Abstract
Background:
Trichomonias is the most common non-viral STI among women worldwide and is associated with serious reproductive morbidity, poor birth outcomes and amplified HIV transmission. Single-dose metronidazole therapy has been the treatment of choice for over three decades. There is mounting evidence, however, of high rates of repeat positives following single-dose metronidazole, and among HIVinfected women, bacterial vaginosis (BV) was found to alter treatment efficacy. The purpose of this study was to examine the effectiveness of single-dose metronidazole compared to 7 day-dose metronidazole for the treatment of trichomoniasis among HIV-uninfected, non-pregnant women and to determine if this effect was modified by BV.
Methods:
This was a randomized, parallel, multi-site, open-label trial of single-dose (2 g one-time) versus 7 day-dose (500 mg twice daily) for the treatment of trichomoniasis. The primary outcome was T. vaginalis infection by arm, per nucleic acid amplification test or culture, four weeks post-completion of treatment, in intentto-treat analyses. This analysis was also stratified by BV status.
Findings:
Of 623 women randomized, those in the 7 day-dose arm were less likely to be T. vaginalis positive at test-of-cure compared to those in the single-dose arm [34/312 (10.9%) vs. 58/311 (18.6%), p=0·001] [R.R. 0.55 (95% C.I. 0.34–0.70)]. Risk was similar by BV status (p=0·17). Self-reported adherence in both arms was > 95%. Side effects were similar by arm.
Interpretation:
In this sample of HIV-uninfected, non-pregnant women with trichomoniasis, compared to single-dose, 7 day-dose metronidazole treatment resulted in 45% fewer treatment failures. The 7 day-dose metronidazole should be the preferred treatment for trichomoniasis among women.
Keywords: Trichomonas vaginalis, metronidazole, treatment failure, women
Introduction:
Trichomoniasis is estimated to be the most common non-viral sexually transmitted infection (STI) in the world and is more prevalent than chlamydia, gonorrhea and syphilis combined.(1) While trichomoniasis is not a reportable disease, there are an estimated 143 million new cases per year globally among women aged 15–29.(1) The prevalence has been found to be 1.8% among women in the United States, though nearly five times higher in African American women,(2) and has been estimated to be 5.0% among women worldwide.(1)
Among women, trichomoniasis has been associated with serious reproductive morbidity (e.g. vaginitis, cervicitis, urethritis, pelvic inflammatory disease)(3) and poor birth outcomes (e.g. premature rupture of membranes, low birth weight, and preterm delivery).(4) Several studies have found that trichomoniasis co-occurs with other STIs(5) and amplifies HIV acquisition.(6)
For over three decades, both the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) have recommended a single 2 g dose of oral metronidazole or tinidazole as first line treatment and a 7 day-dose therapy (400 or 500 mg twice daily for 7 days) as second line treatment for Trichomonas vaginalis infections.(7, 8) In most settings, metronidazole is used more often than tinidazole because of cost considerations.(9)
Evidence, however, has been mounting that the single-dose treatment of metronidazole may be insufficient to treat trichomoniasis. A prior meta-analysis of six published studies found that women who received 7 day-dose metronidazole had 46% fewer treatment failures compared to women who received single-dose and test of cure (TOC) positive rates after single-dose metronidazole ranged from 6·2%−18·0%.(10) All but one of the studies in the meta-analysis concluded that single-dose treatment was comparable to 7 day-dose.(11–15) And all but one of these studies were conducted over 30 years ago when clinical trial methods were not as rigorous as current standards.(16) Most of these studies were not adequately powered to detect meaningful differences, and all were done before the availability of nucleic acid amplification tests (NAAT), which are 3–5 times more sensitive than microscopy for the diagnosis of trichomoniasis.(17–21)
The most recent study included in the meta-analysis, referred to above, used more current clinical trial methods but was conducted among HIV-infected women only.(22) That multi-site study found that, among women attending HIV clinics who had laboratory-diagnosed T. vaginalis, women receiving 7 day-dose metronidazole were half as likely to be T. vaginalis positive at TOC compared to those receiving single-dose treatment.(22) Subsequent analysis, however, found superiority of 7 day-dose arm only among those who had bacterial vaginosis (BV) per Nugent score but not among those who did not have BV, suggesting that altered vaginal flora could have interfered with single-dose treatment.(23)
The purpose of the study reported here was to re-evaluate the efficacy of single-dose metronidazole compared to 7 day-dose metronidazole among HIV-uninfected women using current RCT methodology, a sample size with sufficient power and state-of-the-art T. vaginalis diagnostics. A secondary aim was to examine treatment differences by BV status.
Methods:
Study Design and Participants
This was a randomized, parallel, multi-site, open-label, laboratory-blinded trial comparing the efficacy of 7 day-dose metronidazole (500 mg twice daily for 7 days) to the single-dose metronidazole standard (2 g one time dose) for the treatment of trichomoniasis. An open-label design was used to simulate real world conditions and accommodate for the potential for lack of adherence and sexual re-exposure differences among women who received treatment over multiple days.
The primary outcome was presence of T. vaginalis at TOC (i.e. four weeks postcompletion of treatment) per NAAT or culture. The hypothesis was that women receiving the 7 day-dose metronidazole would be less likely to have a T. vaginalis positive TOC than women receiving single-dose metronidazole. A second hypothesis was that this effect would be observed only among women with BV (Table 2).
Table 2.
