Severe pediatric obesity (class II or higher; body mass index [calculated as weight in kilograms divided by height in meters squared] ≥120% above the 95th percentile or ≥35) is a national public health crisis affecting more than 4.5 million children and adolescents in the United States. The prevalence of severe pediatric obesity has increased from 4.0% in 2000 to 6.0% in 2016.1 For children and adolescents with this disease, metabolic and bariatric surgery (MBS) is effective in achieving long-term weight loss and resolution of comorbidities.2,3 We sought to define contemporary trends in the use of MBS among US children, adolescents, and young adults with severe obesity.
Methods ∣
The Kids’ Inpatient Database (KID) and the National Inpatient Sample (NIS) were used to identify pediatric patients (≤20 years old) undergoing MBS from January 2005 through December 2014 (KID for 2006, 2009, and 2012; NIS for every other year). Patients were identified using procedure codes from the International Classification of Diseases, Ninth Revision, Clinical Modification, for MBS with a confirmatory diagnosis code for obesity. Nonelective procedures were excluded, as well as procedures performed for inflammatory bowel disease, colitis, or gastrointestinal malignancy. Weighted population estimates were calculated, and statistics were obtained for patient characteristics, type of procedure, and in-hospital complications. All analyses were conducted using Stata statistical software (version 13.1; StataCorp) from April through June 2018. This study does not require institutional review board approval given the use of publicly available databases.
Results ∣
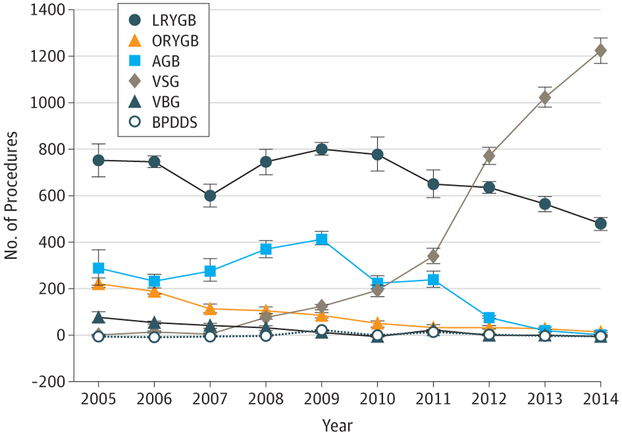
An estimated 14 178 MBS procedures were performed from 2005 through 2014 among pediatric patients 20 years or younger. Patients were predominantly white (59.4%) compared with black (15.4%) and Hispanic (17.5%; P < .001) and predominantly female (78.1%) compared with male (21.9%; P < .001), with a mean age of 18.6 years (95% CI, 18.5-18.7 years). The mean length of stay was 2 days (95% CI, 1.9-2.1 days). These numbers did not significantly change during the 10-year period. In-hospital complications decreased from 8.8% in 2005 to 2.0% in 2014 (P < .001) (Table). No in-hospital deaths occurred except in 2006 (≤10). Vertical sleeve gastrectomy increased from 10 or fewer procedures in 2005 to 1225 by 2014 (70.6%; P < .001), whereas laparoscopic Roux-en-Y gastric bypass decreased from 753 procedures (55.4%) to 480 (27.7%; P < .001). Use of the adjustable gastric band peaked in 2009 at 418 procedures (28.4%) and decreased to 10 or fewer procedures by 2014 (P < .001). Vertical banded gastroplasty and biliopancreatic diversion with a duodenal switch constituted an insignificant proportion of procedures throughout the 10-year period (Figure).
Table.
In-Hospital Complications for Patients 120 Years and Younger Undergoing Elective MBS From 2005 to 2014
| Year | Complications, No. (%)a |
|---|---|
| 2005 | 124 (8.8) |
| 2006 | 101 (7.9) |
| 2007 | 106 (9.6) |
| 2008 | 105 (7.8) |
| 2009 | 84 (5.7) |
| 2010 | 75 (5.9) |
| 2011 | 62 (4.7) |
| 2012 | 75 (4.8) |
| 2013 | 40 (2.4) |
| 2014 | 35 (2.0) |
| All | 807 (5.7) |
Abbreviation: MBS, metabolic and bariatric surgery.
P < .001 for the overall yearly trends. Complications were identified using codes from International Classification of Diseases, Ninth Revision, Clinical Modification, including reoperation (54.12, 54.61, and 54.92), splenic injury (41.2, 41.43, and 41.5), hemorrhage (998.1, 998.11, 998.12, 99.04, and 99.09), anastomotic leak (998.6 and 54.91), wound disruption (998.13, 998.3, 998.30-998.33, and 998.83), infection (998.5, 998.51, 998.59 567.0, 567.1, 567.2, 567.21, 567.22, 567.29, 567.3, 567.31, 567.38, 567.39, and 56.79), small bowel obstruction (560.0-560.3, 560.30-560.32, 560.39, 560.8, 560.81, 560.89, and 560.9), accidental puncture (998.2), conversion to open procedure (V64.4 and V64.41), other (998.4, 998.7, 998.8, 998.89, and 998.9), pulmonary (997.3, 997.31, 997.39, 481, 482.0-3, 482.30-482.32, 482.39, 482.4, 480.40-480.42, 480.49, 480.8, 480.81-480.84, 480.89, 480.9, 485, 486, 518.81, 31.1, and 31.29), cardiac (997.1, 410, and 427), neurologic (997.0, 997.00-02, and 997.09), genitourinary (584.5-9, 599.0, 997.5, and 38.95), deep venous thrombosis or pulmonary embolism (415.1, 415.11-415.13, 415.19, 453.2, 453.4, 453.40-453.42, 453.82, 453.84-453.87, 453.89 and 45.9), and shock/sepsis (998.0 and 995.91-995.94).
