Abstract
Background:
Posttraumatic Stress Disorder (PTSD) is prevalent among patients who survived an acute coronary syndrome, and is associated with adverse outcomes, but the mechanisms underlying these associations are unclear. Individuals with PTSD have enhanced sensitivity of the noradrenergic system to stress which may lead to immune activation. We hypothesized that survivors of a myocardial infarction (MI) who have PTSD would show an enhanced inflammatory response to acute psychological stress compared to those without PTSD.
Methods:
Individuals with a verified history of MI within 8 months and a clinical diagnosis of current PTSD underwent a mental stress speech task. Inflammatory biomarkers including interleukin-6 (IL-6), high-sensitivity C reactive protein (HsCRP), matrix metallopeptidase 9 (MMP-9), intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and monocyte chemoattractant protein (MCP)-1 were measured at rest and 90 min after mental stress.
Results:
Among 271 patients in the study (mean age 51 ± 7 years, 50% female, 60% African-American), the prevalence of PTSD was 12%. Mental stress resulted in a significant increase in IL-6, but the increase was more marked in patients with PTSD (126% increase) than those without (63% increase) (p=0.001). MCP-1 showed a modest increase with stress which was similar in patients with PTSD (9% increase) and without PTSD (6% increase) (p=0.35). CRP did not increase with stress in either group.
Conclusion:
MI patients with current PTSD exhibit enhanced IL-6 response to psychosocial stress, suggesting a mechanistic link between PTSD and adverse cardiovascular outcomes as well as other diseases associated with inflammation.
Keywords: PTSD, mental stress, myocardial infarction, interleukin-6, high-sensitivity C reactive protein, Matrix metallopeptidase 9, Monocyte chemoattractant protein-1
1. INTRODUCTION
Post-traumatic stress disorder (PTSD) has been associated with increased risk for acute cardiac events, including unstable angina (UA) (Edmondson and von Kanel, 2017), myocardial infarction (MI) (Beristianos et al., 2016), and cardiac death (Edmondson et al., 2013), as well as objective evidence of coronary heart disease (CHD) (Vaccarino et al., 2013). The association between PTSD and CHD was found to be independent of traditional CHD risk factors, such as dyslipidemia, diabetes and hypertension (Bedi and Arora; Kubzansky et al.; Sumner et al., 2017; Vaccarino et al., 2013). In addition, PTSD may be the consequence of a life-threatening acute CHD event, such as an MI or stroke, and may adversely affect the risk for subsequent CHD events and mortality in affected patients (Edmondson et al., 2012).
The pathways driving the association between PTSD and CHD remain unclear, but are likely to be multifactorial (Brouwers et al., 2014; O’Donovan, 2016; O’Donovan et al., 2013; Pace and Heim; Plantinga et al., 2013). A major hypothesized mechanism involves alterations in immunity and inflammation. PTSD is characterized by chronic dysregulation of neuro-hormonal systems involved in the psychological stress response, including the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic–adrenal–medullary (SAM) systems (Bremner and Charney, 2010; Bremner et al., 1999; Yehuda, 2002). Repeated activation of both the HPA axis and the SAM system by traumatic reminders or other stressful exposures in PTSD could lead to long-term microvascular dysfunction, endothelial injury and inflammation, eventually increasing CHD risk (Libby, 2002; Passos et al., 2015; Ridker and Luscher, 2014; Vaccarino and Bremner, 2015; Vaccarino and Bremner, 2017; Vaccarino et al., 2016).
Prior work has examined the relationship of acute psychological stress with increased levels of inflammatory biomarkers (Endrighi et al., 2016; Steptoe et al., 2007). In the general population (Marsland et al., 2017) and in subjects with CHD (Hammadah et al., 2017b), acute mental stress is associated with an increase in circulating inflammatory markers, especially interleukin (IL)-6. However, little is known about whether individuals with PTSD differ in their inflammatory response to acute psychological stress. Only one study to date has examined stress reactivity of inflammatory mediators in PTSD; this study showed a significant increase with mental stress for both vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, which are known predictors of ischemic heart disease (Hwang et al., 1997; Luc et al., 2003; von Kanel et al., 2010). This study, however, had a limited sample size and did not evaluate a broader range of inflammatory biomarkers that have been implicated in increased CHD risk (Blankenberg et al.; Blankenberg et al., 2003; de Lemos et al.; Fanola et al.; Held et al.; Ridker; Zakynthinos and Pappa).
The purpose of this study was to assess the relationship between PTSD and inflammatory response to mental stress in patients who survived an MI. We hypothesized that subjects with MI and PTSD would show an increased inflammatory response to acute psychological stress compared with those without PTSD. Specifically, we hypothesized that acute stress would be associated with a greater increase in IL-6 in MI patients with PTSD, as this inflammatory marker is known to be responsive to acute stress (Marsland et al., 2017). Our study also examined other established inflammatory biomarkers associated with CHD progression and/or PTSD, including ICAM-1, VCAM-1, high sensitivity C-reactive protein (HsCRP), matrix metallopeptidase-9 (MMP-9), and monocyte chemoattractant protein (MCP)-1 (Blankenberg et al.; Blankenberg et al., 2003; de Lemos et al.; Fanola et al.; Held et al.; Ridker; Sumner et al., 2017; Zakynthinos and Pappa), given that their relationship with acute stress and with PTSD has not been fully studied in patients with CHD.
2. METHODS AND MATERIALS
2.1. Study Design and Participants
Between June 2011 and March 2016, we enrolled 313 patients with recent MI (159 men, 154 women) in the Myocardial Infarction and Mental Stress Study 2 (MIMS2). The methods of this study were previously described (Vaccarino et al., 2018). The MI cases were recruited from the pool of patients who were admitted with a documented MI in the previous 8 months (index MI) at Emory-affiliated hospitals in Atlanta, Georgia, and who were 18 to 60 years of age at the time of screening. The diagnosis of MI (type 1) was verified by medical record review based on standard criteria of a troponin level increase and ECG changes (Thygesen et al., 2007).
