenic mutations, and clinical characteristics on survival after treatment with anti-PD-1/PD-L1 antibodies versus chemotherapy in non-small cell lung cancer (NSCLC).
Patients and Methods: This meta-analysis included randomized trials comparing anti-PD-1/PD-L1 antibodies with chemotherapy. Hazard ratios (HRs) and 95% confidence interval (CI) for overall survival (OS) for the trial population and prespecified subgroups were extracted. We calculated pooled estimates of treatment efficacy using the fixed-effects or random-effects model when appropriate. All statistical tests were two sided.
Results: Seven trials involving 3871 patients were included. The pooled results showed that anti-PD-1/PD-L1 immunotherapy significantly prolonged OS (HR: 0.73; 95% CI, 0.63 to 0.84) and PFS (HR: 0.84; 95% CI, 0.71 to 0.99) compared to chemotherapy. OS benefit from immunotherapy were observed in all PD-L1 expression subgroups (negative: HR, 0.79; 95% CI, 0.67 to 0.93; weak-positive: HR, 0.80; 95% CI, 0.67 to 0.95; strong-positive: HR, 0.61; 95% CI, 0.47 to 0.78). Strong-positive PD-L1 expression showed a trend towards more benefit compared to weak-positive PD-L1 expression (interaction P = 0.08). KRAS mutant (HR: 0.60; 95% CI, 0.39 to 0.93), EGFR wild-type (HR: 0.73; 95% CI, 0.61 to 0.87) and smoker (HR: 0.70; 95% CI, 0.60 to 0.83) subgroups achieved significant OS benefit from immunotherapy compared to corresponding subgroups. Survival benefit to immunotherapy was not significantly associated with histology, CNS metastases, age, gender and performance status.
Conclusion: This study confirmed that treatment with anti-PD-1/PD-L1 improves overall survival compared with chemotherapy. Benefit was seen, regardless of PD-L1 expression levels; however, PD-L1 strong-positive patients trended to have greatest benefit. Patients with a KRAS mutant or EGFR wild-type tumor have improved survival benefit from immunotherapy compared with KRAS wild-type or EGFR mutant NSCLC, respectively.
KEYWORDS: anti-PD-1, atezolizumab, immunotherapy, nivolumab, non-small cell lung cancer, pembrolizumab
Introduction
Therapeutic blockade of immune checkpoint pathways has become an important paradigm shift in the treatment of non-small cell lung cancer (NSCLC).1 Programmed cell death ligand 1(PD-L1) and programmed cell death receptor 1(PD-1) pathway represent critical immunosuppressive mechanisms to block effector T-cell functions, leading to tumor immune evasion and ultimately resulting in tumor dissemination, relapse, and metastasis.2 Strikingly, antibodies blocking the PD-1/PD-L1 pathway have achieved success in the management of various types of tumors in the clinic.3,4
Anti-PD-1 antibodies (nivolumab and pembrolizumab) and anti-PD-L1 antibody (atezolizumab) are US Food and Drug Administration (FDA) approved and have emerged as the new standard of care of advanced NSCLC in recent years based on clinical trials revealing durable anti-tumor response and survival advantage over chemotherapy.5–9 However, response to PD-1/PD-L1 blockade is observed only in a minority (less than 20%) of overall NSCLC patients with severe adverse events occurring in 20%–40% of these patients.7,9–12 Therefore, the identification of patients who are more likely to benefit from immunotherapy will be pertinent to maximizing efficacy and minimizing toxicity.13
One approach is to select patients based on tumor mutational or neo-antigen burden using next-generation sequencing technologies, which requires large sampling and further prospective validations.14,15 In addition, PD-L1 protein expression in the tumor microenvironment (TME) may serve as a logical biomarker for predicting response to PD-1 pathway inhibition13 and has already been explored in a number of pivotal trials. While some studies5,7,9,16 have revealed that patients with higher PD-L1 expression levels achieved survival advantage, other trials6,11 observed that PD-L1 expression was not a predictive biomarker. In fact, inconsistencies were observed in trials including participants enrolled under similar criteria, treated with the same inhibitor and assayed using the same diagnostic antibody.8,16
Adaptive immune resistance mediated by the PD-1/PD-L1 axis limits the action of cytotoxic T cells. Somatic mutations in tumors have the potential to encode immunogenic neo-antigens, capable of recognition by cytotoxic T cells, and affect the response to immune checkpoint inhibitors.14 For patients harboring epidermal growth factor receptor (EGFR) activating mutations which are characterized by remarkable response to EGFR tyrosine kinase inhibitors, the response rate to anti-PD-1 blockade showed limited efficacy in retrospective analysis.17 Another commonly mutated oncogene in lung cancer is Kirsten Ras viral oncogene homolog (KRAS), which remains an elusive target despite a rapidly-evolving era of targeted therapies. KRAS-driven murine lung cancer models have demonstrated a lack of T cell infiltration in tumors as well as resistance to immunotherapy.18,19 Interestingly, genomic and clinical data of NSCLC patients indicated that, KRAS-activating mutations correlated with higher mutational burden and might serve as a potential biomarker of response to PD-1/PD-L1 blockade.20 Generating KRAS-driven murine lung cancer models with defects in the DNA repair machinery is a novel strategy to increase and mimic high mutation load in human NSCLC. Results from recent clinical trials involving NSCLC patients harboring these two driver mutations may provide insight into these complex relationships. Moreover, we speculate that other confounding variables (or cofactors) such as clinicopathologic characteristics, histology, smoking status and central nervous system (CNS) metastases may be associated with immunotherapy benefits.
