Figure 1.
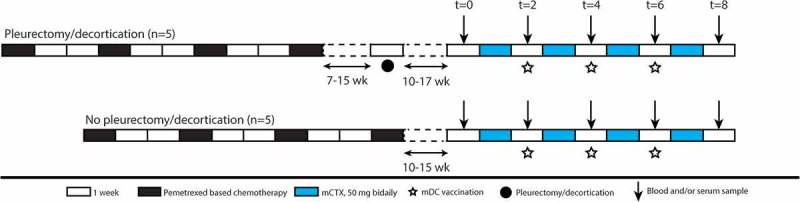
Schematic overview of the clinical trial. Scheme of clinical trial. Patients were included if they had partial response or stable disease after pemetrexed-based chemotherapy. Five patients underwent additional P/D 7–15 weeks after chemotherapy. DC/mCTX therapy started 10–17 weeks after either the last chemotherapeutic treatment or P/D. Blood samples were obtained at t = 0 (baseline); t = 2 (mCTX); t = 4; t = 6; t = 8 (2 wk after DC/mCTX therapy); t = 18.
