Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP) plays an important role in anterior pituitary hormone secretion, neurotransmission, and the control of breathing. Mice lacking PACAP die suddenly mainly in the 2nd postnatal week, coinciding temporally with a critical period of respiratory development uncovered by our laboratory in the rat. The goal of the current study was to test our hypothesis that PACAP expression is reduced during the critical period in normal rats. We undertook immunohistochemistry and optical densitometry of PACAP (specifically PACAP38) in several brain stem respiratory-related nuclei of postnatal days P2–21 rats, and found that PACAP immunoreactivity was significantly reduced at P12 in the pre-Bötzinger complex, nucleus ambiguus, hypoglossal nucleus, and the ventrolateral subnucleus of the nucleus tractus solitarius. No changes were observed in the control, non-respiratory cuneate nucleus at P12. Results imply that the down-regulation of PACAP during normal postnatal development may contribute to the critical period of vulnerability, when the animals’ response to hypoxia is at its weakest.
Keywords: Critical period, hypoglossal nucleus, immunohistochemistry, nucleus ambiguus, PACAP, pre-Bötzinger complex
1. Introduction
The pituitary adenylate cyclase-activating polypeptide (PACAP) is a neuropeptide isolated originally from an extract of ovine hypothalamus (Miyata et al., 1989, 1990). Its N-terminal sequence (1–18) shares 68% homology with the vasoactive intestinal polypeptide (VIP), but it is a thousand times more potent than VIP in stimulating adenylate cyclase in the anterior pituitary, hence its name (Miyata et al., 1989). PACAP has 2 bioactive forms, the C-terminus α-amidated 38 residue peptide, PACAP38, and the N-terminus 27 residue one, PACAP27 (Miyata et al., 1989, 1990). In the rat brain, PACAP38 constitutes the major portion of total PACAP immunoreactivity, whereas the level of PACAP27 is negligibly low (Masuo et al., 1993). PACAP-positive nerve fibers are found throughout the brain as well as in many body organs (reviewed in Arimura, 1992). Besides its action on the anterior pituitary, PACAP is also implicated in neural development, neurotransmission, neuroprotection, neuromodulation, metabolic homeostasis, as well as transcription at central and peripheral targets (Runcie et al., 1995; Morio et al., 1996; Liu et al., 2000; Vaudry et al., 2000).
A role of PACAP in the control of breathing was suspected when its distribution was found in the respiratory tract and several respiratory-related nuclei in the brain stem (Uddman et al., 1991; Légrádi et al., 1994; Kausz et al., 1999). PACAP powerfully stimulates breathing via its activation of arterial chemoreceptors (Runcie et al., 1995), and mice deficient in the PACAP gene die suddenly after birth, but the mortality “does not peak until the second week” (Gray et al., 2001; Wilson and Cummings, 2008), due mainly to reduced respiratory chemoresponse and apnea (Cummings et al., 2004). The abruptness of neonatal death is reminiscent of the human Sudden Infant Death Syndrome (SIDS).
A critical period of respiratory development has been proposed as a major risk factors for SIDS (Filiano and Kinney, 1994). In rats, this period has been found by our laboratory to exist during the 2nd postnatal week (P12–13), when sudden neurochemical changes occur and when the animals’ response to hypoxia is at its weakest (reviewed in Wong-Riley and Liu, 2008). The exact timing of the critical period in mice is not well delineated. In general, however, mice are slightly more precocious than rats in brain development by about 1–2 days (Clancy et al., 2001). The apparent temporal correlation between the peak of PACAP deficiency-induced death in mice and the critical period in rats is intriguing.
The goal of the present study was to test our hypothesis that the level of PACAP is reduced during the critical period of respiratory development in normal rats. We undertook an in-depth immunohistochemical study of PACAP during the first 3 postnatal weeks in several respiratory-related nuclei, such as the pre-Bötzinger complex (PBC), nucleus ambiguus (Amb), the hypoglossal nucleus (XII), and the ventrolateral subnucleus of the nucleus tractus solitarius (NTSVL). The non-respiratory cuneate nucleus (CN) served as a control brain stem nucleus.
2. Methods
2.1. Animals
All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80–23, revised 1996), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee (approval can be provided upon request). All efforts were made to minimize the number of animals used and their suffering.
A total of 96 Sprague-Dawley rats of both sexes (parents purchased from Taconic Biosciences, Germantown, NY) from 8 litters were used in this study. Rat pups were sacrificed at each of postnatal days P2, 3, 4, 5, 7, 10, 11, 12, 13, 14, 17, and 21. They were deeply anesthetized with 0.6% sodium pentobarbital (60 mg/kg IP; Diamondback Drugs, Scottsdale, AZ) and perfused through the aorta with 4% paraformaldehyde-4% sucrose in 0.1 M sodium phosphate buffered saline (PBS), pH 7.4. Brain stems were then removed, postfixed in the same fixative for 3 h at 4°C, cryoprotected by immersion in increasing concentrations of sucrose (10, 20, and 30%) in 0.1 M PBS at 4°C, then frozen on dry ice and stored at −80°C until use.
