ABSTRACT
Vaccines in combination with chemotherapy have been shown to be safe in different tumor types. We investigated the immunological activity of the TroVax® vaccine in combination with pemetrexed-cisplatin chemotherapy in malignant pleural mesothelioma (MPM).
In this first line, open-label, single-arm, phase 2 study, patients with locally advanced or metastatic MPM were enrolled. Eligible patients received up to 9 intramuscular injections of TroVax®, starting two weeks before chemotherapy and continuing at regular intervals during and after chemotherapy to 24 weeks. The primary endpoint was the induction of cellular or humoral anti-5T4 immune response (defined as a doubling of either response at any of six follow-up time points), with a target response rate of 64%.
Of 27 patients, enrolled between Feb 2013-Dec 2014, 23 (85%) received at least three doses of TroVax® and one cycle of chemotherapy and were included in the per-protocol analysis (PPA). 22/23 patients (95.6%) developed humoral or cellular immune response to 5T4. Thus, the study reached its primary endpoint. Disease control was observed in 87% of patients (partial response: 17.4%, stable disease: 69.6%). The median progression-free survival was 6.8 months and median overall survival 10.9 months. Treatment-related adverse events were comparable to those observed in patients with chemotherapy alone. Translational immunology studies revealed a circulating baseline immune signature that was significantly associated with long-term (>20 months in n = 8/23, 34.8%) survival.
In this phase 2 trial, TroVax® with pemetrexed-cisplatin chemotherapy showed robust immune activity, acceptable safety and tolerability to warrant further investigation in a phase 3 setting.
KEYWORDS: Mesothelioma, 5T4 antigen, MVA vaccine, T cell cytokines, immunophenotyping
Introduction
Malignant pleural mesothelioma (MPM) is an incurable and fatal malignancy of the pleural membranes. MPM has a poor prognosis and patients have a median survival of 9–18 months in clinical trials.1,2 To date, only two randomized phase III trials in MPM have shown benefit for one systemic treatment approach over another. In the first of these trials,1 448 patients were randomized to chemotherapy using pemetrexed plus cisplatin or cisplatin alone. Median overall survival (OS) was significantly longer in the pemetrexed-cisplatin arm (12.1 vs. 9.3 months, p < 0.02). As a result of this trial, pemetrexed-cisplatin was established as the chemotherapy standard of care for patients with MPM. For this reason, we chose pemetrexed-cisplatin as the chemotherapy regimen for this trial. Subsequently, the addition of bevacizumab to pemetrexed-cisplatin has been shown to further increase median survival by two to three months compared to chemotherapy alone.2 Despite the benefits seen from chemotherapy with or without the addition of bevacizumab, it is clear that new therapeutic strategies are urgently needed for MPM.
There is significant recent interest in the potential role of immunotherapy in the management of patients with MPM, which has been shown to respond to various immunotherapeutic strategies in animal models and early phase clinical trials.3 Spontaneous regression, associated with improved immune parameters has also been reported.4 Indeed, prognostic significance of intratumoral immune cell subsets in MPM have revealed CD8+ T cells and CD20+ B cells as positive prognostic indicators,5,6 whilst CD163+ macrophages and regulatory T cells (Treg) are negative indicators.6,7 Conversely, very few studies have addressed the significance of peripheral immune parameters to clinical outcome, though proliferating CD8+ T cells, co-expressing Ki678 and dysfunctional dendritic cells9 have been described. Furthermore, blood parameters such as high white blood cell count, neutrophil to lymphocyte ratio (NLR), monocyte numbers and high monocyte to lymphocyte (MLR) ratios have also shown negative prognostic value.10-12 This indicates that patient screening and stratification may improve clinical benefit, particularly in immunotherapy trials.
Current studies are testing a range of immunotherapeutic approaches, such as treatment with a mesothelin-targeting chimeric antibody (amatuximab), type-I interferon delivered by an adenoviral vector, intrapleural viruses and antigen-specific vaccines, such as the Wilms tumor antigen-1 (WT-1) vaccine.13 The WT-1 vaccine has shown evidence of activity in a trial of 40 patients with MPM randomized to WT-1 vaccine or observation after multimodality treatment. Immune checkpoint inhibitors have also been tested in MPM with mixed early results and their further clinical trials – single or combination treatments – are currently ongoing.14,15
5T4 is a 72 kDa oncofoetal glycoprotein that is expressed in many solid tumors but shows minimal or no expression in normal tissues.16 We have shown that 5T4 is widely expressed in mesothelioma tissue and on mesothelioma cell lines.17 Unlike WT-1 and mesothelin, which display subtype-restricted expression, often excluding the more aggressive sarcomatoid variant, 5T4 expression has been shown in all MPM subtypes.17 5T4-specific T cell responses were demonstrated by patients' peripheral blood mononuclear cells (PBMC) and pleural fluid cells (Al-Taei at al., unpublished), making it a valid antigen for targeted therapies, including immunotherapy, in MPM.
TroVax® (Oxford BioMedica, Oxford, UK) is a therapeutic cancer vaccine which consists of a highly attenuated vaccinia virus (modified vaccinia Ankara) containing the 5T4 glycoprotein gene. TroVax® has been administered to more than 500 patients with renal, colorectal and prostate cancer.18 These clinical studies showed that TroVax® is well-tolerated and induced 5T4-specific antibody and/or cellular immune responses in the majority of patients. In addition, TroVax® was also well tolerated when used in combination with chemotherapy in patients with colorectal cancer.19
Pemetrexed-cisplatin chemotherapy is seen as a UK and international standard of care for patients with MPM. We aimed to combine the TroVax® vaccine with first line pemetrexed-cisplatin chemotherapy in MPM patients in a single-arm, single-center, phase II trial (SKOPOS), in order to determine 5T4-specific antibody and/or cellular immune responses, activity, safety and feasibility. Furthermore, we carried out retrospective immunohistochemistry, full blood count (FBC) and immunophenotypic analyses to identify potential immune prognostic indicators.
Results
Patient and treatment details
Between Feb 2013, and Dec 2014, 29 patients were enrolled at Velindre Cancer Centre in Cardiff, UK. 23/29 patients (79%) received at least three doses of TroVax® and one cycle of chemotherapy and were included in the per protocol analysis (PPA) (Fig. 1). Six participants were not included in the PPA as four did not receive the minimum 3 injections, one patient was later found not to have mesothelioma, and one was found to have a co-primary cancer. Median age of the PPA participants was 66 years (IQR 61–70), 20/23 (87%) were male, 20/23 (87%) had epithelioid, 3/23 (13%) had sarcomatoid MPM, and 12/23 (52%) had WHO performance status (PS) of 0 (Table 1).
Figure 1.