Furthermore, individual trials have limited statistical power to validate significant response differences between patient subgroups. As such, we have pooled data from seven clinical trials, totalling 3871 cases, to corroborate the predictive impact of PD-L1 expression levels on the overall survival (OS) benefit after treatment with anti-PD-1 versus conventional chemotherapy. We further examine whether other cofactors including known driver mutations and clinicopathologic characteristics may also contribute to clinical benefit to anti-PD-1 treatment.
Patients and methods
Study eligibility and selection
All eligible studies were randomized trials that compared the survival of anti-PD-1/PD-L1 immunotherapy against chemotherapy in adult patients with advanced NSCLC. In brief, relevant studies were sought, with no language restrictions or limitations on publication year, in Pubmed, Web of Knowledge and Central databases (up to 31 December 2016), using the following terms: “(lung neoplasms OR nonsmall cell lung cancer) AND (pembrolizumab OR Keytruda OR MK-3475 OR SCH 900475 OR nivolumab OR Opdivo OR BMS-936558 OR MDX-1106 OR ONO-4538 OR atezolizumab OR Tecentriq OR MPDL3280A OR RG7446 OR RO5541267 OR PD-1 OR PD-L1) AND trial”. The search was supplemented by searching trial registers, and reference lists of published articles. Abstracts from conference proceedings of the American Society of Clinical Oncology, the European Society for Medical Oncology (ESMO), and the World Lung Cancer Conference were also searched. For studies without survival data, the corresponding authors and study sponsors were contacted.
Data extraction
For each included trial, the study name, year publication or presentation, clinicopathologic characteristics and treatment regimen were collected according to a predefined data extraction form. We further extracted treatment estimates for the following subgroups: age (< 65 v ≥65 years), gender (female v male), Eastern Cooperative Oncology Group performance status (ECOG PS) (0 v 1 and 2), smoking status (never-smoker v current or former smoker), CNS metastasis status (no v yes), EGFR mutation status (mutant v wild-type) KRAS mutation status (mutant v wild-type), histology (non-squamous v squamous), and PD-L1 expression level (negative v weak-positive v strong-positive). Given the fact that the diagnostic antibodies and cutoffs of PD-L1 expression varied with trials, PD-L1 strong-positive was defined as ≥5% tumor cells (TC) staining for the 28–2 assay derived from CheckMate 026 trial21,22, ≥50% TC staining for the 22C3 assay derived from KEYNOTE-024 trial, and ≥5% TC staining and/or tumor-infiltrating immune cells (IC) staining (TC2/3 or IC2/3) for the SP142 assay, respectively. The cutoff of PD-L1 negative and positive expression was 1% in all trials. Data were independently extracted by two authors (Q.Y.H. and H.Z.), and discrepancies were resolved by consensus that included a third author.
Statistical analyses
The primary endpoint was OS, defined as the time from randomization to death of any cause. The hazard ratios (HRs) and its 95% confidence intervals (CIs) for the overall population and subgroups in an individual trial were applied to evaluate the treatment effects, and extracted directly from the article or presentation where possible. HRs and 95% CIs not reported directly were calculated by the methods detailed by Parmar et al.23,24 When heterogeneity was observed (I2 statistic>50%), the random-effects model25 was used to calculate the pooled estimates with 95% CIs, otherwise, the fixed-effect model was used. We used the χ2 Cochran Q test to test the interaction between treatment and patient subgroups when subgroups of a category all benefited from immunotherapy over chemotherapy. In addition, potential sources of heterogeneity were explored through subgroup and meta-regression analysis.
A sensitivity analysis was conducted by excluding the analyses of two first-line trials, which had more strict inclusion criteria (untreated, PD-L1 strong-positive, and EGFR wild-type patients), and used platinum-based doublet chemotherapy as control treatment. Publication bias was assessed by funnel plots and with the use of the Begg's test and the weighted regression test of Egger26 for overall and subgroup populations.
The meta-analysis was conducted in accordance with recommendations of the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement, using the standard software (Stata 12.0, Stata Corporation, College Station, TX). A two-sided P-value of <0.05 was considered significant.
Results
Characteristics of included trials
We screened 729 studies for eligibility and identified seven trials involving 3,871 NSCLC patients for our meta-analysis (Fig. 1). All trials were open label. Anti-PD-1/PD-L1 immunotherapy was the first-line treatment for patients in two studies, CheckMate 026 and KEYNOTE-024, and the second-line or onwards in the remaining five trials. Notably, anti-PD-1 antibodies were examined in five trials (Nivomulab in the CheckMate studies and Pembrolizumab in the KEYNOTE trials), while anti-PD-L1 antibodies (Atezolizumab) were investigated in the POPLAR and OAK trials. Apart from the CheckMate 017 and 057 studies, which recruited only squamous and non-squamous, the remaining studies included mixed histological subgroups. KRAS mutation status was collected and identified in the CheckMate 057 (11%), POPLAR (9.4%) and OAK (7%) trials. Patients with EGFR mutations were excluded in the CheckMate 026 and KEYNOTE-024 studies. The other trials had accessible EGFR mutation status data except for the CheckMate 017. Clinicopathologic characteristics of patients are summarized in Table 1.
Figure 1.
