Abstract
Objectives:
To evaluate the impact of previous local treatment on survival in men with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC).
Methods:
We performed a retrospective study of patients newly diagnosed with mCRPC in the year 2000 or later from eight Veterans Affairs Medical Centers. Patients were categorized based on prior local therapy (none, prostatectomy ± radiation or radiation alone). Overall and cancer specific survival was estimated by the Kaplan-Meier method. Cox proportional hazards regression models were used to test the association between prior local treatment and survival.
Results:
Of 729 patients, 284 (39%) underwent no local treatment, 176 (24%) underwent radical prostatectomy ± radiation and 269 (37%) underwent radiation alone. On multivariable analysis, men with prior prostatectomy had improved overall (HR, 0.71; p=0.005) and cancer specific survival (HR, 0.55; p<0.001) compared to men with no prior local therapy. This improvement in overall (HR, 0.89; p=0.219) and cancer specific survival (HR, 0.87; p=0.170) was not seen in men with prior radiation alone. After further adjusting for comorbidity with Charlson Comorbidity Index, patients with prior prostatectomy still had improved overall survival (HR, 0.70; p=0.003) while this was not seen in patients who received prior radiation alone (HR, 0.88, p=0.185).
Conclusions:
Independent of patient and disease related factors, men with mCRPC who had undergone prior radical prostatectomy have improved overall and cancer-specific survival compared to those with no prior local therapy.
Keywords: local therapy, mCRPC, prostate cancer, radical prostatectomy, radiation therapy
Introduction
Despite significant advances chemotherapy and androgen deprivation therapies, survival for men with metastatic prostate cancer has not significantly improved over the past 20 years.(1) With modern combination systemic therapy, survival rates can be improved. (2, 3) However, despite these combination, the death rate remains high and there remains no cure. As such, has been growing interest in recent years in the role of prior local therapy when the tumor was presumed to be clinically localized on outcomes among men with metastatic prostate cancer. (4)
Earlier studies have shown an increased response to ADT in patients with metastatic disease who had undergone prior local therapy.(5) Previous radical prostatectomy (RP) in patients with metastatic castration-sensitive prostate cancer has also been associated with a decrease in the risk of death. (6, 7) Outside of patients with castration-sensitive disease, the effects of previous local treatment on survival in men with advanced metastatic castration-resistant prostate cancer (mCRPC) disease remains unknown. (8)
To address this gap, we studied the impact of previous local treatment on overall survival (OS) and prostate cancer-specific mortality (CSS) in men with M0/MX CRPC who progressed to mCRPC. To do this, we identified men who were patients at Veterans Affairs (VA) Medical Centers newly diagnosed with mCRPC who had undergone either no prior local therapy, local therapy with RP ± XRT or local therapy with XRT alone. We hypothesized that patients with previous local therapy would have improved OS.
Methods:
Study Cohort
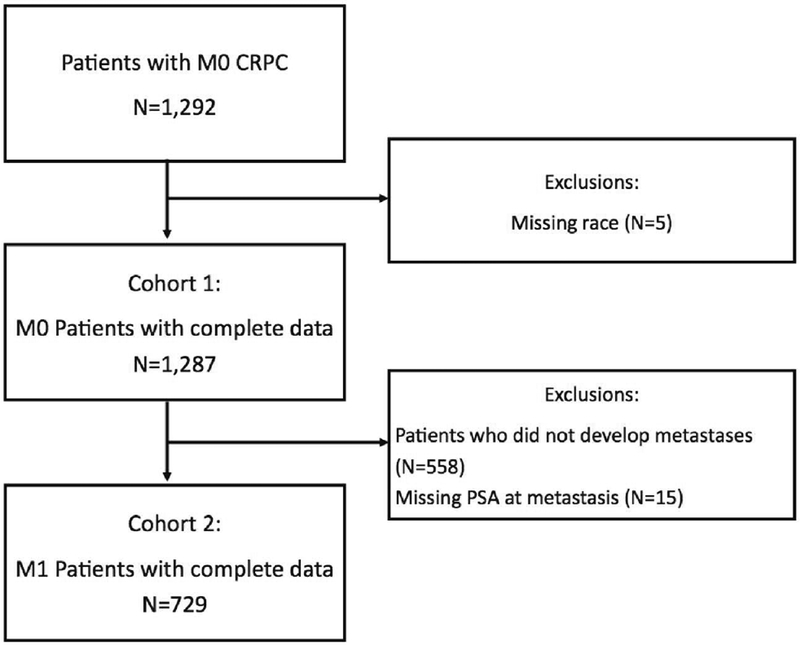
After obtaining Institutional Review Board approval, we collected data on patients who were diagnosed with CRPC, regardless of type of primary treatment, in the year 2000 or later from 8 VA Medical Centers (Durham and Asheville NC; Palo Alto, San Francisco, West Los Angeles, and San Diego, CA; Portland, OR; and Augusta, GA) from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Data abstracted included information on patient age, year of diagnosis, race, prostate-specific antigen (PSA) level, PSA doubling time (PSADT), tumor grade, time from ADT to CRPC, time from CRPC to metastasis and type of metastasis. Comorbidity burden at diagnosis was ascertained using the Deyo-Klabunde modification of the Charlson Comorbidity Index (CCI), utilizing secondary diagnosis codes. Charlson comorbidity scores were calculated for each patient, excluding the diagnosis of prostate cancer. CRPC was defined as a PSA rise of ≥2 ng/mL and 25% from the nadir PSA after ADT, while meeting the biological definition of castration.