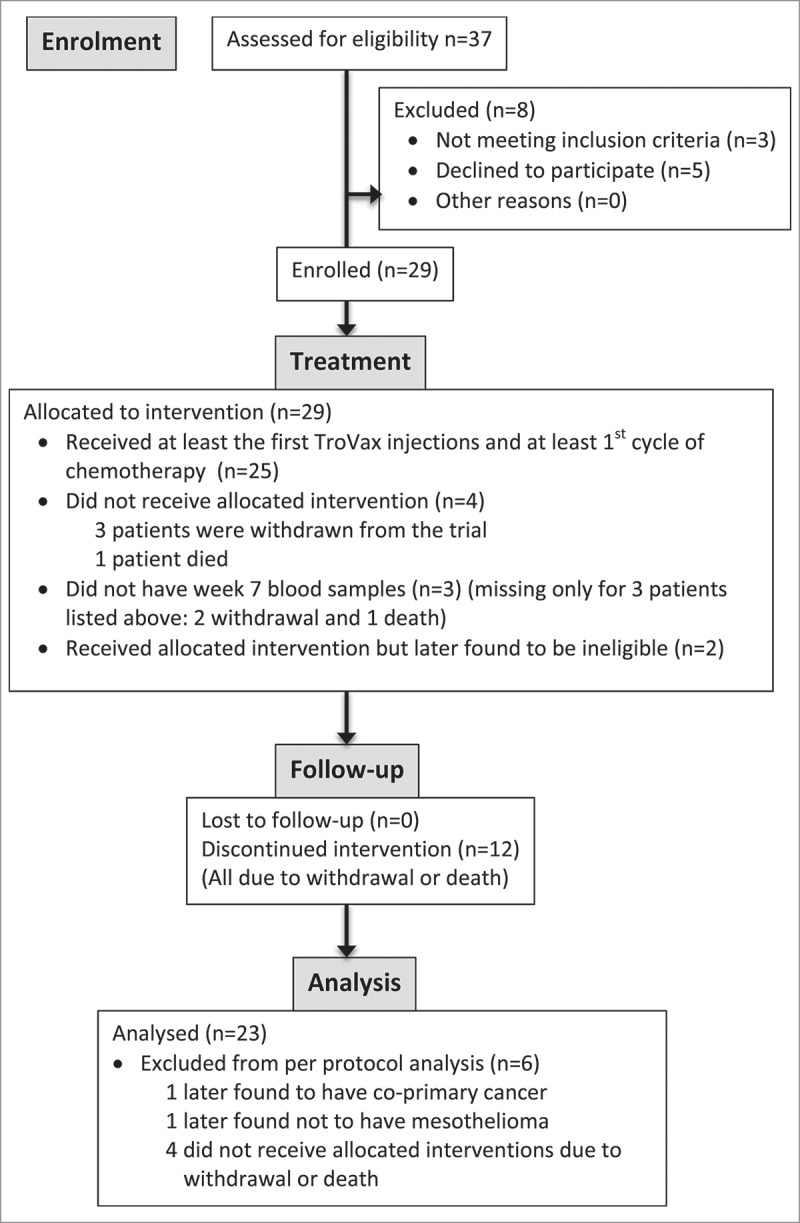
CONSORT Flow diagram of patients allocated for the SKOPOS trial.
Table 1.
Participant baseline characteristics.
| Characteristics | Results (N = 23) |
|---|---|
| Age | 66 (61–70) |
| Sex | |
| Male | 20 (87.0%) |
| Female | 3 (13.0%) |
| WHO performance status | |
| 0 | 12 (52.2%) |
| 1 | 11 (47.8%) |
| Mesothelioma type | |
| Epithelioid | 20 (87.0%) |
| Sarcomatoid | 3 (13.0%) |
| Mesothelioma stage | |
| Stage II | 8 (34.8%) |
| Stage III | 13 (56.5%) |
| Stage IV | 2 (8.7%) |
| Platelets | 351 (250–407) |
| Monocytes | 0.6 (0.4–0.8) |
| Hemoglobin | 13.8 (12.7–14.7) |
– Median (IQR).
The median number of TroVax® injections administered to PPA patients was nine (IQR 7–9): 16/23 (70%) patients received all nine injections, 6/23 (26%) patients received between four and nine injections, and 1/23 (4%) patients received three injections. The reasons that seven patients missed injections are follows: two patients died, two withdrew due to adverse effects (AE), one withdrew due to disease progression, one withdrew to undergo surgery for MPM, and one patient missed the week 11 injection. Four cycles of pemetrexed-cisplatin chemotherapy treatment were successfully administered to 19/23 (83%) patients. Four patients did not complete four cycles of chemotherapy: two died and two withdrew due to AE. The median number of chemotherapy cycles was four (IQR 4–4). Median cisplatin dose intensity (DI) was 90% (IQR 75–100). Median pemetrexed DI was 95% (IQR 75–100).
Primary endpoint – immune responses
Table 2A summarizes the primary endpoint, the generation of cellular and/or humoral immune responses specific for the tumor antigen (5T4) delivered by the vaccine. As 22/23 (96%) patients had an anti-5T4 response (either humoral or cellular), the study reached its primary endpoint. 17/23 (74%) of patients mounted humoral and 20/23 (87%) of patients developed cellular anti-5T4 immune responses, which were defined as doubling of immune responses from baseline. The longitudinal analysis of immune responses is shown on Table 2B and a time-related summary on Fig. 2. The frequency of responders to 5T4 peaked at week 10, followed by a gradual decrease. This may have been due to the accumulated effects of 4 cycles of chemotherapy that started at 4-weeks and ended at 13-weeks. All three patients with sarcomatoid MPM mounted 5T4-specific T cell responses while antibody doubling was observed in 2/3 patients. In an independent preliminary study, involving 27 lung cancer and MPM patients who received pemetrexed-cisplatin chemotherapy without the vaccine, 5T4-specific antibody levels were measured before and after chemotherapy. Only one patient had a doubling of the 5T4-antibody level (data not shown), indicating that if changes in 5T4 antibody levels are observed in the trial, they will be due predominantly to the development of specific immune responses to the vaccine.
Table 2.
Immune responses.
| A. Summary of overall immune responses – primary endpoint | ||||||
|---|---|---|---|---|---|---|
| Measurements |
Results (N = 23) |
|||||
| Humoral 5T4 overall response | 17 (73.9%) | |||||
| Cellular 5T4 overall response | 20 (87.0%) | |||||
| Any overall response (humoral or cellular 5T4 immune response) | 22 (95.7%) | |||||
| B. Detailed immune responses – time kinetics | ||||||
| n/N (%) patients |
||||||
| Immune parameters |
Week 4 |
Week 7 |
Week 10 |
Week 13 |
Week 26 |
Week 34 |
| CD4 IFNγ | 4/21 (19.05%) | 12/22 (54.55%) | 11/22 (50.00%) | 7/18 (38.89%) | 4/15 (26.67%) | 4/11 (36.36%) |
| CD8 IFNγ | 1/21 (4.76%) | 5/22 (22.73%) | 5/22 (22.73%) | 5/18 (27.78%) | 4/15 (26.67%) | 2/11 (18.18%) |
| CD4 TNFα | 2/21 (9.52%) | 3/22 (13.64%) | 5/22 (22.73%) | 1/18 (5.56%) | 4/15 (26.67%) | 3/11 (27.27%) |
| CD8 TNFα | 0/21 (0%) | 0/21 (0%) | 2/22 (9.09%) | 2/18 (11.11%) | 2/15 (13.33%) | 1/11 (9.09%) |
| CD4 IL-2 | 3/21 (14.29%) | 3/22 (13.64%) | 5/22 (22.73%) | 3/18 (16.67%) | 6/15 (40.00%) | 2/11 (18.18%) |
| CD8 IL-2 | 3/21 (14.29%) | 2/22 (9.09%) | 3/22 (13.64%) | 3/18 (16.67%) | 1/15 (6.67%) | 1/11 (9.09%) |
| Humoral 5T4 | 3/23 (13.04%) | 12/23 (52.17%) | 10/22 (45.45%) | 9/19 (47.37%) | 10/16 (62.50%) | 5/12 (41.67%) |
| Cellular 5T4 | 9/21 (42.86%) | 13/22 (59.09%) | 14/22 (63.64%) | 11/18 (61.11%) | 9/15 (60.00%) | 5/11 (45.45%) |
| Any (cellular or humoral 5T4) | 11/23 (47.83%) | 19/23 (82.61%) | 20/22 (90.91%) | 15/19 (78.95%) | 13/16 (81.25%) | 9/12 (75.00%) |
| Humoral MVA | 22/23 (95.65%) | 23/23 (100.00%) | 22/22 (100.00%) | 18/19 (94.74%) | 16/16 (100.00%) | 12/12 (100.00%) |
Number of patients responded (%).
Figure 2.