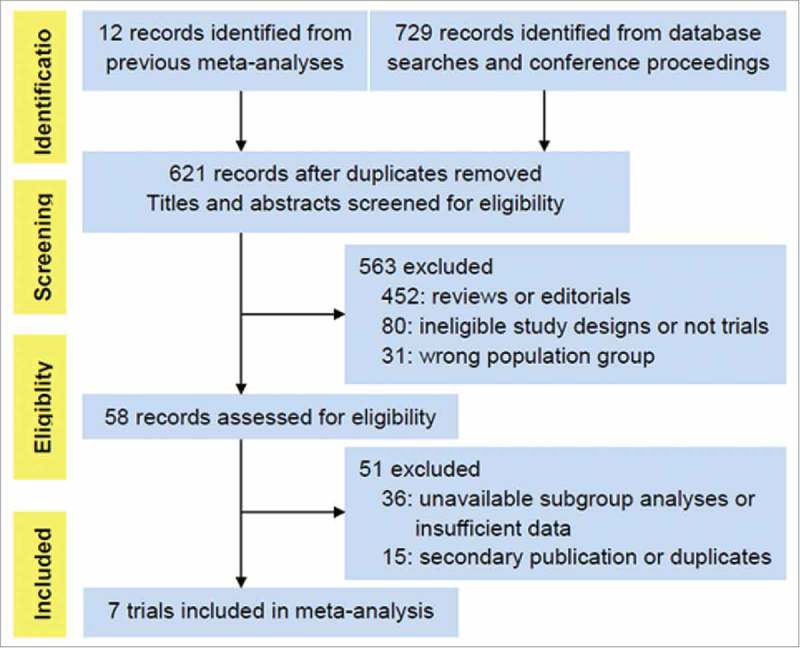
PRISMA flow chart displaying the search and selection process.
Table 1.
Characteristics of patients in constituent trials.
| Study Name | Authors, Year | Treatment Comparison | Median OS (months) | Phase | Line of therapy | No. of Patients | Never-Smoker (%) | Non-Squamous (%) | EGFR Mutation (%) | KRAS Mutation (%) | Age < 65 years (%) | Women (%) | ECOG PS 0 (%) | Central Nervous System Metastasis (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CheckMate 017 | Brahmer et al, 2015 | Nivomulab v Docetaxel | 9.2 v 6.0 | 3 | Second | 272 | 6 | 0 | N/A | N/A | 56 | 24 | 24 | 6 |
| CheckMate 057 | Borghaei et al, 2015 | Nivomulab v Docetaxel | 12.2 v 9.4 | 3 | Second/ third | 582 | 20 | 100 | 14 | 11 | 58 | 93 | 31 | 12 |
| CheckMate 026 | Carbone et al, 2017 | Nivomulab v Platinum-based chemotherapy | 13.7 v 13.8 | 3 | First | 541 | 11 | 76 | 0 | N/A | 52 | 39 | 33 | 13 |
| KEYNOTE-010 | Herbst et al, 2016 | Pembrolizumab v Docetaxel | 10.4 v 12.7 v 8.5 | 2/3 | Second or later | 1034 | 18.4 | 70 | 8 | N/A | 58 | 39 | 34 | 15 |
| KEYNOTE-024 | Reck et al, 2016 | Pembrolizumab v Platinum-based chemotherapy | Not Reached | 3 | First | 305 | 7.9 | 81.6 | 0 | N/A | 46 | 39 | 35 | 9 |
| POPLAR | Fehrenbacher et al, 2016 | Atezolizumab v docetaxel | 12.6 v 9.7 | 2 | Second/ third | 287 | 19.5 | 66 | 6.6 | 9.4 | N/A | 41 | 32 | N/A |
| OAK | Rittmeyer et al, 2016 | Atezolizumab v docetaxel | 13.8 v 9.6 | 3 | Second/ third | 850 | 18 | 74 | 10 | 7 | 53 | 39 | 37 | 10 |
OS and PFS benefit from anti-PD-1/PD-L1 immunotherapy
Of the 3,871 NSCLC patients, 2,112 (55%) were randomized to anti-PD-1/PD-L1 immunotherapy, and 1,759 (45%) were randomly assigned to chemotherapy. Compared to chemotherapy, anti-PD-1/PD-L1 immunotherapy significantly prolonged OS (HR, 0.73; 95% CI, 0.63 to 0.84; P < 0.001, Fig. 2) and PFS (HR, 0.84; 95% CI, 0.71 to 0.99; P = 0.04, Fig. 2). There was evident heterogeneity for both OS (I2 = 61.7%, P = 0.016) and PFS (I2 = 79.2%, P < 0.001). Further subgroup analysis showed therapy line was a potential source of heterogeneity for OS (P= 0.007), and sample size for PFS (P < 0.001), but neither was demonstrated by meta-regression analyses (both P > 0.05, supplementary Table S1).
Figure 2.
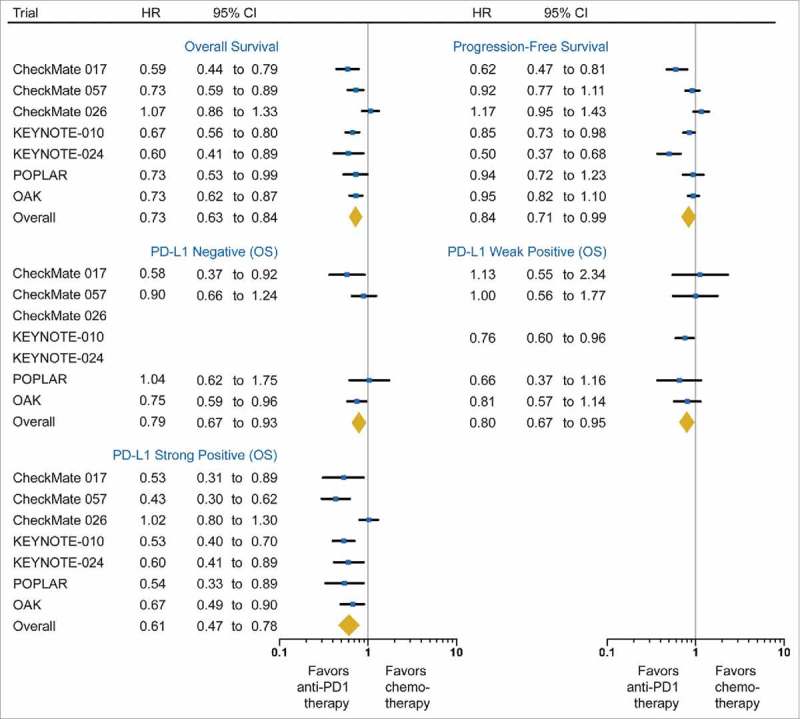
Forest plot of the effect of treatment on overall survival and progression-free survival in all patients, as well as the effect of treatment on overall survival in subgroups of patients according to PD-L1 expression. Hazard ratios (HRs) for each trial are represented by the squares, and the horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis fixed effect. All statistical tests were two sided. PD-L1, programmed death-ligand 1.