Subjects were excluded if they had a severe comorbid medical or psychiatric disorder that could interfere with the study results, such as cancer, renal failure, severe uncontrolled hypertension, current alcohol or substance abuse, bipolar disorder schizophrenia; if they were pregnant or breastfeeding; or if they were currently using immunosuppressant or psychotropic medications other than antidepressants. MI patients were also excluded if they had unstable angina, acute MI or decompensated heart failure within the previous week; if they weighed over 450 pounds (due to weight bearing limits of the nuclear stress test equipment); and if it was deemed to be unsafe by study cardiologists to withhold anti-ischemic medications for 24 hours before the testing.
During the baseline enrollment visit, clinical information including previous cardiovascular events, risk factors for CHD, and coronary angiography results were documented as described below. Patients also underwent mental stress testing following standardized procedures. Medications including beta-blockers, calcium-channel blockers, as well as long-acting nitrates, xanthine derivatives, and caffeine-containing products were withheld for 24 hours prior to stress testing.
Of 313 CHD patients in the MIMS2 dataset, 10 patients had missing PTSD status data. Among the remaining 303 patients, 32 had missing rest plasma samples, 72 had missing post-stress plasma samples, and 32 had both plasma samples missing, due to technical and assay problems. The proportion of patients with missing samples did not differ by PTSD status. Thus, a total of 271 participants were included in the final analysis, who had at least one non-missing value for either rest or post-stress biomarker levels. This research was approved by the Emory University Institutional Review Board. Written informed consent was obtained from all patients enrolled in the study.
2.2. Measurements
Mental Stress Testing Procedure
Patients were tested using a standardized public speaking task after a 30-minute rest period, in a temperature controlled, quiet, and dimly lit room. Briefly, patients were asked to imagine a situation in which a close relative had been mistreated in a nursing home. Patients were given two minutes to prepare and three minutes to deliver a speech in front of an evaluative audience. Blood pressure and heart rate were recorded throughout the test. This mental stress protocol has been validated and widely used in CHD patients (Goldberg et al., 1996; Kim et al., 2003; Ramachandruni et al., 2006; Sheps et al., 2002), and found to be highly reproducible and predictive of mental stress induced myocardial-ischemia and of hemodynamic and vascular responses to stress in our laboratory, as previously reported (Hammadah et al., 2017a; Sullivan et al., 2018).
Hemodynamic monitoring
Hemodynamic parameters, including the systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were recorded every 5 min during the resting period, every 1 min during the mental stress, and every 5 min during the recovery period. Hemodynamic responses to mental stress were calculated as the difference between the maximum value of each hemodynamic parameter during the speech minus the minimum resting value during the rest period.
Measurement of Inflammatory Responses
Inflammatory biomarkers were measured from venous blood samples collected at rest and 90-minutes post mental stress testing, including Interleukin-6 (IL-6), high-sensitivity C-reactive protein (HsCRP), monocyte chemoattractant protein-1 (MCP-1), matrix metallopeptidase-9 (MMP-9), intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1. Plasma collection time points were selected based on prior studies of mental stress testing, and our own pilot testing, indicating that inflammatory response to stress becomes more apparent at 90 minutes after mental stress (Marsland et al., 2017). Venous blood was collected into ice-cooled citrate tubes and immediately centrifuged at 4°C; obtained plasma was snap-frozen at −70°C until further processing. We employed the MesoScale system (Meso Scale Diagnostics Rockville, Maryland) using the SECTOR Imager 2400 to quantitate HsCRP, IL-6, MCP-1, MMP-9, ICAM-1, and VCAM-1 according to the protocols supplied by the manufacturer. The Mesoscale multiplex assay system uses electrochemiluminescence for high sensitivity and broad dynamic range. Lower limits of detection for our experiment were: HsCRP: 1.33 × 10–6 mg/L, IL-6: 0.06 pg/mL, MCP-1: 0.09 pg/mL, ICAM-1: 1.6 ng/mL, VCAM-1: 0.09 ng/mL and MMP-9: 0.011 ng/mL. The inter-assay coefficient of variations for midpoint standards were 2.1% for IL-6, 5.3% for HsCRP, 2.4% for MCP-1, 1.8% for MMP-9, 1.9% for ICAM-1, and 1.7% for VCAM-1.
Other Measurements
Demographic information was obtained using standardized questionnaires. Previous medical history (diabetes, hypertension, previous MI) and medication use (e.g. aspirin, beta blockers) were obtained by study nurses or physicians through medical history, clinical examinations and by reviewing medical records. Current (past month) history of psychiatric disorders (PTSD and major depression) were assessed using the Structured Clinical Interview for DSM-IV (SCID), which provides a clinical diagnosis of psychiatric disorders (First et al., 1995). We assessed depressive symptoms using the Beck Depression Inventory (BDI-II), a 21-item self-administered scale (Beck et al., 1996). PTSD symptoms were assessed using the civilian version of the PTSD Symptom Checklist (PCL-C) a 17-item scale (Blanchard et al., 1996); and general perceived stress with the Perceived Stress Scale (Cohen et al., 1983). The 17-items from the PCL-C scale were further subdivided into three different DSM-IV PTSD symptom clusters as follows: re-experiencing (items 1 to 5); avoidance and numbing (items 6 to 12); arousal (items 13 to 17) (First et al., 1995). Sleep quality was evaluated using the Pittsburgh Sleep Quality Index (PSQI). The PSQI is a self-administered, validated 19-item scale that assesses overall sleep-quality and sleep-related symptoms experienced during the previous 1 month (Buysse et al., 1989). The 19 items yield 7-component scores that reflect the frequency of sleep problems. The sum of the 7 components yields a global score that ranges from 0 to 21, with higher scores indicating poorer sleep quality. Height and weight were measured during the clinical exam and used to calculate body mass index (BMI, kg/m2). Angiographic data were obtained from the most recent coronary angiogram in the patient’s chart. CHD severity was quantified using the Gensini Score (Gensini, 1983).
