Abstract
Introduction:
Post-transplant diabetes mellitus (PTDM), an increasingly recognized complication of solid organ transplantation, is associated with increased morbidity and mortality following liver transplantation. Hepatitis C virus (HCV) infection is a consistent and modifiable risk factor for PTDM. Prior studies have demonstrated improvement in glucose metabolism following sustained virologic response (SVR). However, the effect of SVR on the incidence of PTDM has not been previously investigated in a large cohort of liver transplant (LT) recipients.
Methods:
We performed a single center retrospective cohort study of LT recipients with HCV from 1/1/2010 to 6/30/2015 to compare the risk of sustained PTDM (s-PTDM) prior to and following SVR. SVR was treated as a discrete time varying exposure. s-PTDM was defined as de novo diabetes mellitus following LT of greater than 6-months duration. Univariable and multivariable Cox proportional hazards model were used to compare crude and adjusted time to s-PTDM, prior to and following SVR.
Results:
256 eligible LT recipients were analyzed. Median follow up was 41.2 months. Overall, 31 (12.1%) and 178 (69.5%) patients achieved SVR prior to LT and following LT respectively. During follow up, 71 (27.7%) patients developed s-PTDM. The incidence of s-PTDM was greatest in the first year after LT. After adjustment for potential confounders, SVR was associated with a significantly reduced risk of s-PTDM (HR 0.40, p=0.048).
Conclusions:
Eradication of HCV is independently associated with a reduced incidence of s-PTDM. This benefit appears to be most influenced by pre-LT SVR and persists throughout the post-LT period. Given the association between PTDM and post-transplant morbidity and mortality, these data provide another motivator for pre- or early post-LT treatment of HCV.
Keywords: hepatitis C, liver transplant, diabetes, outcomes, cohort study
Introduction
Through advances in surgical techniques, organ donation, and immunosuppression, liver transplant (LT) recipients have expected 1- and 5-year survival rates greater than 90% and 70%, respectively.(1) However, post-transplant immunosuppression is associated with a number of adverse effects and co-morbidities, including diabetes mellitus. Further, HCV infection independently has been associated with insulin resistance and diabetes mellitus.(2–4) Over the last decade, post-transplant diabetes mellitus (PTDM) has been increasingly recognized as a complication of solid organ transplantation associated with serious morbidity in LT recipients, particularly in patients with HCV infection.(5, 6)
PTDM has previously been identified as an independent risk factor for mortality, graft failure, and cardiovascular events.(7–9) However, these associations have been inconsistent as few investigations of the prognostic impact of PTDM have distinguished de novo PTDM from pre-LT diabetes mellitus persisting after LT. Prior work by our group demonstrated that sustained PTDM (s-PTDM), a persistent state of de novo hyperglycemia related to pancreatic β-cell dysfunction and insulin resistance, is associated with death and major cardiovascular events following LT.(7, 10) In contrast, transient PTDM (t-PTDM), a state of hyperglycemia related to stress response and glucocorticoids, had no association with mortality or major cardiovascular events.(10)
Several prior studies have demonstrated an association between PTDM and chronic hepatitis C virus (HCV) infection.(8, 11–13) In addition, cross-sectional, prospective longitudinal studies, and a meta-analysis have associated HCV infection with an increased risk of type II diabetes mellitus.(3, 4, 14, 15) Further strengthening this association, Kawaguchi et al demonstrated reversibility of derangements in glucose metabolism in a cohort of HCV patients treated with interferon based therapy, in which insulin resistance improved following viral clearance.(2)
With the introduction of safe and highly effective direct acting antiviral (DAA) therapy for HCV infection, viral eradication prior to hepatic decompensation or soon after LT is now standard of care.(16) While viral eradication may improve insulin resistance and glucose metabolism, it is unclear if the incidence of s-PTDM is altered by viral eradication. In this study, we used a contemporary single center cohort of LT recipients to compare the incidence of s-PTDM among patients with HCV related chronic liver disease, with SVR as a time-varying exposure. We hypothesized that SVR is associated with a decreased risk of s-PTDM in LT recipients with HCV cirrhosis.