Association between PD-L1 expression and efficacy
As shown in Table 2, three diagnostic antibodies were used to determine PD-L1 expression and the cutoffs of PD-L1 expression (negative, weak-positive and strong-positive) for each assay were derived from the corresponding trials. The proportion of patients with PD-L1 strong-positive expression in each trial population, approximately one-third, was similar across trials, suggesting that PD-L1 positivity is represented consistently cross the studies examined.
Table 2.
Summary of PD-L1 assay information and scoring system in constituent trials.
| Criteria (Cutoffs) |
|||||||
|---|---|---|---|---|---|---|---|
| Study Name | PD-L1 Antibody Clone | IHC Assay Provider | Interpretative Scoring | Negative | Weak-positive | Strong-positive | Percentage of Strong PD-L1 Expression Subgroup among Evaluable Population |
| CheckMate 017 | 28–8 | Dako North America | TC membrane | <1% | ≥1% and <5% | ≥5% | 81/225, 36.0% |
| CheckMate 057 | 28–8 | Dako North America | TC membrane | <1% | ≥1% and <5% | ≥5% | 181/455, 39.8% |
| CheckMate 026 | 28–8 | Dako North America | TC membrane | ≥1% and <5% | ≥5% | 423/1325, 31.9% | |
| KEYNOTE-010 | 22C3 | Dako North America | TC membrane | <1% | ≥1% and <50% | ≥50% | 633/2222, 28.5% |
| KEYNOTE-024 | 22C3 | Dako North America | TC membrane | ≥50% | 500/1653, 30.2% | ||
| POPLAR | SP142 | Ventana Medical Systems, AZ, USA | TC membrane Infiltrating ICs | <1% | ≥1% and <5% | ≥5% | 105/287, 37% |
| OAK | SP142 | Ventana Medical Systems, AZ, USA | TC membrane Infiltrating ICs | <1% | ≥1% and <5% | ≥5% | 265/850, 31% |
Abbreviation: TC, tumor cell; IC, immune cells
We observed the greatest OS benefit in the PD-L1 strong subgroup (HR, 0.61; 95%CI, 0.47 to 0.78; P < 0.001; I2 = 72.2%). Both PD-L1 weak-positive (HR, 0.80; 95%CI, 0.67 to 0.95; P = 0.01; I2 =0 %) and negative (HR, 0.79; 95% CI, 0.67 to 0.93; P = 0.006; I2 = 18.4%) subgroups also demonstrated superior OS benefit from anti-PD-1/PD-L1 immunotherapy compared to chemotherapy (Fig. 2). Compared with PD-L1 weak-positive subgroup, a trend towards greater benefit of the PD-L1 strong-positive subgroup was observed (interaction P = 0.08). Conversely, no difference in survival benefit was observed between the PD-L1 negative and weak-positive subgroups (interaction P = 0.92).
Of note, by limiting the analyses on second-line trials, sensitivity analysis further confirmed the improved benefit of anti-PD-1/PD-L1 immunotherapy in the PD-L1 strong-positive subgroup (HR, 0.54; 95%CI 0.46 to 0.64; P < 0.001; I2 = 0%; supplementary Fig S1) when compared with the PD-L1 weak-positive subgroup (HR, 0.80; 95%CI, 0.67 to 0.95; P = 0.01; I2 = 0%; interaction P < 0.001).
Subgroup analyses
KRAS mutation, EGFR mutation and smoking status
In trials where OS of KRAS mutation subgroups were reported, 121 patients (8.4%) had KRAS mutant tumors. The pooled HR from anti-PD-1/PD-L1 immunotherapy compared to chemotherapy was 0.60 (95%CI, 0.39 to 0.93; P = 0.02; I2 = 0%) in the KRAS mutant subgroup, and 0.89 (95% CI, 0.68 to 1.17; P = 0.40; I2 = 0%) in the KRAS wild-type subgroup (Fig. 3).
Figure 3.
