Abstract
Background:
Multiple meta-analyses have shown sperm count declines in Western countries spanning eight decades. Secular trends in other parameters remain unclear, as are potential predictors of these trends.
Objective:
To analyze secular trends in semen quality and to evaluate whether factors previously found to be related to semen quality were responsible for these patterns.
Methods:
This is a prospective study including 936 men of couples seeking infertility treatment who provided 1,618 semen samples at a single center (2000-2017). Self-reported demographic, nutritional and reproductive characteristics were collected using standardized questionnaires. Urinary concentrations of bisphenol A, parabens and phthalates were quantified by isotope-dilution tandem mass spectrometry. Semen samples were analyzed for volume, sperm concentration, count, motility and morphology following WHO guidelines. We estimated the differences in semen parameters over time by fitting generalized linear mixed models with random intercepts to account for repeated samples while adjusting for abstinence time. We also adjusted for demographic, nutritional and environmental factors to investigate these as potential predictors of time trends.
Results:
Sperm concentration and count declined by 2.62% per year (95% CI −3.84, −1.38) and 3.12% per year (95% CI: −4.42, −1.80), corresponding to an overall decline of 37% and 42%, respectively, between 2000 and 2017. Decreasing trends were also observed for total motility (per year: −0.44 percentage units, 95% CI −0.71, −0.17) and morphologically normal sperm (per year: −069 percentage units, 95% CI −0.116, −0.023). These decreases reflected relative percentage declines of 15% and 16% over the 17 year study period, respectively. When reproductive factors were included in the model, the downward trends in sperm concentration and sperm count were attenuated by 29% and 26%, respectively, while the trends in motility and morphology were attenuated by 54% and 53%, respectively. Also, the downward trends in both sperm concentration and sperm morphology over time were attenuated by 19% when including the DEHP and non-DEHP metabolites, respectively.
Conclusions:
Sperm concentration, total count, motility and morphology significantly declined between 2000 and 2017 among subfertile men. These negative trends were attenuated when considering simultaneous changes in reproductive characteristics and urinary phthalates during the course of the study.
Keywords: Semen parameters, secular trends, phthalates, predictors, male infertility
1. Introduction
Male factors, defined according to World Health Organization (WHO) reference values for semen quality, account for 40% of infertility cases (Legare et al. 2014). In addition, poor semen quality has been also associated with higher risk of common chronic diseases (Eisenberg et al. 2016; Latif et al. 2017) and mortality (Eisenberg et al. 2014; Jensen et al. 2009), highlighting their public health importance beyond fertility and reproduction. Whether semen quality parameters have declined has been a matter of ongoing research and debate since 1992, when Carlsen et al published a meta-analysis showing a decline in semen quality in Western countries starting in the late 1930s (Carlsen et al. 1992). Others have replicated these findings both in meta-analyses (Swan et al. 1997) and primary analyses from single centers (Feki et al. 2009; Splingart et al. 2011; Sripada et al. 2007). Furthermore, decreasing semen parameters have coincided with an increasing frequency of male reproductive disorders (Skakkebaek et al. 2016). More recently, a rigorous systematic review and meta-regression of 185 studies (42,935 men) excluding subfertile and infertile men confirmed a significant decline in sperm concentration and total sperm count between 1973 and 2011 (Levine et al. 2017).
Despite the consistency across multiple meta-analyses, two major gaps remain in this literature. First, there is little data regarding long-term trends in sperm motility and morphology, important markers of sperm function. Second, while many authors have suggested that these consistent downward trends may be the result of concomitant changes in “environmental factors,” no study to date has formally tested this hypothesis. To address these gaps, we took advantage of an ongoing study recruiting men attending a single fertility center, in which we have used standardized and rigorous methods to assess a wide range of participant characteristics and lifestyle factors. We analyzed data from men recruited between 2000 and 2017 to investigate the secular trends in semen parameters, and evaluate whether demographic, reproductive, nutritional and environmental factors previously related to semen quality, contributed to the observed secular trends in semen quality.
2. Methods
2.1. Study population
Participants were men in couples seeking infertility treatment at the Massachusetts General Hospital (MGH) between 2000 and 2017. Men, aged 18 to 56 years, and without a history of vasectomy were eligible to participate in the study which aimed to identify environmental determinants of fertility (Meeker et al. 2011; Messerlian et al. 2018). Approximately 50% of those contacted by the research nurses were enrolled. Semen quality did not differ between men who enrolled in the study and men who did not enroll (Hauser et al. 2005). After the study procedures were explained and all questions were answered, participants signed an informed consent form. The study was approved by the Human Subject Committees of the Harvard T.H. Chan School of Public Health and MGH. Between 2000 and 2004, each man provided one semen sample. Starting in 2005, consent procedures changed, allowing access to all diagnostic semen samples of men consenting. The final study sample included 936 men who provided a total of 1,618 semen samples, after excluding 22 men who were azoospermic and 15 who did not provide a semen sample. Of these, in 1,482 semen samples morphologically normal sperm was assessed.