TOC positive rates by arm in intent to treat, sensitivity and stratified analyses (n=623)
| 7 daydose | Single-dose | 7 day-dose vs. single-dose rate difference (95% C.I.) | Relative Risk (95% C.I.) Multi- vs. Single | p-value* | |
|---|---|---|---|---|---|
| Primary outcome T. vaginalis per NAAT or Culture | |||||
| Overall per ITT | 34/312 (10·9%) |
58/311 (18·6%) |
−7·8 (−2·2 to −13·3) | 0·55 (0·34 −0·70) | 0.001 |
| Among those with BV at baseline | 16/125 (12·8%) |
26/125 (20·8%) |
−8·0 (−12·8 to −20·8) | 0·59 (0·43–0·80) | 0·001 |
| Among those without BV at baseline | 13/139 (9·4%) |
24/140 (17·1%) |
−7·8 (−0·2 to −15·8) | 0·57 (0·45–0·71) | 0·001 |
| Sensitivity Analyses | |||||
| All missing reclassified as negative | 29/312 (9·3%) |
51/311 (16·4%) |
−7·1 (−1·9 to −12·4) | 0·57 (0·45–0·71) | 0·001 |
| All missing reclassified as positive | 71/312 (22·8%) |
92/311 (29·6%) |
−6·8 (−0·1 to −13·7) | 0·77 (0·70–0·85) | 0·001 |
| Culture as outcome m- ITT*** |
22/269 (8·2%) |
41/270 (15·2%) |
−7·0 (−1·3 to −12·7) | 0·54 (0·39–0·75) | 0·001 |
| Overall NAAT or culture as outcome m-ITT*** | 29/270 (10·7%) |
51/270 (18·9%) |
−8·2 (−2·2 to −14·1) | 0·57 (0·45–0·71) | 0·001 |
Relative Risks and p-values from GEE analysis clustering by site.
missing data imputed using multiple imputations using fully conditional specification
m-ITT (modified ITT using complete case as denominator)
The secondary outcomes were to determine more precisely the origin of a TOC positive result using the results of the genotyping and the sexual histories and to determine if most of the infections are treatment failure, re-exposure, sexual exposure to a new partner or organism lack of susceptibility to MTZ. Results will be presented in a separate report.
Women who attended one sexually transmitted disease clinic in each of three cities (i.e. Birmingham, Alabama; Jackson, Mississippi; and New Orleans, Louisiana) and were T.vaginalis infected per clinical screenings were referred to study staff by their clinical providers.
Inclusion criteria were: female sex, English speaking; age 18 or 19 years depending site requirements for age of majority; T. vaginalis positive per clinical screening (microscopy or NAAT) confirmed by at least one study test (NAAT or culture); agreement to refrain from all alcohol use during treatment and for 24 hours after treatment; willingness to take metronidazole treatment and to be randomized to either arm of the study.
Exclusion criteria included HIV-infection, pregnancy, breast feeding; prior enrollment in the study, incarcerated, any medical contraindications to metronidazole (such as currently taking disulfiram, dilantin or Coumadin, or having a history of alcoholism or known liver damage), have been treated with any medication used to treat trichomoniaisis or BV (including metronidazole, tinidazole, seconidazole, acetarsol, boric acid, furazolidone, and paromomycin) within the previous 14 days, and unable or unwilling to provide informed consent, to be randomized, or unable or unwilling to return for a follow-up visit 4 weeks post treatment completion.
Control arm subjects were treated with 2 g oral metronidazole (4 pills 500 mg each). Intervention arm subjects were treated with 500 mg oral metronidazole (1 pill) taken twice daily for 7 days. Subjects on both arms were asked to take the first dose at the clinic under direct observation therapy and were offered crackers or cookies to prevent nausea. For the 7 day-dose arm, the remaining pills were dispensed in a container with a child-proof cap.
All participants received the standardized counseling by study personnel: to refrain from unprotected sexual intercourse until their symptoms resolved, until they and their partner(s) completed the medication, and to refrain from alcohol consumption while taking the medication and for 24 hours after completion. Participants were informed of the potential to experience metronidazole-related adverse events and of the potential for change in taste sensation and discoloration of urine. Participants in the 7 day-dose arm were also counselled on the importance of taking all doses of the medication.
Subjects in both arms were asked to tell all of their sex partners of their exposure to T. vaginalis and to encourage them to seek treatment. Because of legal or institutional restrictions, none of the participating clinics routinely provided expedited partner treatment (EPT), or the provision of presumptive partner treatment for trichomoniasis without a clinic visit, but some were given EPT at the provider’s discretion.
Randomization and Masking
Subjects were randomized using sealed sequentially numbered envelopes that contained the randomly chosen allocation arm. These envelopes were prepared prior to the start of the study by a non-investigator using SAS in a randomization scheme of blocks of 4 or 6 for each site. A list containing the envelope number and allocation arm was kept in an electronic file that was not accessed until study end. Envelopes were kept at each site and study staff pulled envelopes sequentially and documented the treatment arm, the envelope number, and the lot number of treatment received. All laboratory technicians were blinded to study arm.
Procedures
Behavioral data
The surveys at both baseline and TOC were audio computerassisted self-administered interview (ACASI) to elicit demographics, substance use, vaginal hygiene practices, contraception use, and sexual behavior data detailed for each partner. Symptoms commonly reported with trichomoniasis (including unusual vaginal discharge, vaginal odor, vaginal itching or irritations, painful urination, or pelvic pain)(24) were systematically elicited using ACASI.
The TOC ACASI survey was similar to the baseline survey with additional questions about sexual exposure, treatment adherence, partner treatment, symptoms during follow-up, and side effects of the medication (including, nausea, vomiting or headache). The survey was modelled after surveys from our previous studies.(22, 25)
T. vaginalis nucleic acid amplification testing
Self-collected vaginal swabs were tested for T. vaginalis using the TMA-HPA Assay from Hologic, Inc. (Bedford, MA). At the time of the study, this was an Investigational use only assay provided for research use by Hologic and was performed using the direct tube sampling system. This assay is now available for clinical use.(26) All tests were performed at the laboratory of the Louisiana State University Health Sciences Center (LSUHSC) according to the manufacturer’s instructions.