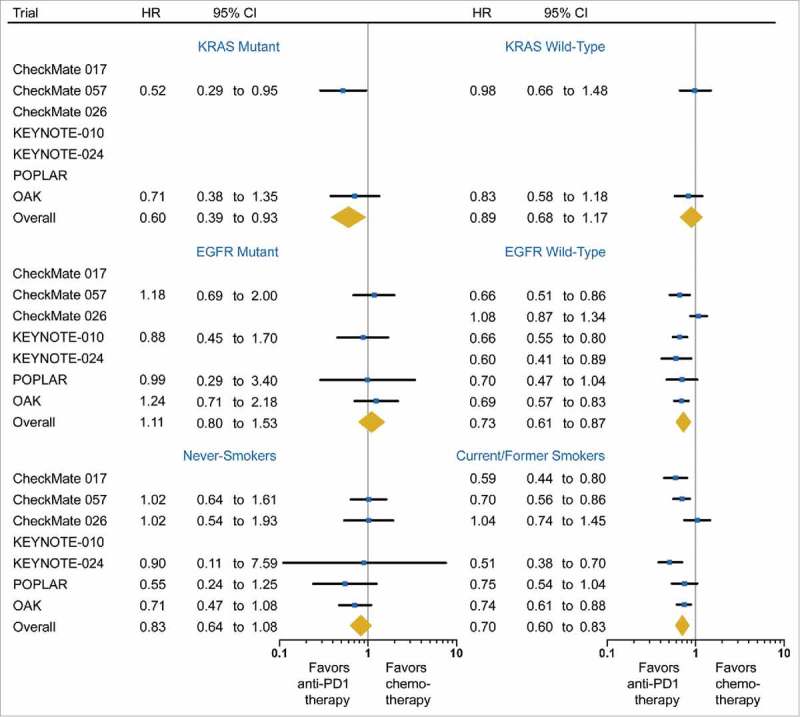
Forest plot of the effect of treatment on overall survival in subgroups of patients according to mutations of KRAS and the epidermal growth factor receptor (EGFR) gene, and smoking status. Hazard ratios (HRs) for each trial are represented by the squares, and the horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis fixed effect. All statistical tests were two sided.
Among 2,907 patients with known EGFR mutation status, 272 patients (9.3%) harbored EGFR activating mutations. EGFR mutant subgroup was not significantly associated with OS benefit from anti-PD-1/PD-L1 compared to chemotherapy (HR: 1.11; 95%CI, 0.80 to 1.53; P = 0.54; I2 = 0%) (Fig. 3). However, the EGFR wild-type subgroup was significantly associated with improved OS (HR: 0.73; 95%CI, 0.61 to 0.87; P = 0.001; I2 = 68.1%) (Fig. 3).
Current or former smokers accounted for 85.3% (n = 2,839) of patients whose smoking status was reported, and significantly benefited from anti-PD-1/PD-L1 immunotherapy over chemotherapy (HR, 0.70; 95%CI, 0.60 to 0.83; P < 0.001; I2 = 55.4%; Fig. 3). Survival advantage of immunotherapy was not observed in the never-smokers (HR, 0.83; 95% CI, 0.64 to 1.08; P = 0.16; I2 = 0%; Fig. 3).
Histology, CNS metastasis, age, gender and PS
The majority of patients (n = 2,785; 74%) had non-squamous lung cancers, while the remaining 998 patients (26%) had squamous disease. Compared to chemotherapy, anti-PD-1/PD-L1 immunotherapy significantly reduced the risk of death in both the non-squamous (HR: 0.73; 95%CI, 0.61 to 0.89; P = 0.002; I2 = 71.2%) and the squamous subgroups (HR: 0.68; 95%CI, 0.58 to 0.80; P < 0.001; I2 = 14.1%; Fig. 4). There was no significant difference in efficacy of anti-PD-1/PD-L1 immunotherapy between two histologic subtypes (interaction P = 0.58).
Figure 4.
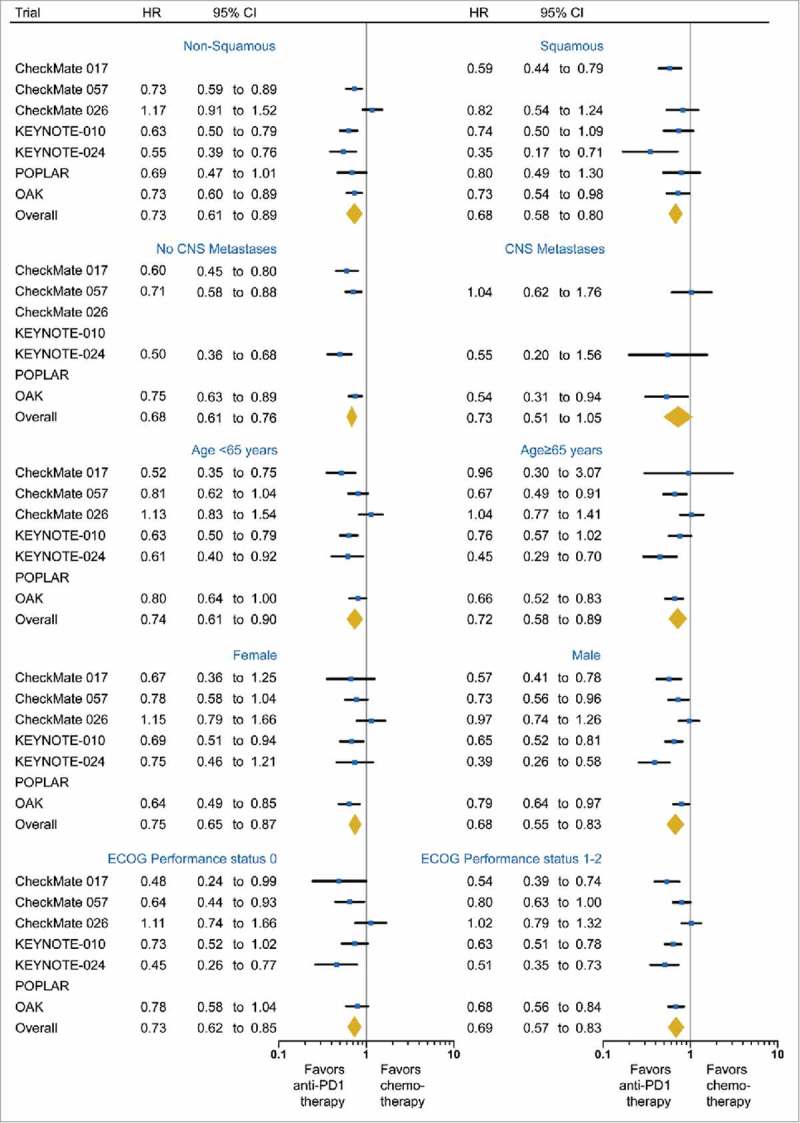
Forest plot of the effect of treatment on overall survival in subgroups of patients according to histology, CNS metastases, age, gender and Eastern Cooperative Oncology Group performance status (ECOG PS). Hazard ratios (HRs) for each trial are represented by the squares, and the horizontal line crossing the square represents the 95% CI. The diamonds represent the estimated overall effect based on the meta-analysis fixed effect. All statistical tests were two sided.