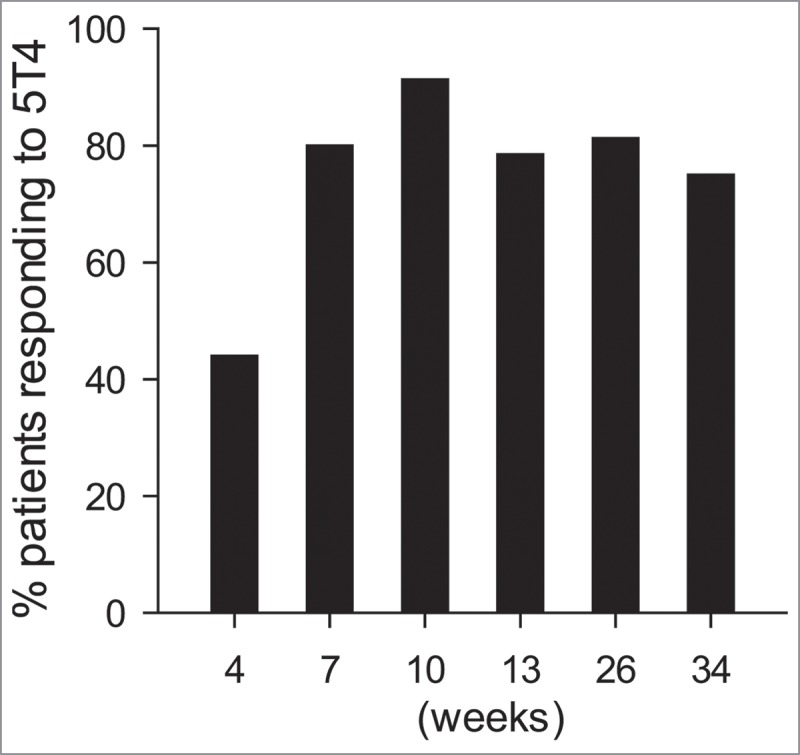
Summary of any 5T4-specific immune response in a longitudinal study. The bars represent the proportions (%) of patients whose immune response was ≥2-fold to any 5T4 peptide group compared to their immune responses at baseline. The six time points are indicated on the X-axis.
Clinical responses
For the 23 patients evaluable for clinical response by 26 weeks, 0/23 (0%) patients achieved a complete response, 4/23 (17%) a partial response, 16/23 (70%) a stable disease and 3/23 (13%) have progressed, giving an overall objective response rate (ORR) of 17% (95% CI 5–39%) and disease control of 87% (95% CI 66–97%; Table 3). The median progression-free survival (PFS) was 6.8 months (95% CI 3.6–8.9) (Fig. 3A). Median OS was 10.9 months (95% CI 8.1–23.5) (Fig. 3B). The mean OS for sarcomatoid patients was 6.6 months. The median length of follow-up for seven patients still alive was 24 months.
Table 3.
Clinical responses.
| Best response (pleural disease, target or non-target tumor) | |
|---|---|
| Complete response | 0/23 (0%) |
| Partial response | 4/23 (17.4%) |
| Stable disease | 16/23 (69.6%) |
| Progressive disease | 2/23 (8.7%) |
| Unknown | 1/23 (4.3%) |
| ORR (CR+PR) | 4/23 (17.4%) |
| Disease Control Rate (CR+PR+SD) | 20/23 (87.0%) |
| Median PFS, months | 6.8 (3.6–8.9) |
| Rate of PFS at 6 months | 60.6% (37.8–77.2%) |
| Rate of PFS at 12 months | 23.3% (8.5–42.2%) |
| Median OS, months | 10.9 (8.1–23.5) |
| Rate OS at 6 months | 82.6% (60.1–93.1%) |
| Rate OS at 12 months | 43.5% (23.3–62.1%) |
| Follow-up, months (alive) | 24.0 (19.3-NR) |
Number of patients n/23 (% responders).
RECIST data is not available for one patient.
months (95% CI).
% of patients (95% CI).
NR –Not reached.
Figure 3.
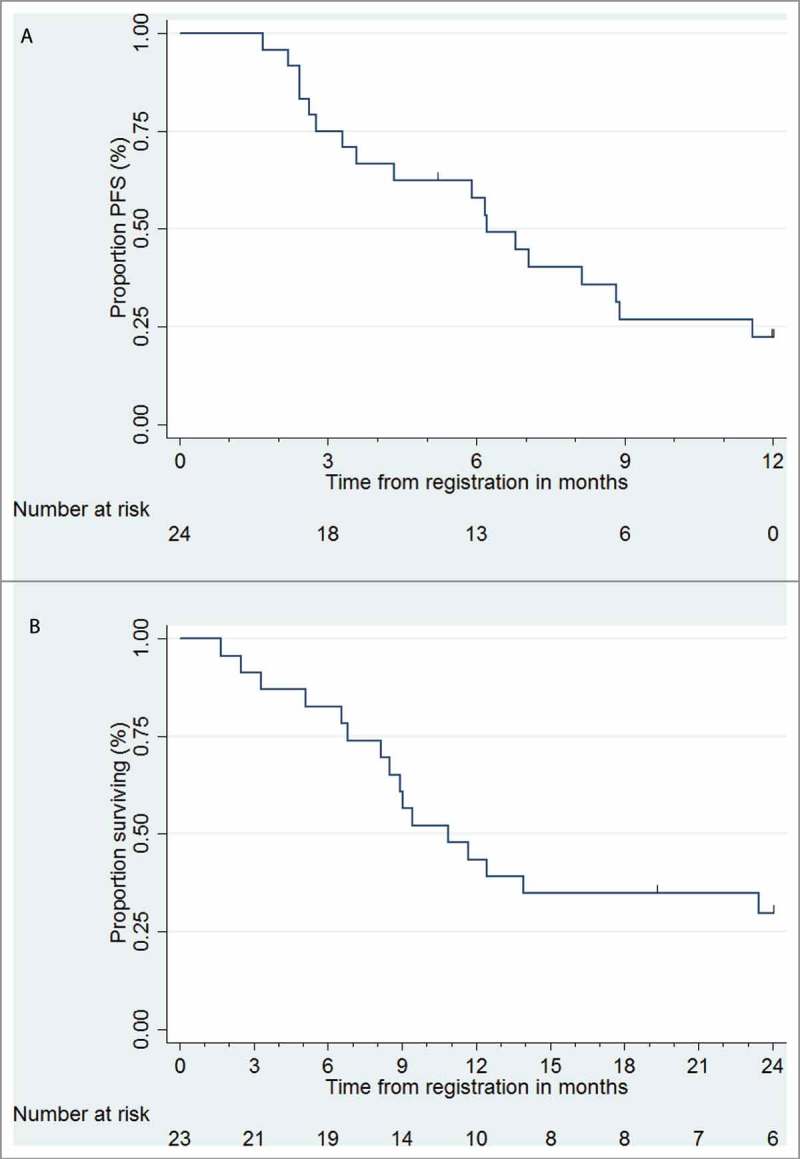
Kaplan-Meier curves for (A) Progression-free survival (PFS), (B) Overall survival (OS).
Adverse effects
Treatment-emergent adverse events experienced are shown in supplementary material Table S2. All 23 patients (100%) had at least one grade 1–2 treatment-emergent AE. Grade 3–4 toxicities were seen in 11/23 (48%) patients: Grade 4 events included one patient with neutropenia and one with pleural effusion. Grade 3 events included three participants each with respiratory tract infection and thromboembolic events, and two participants with hypertension. There were also isolated grade 3 events: pyrexia, fall, cellulitis, phlebitis, and dyspnoea. There were no grade 5 toxicities. Grade 1–2 AE affecting more than 5 patients are summarized in Table S2.
Analysis of circulating immune cells
In this trial, 15 patients died within 14 months (Fig. 3B) while the remaining 8 were still alive at 20 months. The frequency of patients alive in our cohort at 20 months (34.7%) correlates or is somewhat higher than the published frequency (24.8%)1 and can be seen as the tail of the Kaplan Meyer curve (Fig 3B). We carried out a comparative analysis of FBC data and circulating immune cell phenotypes in patients with >20 months survival (8/23 = 34.7%) vs. those with <20 months (15/23 = 65.3%) survival. While there was no evidence that PFS was associated with baseline blood parameters (Table 4A) or that the >20 m patients had any significantly different baseline FBC parameters, we found that NLR and MLR were significantly lower at week 4, when patients have only received 2 doses of vaccine but no chemotherapy, in the >20 m group (Table 4B). Comparative analysis also indicated that more patients mounted T cell responses to 5T4 in the >20 m group (Fig 4A) and T cell responses were generated with a broader 5T4 epitope specificity in the >20 m compared to the <20 m group (Fig 4B).
Table 4A.