T. vaginalis Culture
Self-collected vaginal swabs were placed into the T. vaginalis culture InPouch™ medium, incubated at 37°C and read following the manufacturer’s protocol by trained personnel. Three readings were performed over 7 days. Detection of any live trichomonads were considered positive and after three negative pouch readings, the specimen was considered T. vaginalis-negative.(27).
Gram stains - Gram stains for Nugent score determination were read by an experienced technician at LSUHSC Laboratory. Samples were periodically evaluated for quality assurance following Clinical Laboratory Improvement Amendment regulations.(28) Women were considered to have BV if they had a Nugent score of 7 or greater.(29).
Subject safety
The study was approved by the institutional review boards (IRB) of: Tulane University, Louisiana State University Health Sciences Center, University of Alabama at Birmingham, University of Mississippi Medical Center, Jefferson County Department of Health, and the Mississippi State Department of Health. An independent Data Safety and Monitoring Board comprised of non-investigators monitored the data every six months with a priori stopping rules. An interim analysis was to be done half-way through enrollment. Because of the sensitive nature of the survey, a Certificate of Confidentiality was also obtained from the Department of Health and Human Services. Women who discovered they were pregnant while taking the medication or who had any serious adverse events were followed according to each site’s IRB protocol.
Study enrollment was conducted from October 6, 2014 to April 26, 2017. The study was completed on June 5, 2017. After eligibility screening and informed consent, women were asked to provide urine for pregnancy testing, to self-collect vaginal swabs for T. vaginalis culture, NAAT, and Gram stain testing, and to take a survey. Women were given medication as described above.
A TOC appointment was scheduled for 4 weeks after completion of treatment (i.e. 4 weeks later for single-dose arm and 5 weeks for the 7 day-dose arm) with a window of 3–12 weeks post completion of treatment. Women were not screened before the 3 week TOC because of the potential for false positive NAAT results from remnant DNA.(30) (31) The woman’s contact information was collected, an appointment card was provided and compensation worth $20 was given to subjects. Participants were reminded of their TOC visit using their preferred method of contact. At TOC, data collection included a survey and self-collected vaginal swabs for NAAT, culture and Gram stain; those who completed the TOC visit received compensation worth $50.
Statistical Analysis
The original sample size was based on inputs from our unpublished pilot study among HIV-uninfected women and our prior study of trichomoniasis treatment among HIV-infected women(22) Assuming a TOC infection rate of 16.8% for single-dose and 10.7% for 7 day-dose, at a power of 0.80, and inflating 15% for the potential of inner class correlation (ICC) between three sites and inflating an additional 20% for the potential loss-to-follow-up, the sample size was determined to be 1664.
Selected demographic, clinical and behavioral factors that have been identified in the literature as associated with trichomonas were examined by treatment arm to assess if randomization was effective (Table 1).(2, 32) These same factors were examined by follow-up status to determine if those who were followed were similar to those not followed.
Table 1.
Selected baseline characteristics by study arm (N=623)
| 7 day-dose | Single-dose | |
|---|---|---|
| Demographic | ||
| Age ≥ 30 years | 116/310 (37·4%) | 132/309 (42·7%) |
| African American race | 298/310 (96·1%) | 293/309 (94·8%) |
| Site Jackson New Orleans Birmingham |
77/312 (24·7%) 34/312 (10·9%) 201/312 (64·4%) |
78/311 (25·1%) 34/311 (10·9%) 199/311 (64·0%) |
| Substance use behavior | ||
| Binge drinking in last week | 57/310 (18·4%) | 56/309 (18·1%) |
| Current smoking | 145/310 (46·8%) | 142/308 (46·1%) |
| Sexual behaviors | ||
| >1 male sex partners at baseline | 119/310 (38·4%) | 122/309 (39·5%) |
| Any female partners at baseline | 27/310 (8·7%) | 30/309 (9·7%) |
| Other behaviors | ||
| Vaginal douching ≥ once per month | 117/309 (37·9%) | 114/309 (36·9%) |
| Hormonal contraception use | 60/310 (19·4%) | 58/309 (18·8%) |
| Think it would not be difficult/somewhat difficult/unsure to take 7 day dose | 91/310 (29·4%) | 96/309 (31·1%) |
| Clinical factors | ||
| BV per Nugent at baseline | 146/306 (47·7%) | 148/303 (48·8%) |
| T. vaginalis in last year per self-report | 53/309 (17·2%) | 54/307 (17·6%) |
| BV in last year per self-report | 48/310 (15·5%) | 65/308 (21·1%) |
| Yeast in last year per self-report | 87/301 (28·9%) | 76/297 (25·6%) |
| Baseline T. vaginalis symptoms | 248/311 (79·5%) | 248/311 (79·7%) |
| Expedited partner treatment | 21/307 (6·8%) | 27/309 (8·7%) |
Primary outcome analyses
The primary analysis was the outcome (i.e. T. vaginalis infection at TOC) by arm in ITT analyses. Because the sites did not enroll even numbers of subjects, site differences were non-ignorable.(33) Data were imputed for cases that were lost-to follow-up. This was done with 20 imputation using the fully conditional method in SAS Proc MI. Generalized estimating equation regression methods (GEE) was conducted using an exchangeable correlation matrix and clustering by site. Rate differences and 95% C.I. as well as GEE derived relative risks and 95% C.I. were calculated. Analyses were done for all randomized using ITT and stratified by baseline BV status. (Table 2).