Of the 2,992 patients with reported CNS metastases status, most patients (1,811, 90.9%) were without CNS metastases; 181 (9.1%) were diagnosed with CNS metastases. The pooled HR for OS was 0.68 (95%CI, 0.61 to 0.83; P < 0.001; I2 = 47.5%; Fig. 4) in the subgroup without CNS metastases. The benefit in the subgroup with CNS metastases was borderline significant (HR, 0.73; 95%CI, 0.51 to 1.05; P = 0.09; I2 = 37.4%; Fig. 4). The efficacy of immunotherapy was not significantly different between these two subgroups (interaction P = 0.71).
The benefit of anti-PD-1/PD-L1 immunotherapy compared with chemotherapy was similar in patients <65 years (HR, 0.74; 95%CI, 0.61 to 0.90; P = 0.003; I2 = 64.6%) and ≥65 years (HR, 0.72; 95%CI, 0.58 to 0.89; P = 0.002; I2 = 55.2%; interaction P = 0.85; Fig. 4). Of note, among patients ≥75 years (n=72) whose data were available in the CheckMate 017 and 057 trials, OS of immunotherapy arm was numerically shorter than that of chemotherapy (HR, 1.21; 95%CI, 0.68 to 2.12; P = 0.52; I2 = 33.2%).
The improvement in OS with anti-PD-1/PD-L1 immunotherapy versus chemotherapy did not significantly differ by gender (interaction P = 0.28) and ECOG PS (interaction P = 0.65; Fig. 4).
Publication bias
Funnel plots (not shown), as well as Egger's test and Begg's test, yielded no publication bias in the overall and subgroup populations (all P > 0.05), with the exception in the subgroup without CNS metastases (Begg's P = 0.09; Egger's P = 0.03)
Discussion
Treatment with anti-PD-1/PD-L1 antibodies compared with chemotherapy is associated with 27% reduction in the risk of death, and 16% reduction in the risk of disease progression among the overall population (Fig. 2). When stratifying patients based on PD-L1 expression level, we observed that immunotherapy offered the greatest OS benefit in the strong-positive subgroup, but also significantly improved the OS in the negative and weak-positive subgroups. OS benefit was observed in the KRAS mutant patients, but not in the KRAS wild-type patients. Conversely, OS benefit was observed in the EGFR wild-type patients, but not in the EGFR mutant patients. Current or former smokers had significant survival benefit from immunotherapy, but never-smokers did not. There was no differential treatment effect for OS by histology, CNS metastasis status, age, gender and ECOG PS score.
Early-phase trials demonstrated the positive correlation between objective response rate (ORR) and PD-L1 staining intensity in NSCLC patients.7,10,12 However, the unique radiographic response patterns of anti-PD-1/PD-L1 immunotherapy, including “pseudoprogression”, limit the power of the conventional evaluation criteria to assess its activity.27–29 When using OS, the “gold-standard” endpoint, to evaluate the predictive value of PD-L1 expression in anti-PD-1/PD-L1 immunotherapy, the evidence remains controversial. For instance, the CheckMate 0575 and POPLAR9 trials showed that OS improvement was significant in the patients with higher PD-L1 expression, whereas OS of patients with PD-L1 negative tumors in the anti-PD-1/PD-L1 group was similar to that in the chemotherapy group. In contrast, anti-PD-1/PD-L1 immunotherapy was beneficial to all patients regardless of PD-L1 expression in the CheckMate 0176 and OAK11 trials. To collectively characterize the predictive value of PD-L1 levels in anti-PD-1/PD-L1 immunotherapy, our current study classified all randomized patients as PD-L1 negative, weak-positive and strong-positive subgroups on the basis of trial data, and demonstrated that PD-L1 strong-positive patients gained the greatest OS benefit from PD-1/PD-L1 blockade. Correlation analysis revealed that PD-L1 expression level was a modifier of treatment effect on OS, which was further confirmed by sensitivity analysis when restricted to second-or later-line therapy.