2.2. Assessment of potential predictors
The participant’s date of birth was collected at entry, and weight and height were measured by trained study staff. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. The participants completed a nurse-administered questionnaire that contained additional questions on lifestyle factors, reproductive health, and medical history. Time spent in leisure time physical and sedentary activities was assessed using a validated questionnaire (Wolf et al. 1994). Starting in 2007, diet was assessed using an extensively validated food frequency questionnaire (FFQ) (Yuan et al. 2017) and two data-derived dietary patterns, the ‘Prudent’ and the ‘Western’, were calculated based on reported food intakes using principal component analysis (Gaskins et al. 2012). Urine samples were collected the same day the semen samples were collected. Urinary concentrations of bisphenol A (BPA), methylparaben (MPB), propylparaben (PPB), mono-n-butyl phthalate (mBP), mono-isobutyl phthalate (miBP), monoethyl phthalate (mEP), monobenzyl phthalate (mBzP), mono-2-ethylhexyl phthalate (mEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (mEHHP), mono-2-ethyl-5-oxohexyl phthalate (mEOHP), mono-2-ethyl-5-carboxypentyl phthalate (mECPP) were quantified using tandem mass spectrometry and adjusted for specific gravity as described in detail elsewhere (Hauser et al. 2016; Silva et al. 2007; Ye et al. 2005). For these chemicals, the percentage of urine samples below the limit of detection (LOD) ranged from 1 to 12, with the exception of mEHP that 22% of the urines had concentrations <LOD.
2.3. Semen assessment
Semen samples were collected on site at MGH in a sterile plastic specimen cup following a recommended 48-hour abstinence period (Meeker et al. 2010; Minguez-Alarcon et al. 2017). Of the 936 men in the study, 648 (69%) contributed 1 semen sample, 128 (14%) contributed 2 samples, 63 (7%) contributed 3 samples, and 97 (10%) contributed 4 or more samples (range=4-11). Semen volume (mL) was measured by an andrologist using a graduated serological pipet. Sperm concentration (mil/mL) and motility (% motile) were assessed using a computer-aided semen analyzer (CASA; 10HTM-IVOS, Hamilton-Thorne Research, Beverly, MA). To measure semen concentration and motility, 6 μl of semen was placed into a pre-warmed (37°C) and disposable Leja Slide (Spectrum Technologies, CA, USA). A minimum of 200 sperm cells from at least four different fields were analyzed from each specimen. Total sperm count (mil/ejaculate) was calculated by multiplying sperm concentration by semen volume. Motile spermatozoa were defined as according to the World Health Organization (WHO) four-category scheme: rapid progressive, slow progressive, non-progressive, and immotile (World Health Organization 2010). Sperm morphology (% normal) was assessed on two slides per specimen (with a minimum of 200 cells assessed per slide) via a microscope with an oil-immersion 100× objective (Nikon, Tokyo, Japan). Strict Kruger scoring criteria was used to classify men as having normal or below normal morphology (Kruger et al. 1988). Andrologists were trained in semen analysis and participated in rigorous daily and weekly internal quality control (QC) and external monitoring of within and between observer variation over all the entire study period as required to maintain CLIA certification and accreditation by the College of American Pathologists
2.4. Statistical analysis
Demographic, reproductive, nutritional, urinary concentrations of environmental chemicals, and semen quality parameters were presented using median ± interquartile ranges (IQRs) or percentages. Differences in these factors across years of the study period were evaluated using linear regression models, and random subject effects were used to account for repeated measurements within the same man for abstinence time and environmental factor models. Sperm concentration and total count had skewed distributions and were natural log-transformed before analysis to more closely approximate a normal distribution. Multivariable generalized linear mixed models with to estimate the differences in semen parameters across the years, adjusting for abstinence time (days). Specifically, we used the model
where yij represents individual semen analyses for each man (i) at different times (j), xij represents the vector of covariates for each man at different times, vi represents the random intercept for each individual and eij represents the random error term. The independent variable, year, was used as categorical to report the marginal means per year and continuous to calculate the decrease per year. To allow for better interpretation of the results, population marginal means (Searle et al. 1980) were presented at the mean level for continuous covariates and the weighted frequency for categorical variables. Potential non-linear trends over time were also explored by including year (expressed as year-2000) as a quadratic variable in the models. We evaluated the robustness of the findings by: 1) restricting analyses to one semen sample (first sample) per man, 2) excluding from analyses all samples below the WHO reference limits, and 3) excluding men who did not complete the FFQ and therefore they had missing nutritional data.