Sensitivity analyses of primary outcome
Four sensitivity analyses were also conducted: 1) reclassifying all missing results as negative at TOC and, 2) reclassifying all missing results as positive at TOC (Table 2), 3) using the culture results as the outcome (to remove the possibility of false positives by T. vaginalis NAAT) and 4) modified ITT (m-ITT) using only complete cases in the analysis.
Side effects/adverse events were examined by arm (Table 3). Comparisons in Table 3 was done using Chi-square and Fisher’s Exact. All analyses were done using SAS version 9.4 under the supervision of the team biostatistician.
Table 3:
Adverse Events and Serious Adverse Events by arm (n=540)
| Multi-dose (n=270) | 2 g single-dose (n=270) |
p-value |
|
|---|---|---|---|
| Any Adverse Event during follow-up | 89 (33·0%) | 90(33·5%) | 0·90 |
| Common side effects | |||
| Nausea | 63 (23·3%) | 61 (22·6) | 0·84 |
| Vomiting | 13 (4·8%) | 6 (2·2%) | 0·10 |
| Headache | 23 (8·5%) | 15 (5·6%) | 0·18 |
| Less common side effects | |||
| Dizziness | 6 (2·2%) | 2 (0·7%) | 0·15 |
| Bad/metallic taste in mouth | 4 (1·5%) | 3 (1·1%) | 0·70 |
| Vaginal itching | 3 (1·1%) | 1 (0·4%) | 0·32* |
| Fatigue | 2 (0·7%) | 0 (0·0%) | 0·32* |
| Rash | 2 (0·7%) | 0 (0·0%) | 0·32* |
| Other side effects** | 9 (3·3%) | 9 (3·3%) | 1·00 |
| Serious Adverse Events | |||
| Spontaneous abortion*** | 0 | 2 (0.7%) | 0·32* |
Fisher’s exact
Other side effects assessed included, yeast infection, abdominal pain, numbness, dysuria, loss of appetite, altered mental status, myalgia, generalized itching, lightheadedness, restlessness, verbal intimidation by partner or stiff neck.
The two women tested negative at baseline and discovered they were pregnant during follow-up.
Role of the funding Source
The funder of the study, the National Institute of Health, had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Participant enrollment
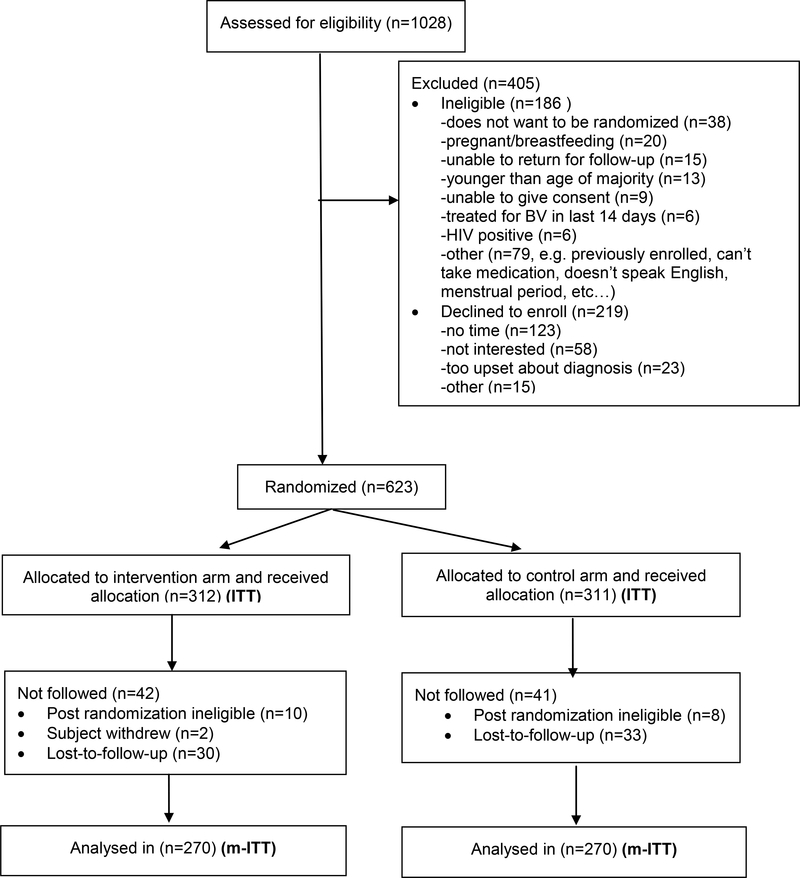
The study was stopped because of funding limitations and we enrolled 623/1664 or 37.4% of our intended sample size. Of 1028 T. vaginalis positive women approached, 623 enrolled in the study. Of the 405 who did not enroll, 186 were ineligible and 219 declined. The most common reasons for ineligibility were not wanting to be randomized (n=38/186, 20·4%) and pregnant or breastfeeding (n=20/186, 10·8%). Most (123/219, 56·2%) of those who declined said it was because they had no time (Figure 1).
Figure 1.
Inclusion and exclusion by randomization arm
Baseline characteristics
Of 623 women enrolled in the study, 311 were randomized to the single-dose arm and 312 were randomized to the 7 day-dose arm. All women received the dose allocated to them. The majority of women were African American (591/619, 95·5%) and the median age was 27·0 (range 18–64). The Birmingham, AL site recruited 64·2% (400/623) of the subjects.
Substance use behaviors included binge drinking in the last week (113/619, 18·3%) and currently smoking any cigarettes (287/618, 46·4%). Sexual behaviors in the last 30 days included having more than one male sex partner (241/619, 38·9%) and any female sex partners (57/618, 9·2%). Self-reported history of T. vaginalis in the past year was 17·4% (95/619), and of BV was 18·3% (104/576). The prevalence of BV at baseline, per Nugent criteria, was 48.3% (294/609), vaginal douching at least once per month was 37·4% (231/618) and hormonal contraception use was 19·1% (118/619). Baseline factors examined (i.e. demographic, substance use, sexual behaviors, other behaviors and clinical factors) were evenly distributed by study arm (Table 1).