Analysis of full blood count data.
| Variable | Hazard Ratio | Lower bound of 95% confidence interval | Upper bound of 95% confidence interval | p-value |
|---|---|---|---|---|
| Platelets | 0.998 | 0.993 | 1.003 | 0.43 |
| Monocytes | 0.693 | 0.073 | 6.536 | 0.75 |
| Haemoglobin | 1.217 | 0.897 | 1.651 | 0.21 |
| Haematocrit*10 | 1.903 | 0.628 | 5.767 | 0.26 |
| Mesothelin | 0.937 | 0.857 | 1.025 | 0.16 |
| Humoral 5T4 | 1.007 | 0.975 | 1.040 | 0.67 |
Exploratory univariate analysis of baseline blood parameters and PFS.
Table 4B.
Retrospective analysis of FBC.
| >20 weeks | <20 weeks | p-value | |
|---|---|---|---|
| Monocytes (B) | 0.587 ± 0.176 | 0.707 ± 0.19 | 0.0833 |
| Platelets (B) | 343.8 ± 126 | 343.5 ± 113 | 0.389 |
| Haematocrit (B) | 0.406 ± 0.047 | 0.413 ± 0.003 | 0.348 |
| NLR (B) | 3.05 ± 1.61 | 3.268 ± 1.55 | 0.378 |
| NLR (W4) | 2.401 ± 1.25 | 3.321 ± 0.98 | 0.041(*) |
| MLR (B) | 0.324 ± 0.099 | 0.415 ± 0.173 | 0.0747 |
| MLR (W4) | 0.268 ± 0.111 | 0.415 ± 0.171 | 0.021(*) |
Patients who survived >20 weeks.
Patients who died before 20 weeks.
P value by one sided t-test
Neutrophil:lymphocyte ratio.
Monocyte:lymphocyte ratio.
B: baseline; W4: 4 weeks after entering trial.
Figure 4.
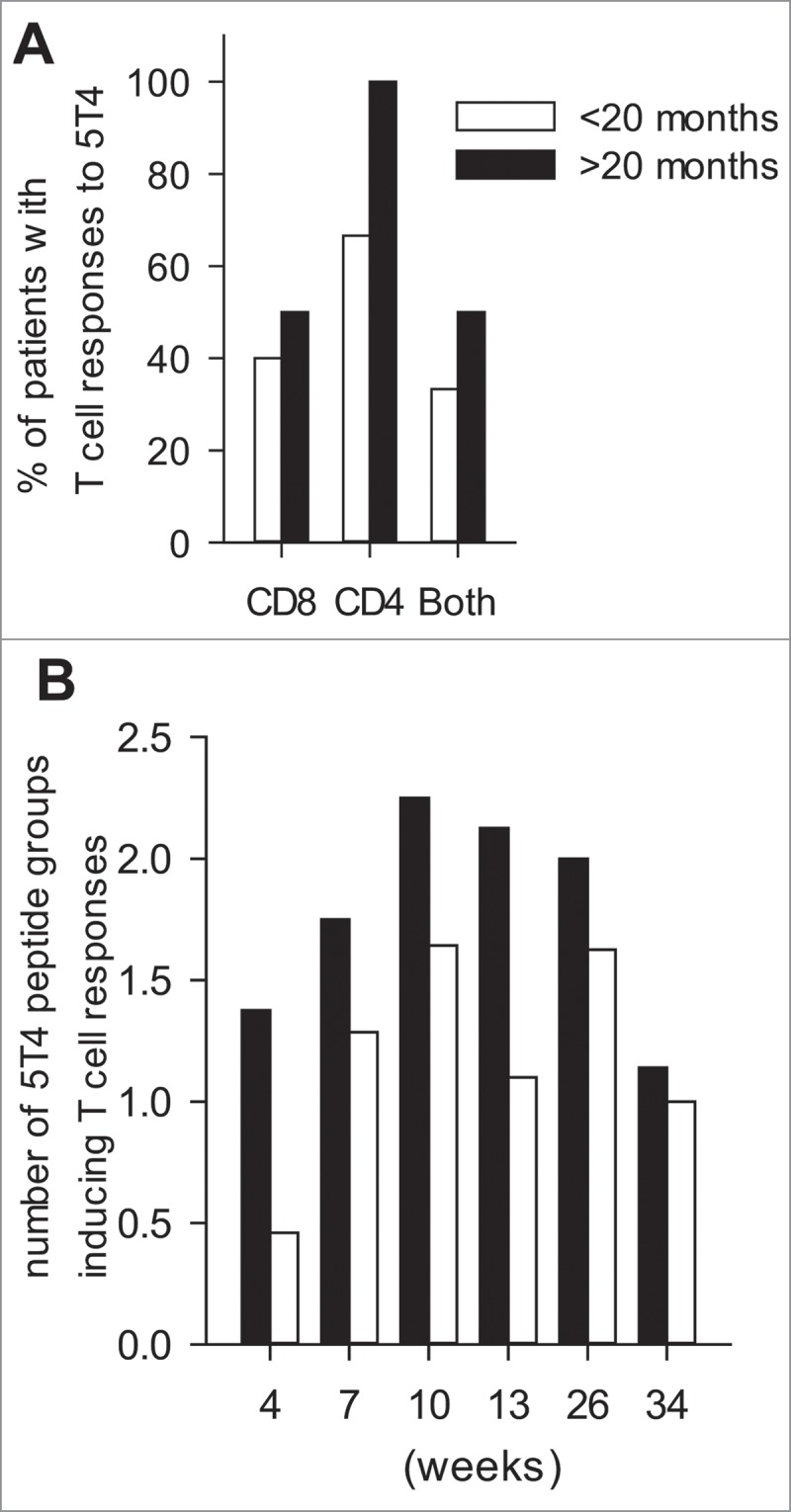
T cell responses to 5T4 peptides by long term vs. short term survivors. (A) The proportions of patients who generated CD4+ or CD8+ or both types of T cell responses to the 5T4 antigen. (B) The average frequencies of peptide groups that T cell responses were generated against at different time points during the trial. (A,B) <20 mo (black) represents patients with less than 20 months, while >20 mo (gray) those with more than 20 months OS.
We also assessed the comparative frequencies of T cell, Treg cell, monocyte and MDSC subsets in baseline samples. As shown in Table 5, the immune parameters, found to be significantly different between the two groups were: higher CD8:CD4 ratios and lower naïve CD4+ T cell frequencies in the >20 m patients. There were no differences between the frequencies of either total or activated Treg cells between the groups, and the frequencies of main myeloid cell subsets were also comparable. These results indicate that elevated peripheral CD8+/CD4+ T cell ratio in MPM patients is a positive prognostic indicator in TroVax® treatment.
Table 5.
Phenotypic analysis of circulating immune cells at baseline.
| T cells | <20 months | >20 months | p-value |
|---|---|---|---|
| CD8:CD4 Ratio | 0.571 ± 0.079 | 0.995 ± 0.389 | 0.035 (*) |
| CD4+CD27+CD45RA- (CM) | 34.07 ± 3.374 | 38.59 ± 6.430 | 0.249 |
| CD4+CD27+CD45RA+ (N) | 59.38 ± 3.957 | 42.93 ± 7.629 | 0.023 (*) |
| CD4+CD27-CD45RA- (EM) | 4.258 ± 0.653 | 6.758 ± 2.375 | 0.104 |
| CD4+CD27-CD45RA+ (TEM) | 2.292 ± 0.948 | 11.72 ± 9.717 | 0.098 |
| CD8+CD27+CD45RA- (CM) | 30.13 ± 3.844 | 22.41 ± 5.384 | 0.127 |
| CD8+CD27+CD45RA+ (N) | 42.10 ± 4.734 | 44.98 ± 8.891 | 0.379 |
| CD8+CD27-CD45RA- (EM) | 5.408 ± 1.098 | 3.919 ± 1.188 | 0.201 |
| CD8+CD27-CD45RA+ (TEM) | 22.37 ± 5.650 | 28.72 ± 9.866 | 0.276 |
| Treg cells | |||
| Treg (CD3+CD4+CD25+Foxp3+) | 5.052 ± 0.368 | 5.938 ± 1.077 | 0.174 |
| CTLA-4+ of Treg | 5.848 ± 0.623 | 6.730 ± 2.291 | 0.319 |
| Ki67+ of Treg | 22.45 ± 2.116 | 20.26 ± 2.049 | 0.256 |
| Myeloid cells | |||
| (DC) CD14- HLA-DR+ | 5.018 ± 0.470 | 6.269 ± 1.149 | 0.123 |
| (MPh) CD14+ HLA-DR+ | 8.682 ± 1.044 | 7.263 ± 1.070 | 0.198 |
| PDL-1+ MPh | 11.10 ± 2.085 | 12.30 ± 3.137 | 0.721 |
| CD200R+ PDL-1+ MPh | 8.613 ± 1.780 | 6.422 ± 1.898 | 0.222 |
| MDSC | |||
| M-MDSC (CD14+ HLA-DR- CD11b+ CD15-) | 4.468+3.079 | 3.317+1.584 | 0.186 |
| G-MDSC (CD14- CD33- CD15- CD11b+) | 0.028+0.011 | 0.276+0.555 | 0.186 |
| E-MDSC (Lineage- CD15- CD11b+ CD33+) | 0.079+0.098 | 0.502+1.01 | 0.058 |
CM: central memory; N: naïve; EM: effector memory; TEM: terminally differentiated effector memory; DC: dendritic cell; MPh: macrophage; MDSC: myeloid derived suppressor cell.