To investigate whether secular trends in semen quality were explained by factors previously related to semen quality in this population, we included each of these factors individually as covariates in the regression models (one at a time, and also in groups). The relative change was calculated as a percentage for each semen parameter, comparing the slope estimate between the adjusted model and the crude model, restricting to just those men with available data for the specific factor(s) evaluated. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Results
Participants were mostly white (85%), 31% had ever smoked and they had a median (interquartile range [IQR]) age of 35.7 (32.7, 39.6) years and BMI of 27.1 (24.8, 29.9) kg/m2 (Table 1). More than half (56%) of the men reported undergoing a previous infertility exam and 77 (10%) had a history of varicocele. Most men’s semen quality parameters were above the WHO 2010 reference levels, although the proportion of men with values below these limits (Cooper et al. 2010) was greater than 10% for all semen parameters (Supplemental Table S1). Some participant characteristics changed significantly over the study period (Table 1). Over the 17 years of enrollment, there was a slight decrease in having a history of previous infertility exam (−0.17% per year) and in the duration of abstinence (−0.10 days per year). Demographic as well as nutritional characteristics (BMI, physical activity, intake of alcohol and caffeine, and summary measures of diet quality) remained stable over time (Table 1). Similarly, urinary concentrations of parabens remained stable over the study period. In contrast, urinary concentrations of BPA and most phthalate metabolites decreased significantly over the study period with the exception of miBP, which increased significantly, and mBP and mCPP which remained stable (Table 1).
Table 1.
Demographic, reproductive history and other lifestyle characteristics among men attending a fertility center in Boston between 2000 and 2017.
| Sample size |
Total cohort median (IQR) or N (%) |
Change per year β, p-value |
||
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 936 | 35.7 (32.7, 39.6) | 0.04 | 0.26 |
| Race, % White | 936 | 798 (85) | 0.02 | 0.26 |
| Ever smoked, % | 936 | 290 (31) | −0.02 | 0.25 |
| Reproductive characteristics | ||||
| Previous infertility exam (yes), % | 915 | 514 (56) | −0.17 | <.0001 |
| Ever made partner pregnant (yes), % | 906 | 358 (40) | 0.01 | 0.37 |
| Varicocele (yes), % | 786 | 77(10) | 0.02 | 0.42 |
| Abstinence time, days | 1,618 | 2.42 (2.00, 3.33) | −0.10 | <.0001 |
| Nutritional factors | ||||
| BMI, kg/m2 | 936 | 27.1 (24.8, 29.9) | 0.01 | 0.65 |
| Physical activity, hrs/day | 436 | 4.00 (0.27, 9.34) | 0.17 | 0.31 |
| Prudent patterna | 276 | −0.20 (−0.72, 0.56) | 0.04 | 0.09 |
| Western patterna | 276 | −0.14 (−0.64, 0.54) | −0.02 | 0.33 |
| Alcohol intake, g/day | 276 | 9.64 (3.07, 19.7) | −0.03 | 0.94 |
| Caffeine intake, mg/day | 276 | 160 (71.2, 271) | 2.40 | 0.40 |
| Environmental factors | ||||
| Urinary concentrations, ng/mL: | ||||
| BPA | 1,108 | 1.12 (0.67,2.00) | −0.06 | 0.07 |
| MPB | 1,066 | 20.3 (8.70, 63.8) | 1.34 | 0.53 |
| PPB | 1,066 | 1.84 (0.52, 10.5) | −0.29 | 0.40 |
| mEP | 1,378 | 52.3 (19.5, 170) | −29.8 | <.0001 |
| mBP | 1,378 | 10.3 (5.6, 17.9) | −3.59 | 0.18 |
| miBP | 1,023 | 6.34 (3.50, 11.1) | 0.55 | 0.0007 |
| mCPP | 1,023 | 2.70 (1.31, 6.98) | 0.72 | 0.19 |
| mBzP | 1,378 | 3.67 (1.80, 7.22) | −0.63 | <.0001 |
| mEHP | 1,378 | 2.66 (1.12, 7.00) | −1.76 | <.0001 |
| mEHHP | 1,154 | 13.3 (6.44, 32.9) | −11.7 | <.0001 |
| mEOHP | 1,154 | 7.93 (3.82, 20.1) | −7.38 | <.0001 |
| mECPP | 931 | 17.6 (9.00, 39.1) | −20.0 | <.0001 |
Abbreviations: BMI, Body Mass Index; BPA, bisphenol A; BPB, methylparaben; PPB, propylparaben; mBP, mono-n-butyl phthalate; miBP, mono-isobutyl phthalate; mEP, monoethyl phthalate; mBzP, monobenzyl phthalate; mEHP, mono-2-ethylhexyl phthalate; mEHHP, mono-2-etliyl-5-hydroxyhexyl phthalate; mEOHP, mono-2-ethyl-5-oxohexyl phthalate; mECPP, mono-2-ethyl-5-carboxypentyl phtlialate; mCPP, mono-3-carboxypropyl phtlialate.
The dietary patterns do not have units since they are calculated using principal component analysis (Gaskins et a., 2012).