Follow-up rates
Of the 623 subjects randomized, 540 (86·7%) returned for their follow-up visit. The mean time from completion of medicine to TOC visit was 4.9 weeks (s.d. 1·6) and there was no difference by arm (p=0·60). There was no difference in the percentage who did not return between intervention versus control arm (42/312, 13·5% vs.41/311, 13·2%, p=0·92) or by site: Jackson vs. New Orleans vs. Birmingham (19/155, 12·3% vs. 13/68, 19·1% vs. 51/400, 12·8%, p=0·33) or by any other factor considered in Table 1. Sexual exposure (to either baseline or new partner) during follow-up was similar for intervention versus control 99/270 (36·7%) versus control arm 89/270 (33·0%), p=0·37.
Adherence and EPT
Adherence rate was lower in the 7 day-dose arm compared to the single-dose arm (253/265, 95·5% vs. 264/266, 99·2%, p< 0·01). Of the 603 women that reported baseline partners, 43 (7·1%) received EPT (2g metronidazole) for their sexual partners with no difference in reception of EPT by arm. (Table 1).
Test–of-Cure by arm
Overall 80/540 (14·8%) were positive at TOC. In ITT analysis with imputation for missing data, women in the 7 day-dose treatment arm had significantly lower TOC positive rates than those in the single-dose arm [34/312 (10.9%) vs. 58/311 (18.6%), p=0·001] [R.R. 0.55 (95% C.I. 0.34–0.70)].
Analysis by BV status
Women who received 7 day-dose metronidazole had consistently lower TOC positive rates irrespective of BV status. When BV was present, women on the 7 day-dose treatment arm were less likely to have a TOC positive result compared to women on the single-dose treatment arm [RR 0·59, (0·43–0·80), p< 0·001]. These findings were similar in the absence of BV [RR 0·57, (0·45–0·71), p< 0·001]. The interaction of BV and arm in GEE analysis was not statistically significant (p=0·17) (Table 2).
Sensitivity analysis
When all missing results were classified as negative, women in the 7 day-dose arm had fewer TOC positives than those in the single-dose arm [RR 0·57, (0·45–0·71), p< 0·001]. Findings were similar when all missing results were classified as positive [RR 0·77, (0·70–0·85), p< 0·001]. When T. vaginalis culture was used as the outcome, women in the 7 day-dose arm were less likely to have TOC positive compared to women in the single-dose arm [RR 0·54, (0·39–0·74), p< 0·001]. In complete case m-ITT, those in the 7 day-dose arm had lower rates than those in the single-dose arm (29/270, 10·7% vs. 51/270, 18·9%, p < 0·001) with a relative risk of 0·57 (95% C.I. 0·45–0·71) (Table 2).
Adverse events
Of those who returned for TOC, 33·2% reported a treatment-related side effect. The most common was nausea (23·0%) followed by headache (7·0%). Rates of these events did not differ by study arm (Table 3). Two spontaneous abortions were reported among women who tested negative for pregnancy at baseline and discovered they were pregnant during follow-up. Both were on the 7 day-dose arm.
Discussion
Trichomonas infection is highly prevalent worldwide and is associated with important reproductive and perinatal morbidities(34) as well as amplified HIV transmission.(1, 6) In this randomized, open-label, multi-centered, parallel trial, 7 day-dose oral metronidazole (500 mg twice daily for 7 days) resulted in 45% fewer TOC positive results compared to single-dose oral (2 g single-dose) metronidazole for the treatment of trichomoniasis among HIV-uninfected non-pregnant women. These findings corroborate a similar RCT conducted among HIV-infected women,(22) and a prior meta-analysis of six previously published studies.(10)
Unlike our prior study among HIV-infected women(23), we did not find treatment differences by BV status (per Nugent score). The reason for this difference is not entirely clear. While it is known that validity of the Nugent score is affected by technician expertise,(29) the same technician read the Gram stains in both studies, so inter-study technician variability is not a likely factor. One possible explanation is that host factors were influential in treatment efficacy. Differences in the vaginal microbiota both with and without BV appear to be different by HIV status and could have contributed to this treatment difference.(35) Future studies are needed to examine how the vaginal microbiota influences the treatment of trichomoniasis.
Concomitant BV is common among women with trichomoniasis.(36) In this sample, 48·3% of the women had BV per Nugent score, yet BV was not diagnosed at by the referring provider. Amsel criteria, which is a clinic based test for BV,(37) was done at the clinics to diagnose BV. Symptomatology for BV and trichomonaisis are often similar(7) and Amsel is known to have low predictability for BV.(38) 7 day-dose metronidazole is first line treatment for BV,(7) and is more effective than single-dose for trichomoniasis, providing further rationale for recommending 7 day-dose metronidazole as first line therapy for the treatment of trichomoniasis.
The high rates of repeat infections in both arms of this study is of concern and even though the 7 day-dose performed better than the single-dose, the repeat infection rate of 10·9% in this group is high and suggests the CDC recommendation to rescreen women treated for T. vaginalis should be upheld even for those receiving the 7 day-dose.
While NAAT has greater sensitivity than culture, it is not clear how long remnant T. vaginalis DNA that is not live parasite stays in vaginal fluids. We chose to perform the TOC visit 4 weeks after completion of treatment because three studies found false positive rates at three weeks between 0%−15%.(30, 31, 39) We also tested women using culture and found a lower rate of T. vaginalis at TOC in both arms, but the relative risk was similar to that using NAAT as the outcome (Table 2). This suggests that false positive by NAAT were not influential in our results.