Tissue immunohistochemistry
19/23 pre-treatment FFPE samples were available for the immunohistochemical analysis of 5T4 expression and CD8+ T cell infiltration. 5T4 expression has been observed in all samples, although at varying degrees. In some cases, distinct expression was only observed on the tumor surface, while in others expression was observed in the tissue or both sites (Fig 5A, B). There was no difference in the frequency of 5T4+ tumor cells in the tissue of long-term and short-term survivors, respectively (32.7 ± 30.3% vs. 46.5 ± 36.2%), however, expression was markedly lower in the sarcomatoid tumors (2.33 ± 2.3%). CD8+ T cell infiltration was also studied. CD8+ cells were either scattered in the tissue or accumulated at the interface of malignant and normal tissues; in some cases both patterns were present (Fig 5A, C). Although there was a trend for higher CD8+ T cell infiltrate in the tissue of long-term vs. short-term survivors (28.4 ± 33.3% vs. 23.1 ± 24.8%), the difference was not significant. In contrast, CD8+ T cell infiltration was markedly lower in sarcomatoid tissues (11.83±15.8%). While the number of patients is insufficient for meaningful statistical analysis, we observed both 5T4 expression and CD8+ T cell infiltration at >10% of the tissue in 50% of long-term survivors, while this was only true in 25% of short-term survivors and in none of the sarcomatoid tumors.
Figure 5.
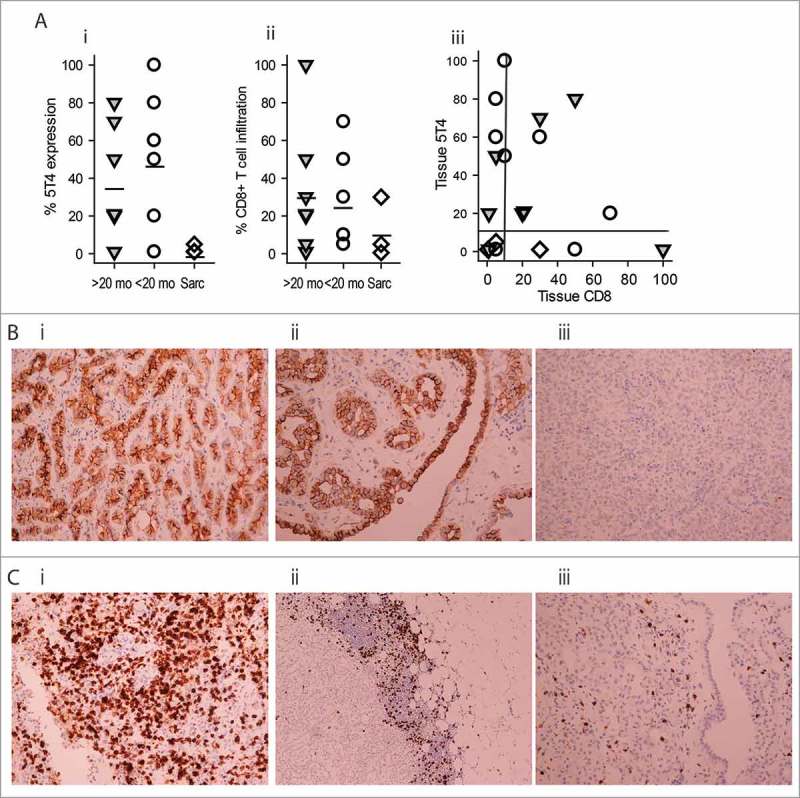
Immunohistochemistry of 5T4 expression and CD8+ T cell infiltration in pre-treatment tissue. (A) Expression levels of 5T4 (i) and CD8 (ii) in the specimens of long term survivors (>20 mo;  ), short-term survivors (<20 mo; ○) or sarcomatoid patients (sarc; ◊). Each symbols represents a different patient. The vertical bars represent mean of expression. (iii) The combination of results from (i) and (ii). Reference lines represent 10% of expression. (B) Representative examples of 5T4 expression in epithelioid tissue. (i) High tissue expression, (ii) high tumor tissue and surface expression, (iii) low tissue expression. (C) representative examples of CD8+ T cell infiltration. (i) high frequency scattered tissue infiltration, (ii) accumulation at the tumour margin, (iii) low level of tissue expression. Magnifications are 200x, except for Cii (100x).
), short-term survivors (<20 mo; ○) or sarcomatoid patients (sarc; ◊). Each symbols represents a different patient. The vertical bars represent mean of expression. (iii) The combination of results from (i) and (ii). Reference lines represent 10% of expression. (B) Representative examples of 5T4 expression in epithelioid tissue. (i) High tissue expression, (ii) high tumor tissue and surface expression, (iii) low tissue expression. (C) representative examples of CD8+ T cell infiltration. (i) high frequency scattered tissue infiltration, (ii) accumulation at the tumour margin, (iii) low level of tissue expression. Magnifications are 200x, except for Cii (100x).
Discussion
In patients with previously untreated MPM, the SKOPOS trial reached its primary endpoint, showing that TroVax® in combination with pemetrexed-cisplatin chemotherapy results in 95.7% of patients developing immune responses against the 5T4 tumor antigen. In the independent preliminary study, pemetrexed-cisplatin chemotherapy alone did not result in immune responses to the 5T4 tumor antigen in 26/27 (96.3%) patients, so the immune responses seen in the trial are overwhelmingly likely to have been generated by the TroVax® vaccine.
The high rate of immune responses generated by the vaccine, despite being given in combination with potentially immunosuppressive chemotherapy, shows that MPM patients are capable of generating or reactivating tumor antigen specific T or B cell responses in first line treatment settings. The combination of TroVax® plus chemotherapy was well tolerated, with no significant additional toxicity seen as a result of the TroVax® vaccine. The proportion of patients getting one or more grade 3 or 4 toxicities was 48%, which compares to 62% reported in the chemotherapy-only arm of the MAPS trial.2
With respect to clinical outcomes in SKOPOS, the median PFS of 6.8 months and median OS of 10.9 months are comparable to the median time to progression of 5.7 months and median OS of 12.6 months reported in the Pemetrexed-cisplatin arm of the Vogelzang trial, demonstrating that the combination with TroVax® has similar clinical activity.1 Importantly, we observed higher disease control (87% vs. 41.3%) than that reported in the Vogelzang trial and a greater proportion of patients achieved >20 months OS than those in the Vogelzang trial (34.7% vs. 24.8%). A feature of some contemporary immunotherapy trials in solid tumors is the observation that the Kaplan-Meier survival curves do not start to separate from the standard chemotherapy arm in the initial months, and clinically meaningful differences only emerge when survival beyond the median timepoint is examined. For example, in the Checkmate 017 trial of the PD-L1 inhibitor nivolumab versus docetaxel chemotherapy in the advanced squamous cell lung cancer second line setting, median PFS was less than one month longer in the nivolumab-treated patients. However, this relatively modest increase in median PFS translated into a more impressive difference in one year PFS: 21% seen with nivolumab compared to 6% with chemotherapy.24 Similar results were seen for OS in the sister Checkmate 057 trial in the second line advanced non-squamous lung cancer setting.25 In a small, non-randomized trial such as SKOPOS it is not possible to draw any definitive conclusions about the significance of 34.7% long-term survivors, but one explanation is that an immune-mediated effect – as seen in the lung cancer Checkmate trials of nivolumab – may be driving a minority of patients to longer disease control and hence survival.