Methods
After approval from the University of Pennsylvania Institutional Review Board, data were obtained from the Penn Data Store, a data repository which supplies clinical, administrative, and pharmacy data through an automated extraction of data elements from multiple patient record systems within the health network. This retrospective cohort study utilized an assembled cohort of adult (>18 years of age) patients with HCV cirrhosis who underwent LT at the Hospital of the University of Pennsylvania from January 1, 2010 to June 30, 2015. Patients entered the cohort on the date of LT. They were censored from the analysis on the date of their last inpatient or outpatient encounter, date of death, or on the study end date, December 31, 2016.
We excluded patients with a history of DM prior to LT. Multi-organ recipients and patients with a solid-organ or hematopoietic stem cell transplant prior to the date of LT were excluded due to their propensity to receive immunosuppressive regimens which differ from the standard regimen used in LT recipients at our institution, as more intensive immunosuppressive regimens and prolonged glucocorticoid exposure may confer an increased risk of PTDM. Finally, LT recipients with less than 6 months of post-LT follow up were excluded due to the inability to adequately assess their post-transplant diabetes status. At our institution, single-organ LT recipients are treated with a relatively homogenous immunosuppressive regimen—combination therapy with tacrolimus and prednisone immediately following LT followed by tapering and cessation of prednisone over the ensuring 3–6 month period. In addition, induction is not used and the decision to add a second immunosuppressive agent, such as mycophenolate mofetil or azathioprine, is individualized on the basis of etiology of liver failure, renal function and prior episodes of rejection.
SVR was ascertained though manual chart review and was treated as a discrete time varying exposure, meaning subjects may only change exposure once. A patient achieved SVR on the date of the first undetectable HCV RNA at least 12 weeks after completing anti-HCV therapy. Patients who achieved SVR prior to LT entered the cohort as “post-SVR”. Data from our specialty pharmacy was used to confirm dates of HCV therapy initiation and completion. Other recipient factors and covariates collected included gender, age at LT, antiviral medications used, HCV genotype, body mass index (BMI) at LT, model for end stage liver disease (MELD) score at LT, hepatocellular carcinoma (HCC) on explant, living vs. deceased donor LT, and calcineurin inhibitor (CNI) use on discharge following LT. Acute cellular rejection (ACR) was ascertained on the date of a liver biopsy demonstrating ACR and the number of rejection episodes per patient was recorded.
The primary outcome was a diagnosis of s-PTDM, defined as de novo diabetes mellitus of at least six months duration following LT.(10) Diabetes mellitus was diagnosed according to the American Diabetes Association definition (Hemoglobin A1c >6.5%, fasting plasma glucose ≥126 mg/dL, a 2-hour plasma glucose level of ≥200 mg/dL or higher during a 75-g oral glucose tolerance test, a random plasma glucose of 200 mg/dL or higher on two occasions, or requiring medication for the management of hyperglycemia).(17) Patient survival, defined as time from LT to death of any cause, was a secondary outcome.
Baseline clinical and demographic characteristics were summarized by medians and interquartile ranges (IQR) for continuous variables or count (%) for categorical variables. Comparisons among patients with and without SVR at the time of LT were performed using chi-square and Kruskal-Wallis tests, when appropriate. Univariable and multivariable Cox proportional hazard models were used to compare crude and adjusted time to s-PTDM by time-varying SVR status. A comprehensive list of demographic and clinical variables was tested for association with s-PTDM and included in the multivariable Cox model if p<0.15. In addition, we included a priori selected demographic and clinical characteristics associated with s-PTDM: age at LT, gender, race, BMI at LT, and ACR events. These methods were repeated to explore associations between SVR, s-PTDM, and patient survival. Following the multivariable Cox proportional hazard model, the adjusted s-PTDM free survival and overall survival curves were plotted by SVR status. We tested Schoenfeld residuals to determine if the proportional hazards assumption was upheld. Two-sided p values <0.05 were considered statistically significant. Analyses were performed using STATA statistical software, version 14.0 (StataCorp, College Station, TX).
Results
From January 1, 2010 to December 31, 2015, 352 patients with HCV cirrhosis underwent LT (Figure 1). A total of 96 patients were excluded (67 patients with pre-LT diabetes mellitus, 16 patients with dual organ transplants, 7 patients with a prior solid organ transplant, and 6 patients with inadequate follow up time). 256 eligible patients were analyzed with a median follow up time of 41.2 months (range 7.6–83.9 months).