In this study we chose to use an open-label design in order to factor in real use conditions, though blinding subjects to treatment arm is often preferred. Because of this choice, there were differences in treatment days and follow-up times from baseline that could have resulted in sexual exposure differences. We did not, however, find differences in sexual exposure to either baseline or new partners. And while it is possible that self-reported sexual behavior is not accurate, we used computer assisted interviews which have been shown to reduce social desirability bias.(40, 41) Furthermore, there is no reason to believe that reporting bias would be different by arm as both arms received similar counseling.
An important limitation of the study was that enrollment was much lower than planned. Low recruitment could have led to reduced power for the primary outcomes and less certainty about the effect measure, particularly for the analysis stratified by BV. It is possible that our sample size calculation was too conservative. The inputs used for the sample size calculation were based on our prior study among HIV-infected women which were more restrictive than we found in the present study. The findings of the ITT and sensitivity analyses were consistent and statistically significant. It is possible we did not have enough power to detect a difference by BV status, but the relative risks by BV status, did not indicate a trend, so it is unlikely that a larger sample size would have led to a different conclusion.
Single-dose treatment for trichomoniasis has long been favored over 7 day-dose because it decreases the issue of adherence, and if directly observed by the provider, can even eliminate this concern. It is worth noting that, despite being willing to be randomized, that 30·2% of the women thought it would be difficult/somewhat difficult/unsure to take the medicine for 7 days. In this RCT, self-reported adherence was indeed higher among single-dose compared to the 7 day-dose (99·2% vs. 95·5%, P <0·01), but adherence in both arms was very high. Providers who have concerns about patient adherence will need to consider the benefits of the 7 day-dose and the convenience of single-dose.
The study population was conducted among non-pregnant, urban, largely African American, symptomatic women, with a high rate of co-occurring BV. While these results may not be generalizable to all women with trichomoniasis, they are likely generalizable to the majority since these are common risk factors for trichomoniasis.(7, 32) While the majority of women with trichomoniasis are asymptomatic,(42) the importance of asymptomatic trichomoniasis on reproductive morbidity is not well known.(7) More studies are needed to evaluate the importance of asymptomatic trichomoniasis on reproductive morbidity.
About one-third of the cohort had side effects that were minimal and did not appear to differ by arm. There were, however, two reported spontaneous abortions among women receiving the 7 day-dose, which, while not statistically different, are noteworthy. While reviews have found that multi-dose metronidazole is safe in pregnancy,(43, 44) providers may wish to monitor this.
Strengths of the study were that randomization appeared to work well, as all factors considered were similar by arm (Table 1), our loss-to-follow up was minimal (13·3%), and the outcome measurement was blinded.(33) Moreover, the ITT, m-ITT and the other sensitivity analyses were highly consistent. This study, combined with our prior work,(45, 46) provides strong evidence that 7 day-dose metronidazole is a better treatment for trichomoniasis in woman than single-dose metronidazole and recommendations should be adapted accordingly.
Research in Context
Evidence for the study
Trichomoniasis is the most common non-viral sexually transmitted infection (STI) worldwide, has been associated with important reproductive and perinatal morbidity, and amplifies HIV acquisition. For over three decades a single 2 g dose of oral metronidazole has been the recommended treatment of trichomoniasis with multidose oral metronidazole (500 mg twice daily for 7 days) as an alternate. A prior metaanalysis found that multi-dose metronidazole resulted in fewer treatment failure than single-dose treatment, but there were only 6 studies included in the meta-analysis, 5 of which were done more than 30 years ago. In our prior trial among HIV-infected women, we found women receiving multi-dose metronidazole were nearly half as likely to have treatment failure compared to those receiving 2 g single-dose treatment, but this difference was mostly found among women who had undiagnosed co-occurring bacterial vaginosis (BV). The purpose of the present study was to conduct a similar trial among HIV-uninfected, non-pregnant women with trichomoniasis and to determine if BV interfered with treatment.
Added values of the study
Our finding adds to the evidence that 7 day-dose metronidazole is superior to single-dose for the treatment of trichomoniasis in women irrespective of BV status. These results can be used to refine treatment guidelines for trichomoniasis in HIV-uninfected women.
Implications of all the available evidence
Multi-dose metronidazole should be the preferred treatment for trichomoniasis among women.
Acknowledgement:
This research was supported by a grant from the National Institute of Health/National Institute of Allergy and Infectious Disease (NIH/NIAID) 1R01AI097080–01A1. Drs. Evan Secor and Peter Augostini from the Centers for Disease Control and Prevention (CDC) provided their services in kind.
Thanks to the Data Safety and Monitoring Board including: Dr. Charlotte Gaydos (Chair), Dr. David Mushatt, Dr. Jeffery Burton and to Dr. Russell Van Dyke who served as a medical and regulatory advisor. Thanks to Dr. Caroline Deal and Dr. Hagit David from NIAID/DMID for their support.
Thanks to the clinical staff and laboratory staff at UAB including Hanna Harbison and Saralyn Richter, Rhonda Whidden, Meghan Whitfield, Christen Press, Jim Alosi, Ann Dillashaw, Charles Rivers, Cheri Aycock and Keonte Graves; at UMC including Melverta Bender, and Jennifer Brumfield; and Camille Fournet at LSU.. Thanks to the laboratory staff at Louisiana State University Health Sciences Center including Catherine Cammarata, Judy Burnett, Denise Diodene. Thanks to the data management staff at Tulane University including Lauren Ostrenga and Scott White.
Study data were collected and managed using REDCap electronic data capture tools hosted at Tulane University.1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the NIH/NIAID or the CDC.