Furthermore, both PD-L1 negative and weak-positive subgroups also had significant survival benefit from anti-PD-1/ PD-L1 immunotherapy, indicating that PD-L1 expression is not a robust biomarker to predict patient response to immunotherapy. Indeed, PD-L1 expression is dynamic and is an inducible biomarker affected by various factors. For instance, activated T cell and innate immune cells in the TME can release interferon gamma (IFN-γ) which increases PD-L1 expression.4 Additionally, PD-L1 expression may be clustered in an area infiltrated with IFN-γ positive T cell; thus, small biopsies of tumor tissues may miss the PD-L1 positive area and produce false negative results.30 This finding also highlights that anti-PD-1/PD-L1 immunotherapy should not be limited to patients with PD-L1 strong-positive tumors. Similar benefits between PD-L1 negative and weak-positive patients were supported by the phase 1 KEYNOTE-001 trial, in which the survival curves of the two groups were close.7 The ongoing trials, including KEYNOTE-042 (NCT02220894), will provide more data on the efficacy of anti-PD-1/PD-L1 immunotherapy for PD-L1 weak-positive or negative patients.31
Activating KRAS mutations are the most frequent genetic alteration in NSCLC and effective therapies for this subclass are lacking.32–35 Strikingly, our finding that anti-PD-1/PD-L1 immunotherapy provided significant OS benefit for patients with KRAS mutant NSCLC is of utmost importance. Clearly, given the lack of success of current therapies in targeting KRAS mutations, these immune checkpoint inhibitors offer a promising option for this subgroup of NSCLC patients. Although the factors that render KRAS mutant NSCLC more sensitive to anti-PD-1/PD-L1 immunotherapy remain largely unknown, potential mechanisms include defective DNA repair, including mismatch repair, with increased mutational load.20 Interestingly, the study by Dong et al. also suggested that patients with TP53 mutations alone or co-occurring TP53/KRAS mutations had increasing PD-L1 expression, reflecting T cell infiltration and augmented tumor immunogenicity, supporting its predictive value for response to PD-1 blockade immunotherapy. Additionally, clinical activity in patients treated with immune checkpoint inhibitors is suggested to be mediated by neo-epitope-reactive T cells.14 Potentially, KRAS-mutant oncoproteins contain single point mutations that serve as neo-epitopes, and can be distinguished from wild-type KRAS by the presence of T cells. Consistently, compared with wild-type, KRAS mutation NSCLC patients had significantly higher candidate neo-antigen burden, supporting their favorable benefit to anti-PD-1 treatment.20 Apart from immune checkpoint blockade, strategies that enhance a T-cell response against mutated tumor antigens may be of clinical benefit in patients with cancer.36,37 Recently, Tran and colleagues reported a case with metastatic colorectal cancer had substantial tumor regression in the lung after adoptive transfer of T cells targeting KRAS G12D.36 Their work further supports the vital role of immunotherapy in targeting mutant KRAS cancers.
On the other hand, consistent with retrospective evidence and previous meta-analysis of three trials17,38, there was no OS advantage of anti-PD-1/PD-L1 immunotherapy compared with chemotherapy among patients harboring EGFR mutations, but there was a 27% reduction in the risk of death among patients with EGFR wild-type NSCLC. Moreover, differential treatment effect for OS was also observed by smoking status. Lower mutational burden and immunogenicity could potentially explain the lack of effectiveness of immunotherapy in patients whose tumors harbor EGFR mutations, many of whom are never-smokers.15,17
A recent study by Stephane Champiat and colleagues showed that older patients (≥65 years) more frequently exhibited hyperprogressive disease, a novel aggressive pattern of progression which was associated with inferior OS in patients.39 This raises concern about treating older patients with anti-PD-1/PD-L1 antibodies. Nevertheless, the benefits of immunotherapy in older and younger patients were almost identical in our pooled analysis (HR, 0.72 vs. 0.74). Interestingly, among patients older than 75 years, survival was numerically shorter with immunotherapy versus chemotherapy, similar to older patients with renal cell carcinoma40, although small sample size should be noted during data interpretation. This may be explained by the age-related immunosenescence, including declining number of CD8+ T cells, modified expression of T cell co-stimulatory/co-inhibitory proteins, and higher levels of immune suppressive inflammatory cytokines.41,42 Special attention should be paid to ≥75-year-old patients in clinical practice and future studies.
CNS metastases are common in patients with advanced NSCLC, and associated with poor survival. Patients with CNS metastases were excluded in some trials, due to uncertainty about the action of these immune modulatory antibodies in the CNS.43 The efficacy of immunotherapy in these patients remains unclear due to the paucity of data. Early analysis of a non-randomized phase 2 trial showed an encouraging activity of Pembrolizumab in NSCLC patients with CNS metastases (ORR 33%); however overall survival data is not yet mature.44 The current study demonstrated the OS benefit of anti-PD-1/PD-L1 immunotherapy over chemotherapy in patients, and the benefit is similar to patients without CNS metastases (interaction P = 0.71). Additional studies are warranted to confirm this benefit and to explore activity of the combination with radiation and other checkpoint inhibitors.45
The major strength of this meta-analysis is that we included all randomized trials comparing anti-PD-1/anti-PD-L1 immunotherapy with chemotherapy in NSCLC from the most up-to-date publications. Both anti-PD-1 and anti-PD-L1 antibodies, of which difference in efficacy was not observed (supplementary Table S1), were eligible for the study. The comprehensive analysis overcomes the problem of inadequate statistical power in individual trials to assess the efficacy among subgroups. Another strength is the use of OS as the primary endpoint, which accurately reflects the durable outcomes of immunotherapy as opposed to PFS and ORR.
There are also limitations in the present study. Outcomes from subgroups in some trials were unavailable for our analyses. This also suggests that future studies should pay close attention to certain populations, such as patients whose tumors harbor KRAS mutations and patients ≥75-year-old. Additionally, the benefit in PD-L1 negative and weak-positive subgroups was derived from second- or later-line therapies, so the benefit in these patients in first-line setting requires further investigation by randomized trials (NCT02477826, NCT02220894 and NCT02409342).
Our findings have significant clinical implications having identified specific sub-populations more or less likely to respond to PD-1/PD-L1 blockade. OS benefit difference existed in the PD-L1 expression subgroups, the oncogenic mutation subgroups and the smoking subgroups. Hence, future studies should obtain these data, and consider them as stratification factors. Lastly, the finding that patients in all PD-L1 expression subgroups benefited from immunotherapy highlights the necessity of exploring novel effective biomarkers. Patient-specific factors (e.g. age) and molecular features (e.g. KRAS, EGFR mutational status) may prove useful in the future to develop tailored treatment regimens using PD-1/PD-L1 blockade.