It is clear that not all tumors respond to immunotherapy. However, a strong correlation between tumor-infiltrating CD8+ T cells and favorable disease prognosis serves as a powerful indirect evidence for immune involvement in MPM.26 Although our study was not statistically powered to analyze correlation between T cell infiltration and clinical benefit, we observed a trend for more CD8 infiltration in patients with longer survival. Despite these observations, to date, trials testing a variety of immunotherapeutic approaches in MPM have reported only modest clinical outcomes. For example, the anti CTLA-4 inhibitor Tremelimumab showed no improvement in overall survival compared to placebo as a later-line therapy in patients with pleural and peritoneal malignant mesothelioma.27 In the single arm Keynote 028 trial, the anti PD-L1 antibody Pembrolizumab reported more encouraging results, with a median PFS of 5.8 months and a disease control rate of 76% in MPM patients previously treated with chemotherapy.28
The biggest limitation of the SKOPOS trial is the single arm design with no control group; no definitive conclusions can be drawn when comparing the clinical outcomes from SKOPOS with published historical controls of patients treated with chemotherapy alone. However, we did show that pemetrexed-cisplatin chemotherapy alone elicited a 5T4 immune response (doubling of antibody response) in only 1/29 (3.4%) lung cancer and mesothelioma patients so we are confident that the immunological activity seen in the SKOPOS trial is predominantly due to the TroVax® vaccine. Another observation we made, albeit only in 3/23 of samples, that patients with sarcomatoid MPM were just as able to mount systemic 5T4 immune responses following the vaccine treatment as the cohort with epithelioid tumors. However, the pre-treatment tissue samples revealed low antigen expression and weak T cell infiltration into the tissue, indicating more powerful immunosuppression or at least lack of immune support in the tumor microenvironment of these patients, potentially contributing to the more rapid disease progression.
Predictive biomarkers are important in providing bespoke cancer treatment for patients. The results from SKOPOS suggest that inducing immune responses against the 5T4 tumor antigen alone does not select patients more likely to have a longer survival – almost all patients in the study developed such an immune response. We have carried out retrospective data-analysis to look for possible differences between those surviving >20 m vs. <20 m. We observed a high CD8:CD4 T cell ratio in long-term survivors, consistent with the positive prognostic significance of CD8+ T cells in MPM patients.26 We also identified elevated levels of CD4+ naïve T cells in the short-term survivor group of patients. This may indicate that MHC Class II antigen presentation was suboptimal in these patients. Although naïve T cells exposed to high levels of TGFβ in the plasma are easily hijacked to become Tregs,29 we did not observe significant differences in Treg frequencies in the circulation of these patients. However, we have not studied the intratumoral frequencies of Tregs that have a known prognostic significance.7
Again, no differences were observed in classical monocyte frequencies (CD14+ HLA-DR+), or in the frequencies of cells expressing M-MDSC, G-MDSC or E-MDSC markers.30
Given the disappointing results of numerous cancer vaccine trials, it seems unlikely that a vaccine alone will have a significant impact on solid tumors, although this type of treatment may be beneficial for some patients. While the combination of a vaccine and chemotherapy may not be optimal, as chemotherapy may damage expanding T cell populations, the combination of a vaccine and a checkpoint inhibitor may lead to synergistic results. Checkpoint inhibitors may also overcome potentially inhibitory signalling pathways that switch off T-cells and dampen the immune response to the tumor antigen. We believe the SKOPOS trial participants are representative of a significant proportion of newly diagnosed MPM patients in the UK, and the results of any subsequent clinical trials using combination treatment schedules would be broadly applicable.
Materials and methods
Study design and participants
We undertook an open-label, single-arm phase II trial (SKOPOS) at Velindre Cancer Centre, Cardiff, UK. Patients with locally advanced or metastatic, histologically or cytologically confirmed, MPM were potentially eligible for the trial. Inclusion criteria included age ≥18 years, World Health Organization (WHO) performance status (PS) 0–1 and an estimated life expectancy of at least six months. Hematological inclusion criteria were hemoglobin ≥10 g/dL, total white cell count ≥3 × 109/L, neutrophil count >1.5 × 109/L, lymphocyte count ≥0.8 × 109/L, monocyte count <1 × 109/L and platelet count 100–500 × 109/L. Patients also had to have adequate renal and liver function.
Exclusion criteria included major surgery, serious infection or radiotherapy (superficial radiotherapy to chest wall sites was permitted) in the four weeks prior to trial entry, previous TroVax® or chemotherapy treatment, cerebral metastases or history of allergic response to previous vaccinia vaccinations.
All patients provided written informed consent before enrolment. Approval from a UK Research Ethics Committee, (Ref: GTAC174), and Medicines and Health Care Products regulatory Committee (EudraCT2010-023230-22) was obtained. The trial was coordinated by the Wales Cancer Trials Unit at Cardiff University and sponsored by Velindre NHS Trust. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the ICH Good Clinical Practice, and registered with ClinicalTrials.gov (NCT01569919).
In order to establish that chemotherapy alone does not elicit an immune response, an independent pre-study was also conducted, in which blood samples from 27 lung cancer and MPM patients were tested for antibody responses to the 5T4 antigen before and after pemetrexed-cisplatin chemotherapy.
Procedures
As summarized on the CONSORT flow diagram (Fig. 1), eligible patients received TroVax® by intramuscular injection at a dose of 1 × 109 TCID50/mL in 1 mL, given on Day 1 or 2 of weeks 1, 3, and then every three weeks to week 24. Patients also received 4 cycles of Pemetrexed (500 mg/m2 over 10 min) and Cisplatin (75 mg/m2 over 1 h), given on day 3 or 4, from week 4. Folic acid, vitamin B12 and corticosteroids were administered according to protocol.
A baseline CT scan of the chest and upper abdomen had to be undertaken with documentation of known measurable or evaluable disease parameters using the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria for mesothelioma.20 WHO PS and toxicity were collected at baseline according to the Common Terminology Criteria for Adverse Events (v4.02). Tumor response was assessed with CT at week 16, 26, 39 and 52 weeks and with RECIST V1.1. Toxicity assessments were carried out at week 4, 7, 10, 13, 16, 26 and 34 with serious adverse events being collected in real time.
Blood sample collection and processing
Venous blood samples were collected into EDTA vacutainers (Greiner #455036) at six time points for immunological testing: baseline (80 mL), week 4, 7, 10, 13, 26 and 34 (50 mL each). PBMC were isolated by density gradient centrifugation on Histopaque (Sigma #H8889), within 30 min of collection. The plasma was frozen in 10 cryovials (Sarstedt #72.377) in 0.5 mL aliquots at −80°C. PBMC were frozen in freezing media comprising 10% DMSO (Sigma #D2650), 20% FBS (Gibco, #26140-079 lot #1233760; batch-tested for low T cell mitogenicity) in RPMI 1640 media (Sigma #BE12-167F), supplemented with 2 mM L-Glutamine (Lonza #BE17-605E), 100 U/ml penicillin/100 μg/mL streptomycin (Lonza #BE17-603E), 25 mM HEPES (Sigma #H0887) and sodium pyruvate (Sigma #S8636). PBMC were frozen as one 2.6 × 107 vial, with the remaining cells in around 1.3 × 107 cells per vials at −80°C in a CoolCell container (VWR) overnight, before transferring to vapor phase of liquid nitrogen. T cell assays were carried out when all longitudinal samples were obtained.