2.2. Immunohistochemistry
The primary antibody used was a rabbit polyclonal anti-PACAP-38 antiserum (T-4473, RRID: AB_519166; Bachem/Peninsula Lab International, Inc., San Carlos, CA). According to the manufacturer, the antigen was the C-terminal end of the PACAP-38 peptide, with the amino acid sequence of HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRVKNK-NH2. They used radioimmunoassay to demonstrate that this antiserum binds to the C-terminal end of PACAP-38 and shows no cross-reactivity with vasoactive intestinal polypeptide, corticotropin-releasing hormone, or adrenocorticotrophic hormone, and only 0.01% cross-reactivity with PACAP-27. Das et al. (2007) performed preadsorption of this antiserum with “10 μM concentration of synthetic PACAP38 (American Peptide Company; catalog 34–0-20; lot T11107T1), molecular weight 4534.3”, and found that it “completely abolished immunoreactivity”.
Coronal sections (12-μm thickness) of frozen brain stems were cut with a Leica CM1850 cryostat (Leica Microsystems, Heidelberger, Nussloch, Germany). Individual sets of serial sections were mounted on gelatin-coated slides. In the same litter, sections from 3 rats at different ages were mounted on the same slides and processed together. Ages were grouped typically as follows: P2–10-21, P3–4-17, P5–7-14, and P11–12-13. All sections from all rats were processed under identical conditions (i.e., time, temperature, and concentration of reagents). They were blocked overnight at 4°C with 5% nonfat dry milk (#170–6404, Bio Rad, Hercules, CA)-5% normal goat serum (Chemicon International, Temecula, CA)-1% Triton X-100 (T9284, Sigma, St. Louis, MO) in 0.1 M PBS (pH 7.4). Sections were then incubated at 4°C for 36 h in the rabbit anti-PACAP-38 primary antibodies diluted at 1:600 in the same solution as used for blocking. Sections were rinsed 3 times, 5 min each, in PBS, then incubated in the secondary antibodies: 1:100 goat anti-rabbit IgG-HRP (Bio-Rad) diluted in the modified blocking solution (without Triton X-100) for 4 h at room temperature. After rinsing twice with PBS and once with 0.1 M ammonium phosphate buffer (APB), pH 7.0, immunoreactivity was detected with 0.05% DAB-0.004% H2O2 in APB for 5 min. The reaction was then stopped with APB for 5 min, rinsed in PBS three times, dehydrated, and coverslipped. Control sections were processed without primary antibodies or with a non-immune serum in place of the primary antibodies. All routine chemicals were from Sigma.
2.3. Semi-quantitative optical densitometry
The immunoreactivity of PACAP in the cell bodies of individual neurons in various nuclei was semi-quantitatively analyzed by optical densitometry performed with a Zeiss Zonax MPM 03 photometer, a ×25 objective, and a 2-μm-diameter measuring spot centered on the cytoplasm of labelled neurons. All lighting conditions were held constant for all of the measurements as described previously (Liu and Wong-Riley, 2002).
The boundaries of the PBC, Amb, XII, NTSVL, and CN were as described previously (Liu and Wong-Riley, 2002, 2005). We used Paxinos and Watson’s “The Rat Brain Atlas” (New York: Academic Press, 1986) as a reference, and the PBC was also identified with the neurokinin-1 receptor labeling (Gray et al., 1999), with its location delineated according to the detailed description of Smith et al. (1991). It is located in the mid portion of the rostral-caudal axis of the ventrolateral medulla, ventral to the nucleus ambiguus, and between the rootlets of the hypoglossal nerve and the vagus nerve. The rostrocaudal extent of the PBC is 300–480 μm from P0 to P21 in the rat (Liu and Wong-Riley, 2002). The part of the nucleus ambiguus chosen was the semicompact formation and the rostral loose formation innervating the upper airway muscles with pharyngolaryngomotor functions (Bieger and Hopkins, 1987). For the remaining nuclei, measurements were taken from the central main portion of each nucleus.
The optical densitometric value of each labeled neuron in the various brain stem nuclei was an average measurement of two to four spots on the cell body (excluding the nucleus). Only those neurons whose nuclei were clearly visible (i.e., sectioned through the middle of the cell body) were measured. To avoid measuring the same neuron more than once, values were taken from cells in sections at least 84 μm apart, as the largest neurons had a maximal diameter of 2530 μm, with a maximal nuclear diameter of only about 10 μm. Between 60 to 100 neurons in each brain stem nucleus in each of 8 rats were measured at each age, making a total of 500–800 neurons at each age for each nucleus. Mean optical density values, standard deviations, and standard errors of the mean in each nucleus at each age were then obtained.
2.4. Statistical analyses
Statistical comparisons were made among the age groups by using one-way analysis of variance (ANOVA) (to control for the type I comparison-wise error rate) and, when significant differences were found, comparisons were made first between successive age groups (e.g., P2 vs. P3, P3 vs. P4) and later between two selected age groups by means of Tukey’s Studentized range test (a post hoc multiple comparisons, to control for the type I experiment-wise error rate). The N was based on the number of animals (8) in each age group, and not on the number of neurons analyzed (in the hundreds). Significance was set at P < 0.01 for one-way ANOVA and P < 0.05 for Tukey’s test.