Figure. Types of Metabolic and Bariatric Surgical Procedures Performed in the Study Population.
Study population includes patients 120 years or younger from 2005 through 2014 in the Kids’ Inpatient Database and National Inpatient Sample database. AGB indicates adjustable gastric band; BPDDS, biliopancreatic diversion with a duodenal switch; LRYGB, laparoscopic Roux-en-Y gastric bypass; ORYGB, open Roux-en-Y gastric bypass; VBG, vertical banded gastroplasty; and VSG, vertical sleeve gastrectomy.
Discussion ∣
This study identified a significant shift in type of MBS procedures performed among pediatric patients with obesity from 2005 through 2014, showing an increasing frequency of vertical sleeve gastrectomy and decreasing frequency of laparoscopic Roux-en-Y gastric bypass and use of the adjustable gastric band, a change that mirrors adult operative trends. In addition, we found a decrease in the in-hospital postoperative complication rates to 2.0%. A recently published study conducting a secondary analysis of data previously collected by the Teen Longitudinal Assessment of Bariatric Surgery and Treatment Options of Type 2 Diabetes in Adolescents and Youth trials4 found adolescents with severe obesity treated with MBS had better weight loss, glycemic control, and improvement of cardiovascular risk markers than those receiving medical treatment alone. Despite these findings, less than 0.04% of children and adolescents with severe obesity are treated with MBS each year.
Prospective studies comparing surgical therapy with medical management of severe pediatric obesity are lacking. However, the promotion of choices targeting improved health for these patients are universally advocated. Referral for multidisciplinary evaluation should be considered early in the disease process, especially among those children and adolescents with type 2 diabetes or other obesity-related comorbidities. The possible causes for the low rate of surgical management among this population are multifactorial and include a likely overestimation of the short- and long-term risks of MBS along with an underrecognition of the long-term detrimental consequences of pediatric obesity.5,6 An additional factor that greatly hinders appropriate referral for surgical evaluation is an inadequate number of multidisciplinary programs willing to treat pediatric patients. In the end, a large population of pediatric patients with severe obesity are not referred to and/or do not have access to the best care for their disease, to include a discussion of MBS. One key limitation of our study is our inability to examine long-term postoperative outcomes and complications with NIS and KID, both of which do not link multiple admissions of 1 patient and instead treat them as separate discharges. We can therefore only comment on in-hospital morbidity and mortality, keeping in mind the former is greatly affected by known coding limitations of all Healthcare Cost and Utilization Project databases. Nevertheless, NIS and KID are known to contain accurate procedure coding; hence they provide a reasonable overview of the current trends in types of surgical procedures, which was the primary goal of this study.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
Cornelia L. Griggs, Department of Surgery, Massachusetts General Hospital, Boston.
Numa P. Perez, Jr, Department of Surgery, Massachusetts General Hospital, Boston.
Robert N. Goldstone, Department of Surgery, Massachusetts General Hospital, Boston.
Cassandra M. Kelleher, Harvard Medical School, Boston, Massachusetts; Department of Surgery, MassGeneral Hospital for Children, Massachusetts General Hospital, Boston.
David C. Chang, Harvard Medical School, Boston, Massachusetts; Codman Center for Clinical Effectiveness in Surgery, Massachusetts General Hospital, Boston.
Fatima Cody Stanford, Division of Endocrinology-Neuroendocrinology, Harvard Medical School, Boston; Massachusetts General Hospital Weight Center, Department of Pediatrics, Division of Endocrinology, Harvard Medical School, Boston.
Janey S. Pratt, Stanford University School of Medicine, Stanford, California; Departments of Surgery and Pediatric Surgery, Lucille Packard Children’s Hospital, Palo Alto, California.
References
- 1.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999-2016. Pediatrics. 2018;24(5):1116–1123. [Google Scholar]
- 2.Pedroso FE, Angriman F, Endo A, et al. Weight loss after bariatric surgery in obese adolescents: a systematic review and meta-analysis. Surg Obes Relat Dis. 2018;14(3):413–422. doi:10.1016/j.soard.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 3.Michalsky MP, Inge TH, Jenkins TM, et al. ; Teen-LABS Consortium. Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics. 2018;141(2):e20172485. doi:10.1542/peds.2017-2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inge TH, Laffel LM, Jenkins TM, et al. ; Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) Consortia. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018;172(5):452–460. doi:10.1001/jamapediatrics.2017.5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal CW, Kumar S, Iqbal AD, Ishitani MB. Perspectives on pediatric bariatric surgery: identifying barriers to referral. Surg Obes Relat Dis. 2009;5(1):88–93. doi:10.1016/j.soard.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 6.Woolford SJ, Clark SJ, Gebremariam A, Davis MM, Freed GL. To cut or not to cut: physicians’ perspectives on referring adolescents for bariatric surgery. Obes Surg. 2010;20(7):937–942. doi:10.1007/s11695-010-0152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]