Measurement of 5T4-specific T cell responses
The intracellular cytokine staining protocol used to assess 5T4 specific T cell responses was optimized prior to the trial21 (and unpublished work). 5T4 peptides (42 in total) spanning the entire 5T4 protein sequence (15-mers, overlapping by 5 amino acids) were synthesised by ProImmune at >85% purity. They were reconstituted in DMSO at a concentration of 10 mg/mL and stored in −20°C. Peptides were pooled into 4 separate groups (peptide pools 1–10, 11–20, 21–31 and 32–42) prior to use. Pools of viral peptides (Class I and II epitopes) representing cytomegalovirus, Epstein-Barr and influenza virus (CTL #CTL-CEF-001 and #CTL-CEFT-001) epitopes were used as positive and DMSO as negative control. Frozen PBMC samples were thawed and viability determined with trypan blue (Sigma #T8154) staining. Any samples with less than 85% viability were not used. PBMC (8 × 105–106) were seeded in 48-well plates (Greiner #677180) in 1 mL supplemented RPMI containing 10% FBS. 5T4 peptide pools (20 μg/mL), viral peptide pool (5 μg/mL) and DMSO (1 μL/mL; negative control) were added to relevant wells in addition to 1000 U/mL IFNα (R&D #11101-2), 20 ng/mL IL-1β and 500 U/mL IL-6 (Peprotech #200-01B and 200–06, respectively). Cells were cultured for 6 days then restimulated using autologous B lymphoblastoid cell lines (BLCL) at 20:1 ratio (5 × 104 BLCL to 1 × 106 PBMC). BLCL were loaded with 10 μg/mL of each 5T4 peptide pool, or 2.5 μg/mL viral peptide pool or volume equivalent of DMSO, for 4 h at 37°C. BLCL were co-incubated with the relevant PBMC in FACS tubes (VWR #352054) for 1 h at 37°C prior to adding Golgi Plug (0.5 μL; BD #555029) and Golgi Stop (0.35 μL; BD #554724) in a final volume of 500 μL followed by a further 12–13 h incubation. Cells were washed in PBS (Lonza #BE17-512F) and labelled with 0.5 μL LIVE/DEAD e-Fluor 780 fixable dye (Affymetrix eBioscience #65-0865-14) in 500 μL at 4°C for 30 min. Cells were fixed in 100 μL Affymetrix eBioscience fixation buffer (#00-8222-49) for 15 min at room temperature. Cells were then washed once in PBS, permeabilized in 100 μL 1x permeabilization buffer (Affymetrix eBioscience #00-8333-56) and labelled with 2.5 μL each of CD3 PE-Cy7 (#25-0038), CD4 APC (#17-0049), CD8a PerCP-Cy5.5 (#45-0088), IFNγ PE (#12-7319), TNFα e-fluor 450 (#48-7349) and IL-2 FITC (# 11–7029; all from Affymetrix eBiosciences) antibodies for 40 min at room temperature in the dark. Samples were then washed in staining buffer, resuspended in 230 μL staining buffer and run on a BD FACSVerse flow cytometer which was normalized daily with CS&T bead tracking. Compensation controls were established with cells labelled with one antibody at a time. Data were acquired with BD FACSuite software. Gating for cytokine-producing T cells was carried out by following the Cancer Immunotherapy Consortium's guidelines.22 This was based on an international assay/gating harmonization exercise in which our laboratory took part (Supplementary Fig. 1). All results were audited and raw data can be provided on request.
Measurement of Antibody Responses
5T4- and MVA-specific antibody responses were determined from longitudinal plasma samples using a validated semi-quantitative Enzyme linked immunosorbant assay (ELISA).23
Immune cell phenotyping
PBMC (2 × 105/tube) were labelled in 100 µL staining buffer for T cells, monocytes, myeloid-derived suppressor cells (MDSC) and Treg cells using antibodies detailed in Table S1. Cells were labelled for surface markers for 40 min on ice. Cells in the Treg panel were then fixed, permeabilized and further labelled with Foxp3 FITC and Ki67 APC, according to the manufacturer's instructions. All cells were run on a BD FACSVerse flow cytometer and analysed with Diva 8 software.
Immunohistochemistry
Formalin fixed paraffin embedded (FFPE) sections were prepared. Antigen retrieval was performed on the Dako Omnis platform by heating slides in retrieval solution at 97°C for 30 min then at room temperature and washing in distilled water. Sections for 5T4 (R&D Systems # MAB 4975) underwent high pH 8.5 EDTA antigen retrieval. Sections for CD8 (Dako Agilent mouse monoclonal clone C8/144B) had pH 6.0 citrate antigen retrieval. 5T4 antibody was used at 1:75 dilution of the stock of 0.5 mg/ml. CD8 antibody was in a ready to use formulation. Both 5T4 and CD8 had primary antibody incubations at 37°C (5T4 for 30 min, CD8 for 20 min) with detection by the DAB Omnis kit. Slides were analyzed on a Nikon eclipse E600 light microscope by an experienced mesothelioma pathologist (RLA). A semi-quantitative evaluation of 5T4 staining was made noting either no, mild, moderate, intense membranous expression of surface and deep tumor tissue. A semi-quantitative evaluation of CD8+ T cells was made noting a nil, mild, moderate or plentiful response within tumor or at the tumor-stromal interface. Percentages and patterns of expression were recorded.
Outcomes
The primary outcome measure was pre-defined as a doubling of anti-5T4 immune responses compared to those at baseline at any of the six time points. Secondary outcome measures included the safety and tolerability of TroVax® in combination with pemetrexed/cisplatin, PFS, ORR, and OS. The study also investigated the relationship between immune and clinical responses, the utility of baseline platelet count, monocyte count, hemoglobin levels, NLR and MLR as predictors of treatment benefit. The latter two were also analyzed at the 4-week time point.
Statistical analysis
We used a Fleming's single arm design with the outcome measure of immune response to the 5T4 antigen. If less than 40% of patients demonstrated an increased response from baseline, then we would not pursue further research. If an increased response was seen in 64% or more of patients then this would justify further research in the vaccine in patients with MPM. Setting α = 0.05 (1-sided) and 80% power, 26 participants were required. If a doubling of 5T4 was seen in at least 16 patients, the null hypothesis that the vaccine does not elicit an immune response could be rejected. A per protocol analysis (PPA) was used where a patient had to receive at least the first three TroVax injections and the first cycle of chemotherapy (at full or reduced dose). PFS was calculated from the day of trial entry to the date of first clinical evidence of local progression or death (of any cause). Patients progression-free and alive were censored at the time last seen. Extended survival time was collected from the site beyond the end of the one-year follow-up after obtaining ethical approval. OS was calculated from the date of trial entry to the day of death (any cause). Those still alive were censored at the time last seen. PFS and OS were presented in time to event format using Kaplan-Meier curves with median time and their corresponding 95% CIs. In a planned exploratory analysis a Cox proportional hazard model was used to explore whether baseline platelet count, baseline monocyte count and baseline hemoglobin predict time to progression. The univariate hazard ratios for each predictor are presented with their corresponding p-value. A logistic regression model was used to explore the effect of hemoglobin, hematocrit, soluble mesothelin and baseline 5T4 antibody level on the post-treatment 5T4 antibody response. The primary endpoint and other secondary categorical endpoints (best response by 26 weeks) were presented as % and 95% CIs. Percentage dose intensity (DI) was calculated as total dose received in mg per m2 (m2)/total expected dose x100. All analysis used STATA 14.0.
Supplementary Material
4305_1_supp_79297_p5wvyf.docx
2017ONCOIMM0966R-s01.docx
Funding Statement
June Hancock Mesothelioma Research Fund. Velindre Cancer Centre Stepping Stones Appeal.
Abbreviations
- AE
adverse events
- BLCL
B lymphoblastoid cell line
- DI
dose intensity
- ELISA
Enzyme linked immunosorbant assay
- FBC
full blood count
- FFPE
formalin fixed paraffin embedded
- MDSC
myeloid derived suppressor cells
- MPM
malignant pleural mesothelioma
- MLR
monocyte:lymphocyte ratio
- NLR
neutrophil:lymphocyte ratio
- ORR
objective response rate
- OS
overall survival
- PBMC
peripheral blood mononuclear cells
- PFS
progression free survival
- PPA
per protocol analysis
- PS
performance status
- RECIST
Response Evaluation Criteria in Solid Tumors
- Treg
Regulatory T cells
- WHO
World Health Organization
- WT-1
Wilms tumor antigen-1
Disclosure of potential conflicts of interest
RH is employed by Oxford BioMedica, the manufacturer of TroVax® who supplied the vaccine for this trial. We declare that no other authors have any conflicts of interest.