Figure 1.
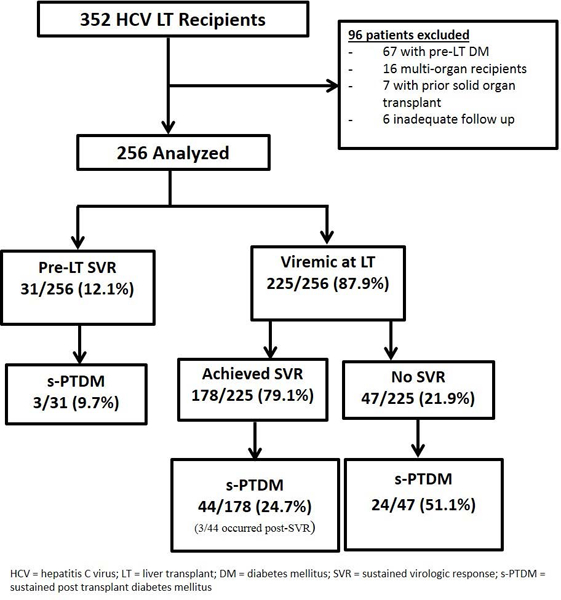
Cohort flow diagram of HCV liver transplant recipients
Baseline patient demographic and clinical characteristics at cohort entry are displayed in Table 1. The median age at LT was 59 years (IQR 54–63). The median physiologic MELD score at LT was 17 (IQR 12–25). Most patients had HCV genotype 1 infection (82.8%) and 167 (65.2%) patients had HCC on explant. All patients were prescribed a calcineurin inhibitor on discharge following LT. 31 (12.1%) patients achieved SVR prior to LT at a median time of 7 months prior to LT (IQR 1–30 month). An additional 178 (69.5%) achieved SVR following LT, at a median time of 23 months (IQR 10–43 months) post-LT. Of those who were viremic at LT, 26.4% (47/178) achieved SVR in the first post-LT year. Patients who achieved SVR following LT had a higher median MELD score at LT, were less likely to have HCC on explant pathology and were more likely to have received DAA therapy than those who achieved SVR pre-LT (Table 1).
Table 1.
Patient Characteristics
| Full Cohort (n = 256) | SVR at OLT (n = 31) |
Viremic at LT (n = 225) |
p value | |
|---|---|---|---|---|
| Males, n (%) | 201(78.5) | 26 (83.9) | 175 (77.8) | 0.44 |
| Age, years (median, IQR) | 59 (54–63) | 59 (55–62) | 59(54–63) | 0.98 |
| Race / Ethnicity | 0.39 | |||
| Caucasian, n (%) | 164 (64.1) | 23(74.2) | 141 (62.7) | |
| Hispanic, n (%) | 14(5.5) | 0(0.0) | 14 (6.2) | |
| African American, n (%) | 52 (20.3) | 6 (19.4) | 46 (20.4) | |
| Asian and Other, n (%) | 26 (10.2) | 2 (6.5) | 24 (10.7) | |
| Body Mass Index at LT | 0.88 | |||
| <25.0, n(%) | 80 (31.2) | 10(32.3) | 70 (31.1) | |
| 25.0–29.9, n (%) | 97 (37.9) | 12(38.7) | 85 (37.8) | |
| 30.0–34.9, n (%) | 45 (17.6) | 4 (12.9) | 41 (18.2) | |
| >35.0, n (%) | 34 (13.3) | 5 (16.1) | 29 (12.9) | |
| MELD score at LT (median,IQR) | 17 (12–25) | 13(9–18) | 18(12–26) | <0.01 |
| Hepatitis C Genotype | 0.34 | |||
| 1 a/b,n(%) | 212 (82.8) | 23 (74.2) | 189 (84.0) | |
| 2, n(96) | 17(6.6) | 3(9.7) | 14(6.2) | |
| 3, n (%) | 23(9.0) | 5 (16.1) | 18 (8.0) | |
| 4, n (%) | 4 (1.6) | 0 (0.0) | 4 (1.8) | |
| HCC on explant, n (%) | 167 (65.2) | 27 (87.1) | 140 (62.2) | 0.01 |
| LDLT, n (%) | 16 (6.25%) | 2(6.5) | 14(6.2) | 0.96 |
| CNI prescribed on discharge, n (%) | 256 (100) | 31 (100) | 225 (100) | 1.00 |
| SVR achieved prior to LT, n (%) | 31 (12.1) | |||
| SVR( achieved following LT, n(%) | 178(69.5) | 178 (79.1) | ||
| SVR achieved with DAA regimen, n (%) | 131(78.4) | 10(33.3) | 118 (89.4) | <0.01 |
IQR=interquartile range; LT=liver transplant; MELD= model for end stage liver disease; HCC=heatocellular carcinoma; LDLT= living donor liver transplant; CNI=calcineurin inhibitor; SVR=sustained virologic response;DAA=direct acting anti-viral
During follow up, 71 (27.7%) patients developed s-PTDM. Median time to the onset of de novo diabetes mellitus was 18 days (IQR 9–28 days) post LT. All patients with s-PTDM required insulin for at least 6 months. Only 3/71 (4.2%) patients were eventually able to discontinue all glucose lowering therapies. This occurred at 11, 17 and 26 months following LT.