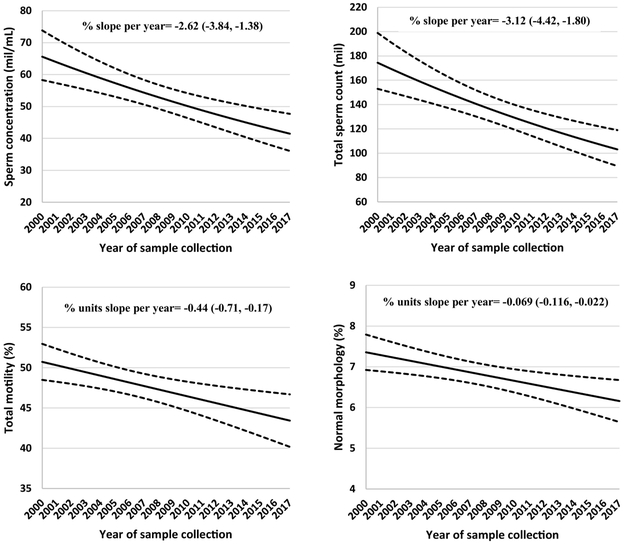
Sperm concentration, total count, total motility and percent morphologically normal sperm decreased significantly over the study period (2000-2017) (Table 2, Figure 1). In models adjusted for abstinence time, sperm concentration and total sperm count declined by 2.62% per year (95% CI −3.84, −1.38) and 3.12% per year (95% CI: −4.42, −1.80), corresponding to an overall decline of 37% and 42%, respectively, between 2000 and 2017 (Figure 1). Decreasing trends were also observed for total motility (per year: −0.44 percentage units, 95% CI −0.71, −0.17) and morphologically normal sperm (per year: −0.069 percentage units, 95% CI −0.116, −0.023) (Figure 1. These decreases reflected relative percentage declines of 15% and 16% over the 17 year study period, respectively. Ejaculate volume remained stable over the study period (Table 2). There was no evidence of non-linearity in the time trends in semen quality (data not shown).
Table 2.
Predicted marginal means (95% CI) for semen parametersa over time among 936 men (contributing 1,618 semen samples) attending a fertility center in Boston between 2000 and 2017.
| Year of sample collection |
N (samples) |
Ejaculate Volume (mL) |
Sperm Concentration (mil/mL) |
Total Sperm Count (mil/ejaculate) |
Total Motility (%) |
Normal Morphologyb (%) |
|---|---|---|---|---|---|---|
| 2000 | 151 | 2.69 (2.46, 2.92) | 72.8 (60.5, 87.6) | 168 (137, 206) | 52.0 (48.3, 55.6) | 7.49 (6.74, 8.25) |
| 2001 | 130 | 3.09 (2.81,3.38)* | 58.5 (47.4, 72.2) | 157 (126, 196) | 49.3 (45.1, 54.6) | 7.17 (6.42, 7.93) |
| 2002 | 93 | 3.43 (3.05, 3.81)* | 65.5 (52.0, 82.5) | 187 (148, 236) | 49.8 (45.1, 54.6) | 7.17 (6.29, 8.05) |
| 2003 | 73 | 3.28 (2.86, 3.71)* | 64.3 (50.3, 82.1) | 182 (141,232) | 48.3 (43.4, 53.2) | 7.16 (6.28, 8.03) |
| 2004 | 55 | 3.58 (3.08,4.07)* | 56.9 (42.6, 75.9) | 174 (132, 231) | 48.8 (43.8, 53.8) | 8.27 (7.01, 9.53) |
| 2005 | 30 | 2.97 (2.37, 3.58) | 57.8 (40.5, 82.5) | 151 (107, 214) | 51.2 (44.8, 57.5) | 5.47 (3.96, 6.99)* |
| 2006 | 76 | 3.13 (2.85, 3.41)* | 71.3 (54.7, 92.9) | 203 (152, 270) | 47.9 (41.5, 54.4) | 6.26 (5.12, 7.40)ǂ |
| 2007 | 71 | 2.95 (2.62, 3.28) | 47.9 (39.5, 58.0)* | 123 (98.9, 152)* | 48.4 (43.5, 53.3) | 6.35 (5.45, 7.25)ǂ |
| 2008 | 121 | 2.90 (2.65, 3.16) | 53.3 (46.3, 61.3)* | 140 (119, 164) | 45.8 (42.0, 49.5)* | 5.40 (4.75, 6.05)* |
| 2009 | 139 | 2.71 (2.49, 2.93) | 52.9 (45.2, 61.9)* | 128 (110, 150)* | 45.4 (41.9, 49.0)* | 6.72 (5.87, 7.57) |
| 2010 | 134 | 2.73 (2.50, 2.96) | 51.4 (44.5, 59.4)* | 124 (106, 144)* | 43.6 (39.5, 47.6)* | 6.39 (5.66, 7.11)* |
| 2011 | 113 | 2.74 (2.51, 2.96) | 48.4 (41.2, 56.8)* | 119 (99.7, 140)* | 42.3 (38.0, 46.6)* | 6.34 (5.58,7.09)* |
| 2012 | 73 | 2.87 (2.60, 3.14) | 46.8 (37.4, 58.4)* | 118 (92.1, 151)* | 41.6 (36.0, 47.1)* | 6.31 (5.50, 7.12)* |
| 2013 | 97 | 2.73 (2.49, 2.97) | 42.2 (35.4, 50.2)* | 99.2 (80.0, 123)* | 41.2 (37.7, 44.7)* | 6.69 (5.84, 7.54) |
| 2014 | 77 | 2.89 (2.59, 3.19) | 45.8 (38.7, 54.1)* | 117 (96.2, 143)* | 48.5 (44.3, 52.8) | 7.58 (6.86, 8.29) |
| 2015 | 72 | 2.62 (2.25, 2.99) | 41.7(33.7, 51.5)* | 91.9 (72.1, 117)* | 48.2 (43.1, 53.2) | 6.21 (5.32, 7.09)* |
| 2016/2017 | 113 | 2.92 (2.61, 3.22) | 49.0 (39.8, 60.2)* | 122 (98.0, 153)* | 45.8 (41.1, 50.6)* | 6.08 (5.36, 6.91)* |
Abbreviations: ml, milliliters; mil, million.