2.3. Statistical Analyses
Descriptive statistics were stratified by PTSD status and differences calculated using t-tests or Mann-Whitley Wilcoxon tests for continuous variables and chi-square tests for categorical variables. Given that all inflammatory biomarkers had skewed distributions, natural log transformations were used in all analyses, and results are presented as geometric means.
We examined concentrations of all inflammatory biomarkers before and after mental stress testing using linear mixed models for repeated measures. To determine whether baseline levels and inflammatory response to stress differed by PTSD status, we included time-by-PTSD interactions in the repeated measures analyses. We estimated linear combinations of the regression coefficients for PTSD and time. We also expressed the IL-6 results in terms of inflammatory response to stress, calculated as (natural log) differences between 90-minutes post mental stress and rest values, and used it as outcome variable in mixed models. Since the IL-6 levels were log-transformed, the antilog of the difference between post stress and rest values is equal to the stress/rest ratio of geometric means. We used this model also when assessing PTSD as a continuous variable.
All analyses were conducted before and after adjusting for possible confounding factors considered a priori, including demographics factors (sex, age, race, years of education), lifestyle and clinical risk factors known to affect inflammation (ever smoking, BMI, diabetes, hypertension, history of MI prior to the index MI, depressive symptoms and perceived stress), as well as medication use (aspirin, statins and antidepressants). We further included plate effect as a random intercept in all of our models. In additional analyses, we used history of major depression in place of depressive symptoms and also checked the interaction between PTSD, major depression and time, since depression is commonly comorbid with PTSD. The significance level for main effects and interaction effects was set at p < 0.05. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Descriptive Characteristics
Among 271 CHD patients in the analytical sample, 11.8% had current diagnosis of PTSD (n = 32). Among these, all but one patient had the onset of PTSD prior to the index MI. Patients with PTSD, compared with those without PTSD, were more likely to be African American, to be less educated, and to have comorbid depression (Table 1). Psychosocial symptom scale scores, including symptoms of depression, PTSD and perceived stress, were all higher in patients with PTSD. However, all CHD risk factors were similar irrespective of PTSD status. There were also no differences in MI severity indicators, and the troponin peak level during the index MI was actually lower in individuals with PTSD than those without PTSD (Table 1).
Table 1.
Characteristics of the Study Population (MIMS-2 Study), n = 271.
| PTSD (−) | PTSD (+) | p-value | ||
|---|---|---|---|---|
| Total Demographics, n | 239 | 32 | ||
| Age, years, mean (SD) | 50.9 (6.6) | 50.25 (7.6) | 0.38 | |
| Female, n (%) | 116 (48.5) | 18 (56.3) | 0.41 | |
| Married, n (%) | 102 (42.7) | 12 (37.5) | 0.58 | |
| African American, n (%) | 145 (60.7) | 27 (84.4) | 0.032 | |
| Years of Education, years, mean (SD) | 13.8 (2.9) | 12.7 (2.2) | 0.043 | |
| Medical History and CHD Risk Factors | ||||
| BMI, kg/m2, mean (SD) | 31.3 (7.3) | 32.1 (8.9) | 0.58 | |
| Lifetime History Major Depression, n (%) | 71 (29.7) | 25 (78.1) | <.0001 | |
| Beck Depression Inventory (BDI), median (IQR) | 8 (12.0) | 20 (12.5) | <.0001 | |
| Perceived Stress Scale (PSS), median (IQR) | 15 (13) | 23 (8) | <.0001 | |
| PTSD Checklist (PCL), median (IQR) | 25 (14) | 54 (23) | <.0001 | |
| Lifetime History of Smoking, n (%) | 128 (54.7) | 17 (53.1) | 0.87 | |
| Diabetes, n (%) | 77 (32.2) | 12 (37.5) | 0.55 | |
| Hypertension, n (%) | 193 (80.8) | 29 (90.6) | 0.17 | |
| Dyslipidemia, n (%) | 192 (80.3) | 26 (81.3) | 0.90 | |
| History of MI prior to index MI, n (%) | 44 (18.4) | 9 (29.0) | 0.16 | |
| Heart Failure, n (%) | 18 (7.5) | 4 (12.9) | 0.30 | |
| CABG prior to index, n (%) | 47 (19.7) | 8 (25.0) | 0.48 | |
| PTCA prior to index, n (%) | 168 (70.3) | 21 (65.6) | 0.59 | |
| Cardiovascular Disease Severity | ||||
| Type of Index MI: STEMI, n (%) | 69 (28.9) | 9 (28.1) | 0.93 | |
| Summed Rest Score, mean (SD) | 3.7 (6.1) | 3.7 (6.9) | 0.62 | |
| Ejection Fraction, %, mean (SD) | 50 (11.9) | 51 (11.7) | 0.69 | |
| Index MI Troponin T Peak, µg/L, median (IQR) | 6.6 (28.6) | 1.6 (8.9) | 0.02 | |
| Gensini score, median (IQR) | 32 (46) | 25 (72) | 0.37 | |
| Medications | ||||
| Aspirin, n (%) | 198 (83.2) | 22 (68.8) | 0.06 | |
| Clopidogrel, n (%) | 172 (72.3) | 19 (59.4) | 0.15 | |
| Beta Blocker, n (%) | 203 (85.3) | 28 (87.5) | 0.79 | |
| ACE Inhibitors, n (%) | 104 (45.7) | 20 (62.5) | 0.06 | |
| Anti-Depressant, n (%) | 38 (16.0) | 7 (21.9) | 0.45 | |
| Statins, n (%) | 206 (86.6) | 23 (71.9) | 0.04 |
Abbreviations: SD: Standard Deviation; BMI: Body Mass Index; CABG: Coronary Artery Bypass Graft; MI: Myocardial Infarction; STEMI: ST Segment Elevation MI; BP: Blood Pressure; RPP: Rate Pressure Product; ACE: Angiotensin Converting Enzyme.
Patients with PTSD showed a similar hemodynamic reactivity to mental stress when compared with patients without the disorder (Table 2). On average, there was a 20% increase in systolic blood pressure, a 25% increase in heart rate and a 50% increase in the rate pressure product in response to stress in both groups.
Table 2.