3. Results
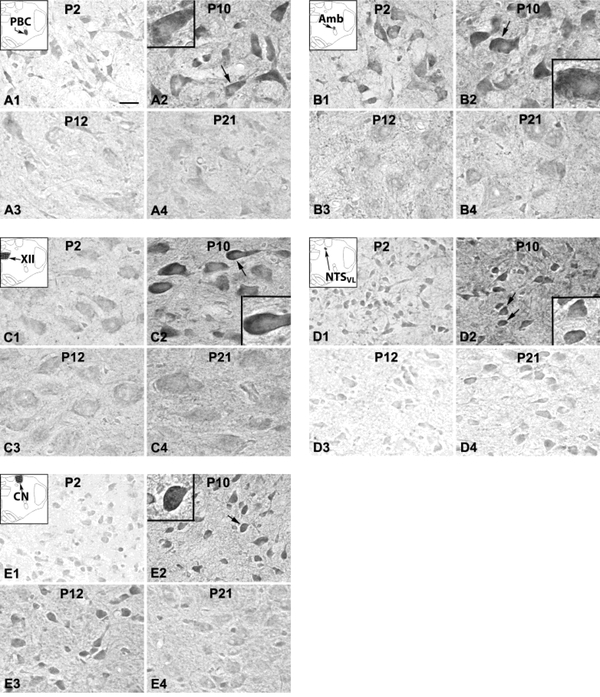
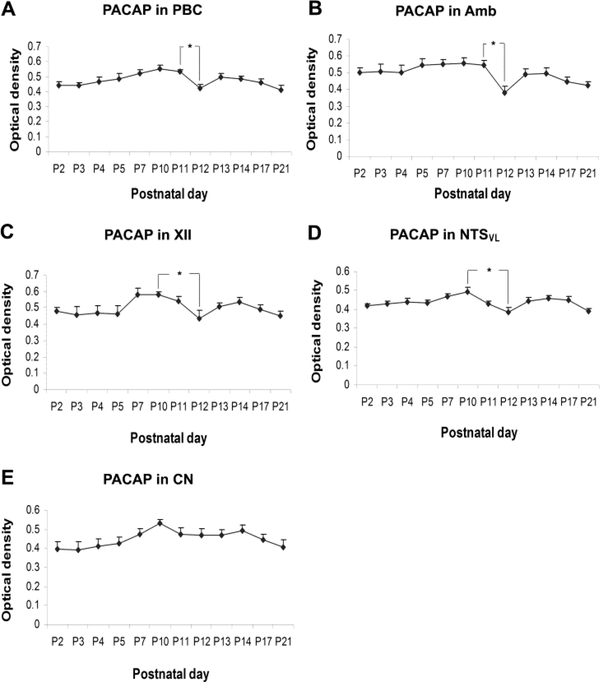
PACAP immunoreactivity (ir) was clearly visible in subpopulations of neurons in all five brain stem nuclei examined (Fig. 1). Immunoreaction product was present in the cytoplasm and proximal dendrites of labeled neurons as well as in the neuropil (including dendritic processes). Plasma membrane labeling was also observed in about 5 – 10% of PACAP-ir neurons from P2 to P11 (see all insets for P10 in Fig. 1), but was less distinct thereafter. Control sections without any primary antibodies had no specific immunoreactivity above background (data not shown). ANOVA indicated significant differences (P < 0.01) in PACAP-ir neurons among the ages in the PBC, Amb, XII, and NTSVL, but not in the CN. Tukey’s Studentized range test that compared one age group with its adjacent younger age group revealed a significant fall in immunoreactivity at P12 in the PBC and Amb when compared to that at P11 in both nuclei (P < 0.05). The fall was more gradual for the XII and NTSVL from P10 through P11 to P12, hence Tukey’s test showed a significant difference between P10 and P12 in these two nuclei (P < 0.05) (Fig. 2). No significant fall was observed in the CN at any age during the first three postnatal weeks.
Figure 1.
PACAP-ir neurons and neuropil in the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) at P2 (A1 – E1), P10 (A2 – E2), P12 (A3 – E3), and P21 (A4 – E4). In the PBC, XII, and NTSVL, PACAP immunoreactivity was relatively low at P2, markedly increased at P10, then significantly reduced at P12, followed by a rise and a plateau at P21. The level of immunoreactivity in Amb was relatively high from P2 to P11, but was significantly reduced at P12, followed by a rise and a plateau at P21. PACAP expression in the CN was relatively low at P2, followed by a gradual climb to a peak level at P10, then remained constant until P21. In about 5 – 10% of labeled neurons, the plasma membrane was also clearly labeled at P2 (not shown) and at P10 (arrows in A2 to E2, with higher magnifications shown in respective insets), but was less distinct thereafter. The insets at the upper left corners of A1 -E1 are schematic diagrams of the location of each of the five brain stem nuclei. Scale bar in A1: 20 μm for all low magnifications, and 8.33 μm for all high magnifications in insets.