Funding: National Institute of Health
Footnotes
Disclosures:
All investigator received a portion of their salary, via their institutions from the NIH/NIAHD 1R01AI097080–01A1.
Patricia Kissinger, Norine Schmidt, Rebecca A. Lillis, Stephanie N. Taylor, Laura Beauchamps, Leann Myers, Jane M. Carlton, Martina Bradic, Evan Secor, Peter Augostini have no other disclosures.
Leandro Mena and Laura Beauchamps received salary support from his University from Hologic, Becton Dickinson and Company, Gilead Science, ViiV Healthcare and Consulting fees paid to him from Gilead Science ad ViiV Healthcare.
Stephanie N. Taylor has received research support from Becton-Dickinson, Hologic, Cepheid, Beckman-Coulter, Roche, ELITech, GlaxoSmithKline, Melinta and Entasis. Consulting and peer educator honoraria, and advisory board honoraria from Hologic and GlaxoSmithKline.
Christina Muzny has been a consultant for Lupin Pharmaceuticals.
Jane R. Schwebke received research support from Hologic, Becton Dickinson and Company, Cepheid, and Symbiomix.
David H. Martin has served as a consultant for BioFire Diagnostics and GlaxoSmithKline.
Assurances:
The study was registered with the Federal Drug Administration (IND118276) and Clinicaltrials.gov (NCT01018095).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patricia Kissinger, Tulane University School of Public Health and Tropical Medicine – Department of Epidemiology.
Christina A. Muzny, University of Alabama at Birmingham, Division of Infectious Diseases
Leandro Mena, University of Mississippi Medical Center– Department of Medicine and the John D. Bower School of Population Health, Department of Population Health Sciences
Rebecca A. Lillis, Louisiana State University Health Sciences Center – Section of Infectious Diseases
Jane R. Schwebke, University of Alabama at Birmingham, Division of Infectious Diseases C.A..
Laura Beauchamps, University of Mississippi Medical Center– Department of Medicine and the John D. Bower School of Population Health, Department of Population Health Sciences
Stephanie N. Taylor, Louisiana State University Health Sciences Center – Section of Infectious Diseases
Norine Schmidt, Tulane University School of Public Health and Tropical Medicine – Department of Epidemiology
Leann Myers, Tulane University School of Public Health and Tropical Medicine – Department of Global Biostatistics and Data Science.
Peter Augostini, Centers for Disease Control and Prevention, Division of Parasitic Diseases and Malaria
William E. Secor, Centers for Disease Control and Prevention, Division of Parasitic Diseases and Malaria
Martina Bradic, Center for Genomics and Systems Biology, Department of Biology, New York University
Jane M. Carlton, Center for Genomics and Systems Biology, Department of Biology, New York University.
David H. Martin, Louisiana State University Health Sciences Center – Section of Infectious Diseases.
References:
- 1.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PloS one. 2015;10(12):e0143304 Epub 2015/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and Correlates of Trichomonas vaginalis Infection Among Men and Women in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018. Epub 2018/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherpes TL, Wiesenfeld HC, Melan MA, Kant JA, Cosentino LA, Meyn LA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sexually transmitted diseases. 2006;33(12):747–52. [DOI] [PubMed] [Google Scholar]
- 4.Silver BJ, Guy RJ, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sexually transmitted diseases. 2014;41(6):369–76. Epub 2014/05/16. [DOI] [PubMed] [Google Scholar]
- 5.Allsworth JE, Ratner JA, Peipert JF. Trichomoniasis and other sexually transmitted infections: results from the 2001–2004 National Health and Nutrition Examination Surveys. Sexually transmitted diseases. 2009;36(12):738–44. Epub 2009/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sexually transmitted infections. 2013;89(6):426–33. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2015;64(RR-03):1–137. Epub 2015/06/05. [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization W. Guidelines for the treatment of sexually transmitted infections. 2004.
- 9.Johnson G. Tinidazole (Tindamax) for Trichomoniasis and Bacterial Vaginosis. Am Fam Physician. 2009;79(2):102–5. [Google Scholar]
- 10.Howe K, Kissinger PJ. Single-Dose Compared With Multidose Metronidazole for the Treatment of Trichomoniasis in Women: A Meta-Analysis. Sexually transmitted diseases. 2017;44(1):29–34. Epub 2016/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aubert JM, Sesta HJ. Treatment of vaginal trichomoniasis. Single, 2-gram dose of metronidazole as compared with a seven-day course. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 1982;27(12):743–5. [PubMed] [Google Scholar]
- 12.Csonka GW. Trichomonal vaginitis treated with one dose of metronidazole. British Journal of Venereal Diseases. 1971;47(6):456–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hager WD, Brown ST, Kraus SJ. Metronidazole for vaginal trichomoniasis. Seven-day vs single-dose regimens. Journal of the American Medical Association. 1980;244(11):1219–20. [PubMed] [Google Scholar]
- 14.Thin RN, Symonds MAE, Booker R. Double-blind comparison of a single dose and a five-day course of metronidazole in the treatment of trichomoniasis. British Journal of Venereal Diseases. 1979;55(5):354–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodcock KR. Treatment of trichomonal vaginitis with a single oral dose of metronidazole. British Journal of Venereal Diseases. 1972;48(1):65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Bmj. 2010;340:c332 Epub 2010/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth AM, Williams JA, Ly R, Curd K, Brooks D, Arno J, et al. Changing sexually transmitted infection screening protocol will result in improved case finding for trichomonas vaginalis among high-risk female populations. Sexually transmitted diseases. 2011;38(5):398–400. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 18.Hollman D, Coupey SM, Fox AS, Herold BC. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. Journal of pediatric and adolescent gynecology. 2010;23(5):312–6. Epub 2010/05/25. [DOI] [PubMed] [Google Scholar]