Acknowledgments
Oxford BioMedica (vaccine lead: Dr Richard Harrop) provided the vaccine for the trial. We thank current and former staff of Velindre NHS Trust and WCTU for supporting the development and running of this trial; the patients' representative; research nurses and pharmacy staff at Velindre Cancer Centre; members of the independent data-monitoring committee and trial steering committee. Finally, we thank all patients who participated in the trial.
Contributors
JL, GG, AC, RH and ZT designed and developed the study and wrote and reviewed the protocol. JL played a part in day-to-day running of the study. LK and AC did statistical analyses. JL reviewed serious adverse events during the study. SA-T and ZT undertook the laboratory work. RLA directed the IHC staining and conducted the tissue analysis. All authors contributed to the writing of the manuscript.
Role of the funding source
The funder and sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. AC and LK had full access to all the data in the study. All authors had final responsibility for the decision to submit for publication.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al.. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. PMID:12860938. [DOI] [PubMed] [Google Scholar]
- 2.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, et al.. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14. doi: 10.1016/S0140-6736(15)01238-6. PMID:26719230. [DOI] [PubMed] [Google Scholar]
- 3.Wong RM, Ianculescu I, Sharma S, Gage DL, Olevsky OM, Kotova S, Kostic MN, Grundfest WS, Hou D, Cameron RB. Immunotherapy for malignant pleural mesothelioma. Current status and future prospects. Am J Respir Cell Mol Biol. 2014;50:870–5. doi: 10.1165/rcmb.2013-0472TR. PMID:24450537. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BW, Robinson C, Lake RA. Localised spontaneous regression in mesothelioma – possible immunological mechanism. Lung Cancer. 2001;32:197–201. doi: 10.1016/S0169-5002(00)00217-8. PMID:11325491. [DOI] [PubMed] [Google Scholar]
- 5.Yamada N, Oizumi S, Kikuchi E, Shinagawa N, Konishi-Sakakibara J, Ishimine A, Aoe K, Gemba K, Kishimoto T, Torigoe T, et al.. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother. 2010;59:1543–9. doi: 10.1007/s00262-010-0881-6. PMID:20567822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ujiie H, Kadota K, Nitadori JI, Aerts JG, Woo KM, Sima CS, Travis WD, Jones DR, Krug LM, Adusumilli PS. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology. 2015;4:e1009285. doi: 10.1080/2162402X.2015.1009285. PMID:26155428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ireland DJ, Kissick HT, Beilharz MW. The Role of Regulatory T Cells in Mesothelioma. Cancer Microenviron. 2012;5:165-72. doi: 10.1007/s12307-012-0100-4. PMID:22302659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy MJ, Nowak AK, van der Most RG, Dick IM, Lake RA. Peripheral CD8(+) T cell proliferation is prognostic for patients with advanced thoracic malignancies. Cancer Immunol Immunother. 2013;62:529-39. doi: 10.1007/s00262-012-1360-z. PMID:23069871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornwall SM, Wikstrom M, Musk AW, Alvarez J, Nowak AK, Nelson DJ. Human mesothelioma induces defects in dendritic cell numbers and antigen-processing function which predict survival outcomes. Oncoimmunology. 2016;5:e1082028. doi: 10.1080/2162402X.2015.1082028. PMID:27057464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–13. doi: 10.1158/1078-0432.CCR-10-2245. PMID:20956618. [DOI] [PubMed] [Google Scholar]
- 11.Tanrikulu AC, Abakay A, Komek H, Abakay O. Prognostic value of the lymphocyte-to-monocyte ratio and other inflammatory markers in malignant pleural mesothelioma. Environ Health Prev Med. 2016;21:304–11. doi: 10.1007/s12199-016-0530-6. PMID:27068290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang A, Cao S, Jin S, Cao J, Shen J, Pan B, Zhu R, Yu Y. Elevated aspartate aminotransferase and monocyte counts predict unfavorable prognosis in patients with malignant pleural mesothelioma. Neoplasma. 2017;64:114–22. doi: 10.4149/neo_2017_114. PMID:27881012. [DOI] [PubMed] [Google Scholar]
- 13.Zauderer GM, Dao T, Rusch V, Ginsberg M, Tsao A, Panageas K, al. e Randomized Phase II study of adjuvant WT1 vaccine (SLS-001) for malignant pleural mesothelioma (MPM) after multimodal therapy. J Clin Oncol. 2016;34:Abstract 8519. [Google Scholar]
- 14.https://clinicaltrials.gov/ct2/show/NCT02716272?term = mesothelioma+nivolumab&rank = 1.
- 15.https://clinicaltrials.gov/ct2/show/NCT02399371?term = mesothelioma+pembrolizumab&rank = 1.
- 16.Hole N, Barton-Hanson N, Berwick S, Stern P. Human trophoblast glycoproteins defined by monoclonal antibody 1D2. Exp Cell Biol. 1988;56:39–48. PMID:3053282. [DOI] [PubMed] [Google Scholar]
- 17.Al-Taei S, Salimu J, Lester JF, Linnane S, Goonewardena M, Harrop R, Mason MD, Tabi Z. Overexpression and potential targeting of the oncofoetal antigen 5T4 in malignant pleural mesothelioma. Lung Cancer. 2012;77:312–8. doi: 10.1016/j.lungcan.2012.03.008. PMID:22498111. [DOI] [PubMed] [Google Scholar]
- 18.Harrop R, Shingler W, Kelleher M, de Belin J, Treasure P. Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother. 2010;33:999–1005. doi: 10.1097/CJI.0b013e3181f5dac7. PMID:20948436. [DOI] [PubMed] [Google Scholar]
- 19.Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, et al.. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–86. doi: 10.1007/s00262-007-0428-7. PMID:18060404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–60. doi: 10.1093/annonc/mdh059. PMID:14760119. [DOI] [PubMed] [Google Scholar]
- 21.Coleman S, Clayton A, Mason MD, Jasani B, Adams M, Tabi Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005;65:7000–6. doi: 10.1158/0008-5472.CAN-04-3792. PMID:16061686. [DOI] [PubMed] [Google Scholar]
- 22.McNeil LK, Price L, Britten CM, Jaimes M, Maecker H, Odunsi K, Matsuzaki J, Staats JS, Thorpe J, Yuan J, et al.. A harmonized approach to intracellular cytokine staining gating: Results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI). Cytometry A. 2013;83:728–38. doi: 10.1002/cyto.a.22319. PMID:23788464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrop R, Shingler WH, McDonald M, Treasure P, Amato RJ, Hawkins RE, Kaufman HL, de Belin J, Kelleher M, Goonewardena M, et al.. MVA-5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother. 2011;60:829–37. doi: 10.1007/s00262-011-0993-7. PMID:21387109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. PMID:26028407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al.. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. PMID:26412456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anraku M, Cunningham KS, Yun Z, Tsao MS, Zhang L, Keshavjee S, Johnston MR, de Perrot M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2008;135:823–9. doi: 10.1016/j.jtcvs.2007.10.026. PMID:18374762. [DOI] [PubMed] [Google Scholar]
- 27.Facchinetti F, Marabelle A, Rossi G, Soria JC, Besse B, Tiseo M. Moving Immune Checkpoint Blockade in Thoracic Tumors beyond NSCLC. J Thorac Oncol. 2016;11:1819–36. doi: 10.1016/j.jtho.2016.05.027. PMID:27288978. [DOI] [PubMed] [Google Scholar]
- 28.Kindler HL, Scherpereel A, Calabro L, Aerts JG, Cedres Perez S, Bearz A. Tremelimumab as second- or third-line treatment of unresectable malignant mesothelioma (MM): Results from the global, double-blind, placebo-controlled DETERMINE study. J Clin Oncol. 2016;34:Abstract 8502. [Google Scholar]
- 29.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. PMID:14676299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al.. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. PMID:27381735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
4305_1_supp_79297_p5wvyf.docx
2017ONCOIMM0966R-s01.docx