Figure 2.
Optical densitometric measurements of PACAP immunoreaction product in individual neurons of the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) from P2 to P21. Data points represent the mean ± SEM. ANOVA revealed significant differences among the ages in the PBC, Amb, XII, and NTSVL (P < 0.01), but not in the CN. Tukey’s Studentized tests showed a significant drop in immunoreactivity from P11 to P12 in the PBC and Amb, and from P10 to P12 in the XII and NTSVL. Other significant differences are indicated in the text. *P < 0.05.
3.1. PACAP-ir neurons in the Pre-Bötzinger complex (PBC)
PACAP-ir was observed in ~ 40% - 45% of the PBC neurons. They were multipolar, granular, or fusiform in shape and small and medium in size (Fig. 1A). The size of small neurons ranged from 4.5 to 7 μm in diameter at P2 to 7 – 10 μm at P21, and medium-sized neurons ranged from 8.5 – 12 μm at P2 to 12 – 23 μm at P21. The expression of PACAP increased gradually from P2 to P10 (P < 0.05 for Tukey’s test between P2 and P10) and plateaued at P11. It then fell significantly at P12 (P < 0.05) followed by a rise (P > 0.05) at P13 and a gradual decline until P21 (Fig. 2A). P12 was the only time point in the first 3 postnatal weeks when a day-to-day significance was found. Tukey’s test also revealed that the value at P10 was significantly higher than those at P3, P12, and P21 (P < 0.05 for all).
3.2. PACAP-ir neurons in the nucleus ambiguus (Amb)
About 65% - 70% of Amb neurons demonstrated PACAP-ir. These neurons were multipolar or oval in shape and mainly medium or small in size (Fig. 1B). The size of small neurons ranged from 6.5 to 8 μm in diameter at P2 to 8 – 13 μm at P21, and medium-sized neurons ranged from 12 – 15.5 μm at P2 to 15.5 – 22 μm at P21. Occasionally, a few of the large neurons (24 – 28 μm in diameter) could be observed at P21. PACAP-ir exhibited a relative plateau from P2 to P11, then a significant fall at P12 (P < 0.05) followed by a slight rise at P13 and a plateau thereafter (Fig. 2B). Tukey’s test also yielded significant differences between P12 and each individual day from P5 to P10 (P < 0.05 for all).
3.3. PACAP-ir neurons in the hypoglossal neurons (XII)
PACAP-ir was present in ~ 70% - 90% of XII neurons. They were multipolar, oval, or fusiform in shape and mainly medium or large in size (Fig. 1C). Medium-sized neurons ranged from 10.5 to 14 μm in diameter at P2 to 13.5 – 21 μm at P21, and large neurons ranged from 16 – 21.5 μm at P2 to 24.5 – 28.5 μm at P21. The developmental trend of PACAP-ir exhibited a plateau from P2 to P5, then a nonsignificant rise at P7 followed by a decline from P10 to P12, a slight rise at P13 and P14, and finally a slight decline at P17 and P21 (Fig. 2C). Tukey’s test revealed a significant decrease in immunoreactivity from P10 to P12 and from P7 to P12 (P < 0.05 for both) (Fig. 2C).
3.4. PACAP-ir neurons in the ventrolateral subnucleus of the nucleus tractus solitarius (NTSVL)
PACAP-ir was observed in about 30% - 40% of the NTSVL neurons. They were multipolar, granular, oval, or fusiform in shape and mainly small in size (Fig. 1D). The small neurons ranged from 5 – 7.5 μm in diameter at P2 to 7 – 12 μm at P21. Occasionally, a few medium-sized neurons (14.5 – 18 μm in diameter) could be observed at P17 and P21. The expression of PACAP in the NTSVL showed a plateau from P2 to P5, then a slight rise at P7 and P10 followed by a decline at P11 and again at P12, a slight rise at P13 and P14 and a mild decline at P17 and P21 (Fig. 2D). Tukey’s test revealed a significant fall in immunoreactivity from P10 to P12, as well as from P7 to P12 (P < 0.05 for both) (Fig. 2D).
3.5. PACAP-ir neurons in the cuneate nucleus (CN)
About 50% - 70% of neurons in the CN demonstrated PACAP-ir (Fig. 1E). These labeled neurons were oval, multipolar, or granular in shape and mainly small in size. Small neurons ranged from 5 – 7.5 μm in diameter at P2 to 7.5 – 11.5 μm at P21, and medium-sized labeled neurons ranged from 9.5 – 12 μm at P2 to 14 – 18.5 μm at P21. The expression of PACAP was relatively low at P2, then increased gradually to peak at P10, followed by a relative plateau until P21 (Fig. 2E). Tukey’s test did not show any significant difference between any two age groups.
4. Discussion
The present study documented that the expression of PACAP (indicated by PACAP38 immunoreactivity) remained relatively stable throughout the first three postnatal weeks in multiple brain stem nuclei in the rat, except for a distinct fall at P12 in four respiratory-related nuclei (PBC, Amb, NTSVL, and XII), but not in the non-respiratory CN. The fall was more abrupt from P11 to P12 in the PBC and Amb, but was more gradual from P10 through P11 to P12 in the NTSVL and XII. Tukey’s test showed a significant difference between P11 and P12 in the PBC and Amb, and between P10 and P12 in the NTSVL and XII (P < 0.05 for all). On the other hand, the expression of PACAP in the non-respiratory CN remained at a relative plateau throughout the first three postnatal weeks.