In summary, anti-PD-1/PD-L1 immunotherapy significantly prolonged the OS and PFS of patients with advanced NSCLC compared with chemotherapy. Although patients with PD-L1 strong-positive NSCLC gained greatest OS benefit, patients with PD-L1 negative or weak-positive NSCLC also benefited from PD-1/PD-L1 blockade. OS benefit of anti-PD-1/PD-L1 immunotherapy compared with chemotherapy was also observed in KRAS mutant, EGFR wild-type and current or former smoker subgroups, but not in the corresponding subgroups. The OS benefit of immunotherapy was observed in all subgroups of histology, CNS metastases status, age, gender and ECOG PS.
Supplementary Material
Disclosure of potential conflicts of interest
None
Acknowledges
The authors thank Dr. Russell W. Jenkins for helpful discussions on the article. This work was supported by NIHR Imperial Biomedical Research Centre (BRC), NIHR (J.S.) and Action Against Cancer.
Author contributions
All authors
Conception and design
All authors
Collection and assembly of data
All authors
Data analysis and interpretation
All authors
Manuscript writing
All authors
Final approval of manuscript
All authors
References
- 1.Fusi A, Festino L, Botti G, Masucci G, Melero I, Lorigan P, Ascierto PA. PD-L1 expression as a potential predictive biomarker. Lancet Oncol. 2015;16:1285–87. doi: 10.1016/S1470-2045(15)00307-1. [DOI] [PubMed] [Google Scholar]
- 2.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med. 2016;375:1767–78. doi: 10.1056/NEJMra1514296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. PMID:2693650826068851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 8.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al.. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al.. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibney GT, Weiner LM, Atkins MB: Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al.. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. PMID:26068851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al.. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 17.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al.. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22:4585–93. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al.. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343–54. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM, Denning WL, et al.. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong ZY, Zhong W, Zhang XC, Su J, Xie Z, Liu SY, Tu HY, Chen HJ, Sun YL, Zhou Q, et al.. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017;23:3012–24. [DOI] [PubMed] [Google Scholar]
- 21.Socinski M, Creelan B, Horn L, Reck M, Paz-Ares L, Steins M, Felip E, van den Heuvel M, Ciuleanu TE, Badin F, et al.. NSCLC, metastaticCheckMate 026: A phase 3 trial of nivolumab vs investigator's choice (IC) of platinum-based doublet chemotherapy (PT-DC) as first-line therapy for stage iv/recurrent programmed death ligand 1 (PD-L1)–positive NSCLC. Annals of Oncology. 2016. 27:LBA7_PR–LBA7_PR. doi: 10.1093/annonc/mdw435.39. [DOI] [Google Scholar]
- 22.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, et al.. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR: Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. PMID:17555582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24%3c2815::AID-SIM110%3e3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, Hodi FS, Awad MM. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. PMID:28018599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al.. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. doi: 10.1158/1078-0432.CCR-09-1624. PMID:19934295 [DOI] [PubMed] [Google Scholar]
- 29.Shukuya T, Mori K, Amann JM, Bertino EM, Otterson GA, Shields PG, Morita S, Carbone DP.. Relationship between Overall Survival and Response or Progression-Free Survival in Advanced Non-Small Cell Lung Cancer Patients Treated with Anti-PD-1/PD-L1 Antibodies. J Thorac Oncol. 2016;11:1927–1939. doi: 10.1016/j.jtho.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. PMID:22461641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mok T, Wu Y-L, Patricia A W, Zhang J, Rangwala RA, Lopes G.. Phase 3 KEYNOTE-042 trial of pembrolizumab (MK-3475) versus platinum doublet chemotherapy in treatment-naive patients (pts) with PD-L1–positive advanced non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2015;33:TPS8105–TPS8105 [Google Scholar]
- 32.Blumenschein GR Jr., Smit EF, Planchard D, Kim DW, Cadranel J, De Pas T, Dunphy F, Udud K, Ahn MJ, Hanna NH, et al.. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)dagger. Ann Oncol. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter CA, Rajan A, Keen C, Szabo E, Khozin S, Thomas A, Brzezniak C, Guha U, Doyle LA, Steinberg SM, et al.. Selumetinib with and without erlotinib in KRAS mutant and KRAS wild-type advanced nonsmall-cell lung cancer. Ann Oncol. 2016;27:693–9. doi: 10.1093/annonc/mdw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh H, Longo DL, Chabner BA: Improving Prospects for Targeting RAS. J Clin Oncol. 2015;33:3650–9. doi: 10.1200/JCO.2015.62.1052. [DOI] [PubMed] [Google Scholar]
- 35.Khleif SN, Abrams SI, Hamilton JM, Bergmann-Leitner E, Chen A, Bastian A, Bernstein S, Chung Y, Allegra CJ, Schlom J. A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother. 1999;22:155–65. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, et al.. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255–62. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.June CH: Drugging the Undruggable Ras - Immunotherapy to the Rescue? N Engl J Med. 2016;375:2286–89. doi: 10.1056/NEJMe1612215. [DOI] [PubMed] [Google Scholar]
- 38.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. 2017;12:403–407 [DOI] [PubMed] [Google Scholar]
- 39.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al.. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2016. PMID:27827313 [DOI] [PubMed] [Google Scholar]
- 40.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al.. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawelec G, Derhovanessian E, Larbi A: Immunosenescence and cancer. Crit Rev Oncol Hematol. 2010;75:165–72. doi: 10.1016/j.critrevonc.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Goronzy JJ, Weyand CM: Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–36. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waqar SN, Morgensztern D, Govindan R: Systemic Treatment of Brain Metastases. Hematol Oncol Clin North Am. 2017;31:157–176. doi: 10.1016/j.hoc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, et al.. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–83. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johanns T, Waqar SN, Morgensztern D: Immune checkpoint inhibition in patients with brain metastases. Ann Transl Med. 2016;4:S9. doi: 10.21037/atm.2016.09.40. PMID:27867977 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