Adjusted for abstinence time (days).
Morphologically nonnal sperm was assessed in 1,482 semen samples.
p-value≤0.05 when compared against samples obtained in 2000.
p-value<0.10 when compared against samples obtained in 2000.
Figure 1. Secular trends in sperm concentration, total sperm count, sperm motility and normal morphology among men attending a fertility center in Boston between 2000 and 2017.
The solid lines represent the adjusted mean for semen parameters by year of sample collection, the dotted lines are the upper and lower 95% confidence intervals for the adjusted means. Adjusted means are presented for average abstinence time (2 days). N=936 men and 1,618 semen samples for all parameters except sperm morphology, which was assessed in 1,482 semen samples.
We tested the robustness of our findings in a series of sensitivity analyses. Downward trends were still observed for sperm concentration, total count, motility and morphology when analyses were restricted to the first semen sample per man, when samples with values below WHO 2010 reference limits were excluded, and in analyses restricted to men who had completed dietary assessments (Supplemental Table S2). The downward trends for all parameters were of similar magnitude in the analyses restricted to men with diet data, steeper in analyses restricted to one sample per man, and less steep when excluding men below WHO 2010 reference limits.
Lastly, we investigated whether demographic, reproductive, nutritional and environmental exposures previously related or hypothesized to be related to semen quality could explain the secular trends in semen quality. When reproductive factors were included in the model, the downward trends in sperm concentration and sperm count were attenuated by 29% and 26%, respectively, while the trends in motility and sperm morphology were attenuated by 54% and 53%, respectively (Table 3). Most of the attenuation resulted from an increase in the proportion of men with a previous infertility exam (Supplemental Table S3). The observed trends were also attenuated when including urinary concentrations of phthalates in the models. Specifically, the downward trends in both sperm concentration and morphology over time were attenuated by 19% when including the DEHP and non-DEHP metabolites, respectively (Table 3). When individual metabolites were examined, urinary mECPP accounted for most of the contribution of DEHP metabolites towards decreasing sperm concentration, and urinary concentrations of miBP and mCPP accounted for most of the contribution of non-DEHP metabolites to the decreasing trend in morphologically normal sperm (Supplemental Table S3). The observed trends in sperm counts (sperm concentration and total count) were not substantially attenuated by including any of the demographic, nutritional or other environmental exposures considered (BPA and parabens). (Table 3, Supplemental Table S3).
Table 3.
Decrease in sperm parameters (per year) over time after adjusting for different factors among men attending a fertility center in Boston between 2000 and 2017.