Timing of viral eradication had an impact on the risk of s-PTDM. Of the 31 patients who achieved SVR prior to LT, 3 (9.67%) developed s-PTDM. In comparison, patients who were viremic at LT were more likely to develop s-PTDM (68/225; Chi squared =5.74, p=0.02). In univariable Cox regression, pre-LT SVR was associated with a reduced risk of s-PTDM (HR 0.30, p=0.046). Following adjustment for pertinent potential confounders present at the time of LT, include age, gender, race/ ethnicity, BMI, MELD, and presence of HCC, pre-LT SVR maintained a trend towards reduced incidence of s-PTDM (HR 0.36, p=0.09). Of the 225 subjects who were viremic at LT, 178 (79.1%) achieved SVR during the follow up period, but 41 (23.0%) patients developed s-PTDM prior to SVR. Only three of the remaining 137 patients (2.2%) developed s-PTDM following SVR (Figure 1). Furthermore, when excluding the 31 patients who had achieved SVR prior to LT, patients with SVR in the first post-LT year had a trend towards reduced risk of s-PTDM (HR 0.50, p=0.06).
In unadjusted analysis, Hispanic ethnicity, MELD score at LT, and SVR were associated with a reduced risk of s-PTDM (Table 2). A Cox multivariable regression model, with a priori selected covariables and variables with p<0.15 in univariable analysis, was fit (Table 2). Variables included in the model were gender, race/ethnicity, age at LT, BMI at LT, ACR, SVR and MELD score at LT (p<0.15 in univariable analysis). SVR was independently associated with a significantly reduced risk of s-PTDM (HR 0.40, p=0.048). Hispanic ethnicity was the only other variable found to be independently associated with s-PTDM (HR 2.78, p=0.01). The Cox adjusted s-PTDM free survival by SVR status is depicted in Figure 2. The 1, 3 and 5-year cumulative incidence of s-PTDM was 8.1%, 9.6% and 11.7% and 18.4%, 21.4%, and 25.1% for patients with and without SVR respectively. The test of Schoenfeld residuals indicate that the assumption of proportional hazards was not violated (p=0.15).
Table 2.