- 19.Schwebke JR, Hobbs MM, Taylor SN, Sena AC, Catania MG, Weinbaum BS, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. Journal of clinical microbiology. 2011;49(12):4106–11. Epub 2011/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwebke JR, Gaydos CA, Davis T, Marrazzo J, Furgerson D, Taylor SN, et al. Clinical Evaluation of the Cepheid Xpert TV Assay for Detection of Trichomonas vaginalis with Prospectively Collected Specimens from Men and Women. Journal of clinical microbiology. 2018;56(2). Epub 2017/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Pol B, Williams JA, Taylor SN, Cammarata CL, Rivers CA, Body BA, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. Journal of clinical microbiology. 2014;52(3):885–9. Epub 2014/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissinger P, Mena L, Levison J, Clark RA, Gatski M, Henderson H, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. Journal of acquired immune deficiency syndromes. 2010;55(5):565–71. Epub 2011/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatski M, Martin DH, Levison J, Mena L, Clark RA, Murphy M, et al. The influence of bacterial vaginosis on the response to Trichomonas vaginalis treatment among HIV-infected women. Sexually transmitted infections. 2011;87(3):205–8. Epub 2011/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. Journal of reproductive immunology. 2009;83(1–2):185–9. Epub 2009/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kissinger P, Schmidt N, Mohammed H, Leichliter JS, Gift TL, Meadors B, et al. Patientdelivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sexually transmitted diseases. 2006;33(7):445–50. Epub 2006/03/15. [DOI] [PubMed] [Google Scholar]
- 26.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. American journal of obstetrics and gynecology. 2009;200(2):188 e1–7. Epub 2009/02/03. [DOI] [PubMed] [Google Scholar]
- 27.Biomed Diagnostics I. 2015; Available from: http://biomeddiagnostics.com/resources/files/100-001%20IFU%20InPouch%20TVRev_M.PDF.
- 28.FDA. Clinical Laboratory Improvement Amendments (CLIA). Available from: https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/IVDRegulatoryAssistance/ucm124105.htm.
- 29.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of clinical microbiology. 1991;29(2):297–301. Epub 1991/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. Journal of Infectious Diseases. 2005;192(12):2039–44. [DOI] [PubMed] [Google Scholar]
- 31.Williams JA, Ofner S, Batteiger BE, Fortenberry JD, Van Der Pol B. Duration of polymerase chain reaction-detectable DNA after treatment of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections in women. Sexually transmitted diseases. 2014;41(3):215–9. Epub 2014/02/14. [DOI] [PubMed] [Google Scholar]
- 32.Kissinger P Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC infectious diseases. 2015;15:307 Epub 2015/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feaster DJ, Mikulich-Gilbertson S, Brincks AM. Modeling site effects in the design and analysis of multisite trials. The American Journal of Drug and Alcohol Abuse. 2011;37(5):38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meites E, Gaydos CA, Hobbs MM, Kissinger P, Nyirjesy P, Schwebke JR, et al. A Review of Evidence-Based Care of Symptomatic Trichomoniasis and Asymptomatic Trichomonas vaginalis Infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61 Suppl 8:S837–48. Epub 2015/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spear GT, Sikaroodi M, Zariffard MR, Landay AL, French AL, Gillevet PM. Comparison of the Diversity of the Vaginal Microbiota in HIV-Infected and HIV-Uninfected Women with or without Bacterial Vaginosis. The Journal of infectious diseases. 2008;198(8):1131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torrone EA, Morrison CS, Chen PL, Kwok C, Francis SC, Hayes RJ, et al. Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies. PLoS medicine. 2018;15(2):e1002511 Epub 2018/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. The American journal of medicine. 1983;74(1):14–22. Epub 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 38.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virusinfected women. Journal of clinical microbiology. 2005;43(9):4607–12. Epub 2005/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin D, Burnett J, Taylor S. O10.2 Trichomonas vaginalis nucleic acid clearance following treatment of hiv negative women. Sexually transmitted infections. 2015;91(Suppl 2):A47–A. [Google Scholar]
- 40.Kissinger P, Rice J, Farley T, Trim S, Jewitt K, Margavio V, et al. Application of computer-assisted interviews to sexual behavior research. American journal of epidemiology. 1999;149(10):950–4. Epub 1999/05/26. [DOI] [PubMed] [Google Scholar]
- 41.Richman WL, Kiesler S, Weisband S, Drasgow F. A meta-analytic study of social desirability distortion in computer-administered questionnaires, traditional questionnaires, and interviews. Journal of Applied Psychology. 1999;84(5):754–75. [Google Scholar]
- 42.Sutton M, Sternberg M, Koumans EH, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(10):1319–26. Epub 2007/10/31. [DOI] [PubMed] [Google Scholar]
- 43.Sheehy O, Santos F, Ferreira E, Berard A. The use of metronidazole during pregnancy: a review of evidence. Current drug safety. 2015;10(2):170–9. Epub 2015/05/20. [DOI] [PubMed] [Google Scholar]
- 44.Koss CA, Baras DC, Lane SD, Aubry R, Marcus M, Markowitz LE, et al. Investigation of metronidazole use during pregnancy and adverse birth outcomes. Antimicrobial agents and chemotherapy. 2012;56(9):4800–5. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatski M, Martin DH, Clark RA, Harville E, Schmidt N, Kissinger P. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis among HIV-positive women. Sexually transmitted diseases. 2011;38(3):163–6. Epub 2010/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin TL, Monif GR. Trichomonas vaginalis and bacterial vaginosis. Coexistence in vaginal wet mount preparations from pregnant women. The Journal of reproductive medicine. 2000;45(2):131–4. Epub 2000/03/11. [PubMed] [Google Scholar]