| N (men /samples) |
Sperm concentratio n (mil/mL) |
Total sperm count (mil) |
Total motility (%) |
Normal morphology (%) |
|
|---|---|---|---|---|---|
| Models | % 95% CI |
% 95% CI |
% units 95% CI |
%units 95% CI |
|
| Main analysis | 936 / 1,618 | −2.62(−3.84, −1.38) | −3.12(−4.42, −1.80) | −0.44(−0.71, −0.17) | −0.069(−0.116, −0.022) |
| + Demographics | 936 / 1,618 | −2.70 (−3.92, −1.47) |
−3.10 (−4.39, −1.79) |
−0.43 (−0.70, 0.17) |
−0.069 (−0.116, −0.022) |
| + Reproductive | 764 / 1,408 | −1.84 (−3.26, −0.40) |
−2.09 (−3.62, −0.55) |
−0.31 (−0.61, −0.01) |
−0.025 (−0.076, 0.026) |
| + Energy balance | 436 / 1,118 | −2.41 (−4.80, 0.03) | −3.30 (−5.81,−0.73) | −0.21 (−0.74, 0.32) | 0.055 (−0.038, 0.148) |
| + Diet quality | 276 / 765 | −2.57 (−5.55, 0.50) | −2.47 (−6.00, 1.20) | −0.49 (−1.18, 0.21) | 0.051 (−0.069, 0.171) |
| + BPA | 596 / 1,108 | −2.46 (−4.40, −0.85) | −2.23 (−4.01, −0.43) | −0.27 (−0.61, 0.08) | −0.063 (−0.129, 0.002) |
| + Parabens | 576 / 1,066 | −2.49 (−4.08, −0.88) | −2.24 (−4.03, −0.43) | −0.26 (−0.61, 0.09) | −0.067 (−0.133, −0.001) |
| + DEHP | 419 / 931 | −1.57 (−4.16, 1.08) | −2.95 (−5.69, −0.13) | 0.03 (−0.51, 0.57) | 0.008 (−0.098, 0.114) |
| + non-DEHP | 511 / 1,023 | −2.13 (−4.04, 0.18) | −3.43 (−5.45, −1.36) | −0.18 (−0.62, 0.26) | −0.017 (−0.096, 0.063) |
Main analysis: abstinence time.
Demographics: main analysis + age, race and smoking.
Reproductive characteristics: main analysis + previous infertility exam, ever made partner pregnant, varicocele.
Energy balance: main analysis + BMI and physical activity.
Diet quality: main analysis + Prudent and Western diet patterns.
BPA: main analysis + BPA
Parabens: main analysis + MPB+PPB
DEHP: main analysis + mEHP+mEHHP+mEOHP+mECPP
Non-DEHP: main analysis + miBP+mBP+mBzP+mEP+mCPP
Note: Each model included the semen samples with available data for all the covariates in that specific model.
Abbreviations: BMI, Body Mass Index; BPA, bisphenol A; DEHP, di(2-ethylhexyl)phthalate; MPB, methylparaben; PPB, propylparaben; mBP, mono-n-butyl phthalate; miBP, mono-isobutyl phthalate; mEP, monoethyl phthalate; mBzP, monobenzyl phthalate; mEHP, mono-2-ethylhexyl phthalate; mEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate; mEOHP, mono-2-ethyl-5-oxohexyl phthalate; mECPP, mono-2-ethyl-5-carboxypentyl phthalate; mCPP, mono-3-carboxypropyl phthalate.
4. Discussion
We observed significant downward trends in sperm concentration, total count, total motility and morphologically normal sperm in semen samples of men attending a fertility center in Boston, MA between 2000 and 2017. Yearly decrease in semen parameters were attenuated up to 54% when including reproductive characteristics in the models. The downward trends in sperm concentration and morphology also were attenuated by 19% when including urinary phthalate concentrations in the models. Including several demographic, nutritional and other environmental factors previously related to semen quality in this and other studies (Chavarro et al. 2010; Cutillas-Tolin et al. 2015; Gaskins et al. 2014; Gaskins and Chavarro 2018; Meeker et al. 2010; Meeker et al. 2011), did not substantially change the secular trends in this study population of men attending a fertility center. If replicated, these results may help to reduce economic burden of male infertility and the associated morbility and mortality in men (Eisenberg et al. 2014; Eisenberg et al. 2016; Hauser et al. 2015; Latif et al. 2017).
Our results are consistent with three meta-analyses, collectively covering the period between 1934 and 2013, that have documented downward trends in sperm concentration and total sperm county. In the first of these meta-analyses on 61 studies, which excluded subfertile men, Carlsen et al reported a decline in total sperm concentration of 1% per year between 1940 and 1990 (Carlsen et al. 1992). Swan and colleagues (Swan et al. 1997) extended this meta-analysis by including 101 studies and reported a downward trend in sperm concentration (0.94 mil/mL per year). More recently, Levine et al conducted an even larger meta-analysis including 185 studies and 42,935 men, again excluding subfertile and infertile men. This meta-analysis confirmed significant declines in sperm concentration and total sperm count between 1973 and 2011 (−0.75% per year and 28.5% overall, for both outcomes (Levine et al. 2017), which were particularly pronounced among Western men.
While compelling, these meta-analyses were limited in their ability to examine secular trends in sperm motility and morphology. They also excluded studies conducted in fertility centers and were unable to adjust for potential time trends in factors that may impact semen quality. In contrast, our single fertility center study, albeit smaller in sample size, was able to consider sperm motility and morphology and adjust for some environmental and nutritional trends. Other studies have also explored trends using data on infertile couples and included measures of motility and morphology. Overall declines in sperm concentration and morphology were reported among French male partners of women with bilateral tubal blockage between 1989 and 2005, although no decrease in motility was observed (Rolland et al. 2013). Sperm concentration also decreased significantly among men seeking evaluation for infertility in Vienna between 1986 and 2003, but no secular trends were observed for motility or normal morphology (Lackner et al. 2005). No evidence of deteriorating semen quality based on sperm counts were found among infertile men in Northeastern Spain between 1960 and 1996 (Andolz et al. 1999) or among Swedish men in infertile couples between 1985 and 1995 (Berling and Wolner-Hanssen 1997). In addition, one recent study including over 6,000 young men attending the military system in Copenhagen (Denmark) found, overall, no persistent temporal trends in semen quality, testicular volume or levels of follicle-stimulating hormone over the 21 years studied (1996-2016) (Priskorn et al. 2018).