Differences in Hemodynamic Reactivity Parameters in Response to Mental Stress by PTSD status, n=271.
| PTSD (−) (n=239) |
PTSD (+) (n=32) |
p-value | |
|---|---|---|---|
|
Systolic Blood Pressure (mmHg)a |
|||
| Rest | 134 (21.1) | 139 (23.8) | 0.25 |
| Stress | 163 (27.1) | 167 (27.3) | 0.45 |
| Systolic Blood Pressure Reactivity |
40 (16.7) | 39 (14.6) | 0.72 |
| Heart Rate (beats/min)a | |||
| Rest | 67 (11.3) | 68 (13.0) | 0.56 |
| Stress | 83 (15.9) | 84 (19.7) | 0.58 |
| Heart Rate Reactivity | 23 (13.9) | 23 (17.6) | 0.92 |
|
Rate Pressure Product (RPP) (1000*mmHg*beats/min)a |
|||
| Rest | 9 (2.3) | 9 (2.7) | 0.26 |
| Stress | 14 (3.6) | 14 (5.2) | 0.28 |
| RPP Reactivity | 5 (3.1) | 5 (3.8) | 0.67 |
Continuous variables are reported as mean (standard deviation). Average values during rest and stress conditions were calculated for both PTSD positive and negative patients. Values for systolic blood pressure, heart rate, and rate pressure product reactivity were calculated by taking the difference between the average stress and rest values for each parameter.
3.2. Biomarker Levels at Rest and Post Mental Stress by PTSD Status
At rest, there were minor differences in inflammatory marker levels between MI patients with and without PTSD; the only biomarkers that tended to be higher in patients with PTSD at baseline were MCP-1 (8% higher, p=0.06) and ICAM-1 (11%, p=0.046) (Figure 1 and Supplemental Table 1). Overall, several biomarkers increased significantly with mental stress at 90 minutes, including IL-6 (68% higher, p=0.01), MCP-1 (6% higher, p=0.02) and MMP-9 (11% higher, p=0.05). When examined by PTSD status, PTSD patients displayed greater biomarker levels for IL-6 (42% higher, p=0.02) compared with patients without PTSD. The difference in slopes between individuals with and without PTSD, as determined by the interaction term between PTSD and time, was statistically significant for IL-6 (p=0.002) (Figure 1). Unlike IL-6, the slopes by PTSD status for ICAM-1, VCAM-1, HsCRP, MCP-1, and MMP-9 were not significantly different (Figure 1 and Supplemental Table 1).
Figure 1. Unadjusted Geometric Mean Plasma Concentrations and 95% Confidence Intervals of IL-6, HsCRP, MCP-1, MPP-9, ICAM-1 and VCAM-1 by PTSD Status and Time.
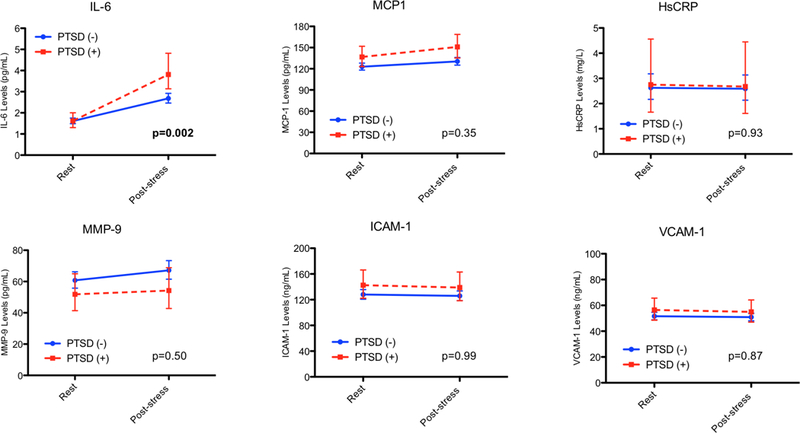
Repeated measures models were used to investigate differences across time by testing for the interaction of PTSD status with time. Natural log values were modeled and presented as geometric means. P values for slope differences represent the PTSD by time interaction.
In multivariable analyses that progressively adjusted for demographic factors, lifestyle and clinical risk factors, the post-stress difference in IL-6 by PTSD status remained significant, as did the interaction between PTSD and time (Table 3). Given the frequent co-morbidity between depression and PTSD, we repeated this analysis adjusting for history of major depression instead of depressive symptoms and the results remained similar. Furthermore, we assessed the interaction between PTSD, depression and time as a predictor of IL-6 response to mental stress in a separate model. We found that both the PTSD*depression*time and the depression*time interaction terms were not significant (p=0.18 and 0.29, respectively). However, the PTSD*time interaction term remained significant in this model (p=0.0028). Finally, sleep disturbances are extremely prevalent in those with PTSD, being also associated with increases in inflammation (Maher et al., 2006; Rohleder et al., 2012). Therefore, as an exploratory analysis, we added the Pittsburgh Sleep Quality Index total score to our models and it did not change the parameter estimates for PTSD which remained statistically significant (p=0.0038).
Table 3.
Unadjusted and Adjusted Geometric Mean Plasma Concentrations of IL-6 by PTSD Status and Time*.
| Rest | 90-Minutes Post Stress | PTSD*Time | |||||
|---|---|---|---|---|---|---|---|
| PTSD (−) Geometric Mean (95% CI) |
PTSD (+) Geometric Mean (95% CI) |
p-value | PTSD (−) Geometric Mean (95% CI) |
PTSD (+) Geometric Mean (95% CI) |
p-value |
p-value for the Interaction |
|
| IL-6 (pg/mL) | |||||||
| Unadjusted | 1.6 (1.5, 1.8) | 1.6 (1.3, 2.0) | 0.80 | 2.7 (2.5, 3.0) | 3.8 (3.1, 4.8) | 0.01 | 0.002 |
| Adjusted Model 1 | 1.7 (1.5, 2.0) | 1.5 (1.2, 1.9) | 0.52 | 2.8 (2.5, 3.1) | 3.6 (2.9, 4.6) | 0.02 | 0.002 |
| Adjusted Model 2 | 1.7 (1.5, 2.0) | 1.6 (1.3, 2.0) | 0.48 | 2.8 (2.4, 3.3) | 3.8 (3.0, 4.8) | 0.02 | 0.001 |
| Adjusted Model 3 | 1.7 (1.5, 2.0) | 1.6 (1.3, 2.0) | 0.53 | 2.8 (2.4, 3.3) | 3.8 (3.0, 4.9) | 0.02 | 0.001 |
Abbreviations: CI: confidence interval; IL-6: interleukin-6.