Univariable and Multivariable Models
| univariable Model | multivariable Model | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p value | HR | 95% CI | p value |
| Gender1 | ||||||
| Female | Ref | -- | -- | Ref | -- | -- |
| Male | 1.44 | 0.77–2.68 | 0.25 | 1.52 | 0.79–2.91 | 0.21 |
| Race/ Ethnicity1 | ||||||
| Caucasian. non. Hispanic | Ref | -- | - | ref | -- | -- |
| African American | 1.31 | 0.73–2.34 | 0.37 | 1.20 | 0.65–2.21 | 0.56 |
| Hispanic | 3.16 | 1.47–6.78 | <0.01 | 2.78 | 1.28–6.10 | 0.01 |
| Asian and tither | 1.32 | 0.62–2.83 | 0.47 | 1.30 | 0.60–2.80 | 0.50 |
| Ago at LT1 | 0.99 | 0.95–1.03 | 0.58 | 1.00 | 0.96–1.04 | 0.96 |
| HCC | 0.72 | 0.45–1.16 | 0.18 | -- | -- | -- |
| LDLT | 1.31 | 0.53–3.24 | 0.56 | -- | -- | -- |
| MELD score at LT2 | 1.02 | 1.00–1.05 | 0.03 | 1.04 | 0.99–1.09 | 0.11 |
| BMI at LT1 | 1.00 | 0.96–1.04 | 0.99 | 0.99 | 0.95–1.03 | 0.69 |
| CNI at discharge | 1.00 | -- | -- | -- | -- | -- |
| ACR events1 | 4.93 | 0.58–1.48 | 0.75 | 1.13 | 0.69–1.84 | 0.63 |
| SVR1 | 0.34 | 0.14–0.84 | 0.02 | 0.40 | 0.16–0.99 | 0.048 |
variable selected a priori for inclusion in the final multivariable model
Variables included in Multivariable model if p<0.15 in unvariable regression model
HR = hazard ratio; CI = confidence interval; LT= liver transplant; HCC = hepatocellular carcinoma; LDLT = living donor liver transplant; MELD= Model for End Stage Liver Disease; BMI = body mass index; CNI = calcineurin inhibitor; ACR = acute cellular rejection; SVR = sustained virologic response
Figure 2.
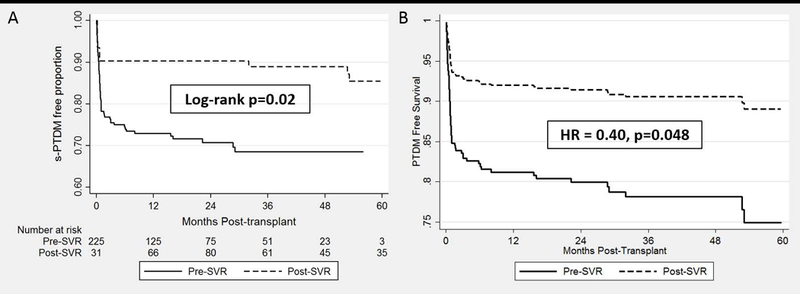
Kaplan Meier and Cox Adjusted s-PTDM Free Survival Estimates by SVR Status
A total of 34 (13.3%) patients died during follow up. Hispanic ethnicity (HR 3.04, p=0.048), African-American race (HR 2.42, p=0.02), MELD score at LT (HR 1.05, p=0.01) were associated with mortality, while HCC on explant (HR 0.48, p=0.03), and SVR (HR 0.30, p=0.01) were all associated with a survival benefit. There was a trend towards increased risk of death in patients with s-PTDM (HR 1.91, p=0.07). Following Cox multivariable regression model adjusting for a priori selected covariables (gender, age, race/ethnicity, and BMI) and variables with p<0.15 in univariable analysis, SVR was the only variable independently associated with post-LT survival (HR 0.38, p=0.04). The Cox adjusted overall survival by SVR status is illustrated in Figure 3.
Figure 3.
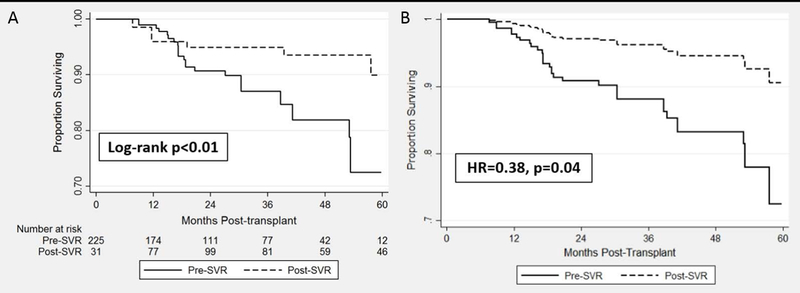
Kaplan Meier and Cox Adjusted Overall Survival Estimates by SVR Status
Discussion
The advent of DAA therapy for HCV has revolutionized the landscape of liver transplantation for HCV related chronic liver disease. Since the introduction of DAA therapy, there has been a significant decline in the number and percentage of LT waitlist registrants with HCV cirrhosis and percentage of LT surgeries performed for HCV.(18, 19) Post-LT SVR rates have risen from approximately 25% with interferon-based therapy to greater than 90% with DAA.(20, 21) By preventing the rapid progression of liver fibrosis associated with post-LT HCV infection, we will undoubtedly witness improvement in graft and patient survival for LT recipients with HCV. While the benefits of SVR in a post-transplant population may no longer be disputed, the impact of SVR on post-transplant metabolic complications is of interest. We explored the association of HCV viral eradication with the incidence of s-PTDM, a major co-morbidity following solid organ transplantation with few known modifiable risk factors. This large single center cohort study allowed the unique opportunity to use granular data to carefully examine variables which could affect the incidence of s-PTDM in a period spanning the introduction of DAA therapy, data which is lacking in larger data sets such as UNOS/OPTN.