Studies evaluating secular trends in semen quality conclude that such trends may be explained by poorly specified environmental factors (Carlsen et al. 1992; Levine et al. 2017; Swan et al. 1997). However, to our knowledge, no study to date has tested the hypothesis that specific environmental factors are explanations for a downward trend in semen quality. Including environmental factors, such as BPA and parabens, and energy/nutritional factors that we have previously found related to or hypothesized to impact semen quality in our cohort (Chavarro et al. 2010; Gaskins et al. 2014; Meeker et al. 2010; Meeker et al. 2011) did not substantially change the downward trends in semen parameters. However, the downward trends in sperm concentration and normal morphology were attenuated when including urinary concentrations of phthalates in the models. Our results do not rule out the possibility that these consistent trends in semen quality may be explained by yet to be determined environmental factors.
The most salient strength of the study was our ability to evaluate multiple environmental correlates of semen quality as potential contributors to the downward trends in semen quality, which has not been addressed in any previous study and that was made possible by the long-term, consistent and standardized assessment of environmental, nutritional and lifestyle factors in our study. In addition and in contrast to the meta-analyses, we were able to evaluate long-term trends in sperm motility and morphology. Although there are important strengths imparted by studying an infertility clinic population, the choice of study population also represents a limitation. It is uncertain whether our findings can be generalized to men in the general population and in non-Western countries. However, our findings are comparable to those observed in studies which excluded men with known fertility issues. This may be because men in our study tended to have good semen quality compared to international reference standards (World Health Organization 2010) and fertile men (Levine et al. 2017) and findings remained unchanged when samples below WHO reference limits were excluded. Another limitation is the lack of data on all potential predictors in all study participants over the study period, which prevented us from evaluating potential contributors to the trends in semen quality simultaneously adjusting for all potential predictors.
5. Conclusion
Overall declines in sperm concentration, total count, total motility and morphologically normal sperm were observed among men attending a fertility center in Boston between 2000 and 2017. These decreases in temporal trends were attenuated when reproductive characteristics and urinary phthalate concentrations were considered in the models. Further research is needed to confirm these results in our study and others, given their potential public health consequences for fertility and overall male health.
Supplementary Material
Highlights.
-
1)
Sperm concentration and total count declined 37% and 42%, respectively, between 2000 and 2017 among men attending a fertility center in Boston.
-
2)
Total motility and morphologically normal sperm declined 15% and 16% over the 17 year study period, respectively.
-
3)
When reproductive factors were included in the model, the downward trends in sperm concentration and sperm count were attenuated by 29% and 26%, respectively, while the trends in motility and morphology were attenuated by 54% and 53%, respectively.
-
4)
The downward trends in both sperm concentration and sperm morphology over time were attenuated by 19% when including the DEHP and non-DEHP metabolites, respectively.
Acknowledgments:
The authors gratefully acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research staff Jennifer Ford and Myra Keller, and the physicians and staff at Massachusetts General Hospital fertility center. A special thank you to all of the study participants.
Funding: The project was funded by grants R01ES022955 and R01ES009718, P30ES000002, P30DK06200 and K99ES026648 from the National Institutes of Health.