The PBC is part of the rostroventral respiratory group and is a medullary kernel for respiratory rhythmogenesis (Smith et al., 1991; 2000; Rekling and Feldman, 1998). The Amb receives input from the central respiratory network and innervates upper airway muscles (larynx and pharynx) (Nunez-Abades et al., 1992; Jordon, 2001). The NTSVL is a key subnucleus of NTS, a major representation of the dorsal respiratory group that receives direct projections from the carotid body (Hilaire et al., 1990; Finley and Katz, 1992). The XII innervates the tongue and pharyngeal muscles to keep the airway patent during respiration (Lowe, 1980). There are many interconnections between and among these as well as other respiratory-related nuclei in the brain stem (Wong-Riley and Liu, 2013). The fact that the level of PACAP is relatively high among these nuclei throughout the first three neonatal weeks indicates that this neuropeptide is involved in the normal functioning of these neurons. Intriguingly, the fall at P12 occurs only in respiratory-related, but not in the non-respiratory, nuclei.
PACAP was first recognized as a potent activator of adenylate cyclase in anterior pituitary cell cultures (Miyata et al., 1989). Later, it was found to also modulate glutamatergic, catecholaminergic, and cholinergic neurotransmission, with neuroprotective and homeostatic functions (Hannibal et al., 2000; Liu et al., 2000; Hamelink et al., 2002; MacDonald et al., 2005). Its role in the control of breathing is validated both physiologically via its stimulation of arterial chemoreceptors and the maintenance of normal pulmonary vascular tone (Runcie et al., 1995; Vaudry et al., 2009), as well as anatomically by its widespread distribution throughout the respiratory tract and brain stem respiratory-related nuclei (Uddman et al., 1991; Dun et al., 1996; Kausz et al., 1999). PACAP38 is the major contributor to the total PACAP immunoreactivity in the brain, with negligible amount from PACAP27 (Masuo et al., 1993). Our findings of robust PACAP38 immunoreactivity in the PBC, Amb, NTSVL, and XII are consistent with previous reports, and highlight PACAP’s presence during the first three postnatal weeks of brain stem development in the rat.
A striking feature of PACAP is that, in its absence, the mice experience sudden death peaking during the second postnatal week (Gray et al., 2001; Wilson and Cummings, 2008). The null animals have blunted responses to both hypoxia and hypercapnia, and their baseline ventilation is significantly reduced even at P4 (Cummings et al., 2004). In another study, PACAP−/− mice exhibit respiratory arrest to hypoxia at P7, but their response to hypercapnia is the same as controls (Arata et al., 2013). The high mortality rate is attributable to defects in respiratory control (Cummings et al., 2004; Arata et al., 2013). With mild reduction in the ambient temperature, the PACAP knockout mice are more prone to prolonged apnea, and their mortality rate is significantly increased, especially during the second postnatal week (Cummings et al., 2008; Wilson and Cummings, 2008). The unexpected and abruptness of death is reminiscent of Sudden Infant Death Syndrome (SIDS). Indeed, mutations in the PACAP gene have been proposed as one of the causes of disrupted homeostatic control of cardiovascular, respiratory, and metabolic functions, as well as eventual death in SIDS victims (Cummings et al., 2003, 2004; Prandota, 2004). Evidence of some abnormality in the PACAP system has also been reported recently in SIDS brains (Huang et al., 2017a), and PACAP gene polymorphism has been associated with SIDS in African Americans (Cummings et al., 2009; Barrett et al., 2013). Moreover, piglets exposed to acute intermittent hypercapnic hypoxia exhibit reduced levels of PACAP in their brain stem nuclei (Huang et al., 2017b).