A natural log transformation was used for biomarker values as outcome using repeated measures analyses.
Model 1 adjusted for sex, race, age, years of education, plate effect.
Model 2 adjusted for model 1 covariates + hypertension, History of MI prior to index MI, body mass index (continuous), diabetes, smoking, aspirin and statin use.
Model 3 adjusted for model 2 covariates + beck depression inventory, perceived stress scale and anti-depressant use.
When IL-6 data were expressed as the difference between post stress values and rest values (Table 4), the IL-6 geometric mean increased 63% for patients without PTSD after mental stress, while it increased 126% (more than double) for those with PTSD (p=0.008). These differences changed minimally after adjusting for demographic and CHD risk factors (p=0.001).
Table 4.
Unadjusted and Adjusted Geometric Means of IL-6 Response (Stress Level Minus Rest Level) by PTSD status, with Inflammatory Response Modeled as the Outcome*.
| Outcome: Inflammatory Response Post Stress Value/Rest Value |
|||
|---|---|---|---|
| PTSD (−) | PTSD (+) | ||
|
Geometric Mean (95% CI) |
Geometric Mean (95% CI) |
p-value | |
| Unadjusted | 1.6 (1.5, 1.7) | 2.26 (1.9, 2.7) | 0.001 |
| Adjusted Model 1 | 1.6 (1.5, 1.8) | 2.19 (1.8, 2.7) | 0.003 |
| Adjusted Model 2 | 1.6 (1.4, 1.8) | 2.23 (1.8, 2.8) | 0.002 |
| Adjusted Model 3 | 1.6 (1.5, 1.8) | 2.20 (1.7, 2.8) | 0.008 |
Abbreviations: CI: confidence interval; IL-6: interleukin-6.
A natural log transformation was used for biomarker values in analyses. Inflammatory response calculated as geometric means: exp(loge(post stress values) – loge(rest values)) = Post stress value /rest value.
Model 1 adjusted for sex, race, age, years of education, plate effect.
Model 2 adjusted for model 1 covariates + hypertension, history of MI prior to index MI, body mass index (continuous), diabetes, smoking, aspirin and statin use.
Model 3 adjusted for model 2 covariates + beck depression inventory, perceived stress scale and anti-depressant use.
3.3. Relationship Between PTSD Symptoms and Inflammatory Response to Stress
Higher PTSD symptom severity as a continuous variable was also significantly associated with an enhanced inflammatory response to IL-6 (Table 5). For each 5-point increase in total PTSD symptom severity assessed by the PCL-C scale score, there was a 5.2% increase in IL-6 response (p=0.01). This association remained significant after adjusting for sociodemographic and clinical risk factors.
Table 5.
Unadjusted and Adjusted Percent Change in IL-6 Plasma Concentration with Mental Stress for Each 5-point Increase in PTSD Symptom Severity*.
| Percent Change in IL-6 (per 5-point increase in PTSD symptom severity) |
||
|---|---|---|
| % (95% Confidence Interval) | p-value | |
| Total PTSD Score | ||
| Unadjusted | 5.2 (4.8, 5.6) | 0.01 |
| Model 1 | 4.9 (4.5, 5.3) | 0.02 |
| Model 2 | 5.2 (4.8, 5.7) | 0.03 |
| Re-Experiencing | ||
| Unadjusted | 10.8 (9.4, 12.1) | 0.001 |
| Model 1 | 10.2 (8.8, 11.6) | 0.003 |
| Model 2 | 10.5 (9.1, 12.0) | 0.003 |
| Avoidance & Numbing | ||
| Unadjusted | 5.0 (4.1, 6.0) | 0.04 |
| Model 1 | 4.9 (3.9, 6.0) | 0.06 |
| Model 2 | 5.2 (4.1, 6.2) | 0.05 |
| Arousal | ||
| Unadjusted | 3.3 (1.3, 5.2) | 0.18 |
| Model 1 | 2.2 (0.2, 4.2) | 0.37 |
| Model 2 | 2.6 (0.6, 4.6) | 0.31 |
Abbreviations: CI: confidence interval; IL-6: interleukin-6.
PTSD symptoms from PCL-C scale are divided here in DSM-IV clusters: re-experience, avoidance and numbing and arousal.
Model 1 adjusted for sex, race, age, years of education, plate effect.
Model 2 adjusted for model 1 covariates + hypertension, history of MI prior to index MI, body mass index (continuous), diabetes, smoking, antidepressants, aspirin and statin use.
We also analyzed PTSD symptoms according to three different DSM-IV symptom clusters, namely: re-experiencing; avoidance & numbing; and arousal. Re-experiencing of trauma was the symptom cluster most robustly associated with an enhanced inflammatory response to stress, with 11% increase in IL-6 with mental stress for each 5-point increase in re-experiencing symptom severity (p=0.001). This association remained significant in subsequent multivariate models. Avoidance & numbing symptoms were also associated with IL-6 response, albeit more weakly (5% increase for each 5-point increase in symptom severity, p=0.04). Arousal symptoms were not significantly associated with an enhanced inflammatory response.
4. DISCUSSION
This study showed that acute mental stress is associated with an increase in the inflammatory marker IL-6 in MI patients with PTSD. Consistent with prior reports, stress increased IL-6 in all patients, but the magnitude of the effect was more than double in MI patients with PTSD compared with those without PTSD. Importantly, these findings were independent of clinical and behavioral factors associated with increased inflammation, including comorbid depression which is common in PTSD patients. The association persisted when we considered PTSD symptom severity as a continuous variable. The association between PSD and heightened inflammatory response to stress was specific for IL-6, as it was not observed for the other inflammatory biomarkers evaluated in this study, namely ICAM-1, VCAM-1, hsCRP, MCP-1, and MMP-9.