Footnotes
Competing financial interests: The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andolz P, Bielsa MA, Vila J. 1999. Evolution of semen quality in north-eastern spain: A study in 22,759 infertile men over a 36 year period. Human reproduction (Oxford, England) 14:731–735. [DOI] [PubMed] [Google Scholar]
- Berling S, Wolner-Hanssen P. 1997. No evidence of deteriorating semen quality among men in infertile relationships during the last decade: A study of males from southern sweden. Human reproduction (Oxford, England) 12:1002–1005. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. 1992. Evidence for decreasing quality of semen during past 50 years. BMJ (Clinical research ed) 305:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. 2010. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertility and sterility 93:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. 2010. World health organization reference values for human semen characteristics. Human reproduction update 16:231–245. [DOI] [PubMed] [Google Scholar]
- Cutillas-Tolin A, Minguez-Alarcon L, Mendiola J, Lopez-Espin JJ, Jorgensen N, Navarrete-Munoz EM, et al. 2015. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Human reproduction (Oxford, England) 30:2945–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, et al. 2014. Semen quality, infertility and mortality in the USA. Human reproduction (Oxford, England) 29:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Cullen MR, Baker LC. 2016. Increased risk of incident chronic medical conditions in infertile men: Analysis of united states claims data. Fertility and sterility 105:629–636. [DOI] [PubMed] [Google Scholar]
- Feki NC, Abid N, Rebai A, Sellami A, Ayed BB, Guermazi M, et al. 2009. Semen quality decline among men in infertile relationships: Experience over 12 years in the south of tunisia. Journal of andrology 30:541–547. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. 2012. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. Jama 307:491–497. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. 2012. Dietary patterns and semen quality in young men. Human reproduction (Oxford, England) 27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Afeiche MC, Hauser R, Williams PL, Gillman MW, Tanrikut C, et al. 2014. Paternal physical and sedentary activities in relation to semen quality and reproductive outcomes among couples from a fertility center. Human Reproduction 29:2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Chavarro JE. 2018. Diet and fertility: A review. American journal of obstetrics and gynecology 218:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. 2018. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007–2008 to 2015–2016. Jama 319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Godfrey-Bailey L, Chen Z. 2005. Does the potential for selection bias in semen quality studies depend on study design? Experience from a study conducted within an infertility clinic. Human reproduction (Oxford, England) 20:2579–2583. [DOI] [PubMed] [Google Scholar]
- Hauser R, Skakkebaek NE, Hass U, Toppari J, Juul A, Andersson AM, et al. 2015. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the european union. The Journal of clinical endocrinology and metabolism 100:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. 2016. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the earth study. Environmental health perspectives 124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. 2009. Good semen quality and life expectancy: A cohort study of 43,277 men. American journal of epidemiology 170:559–565. [DOI] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. 1988. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertility and sterility 49:112–117. [DOI] [PubMed] [Google Scholar]
- Lackner J, Schatzl G, Waldhor T, Resch K, Kratzik C, Marberger M. 2005. Constant decline in sperm concentration in infertile males in an urban population: Experience over 18 years. Fertility and sterility 84:1657–1661. [DOI] [PubMed] [Google Scholar]
- Latif T, Kold Jensen T, Mehlsen J, Holmboe SA, Brinth L, Pors K, et al. 2017. Semen quality as a predictor of subsequent morbidity: A danish cohort study of 4,712 men with long-term follow-up. American journal of epidemiology 186:910–917. [DOI] [PubMed] [Google Scholar]
- Legare C, Droit A, Fournier F, Bourassa S, Force A, Cloutier F, et al. 2014. Investigation of male infertility using quantitative comparative proteomics. Journal of proteome research 13:5403–5414. [DOI] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. 2017. Temporal trends in sperm count: A systematic review and meta-regression analysis. Human reproduction update 23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. 2010. Semen quality and sperm DNA damage in relation to urinary bisphenol a among men from an infertility clinic. Reproductive toxicology (Elmsford, NY) 30:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. 2011. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environmental health perspectives 119:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, et al. 2018. The environment and reproductive health (earth) study: A prospective preconception cohort. Human Reproduction Open 2018:hoy001-hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Afeiche MC, Williams PL, Arvizu M, Tanrikut C, Amarasiriwardena CJ, et al. 2017. Hair mercury (hg) levels, fish consumption and semen parameters among men attending a fertility center. International journal of hygiene and environmental health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priskorn L, Nordkap L, Bang AK, Krause M, Holmboe SA, Egeberg Palme DL, et al. 2018. Average sperm count remains unchanged despite reduction in maternal smoking: Results from a large cross-sectional study with annual investigations over 21 years. Human reproduction (Oxford, England) 33:998–1008. [DOI] [PubMed] [Google Scholar]
- Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. 2013. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in france. Human reproduction (Oxford, England) 28:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. 1980. Population marginal means in the linear model: An alternative to least square means. Am Stat 34:216–221. [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. 2013. Bmi in relation to sperm count: An updated systematic review and collaborative meta-analysis. Human reproduction update 19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 860:106–112. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. 2016. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiological reviews 96:55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splingart C, Frapsauce C, Veau S, Barthelemy C, Royere D, Guerif F. 2011. Semen variation in a population of fertile donors: Evaluation in a french centre over a 34-year period. International journal of andrology. [DOI] [PubMed] [Google Scholar]
- Sripada S, Fonseca S, Lee A, Harrild K, Giannaris D, Mathers E, et al. 2007. Trends in semen parameters in the northeast of scotland. Journal of andrology 28:313–319. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. 1997. Have sperm densities declined? A reanalysis of global trend data. Environmental health perspectives 105:1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. 1994. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology 23:991–999. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2010. Who laboratory manual for the examination and processing of human semen, 5th edition. Geneva, Switzerland:WHO Press. [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Automated on-line column-switching hplc-ms/ms method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry 77:5407–5413. [DOI] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. 2017. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records compared with urinary recovery and plasma concentration biomarkers: Findings for women. American journal of epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.