The temporal coincidence of death peaking during the second postnatal week in PACAP null mice and a critical period of respiratory development in the rat that our laboratory has uncovered is particularly intriguing. A critical period of postnatal development has been proposed by Filiano and Kinney (1994) as one of the three risk factors for SIDS. The underlying mechanism was poorly understood. By undertaking daily studies of the first three postnatal weeks of development in the rat, we found that significant neurochemical, ventilatory, and electrophysiological changes occur in the brain stem respiratory system toward the end of the second postnatal week, at P12–13, during which the animals’ response to acute hypoxia is at its weakest (reviewed in Wong-Riley and Liu, 2005, 2008, 2013). Specifically, the expressions of excitatory neurochemicals (glutamate and NMDA receptor subunits 1 and 2A) and the metabolic marker cytochrome oxidase plummet, while those of inhibitory neurochemicals (GABA, GABAB receptors and glycine receptors) surge in multiple brain stem respiratory-related nuclei within the narrow window (Liu and Wong-Riley, 2002, 2003, 2005, 2010c). An imbalance between heightened inhibition and suppressed excitation is also evident electrophysiologically in XII and NTSVL during that time (Gao et al., 2011, 2015). When exposed to acute hypoxia (10% oxygen for 5 min), the hypoxic to normoxic ratios for frequency (f), tidal volume (VT) and minute ventilation (VE) all exhibit a sudden and significant fall at P13 (Liu et al., 2006), as does the metabolic rate (Liu et al., 2009). In addition, the expressions of several serotonergic neurochemicals are downregulated at P12, and they either return to P11 values or remain low until P21 (Liu and Wong-Riley, 2008, 2010a,b). There is an apparent switch in receptor subunits for GABAA from the neonatal α3 to the more mature α1 (Liu and Wong-Riley, 2004, 2006), for the glycine receptors from the neonatal GlyRα2/α3 to the adult GlyRα1 (Liu and Wong-Riley, 2013b), and for the NMDA receptors from GluN2D with low-conductance to GluN3B with faster kinetics and reduced Ca2+ permeability around the same time (Liu and Wong-Riley, 2010c). There is also a switch in dominance from the Cl- importer Na+-K+−2Cl- cotransporter 1 (NKCC1) to the Cl- exporter K+-Cl- cotransporter 2 (KCC2) around P12 (Liu and Wong-Riley, 2012). These changes all help to enhance inhibition in the respiratory network at P12–13, the critical period of respiratory development in the rat (Wong-Riley and Liu, 2005, 2008, 2013).
In probing for the basis of the underlying inhibitory-excitatory imbalance further, we found that the expressions of brain-derived neurotrophic factor (BDNF) and its high-affinity receptor (TrkB) are significantly reduced in a number of brain stem respiratory-related nuclei at P12 (Liu and Wong-Riley, 2013a). BDNF normally enhances glutamatergic neurotransmission and attenuates GABAergic and glycinergic ones (Wardle and Poo, 2003; Bardoni et al., 2007). Its downregulation during the critical period would contribute to the inhibitory-excitatory imbalance within the respiratory network. Moreover, BDNF plays an important role in the development of normal respiratory rhythm and ventilatory control, and BDNF knockout mice exhibit abnormal respiratory pattern and apnea, dying typically within 1 – 2 weeks of birth (Erickson et al., 1996; Balkowiec and Katz, 1998). Significantly, PACAP is known to stimulate BDNF expression by potentiating glutamatergic action (Pelligri et al., 1998), and PACAP directly enhances synaptic NMDA and evoked NMDA receptor currents (MacDonald et al., 2005). The fact that NMDA receptors, BDNF, and PACAP in multiple brain stem respiratory-related nuclei are all downregulated during the critical period in the rat indicates that the three neurochemicals are positively correlated to bring about suppressed excitation and, in the case of reduced BDNF, enhanced inhibition as well. Whether PACAP plays a leading role in initiating the inhibition-excitation imbalance remains to be determined.
Is a critical period inevitable during normal respiratory development? Carotid body denervation simply postpones and delays, but not eliminate the critical period in the rat (Liu et al., 2003). Thus, this period seems to be innately determined. One possible rationale for the existence of this period is that excitatory synapses develop and mature earlier than inhibitory ones (Gao et al., 2011), but refinement of this system requires the growth and maturation of inhibitory synapses. BDNF and PACAP are needed for the initial growth and development of excitatory neural connections, but their levels have to be briefly curtailed to allow for the full maturation of inhibitory synapses. Thus, the resulting abrupt, transient period of inhibitory-excitatory imbalance may be necessary for the proper development of the respiratory system. Both BDNF and PACAP play important roles. Indeed, an agonist of TrkB receptors partially reverses, but a TrkB antagonist accentuates, the imbalance during the critical period (Gao et al., 2014, 2015). Whether agonist and antagonist of PACAP or its receptors would affect the imbalance awaits future investigation.
Highlights.
Pituitary adenylate cyclase-activating polypeptide is known to control breathing.
A critical period of respiratory development exists in rats at postnatal days 12–13.
PACAP immunoreactivity is markedly reduced at P12 in brain stem respiratory nuclei.
PACAP down-regulation at P12 may contribute to vulnerability in the critical period.
Acknowledgments
This work was supported in part by the National Institutes of Health grant R01 HD048954.