We compared the incidence of s-PTDM among LT recipients with HCV related cirrhosis and no prior history of diabetes mellitus, using SVR as a time varying exposure. We observed that viral clearance was independently associated with a significantly reduced risk of s-PTDM and death. Timing of SVR plays an intricate role in this association. Pre-LT SVR, which was associated with a 70% reduction in the risk of s-PTDM in univariable analysis, appeared to have a significant influence on the development of de novo diabetes mellitus early after transplant. This is illustrated by the rapid divergence in the estimated s-PTDM free survival curves in Figure 2B. While the benefit of pre-LT SVR on s-PTDM was more evident, there also seemed to be an advantage to early post-LT SVR. Of those viremic at transplant, SVR in the first post-LT year was associated with a trend towards a reduced risk of s-PTDM.
This single center study has several strengths. First, we have robust data with long-term follow up of over 250 LT recipients over a period of time in which DAA use became standard of care. Second, use of automated data extraction techniques from the health system data warehouse allowed use of granular data elements including inpatient and outpatient encounter diagnoses, procedures codes, laboratory results, and medications prescribed. This facilitated a rigorous characterization of patient diabetes status according to the standard definition of the World Health Organization/American Diabetes Association, reducing the risk of misclassification which commonly occurs when using large registry data and allowing for differentiation between transient hyperglycemia following LT and s-PTDM. Finally, by using a time-varying definition for SVR, we could accurately establish a temporal relationship between the exposure of interest and the outcome.
The results presented should be interpreted with some limitations in mind. Use of SVR12 as a surrogate for viral eradication, rather than actual HCV RNA quantification, may impart up to 24 weeks of potential misclassified time for subjects on HCV therapy in which they are classified as “viremic” because SVR12 has not yet been achieved despite actual viral clearance shortly after initiating DAA therapy. However, if HCV eradication does indeed decrease the risk of s-PTDM, this misclassified period from the time of actual viral clearance to the observation of SVR12 would bias the results toward the null hypothesis. Second, while we were able to adjust for number of incident ACR events which may account for some heterogeneity in immunosuppressive therapy, our database lacked consistent longitudinal patient data on immunosuppressive management. Nonetheless, we do not think this biases our findings substantially because, being a single center study, single-organ LT recipients are treated in a relatively homogenous manner. This is demonstrated by 100% of the cohort receiving a calcineurin inhibitor on discharge following the transplant episode. Finally, as we did not have data pertaining to hemoglobin A1c, we could not discern the association between HCV therapy and glucose homeostasis in those patients who developed s-PTDM prior to viral eradication.
In summary, in this large single center experience, we have demonstrated s-PTDM to be a common complication following LT in patients with HCV. SVR is associated with a reduced risk of s-PTDM, independent of other classic risk factors for PTDM, including age, gender, race and ethnicity, BMI, and number of ACR episodes. Timing of SVR likely plays a vital role in risk reduction with pre-LT SVR having the most biologically plausible influence on post-LT de novo diabetes. HCV, a consistently reported risk factor for PTDM, is one of the only modifiable risk factors for s-PTDM. These findings indicate a potential non-graft related benefit to pre-transplant HCV therapy. However, this benefit must be appraised in the context of factors which influence the probability of the patient successfully undergoing liver transplantation, namely long wait time, MELD score, and the availability and use of HCV positive grafts in a given region. At this time, further investigations in larger and more contemporary cohorts are needed to confirm these findings and to investigate the impact of early HCV eradication on other post-transplant outcomes including renal function, graft rejection, and components of the metabolic syndrome.