Inflammation has been implicated as an important mechanism linking PTSD to atherosclerosis and increased CHD risk (Brouwers et al., 2014; Edmondson and von Kanel, 2017; Pace and Heim, 2011; Ridker and Luscher, 2014). In a recent systematic review and meta-analysis of inflammatory responses to acute mental stress in healthy subjects and subjects with physical or mental health conditions, IL-6 demonstrated the most robust and consistent associations with stress amongst a number of cytokines (Marsland et al., 2017). Only two studies evaluated the effects of acute psychological stress on circulating inflammatory markers in CHD patients. Kop et al. (2008) demonstrated that IL-6 increases after mental stress testing as well as after physical stress (treadmill) (Kop et al., 2008). Furthermore, a recent report from our group showed, in a similar but independent patient population from the current study, that mental stress is associated with significant increases in IL-6, MMP-9, and MCP-1 levels, but not hsCRP. However, none of the changes in inflammatory marker levels predicted mental stress-induced myocardial ischemia (Hammadah et al., 2017b). Our current results, using a larger biomarker panel, are consistent with these previous studies in CHD patients in showing that some inflammatory biomarkers but not others are acutely responsive to stress (within 90 minutes).
Only one previous study has assessed stress reactivity of inflammatory mediators in CHD patients with and without PTSD. In a study of post-MI patients, half of whom had developed PTSD after the acute MI event, von Känel et al. (2010) found that patients who developed PTSD, compared with those who did not, had higher plasma levels of soluble ICAM-1 and VCAM-1 at rest and in response to the administration of the Clinician-Administered PTSD Scale (CAPS) interview, while levels of soluble P-selectin, another inflammatory biomarker, were not different between the two groups (von Kanel et al., 2010). This study, however, was limited by a small sample size of 44 CHD patients and the lack of a standardized and validated mental stress test. In our data, we did not find any significant differences of ICAM-1 or VCAM-1 with mental stress by PTSD status. However, our results are difficult to compare with the aforementioned study given that the latter didn’t use a validated mental stress test.
In our study, we also found that the resting levels of cytokines, with the exception of ICAM-1, did not differ by PTSD status. On the other hand, a recent meta-analysis showed a significant relationship between PTSD and increased resting IL-6 levels (Passos et al., 2015), which was not observed here. This divergence could be related to the intrinsic differences in study populations. All our participants had a recent MI, which in itself is associated with a higher pro-inflammatory state. Therefore, this could have attenuated baseline differences in inflammatory markers between participants with and without PTSD. The average resting IL-6 levels in our study are comparable to prior reports of IL-6 in patients with CHD (Hammadah et al., 2017b; Marsland et al., 2017). Our finding of increased resting ICAM-1 levels in patients with PTSD is consistent with a recent report in a sample of middle-aged women with PTSD (Sumner et al., 2017). Our study is the first to assess differences in MCP-1 and MMP-9 by PTSD status in response to mental stress. We demonstrated a small increase in MCP-1 levels post stress for the PTSD group, which paralleled baseline differences with no significant differences in the slopes of the two groups. For MMP-9, we found no difference whatsoever between rest or post-stress levels.
The mechanisms underlying the enhanced IL-6 response to stress in PTSD patients are not yet established. However, alterations in the SAM system and/or HPA activity in PTSD are potentially implicated. The inflammatory and HPA axis systems are involved in a complex inter-regulation. PTSD has been associated with both a decrease in resting cortisol and an increase in corticotropin releasing factor (Baker et al., 2005; Bremner et al., 1997; de Kloet et al., 2008), both of which are associated with higher levels of IL-6 (Swolin-Eide and Ohlsson, 1998; Venihaki et al., 2001). PTSD is also associated with activation of the SAM system (Bremner and Pearce, 2016) which is linked to increased IL-6 (Okamoto et al., 2015). Psychological stress, via SAM system activation, is known to increase binding activity of the nuclear factor-kappa B (NF-kB), an essential transcription factor involved in the activation of immune and inflammatory responses (Bierhaus et al., 2003). Interestingly, we did not find significant differences in hemodynamic responses to stress between patients with and without PTSD. These results could be partially explained by the fact that CHD patients can display a blunted response to mental stress that may render difficult detecting such differences (Hammadah, 2017). Furthermore, low cardiovascular reactivity to mental stress has been linked to chronic stress and depression, as well as to poor health behaviors that are associated with PTSD and CHD, such as smoking, substance abuse, and obesity (Phillips et al., 2013). Taken together, changes in the SAM system, HPA axis and cortisol responses could explain an enhanced inflammatory response in PTSD, although the link between stress, PTSD, the SAM system and hemodynamic reactivity is complex among CHD patients.
Epigenetic changes in PTSD may also represent a mechanism for increased IL-6 response to stress in PTSD (Smith et al., 2011). A prior report demonstrated that PTSD patients have more unmethylated genes linked to inflammation compared to healthy controls, potentially contributing to an enhanced inflammatory reaction during stress through changes in gene expression (Rusiecki et al., 2013). In accordance with these findings, Rohleder et al. (2004) reported increased lipopolysaccharide-induced IL-6 and tumor necrosis factor-α (TNF-α) production in whole blood collected from Bosnian war refugees with PTSD compared to healthy controls (Rohleder et al., 2004).