Abbreviations:
- XII
hypoglossal nucleus
- Amb
nucleus ambiguous
- CN
cuneate nucleus
- NTSVL
nucleus tractus solitarius ventrolateral subnucleus
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PBC
pre-Bötzinger complex
- VIP
vasoactive intestinal polypeptide
Footnotes
Declaration of interests
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arata S, Nakamachi T, Onimaru H, Hashimoto H, Shioda S, 2013. Impaired response to hypoxia in the respiratory center is a major cause of neonatal death of the PACAP-knockout mouse. Eur. J. Neurosci 37, 407–416. [DOI] [PubMed] [Google Scholar]
- Arimura A, 1992. Pituitary adenylate cyclase activating polypeptide (PACAP): Discovery and current status of research. Regul. Pept 37, 287–303. [PubMed] [Google Scholar]
- Balkowiec A, Katz DM, 1998. Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J. Phyiol 510 (Pt 2), 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Salio C, Prandini M, Merighi A, 2007. BDNF-mediated modulation of GABA and glycine release in dorsal horn lamina II from postnatal rats. Dev. Neurobiol 67, 960–975. [DOI] [PubMed] [Google Scholar]
- Barrett KT, Rodikova E, Weese-Mayer DE, Rand CM, Marazita ML, Cooper ME, BerryKravis EM, Bech-Hansen NT, Wilson RJA, 2013. Analysis of PAC1 receptor gene variants in Caucasian and African American infants dying of sudden infant death syndrome. Acta Paediatr. 102, e546–e552. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA, 1987. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J. Comp. Neurol 262, 546–562. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL, 2001. Translating developmental time across mammalian species. Neurosci. 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Pendlebury JD, Sherwood NM, Wilson RJ, 2003. Sudden neonatal death in PACAP-deficient mice is associated with reduced respiratory chemoresponse and susceptibility to apnoea. J. Physiol 555, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Pendlebury JD, Jirik FR, Sherwood NM, Wilson RJ, 2004. A SIDS-like phenotype is associated with reduced respiratory chemoresponses in PACAP deficient neonatal mice. Adv. Exp. Med. Biol 551, 77–83. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Willie C, Wilson RJ, 2008. Pituitary adenylate cyclase-activating polypeptide maintains neonatal breathing but not metabolism during mild reductions in ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol 294, R959–965. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Klotz C, Liu W-Q, Weese-Mayer DE, Marazita ML, Cooper ME, Berry-Kravis EM, Tobias R, Goldie C, Bech-Hansen NT, Wilson RJA, 2009. Sudden infant death syndrome (SIDS) in African Americans: polymorphisms in the gene encoding the stress peptide pituitary adenylate cyclase-activating polypeptide (PACAP). Acta Paediatr. 98, 482–489. [DOI] [PubMed] [Google Scholar]
- Das M, Vihlen CS, Legradi G, 2007. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J. Comp. Neurol 500, 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun NJ, Miyazaki T, Tang H, Dun EC 1996. Pituitary adenylate cyclase activating polypeptide immunoreactivity in the rat spinal cord and medulla: implication of sensory and autonomic functions. Neurosci. 73, 677–686. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM, 1996. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci 16, 5361–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC, 1994. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol. Neonate 65, 194–197. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Katz DM, 1992. The central organization of carotid body afferent projection to the brain stem of the rat. Brain Res. 572, 108–116. [DOI] [PubMed] [Google Scholar]
- Gao XP, Liu QS, Liu Q, Wong-Riley MTT, 2011. Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat. J. Physiol 589, 1991–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XP, Liu Q, Nair B, Wong-Riley MTT, 2014. Reduced levels of brain-derived neurotrophic factor contribute to synaptic imbalance during the critical period of respiratory development in rats. Eur. J. Neurosci 40, 2183–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XP, Zhang H, Wong-Riley MTT, 2015. Role of brain-derived neurotrophic factor in the excitatory-inhibitory imbalance during the critical period of postnatal respiratory development in the rat. Physiol. Rep 3, e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL, 1999. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the pre-Bötzinger complex. Science 286, 1566–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Cummings KJ, Jirik FR, Sherwood NM, 2001. Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol. Endocrinol 15, 1739–1747. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE, 2002. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc. Natl. Acad. Sci. USA 99, 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J, 2000. PACAP and glutamate are costored in the retinohypothalamic tract. J. Comp. Neurol 418, 147–155. [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Gauthier P, Rega P, Morin D, 1990. Functional significance of the dorsal respiratory group in adult and newborn rats: in vivo and in vitro studies. Neurosci. Lett 111, 133–138. [DOI] [PubMed] [Google Scholar]
- Huang J, Waters KA, Machaalani R, 2017a. Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor 1 (PAC1) in the human infant brain and changes in the Sudden Infant Death Syndrome (SIDS). Neurobiol. Dis 103, 70–77. [DOI] [PubMed] [Google Scholar]
- Huang J, Waters KA, Machaalani R, 2017b. Hypoxia and nicotine effects on Pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor 1 (PAC1) in the developing piglet brainstem. NeuroToxicology 62, 30–38. [DOI] [PubMed] [Google Scholar]
- Jordan D, 2001. Central nervous pathways and control of the airways. Respir. Physiol 125, 67–81. [DOI] [PubMed] [Google Scholar]
- Kausz M, Murai Z, Arimura A, Koves K, 1999. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactive elements in the brain stem of rats studied by immunohistochemistry. Neurobiology (Bp) 7, 19–31. [PubMed] [Google Scholar]
- Légrádi G, Shioda S, Arimura A, 1994. Pituitary adenylate cyclase-activating polypeptide-like immunoreactivity in autonomic regulatory areas of the rat medulla oblongata. Neurosci. Lett 176, 193–196. [DOI] [PubMed] [Google Scholar]
- Liu DM, Cuevas J, Adams DJ, 2000. VIP and PACAP potentiation of the nicotinic ACh-evoked currents in rat parasympathetic neurons is mediated by G-protein activation. Eur. J. Neurosci 12, 2243–2251. [DOI] [PubMed] [Google Scholar]