Acknowledgments
Grants and financial support: This study was supported by the NIH T32-DK007740–19. This funding agency played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations
- ACR
acute cellular rejection
- CNI
calcineurin inhibitor
- DAA
direct acting anti-viral
- HCV
hepatitis C virus
- IQR
interquartile range
- LT
liver transplant
- HR
hazard ratio
- MELD
Model for end-stage liver disease
- PTDM
post-transplant diabetes mellitus
- s-PTDM
sustained post-transplant diabetes mellitus
- SVR
sustained virologic response
- t-PTDM
transient post-transplant diabetes mellitus
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement Transplant Network database
Footnotes
Conflicts of interest: KRR: Advisory Board: Abbvie, Merck, Gilead, Shionogi: Research support (Paid to the University of Pennsylvania): Abbvie, Gilead, Merck, Conatus, Intercept, Mallinckrodt; DSMD: Novartis.
None for others
References
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2015 annual data report: Liver. Am J Transplant. 2017. January;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007. March;102(3):570–6. [DOI] [PubMed] [Google Scholar]
- 3.Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: Meta-analysis. World J Gastroenterol. 2012. April 14;18(14):1642–51. PMCID: PMC3325531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007. July 15;166(2):196–203. [DOI] [PubMed] [Google Scholar]
- 5.Honda M, Asonuma K, Hayashida S, Suda H, Ohya Y, Lee KJ, et al. Incidence and risk factors for new-onset diabetes in living-donor liver transplant recipients. Clin Transplant. 2013. May-Jun;27(3):426–35. [DOI] [PubMed] [Google Scholar]
- 6.Sharif A, Cohney S. Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol. 2016. April;4(4):337–49. [DOI] [PubMed] [Google Scholar]
- 7.Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: Long-term follow up. Transplantation. 2006. December 27;82(12):1625–8. [DOI] [PubMed] [Google Scholar]
- 8.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, et al. Posttransplant diabetes mellitus in liver transplant recipients: Risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001. September 27;72(6):1066–72. [DOI] [PubMed] [Google Scholar]
- 9.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012. March;18(3):370–5. [DOI] [PubMed] [Google Scholar]
- 10.Roccaro GA, Goldberg DS, Hwang WT, Judy R, Thomasson A, Kimmel SE, et al. Sustained posttransplantation diabetes is associated with long-term major cardiovascular events following liver transplantation. Am J Transplant. 2018. January;18(1):207–15. PMCID: PMC5740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: A systematic review and meta-analysis. Am J Transplant. 2004. April;4(4):583–95. [DOI] [PubMed] [Google Scholar]
- 12.Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, et al. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection : An observational multicenter study. Liver Transpl. 2007. January;13(1):136–44. [DOI] [PubMed] [Google Scholar]
- 13.Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the organ procurement and transplant network/united network for organ sharing database. Transplantation. 2010. May 15;89(9):1134–40. [DOI] [PubMed] [Google Scholar]
- 14.Negro F, Alaei M. Hepatitis C virus and type 2 diabetes. World J Gastroenterol. 2009. April 7;15(13):1537–47. PMCID: PMC2669937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003. July;38(1):50–6. [DOI] [PubMed] [Google Scholar]
- 16.Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS,Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017. October;66(4):1090–101. PMCID: PMC5756478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010. January;33 Suppl 1:S62–9. PMCID: PMC2797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017. April;152(5):1090,1099.e1. PMCID: PMC5367965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the united states. Clin Gastroenterol Hepatol. 2017. December 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CS, Ko HH, Yoshida EM, Marra CA, Richardson K. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: A review and quantitative analysis. Am J Transplant. 2006. July;6(7):1586–99. [DOI] [PubMed] [Google Scholar]
- 21.Belli LS, Duvoux C, Berenguer M, Berg T, Coilly A, Colle I, et al. ELITA consensus statements on the use of DAAs in liver transplant candidates and recipients. J Hepatol. 2017. September;67(3):585–602. [DOI] [PubMed] [Google Scholar]