4.1. Clinical Implications
The clinical significance of an enhanced inflammatory response to mental stress in CHD patients with PTSD is not fully understood. There is some evidence showing that individuals who mount an increased inflammatory response, in the long run, are prone to the development of a sustained chronic pro-inflammatory state (Marsland et al., 2017) which is a well-established risk factor for atherosclerosis, CHD (Ridker, 2003) and sudden cardiac death (Hussein et al.). MI patients with PTSD may undergo repeated episodes of mental stress and neurohormonal activation during daily life through re-experiencing symptoms, which may result in increased reactivity of the inflammatory system to stress in this disorder. This hypothesis is consistent with our findings showing that, among the three main PTSD symptom sub-clusters, reexperiencing symptoms had the most robust association with IL-6 response. Whatever the cause, heightened activation of the inflammatory system could increase their CHD risk for recurrent events for the reasons described above. Our findings may inform strategies for treatment and secondary prevention in patients with CHD and comorbid PTSD. For example, CHD patients with PTSD may potentially benefit from anti-inflammatory therapies for secondary prevention (Ridker et al., 2017).
4.2. Strengths and Limitations
One of the main limitations of the current study is the lack of long-term follow-up data; therefore, the prognostic significance of our findings needs further investigation. Furthermore, because all study participants had a previous MI, our results should not be generalized to individuals without CHD. The neurobiological and cardiovascular physiology of PTSD comorbid with CHD are incompletely understood, and likely vary from PTSD in the absence of CHD. Therefore, our results cannot be generalized to all PTSD patients. Another potential limitation is that the 90 minute-timeframe could be inadequate to capture the peak increase for some biomarkers. However, in most previous studies the largest effect for IL-6 following stress was at 90 minutes post stress (Marsland et al., 2017), which supports our plasma collection time point at 90 minutes. Lastly, our sample had a relatively small proportion of patients diagnosed with PTSD. Despite these limitations, our study has several strengths, including the large sample size of well-characterized young post-MI patients following an experimental design. Other strengths are the comprehensive panel of biomarkers evaluated and the diversity of the population studied. Prior studies evaluating CHD risk in subjects with PTSD focused mostly on Caucasian male military veterans (Edmondson and von Kanel, 2017); in contrast, our study utilized a well-balanced civilian population with the majority of patients with PTSD being women and African-Americans.
5. CONCLUSION
In a well-characterized sample of survivors of a recent MI, PTSD was associated with an enhanced IL-6 response to acute mental stress. Our results are consistent with the possibility that an increased inflammatory response to stress plays a role in the link between PTSD and CHD. Future studies should examine if an enhanced inflammatory response to mental stress has prognostic value in post-MI patients with co-morbid PTSD, and if targeting these inflammatory pathways with appropriate therapies translates into better outcomes for these individuals. Finally, exploring the molecular and cellular mechanisms of the interplay between PTSD, stress response and CHD, may inform better treatment and prevention strategies for this population.
Supplementary Material
HIGHLIGHTS.
MI patients with PTSD show enhanced IL-6 response to stress than those without PTSD
This effect is mainly driven by the re-experiencing symptom cluster of PTSD
Our data suggest a mechanistic link between PTSD and poor cardiovascular outcomes
6. ACKNOWLEGEMENTS
This work was supported by the NIH, through the following grants: R01 HL109413, R01HL109413-02S1, R01 HL125246, R01 HL136205, R01 HL088726, P01 HL101398, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, and THL130025A.
This work was supported by the e National Institutes of Health Grant Nos. P01HL101398, P20HL113451, P01HL086773-06A1, R56HL126558, R01HL109413, R01HL109413-02S1, R01HL125246, UL1TR000454, KL2TR000455, K24HL077506, K24 MH076955, K23HL127251, and THL130025A.
Abbreviations:
- PTSD
posttraumatic stress disorder
- MI
myocardial infarction
- IL-6
interleukin-6
- HsCRP
high-sensitivity C reactive protein
- MMP-9
matrix metallopeptidase-9
- MCP-1
monocyte chemoattractant protein-1
- ICAM-1
intercellular adhesion molecule
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE
The authors report no biomedical financial interests or potential conflicts of interest.
7. REFERENCES
- Baker DG, Ekhator NN, Kasckow JW, Dashevsky B, Horn PS, Bednarik L, Geracioti TD, 2005. Higher levels of basal serial CSF cortisol in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry 162, 992–994. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. BDI-II. Beck Depression Inventory: Second Edition. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bedi US, Arora R, 2007. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc 99, 642–649. [PMC free article] [PubMed] [Google Scholar]
- Beristianos MH, Yaffe K, Cohen B, Byers AL, 2016. PTSD and Risk of Incident Cardiovascular Disease in Aging Veterans. Am J Geriatr Psychiatry 24, 192–200. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP, 2003. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U. S. A 100, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA, 1996. Psychometric properties of the PTSD checklist (PCL). Behavioral Research & Therapy 34, 669–673. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J, 2001. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 104, 1336–1342. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, AtheroGene I, 2003. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 107, 1579–1585. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Charney DS, 2010. Neural circuits in fear and anxiety. In: Stein DJ, Hollander E, Rothbaum BO, eds. Textbook of anxiety disorders Arlington, VA: American Psychiatric Publishing; pp. 55–71. [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens M, Southwick SM, Nemeroff CB, Charney DS, 1997. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry 154, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Pearce B, 2016. Neurotransmitter, neurohormonal, and neuropeptidal function in PTSD. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment Wiley-Blackwell, Hoboken, New Jersey, pp. 181–232. [Google Scholar]
- Bremner JD, Southwick SM, Charney DS, 1999. The neurobiology of posttraumatic stress disorder: An integration of animal and human research. In: Saigh PA, Bremner JD (Eds.), Posttraumatic Stress Disorder: A Comprehensive Text Allyn & Bacon, New York, pp. 103–143. [Google Scholar]
- Brouwers CJ, Wolf JM, von Känel R, 2014. Inflammatory Markers in PTSD. In: Martin CR, Preedy VR, Patel VB (Eds.), Comprehensive Guide to Post-Traumatic Stress Disorder Springer International Publishing, Cham, pp. 1–13. [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Lentjes EG, Heijnen CJ, Stalla GK, Westenberg HG, 2008. Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Prog. Brain Res 167, 287–291. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E, 2003. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 107, 690–695. [DOI] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM, 2013. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J 166, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y, 2012. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One 7, e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, von Kanel R, 2017. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 4, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrighi R, Hamer M, Steptoe A, 2016. Post-menopausal Women Exhibit Greater Interleukin-6 Responses to Mental Stress Than Older Men. Ann Behav Med 50, 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, Hochman JS, Goodrich EL, Braunwald E, O’Donoghue ML, 2017. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) Trial. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbons M, 1995. Structured clinical interview for DSM-IV-Patient version New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Gensini GG, 1983. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 51, 606. [DOI] [PubMed] [Google Scholar]