- Liu Q, Fehring C, Lowry TF, Wong-Riley MTT, 2009. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J. Appl. Physiol 106,1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kim J, Cinotte J, Homolka P, Wong-Riley MTT, 2003. Carotid body denervation effect on cytochrome oxidase activity in pre-Botzinger complex of developing rats. J. Appl. Physiol 94, 1115–1121. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MTT, 2006. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J. Physiol 577, 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2002. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Botzinger complex. J. Appl. Physiol 92, 923–934. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2003. Postnatal changes in cytochrome oxidase expressions in brain stem nuclei of rats: implications for sensitive periods. J. Appl. Physiol 95, 2285–2291. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2004. Developmental changes in the expression of GABAA receptor subunits α1, α2, and α3 in the rat pre-Bötzinger complex. J. Appl. Physiol 96, 1825–1831. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2005. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J. Appl. Physiol 98, 1442–1457. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2006. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 1098, 129–138. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2008. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J. Appl. Physiol 104, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2010a. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neurosci. 165, 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2010b. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J. Comp. Neurol 518, 1082–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2010c. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D, and 3B immunoreactivity in brain stem respiratory nuclei of the rat. Neurosci. 171, 637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2012. Postnatal development of Na(+)-K(+)-2Cl(−) co-transporter 1 and K(+)-Cl(−) co-transporter 2 immunoreactivity in multiple brain stem respiratory nuclei of the rat. Neurosci. 210, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2013a. Postnatal development of brain-derived neurotrophic factor (BDNF) and tyrosine protein kinase B (TrkB) receptor immunoreactivity in multiple brainstem respiratory-related nuclei of the rat. J. Comp. Neurol 521, 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT, 2013b. Postnatal development of glycine receptor subunits α1, α2, α3, and β immunoreactivity in multiple brain stem respiratory-related nuclei of the rat. Brain Res. 1538, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AA, 1980. The neural regulation of tongue movements. Prog. Neurobio 15, 295–344. [DOI] [PubMed] [Google Scholar]
- MacDonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF, 2005. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J. Neuroaci 25, 1137–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M, 1993. Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res. 602, 57–63. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH, 1989. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Comm 164, 567–574. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A, 1990. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem. Biophys. Res. Comm 170, 643–648. [DOI] [PubMed] [Google Scholar]
- Morio H, Tatsuno I, Hirai A, Tamura Y, Saito Y, 1996. Pituitary adenylate cyclaseactivating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 741, 82–88. [DOI] [PubMed] [Google Scholar]
- Nunez-Abades PA, Pasaro R, Bianchi AL, 1992. Study of the topographical distribution of different populations of motoneurons within rat’s nucleus ambiguus, by means of four different fluorochromes. Neurosci. Lett 135, 103–107. [DOI] [PubMed] [Google Scholar]
- Pelligri G, Magistretti PJ, Martin JL, 1998. VIP and PACAP potentiate the action of glutamate on BDNF expression in mouse cortical neurons. Eur. J. Neurosci, 10, 272–280. [DOI] [PubMed] [Google Scholar]
- Prandota J, 2004. Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am. J. Ther 11, 517–546. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL, 1998. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol 60, 385405. [DOI] [PubMed] [Google Scholar]
- Runcie MJ, Ulman LG, Potter EK, 1995. Effects of pituitary adenylate cyclase-activating polypeptide on cardiovascular and respiratory responses in anesthetized dogs. Regul. Pept 60, 193–200. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL, 1991. Pre-Bötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science 254, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Negro CD, Wilson CG, Johnson SM, 2000. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir. Physiol 122, 131–147. [DOI] [PubMed] [Google Scholar]
- Uddman R, Luts A, Arimura A, Sundler F, 1991. Pituitary adenylate cyclase-activating polypeptide (PACAP), a new vasoactive intestinal peptide (VIP)-like peptide in the respiratory tract. Cell Tissue Res. 265, 197–201. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H, 2000. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to function. Pharmacol. Rev 52, 269–324. [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H, 2009. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmcol. Rev 61, 283–357. [DOI] [PubMed] [Google Scholar]
- Wardle RA, Poo MM, 2003. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J. Neurosci 23, 8722–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJA, Cummings KJ, 2008. Pituitary adenylate cyclase-activating polypeptide is vital for neonatal survival and the neuronal control of breathing. Resp. Physiol. Neurobiol 164, 168–178. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q, 2005. Neurochemical development of brain stem nuclei involved in the control of respiration. In Special Issue on “Development of Respiratory Control”. Bavis R and Carroll J (eds.). Respir. Physiol. Neurobiol 149, 83–98. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q, 2008. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir. Physiol. Neurobiol 164, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q, Gao X-P, 2013. Peripheral-central chemoreceptor interaction and the significance of a critical period in the development of respiratory control. Invited review. In Special Issue on “Development of the Carotid Body”. Carroll JL, Donnelly D, and Bairm A (eds.) Respir. Physiol. Neurobiol 185, 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]