- Goldberg AD, Becker LC, Bonsall R, Cohen JD, Ketterer MW, Kaufman PG, Krantz DS, Light KC, McMahon RP, Noreuil T, Pepine CJ, Raczynski J, Stone PH, Strother D, Taylor H, Sheps DS, 1996. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 94, 2402–2409. [DOI] [PubMed] [Google Scholar]
- Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA, 2017a. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol 243, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, Tahhan AS, O’Neal WT, Obideen M, Alkhoder A, Abdelhadi N, Mohamed Kelli H, Ghafeer MM, Pimple P, Sandesara P, Shah AJ, Hosny KM, Ward L, Ko YA, Sun YV, Weng L, Kutner M, Bremner JD, Sheps DS, Esteves F, Raggi P, Vaccarino V, Quyyumi AA, 2017b. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun [DOI] [PMC free article] [PubMed]
- Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L, Investigators S, 2017. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences From the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, DeFilippi C, See V, Deo R, Siscovick D, Stein PK, Lloyd-Jones D, 2013. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm 10, 1425–1432. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr., Boerwinkle E, 1997. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 96, 4219–4225. [DOI] [PubMed] [Google Scholar]
- Kim CK, Bartholomew BA, Mastin ST, Taasan VC, Carson KM, Sheps DS, 2003. Detection and reproducibility of mental stress-induced myocardial ischemia with Tc-99m sestamibi SPECT in normal and coronary artery disease populations. J Nucl Cardiol 10, 56–62. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Glaes SB, Krantz DS, Gottdiener JS, Tracy RP, 2008. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol 101, 767–773. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A 3rd, Vokonas PS, Sparrow D, 2007. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry 64, 109–116. [DOI] [PubMed] [Google Scholar]
- Libby P, 2002. Inflammation in atherosclerosis. Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- Luc G, Arveiler D, Evans A, Amouyel P, Ferrieres J, Bard JM, Elkhalil L, Fruchart JC, Ducimetiere P, Group PS, 2003. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis 170, 169–176. [DOI] [PubMed] [Google Scholar]
- Maher MJ, Rego SA, Asnis GM, 2006. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs 20, 567–590. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun [DOI] [PMC free article] [PubMed]
- O’Donovan A, 2016. PTSD is associated with elevated inflammation: any impact on clinical practice? Evid Based Ment Health 19, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Slavich GM, Epel ES, Neylan TC, 2013. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev 37, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, Garland EM, Black BK, Farley G, Diedrich A, Biaggioni I, 2015. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. American Journal of Physiology - Heart and Circulatory Physiology 309, H2098–H2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Heim CM, 2011. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun 25, 6–13. [DOI] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhaes PV, Kapczinski F, Kauer-Sant’Anna M, 2015. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM, 2013. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int J Psychophysiol 90, 1–7. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V, 2013. Association between posttraumatic stress disorder and inflammation: a twin study. Brain. Behav. Immun 30, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS, 2006. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol 47, 987–991. [DOI] [PubMed] [Google Scholar]
- Ridker PM, 2003. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107, 363–369. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT, 2017. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Luscher TF, 2014. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J 35, 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Aringer M, Boentert M, 2012. Role of interleukin-6 in stress, sleep, and fatigue. Ann N Y Acad Sci 1261, 88–96. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C, 2004. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 55, 745–751. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Byrne C, Galdzicki Z, Srikantan V, Chen L, Poulin M, Yan L, Baccarelli A, 2013. PTSD and DNA Methylation in Select Immune Function Gene Promoter Regions: A Repeated Measures Case-Control Study of U.S. Military Service Members. Front Psychiatry 4, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG, 2002. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 105, 1780–1784. [DOI] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ, 2011. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 156B, 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 21, 901–912. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V, 2018. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arterioscler Thromb Vasc Biol 38, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, Glymour MM, Tworoger SS, Koenen KC, Kubzansky LD, 2017. Cross-Sectional and Longitudinal Associations of Chronic Posttraumatic Stress Disorder With Inflammatory and Endothelial Function Markers in Women. Biol Psychiatry 82, 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swolin-Eide D, Ohlsson C, 1998. Effects of cortisol on the expression of interleukin-6 and interleukin-1â in human osteoblast-like cells. J. Endocrinol 156, 107–114. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, White HD, 2007. Universal definition of myocardial infarction. J. Am. Coll. Cardiol 50, 2173–2195. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD, 2015. Posttraumatic Stress Disorder and Risk of Cardiovascular Disease. In: Alvarenga M, Byrne D (Eds.), Handbook of Psychocardiology Springer, Singapore. [Google Scholar]
- Vaccarino V, Bremner JD, 2017. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci. Biobehav. Rev 74, 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD, 2013. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol 62, 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Mayer E, Bremner JD, 2016. Stress and Health. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment Wiley-Blackwell Press, Hoboken, N.J. [Google Scholar]
- Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P, 2018. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation 137, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venihaki M, Dikkes P, Carrigan A, Karalis KP, 2001. Corticotropin-releasing hormone regulates IL-6 expression during inflammation. J. Clin. Invest 108, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Abbas CC, Begre S, Saner H, Gander ML, Schmid JP, 2010. Posttraumatic stress disorder and soluble cellular adhesion molecules at rest and in response to a trauma-specific interview in patients after myocardial infarction. Psychiatry Res 179, 312–317. [DOI] [PubMed] [Google Scholar]
- Yehuda R, 2002. Post-traumatic stress disorder. N. Engl. J. Med 346, 108–114. [DOI] [PubMed] [Google Scholar]
- Zakynthinos E, Pappa N, 2009. Inflammatory biomarkers in coronary artery disease. J Cardiol 53, 317–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