(9) Castration was defined as a serum testosterone level <50 ng/dL, bilateral orchiectomy, or continuous receipt of luteinizing-hormone releasing-hormone (LHRH) agonist or antagonist. In total, 1292 men had documented CRPC with an absence of a positive imaging test for distant metastases before a CRPC diagnosis (ie. M0 CRPC). The rationale to exclude men with metastasis prior to CRPC is to generate a cohort of men who all had initial diagnosis of mCRPC without having had metastases prior, thereby controlling for metastatic tumor burden as best as possible. Of these 1292 men with non-metastatic CRPC, 558 (43.2%) did not develop metastatic disease during follow-up and were excluded. We excluded patients who were missing data on race (n=5) and PSA (n=15) which left a total study cohort of 729 patients (Figure 1).
Figure 1:
Consort diagram showing derivation of study cohort
Statistical Analysis
Characteristics were compared among patients who had either no previous local treatment, RP with or without post-operative XRT or XRT alone using Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables. Univariable and multivariable Cox proportional hazards models were used to test the association between prior local treatment (none, RP ± XRT or XRT) and our primary endpoint of the time from diagnosis of mCRPC to OS and our secondary endpoint of CSS. Death from prostate cancer was defined as having metastases showing progression after hormonal therapy without another obvious cause of death. Multivariable models were adjusted for age at mCRPC diagnosis (continuous), year of mCRPC diagnosis (continuous), biopsy grade group (1 vs 2–3 vs 4–5 vs unknown/no biopsy), months from ADT to CRPC (continuous), months from CRPC to metastases (continuous), PSA at mCRPC (continuous, log-transformed), and PSADT at mCRPC diagnosis (>9 months vs <9 months vs missing), type of metastases (bone vs. soft tissue vs. bone and soft tissue vs. unknown) and treatment center. Kaplan-Meier curves were created for each endpoint, and differences between previous local therapy and the various outcomes were tested using the log-rank test. In order to assess for possible differences in OS and CSS in patients who had received RP alone versus those who had received RP ± XRT, we repeated the analysis using four groups, no prior local therapy, RP, RP± XRT and XRT. Stata version 14.0 (Stata Corp, College Station, Tex) was used for all statistical analyses. P < .05 was the threshold for statistical significance.
Results
Baseline Characteristics at Time of Metastases
Of the 729 patients with mCRPC, there were 284 (39%) that underwent no local treatment, 176 (24%) that had prior RP ± XRT and 269 (37%) had prior XRT alone (Table 1). All patients who underwent local therapy did so prior to development of CRPC. A total of 99 patients underwent RP + XRT (56%) and among these patients, 94 (95%) underwent either adjuvant or salvage XRT and 5 (5%) underwent salvage RP. A total of 364 patients underwent radiation therapy. Of these patients, 297 (81.6%) underwent external beam radiation therapy (EBRT), 21 (5.8%) underwent brachytherapy alone, 19 (5.2%) patients underwent a combination of brachytherapy and EBRT. Radiation technique is unknown for 27 (7.4%) patients. At the time of mCRPC diagnosis, patients with no prior local therapy were older than patients with prior local therapy with RP ± XRT or prior XRT alone (80 vs. 72 vs. 77 years; p<0.001), were diagnosed with metastases in earlier years (2007 vs. 2008 vs. 2009; p=0.001), had higher PSA values at metastases diagnosis (51.3 vs. 20.6 vs. 34.1 ng/ml; p<0.001), had high-grade tumors (36% vs. 24% vs. 30% grade group 4–5; p=0.033) and had longer time from ADT to CRPC (50 vs. 40 vs. 32 months; p=0.001). Among patients who received primary treatment, median time to ADT from primary localized treatment was 34 months in patients receiving RP ± XRT and 27 months in patients receiving XRT alone (p=0.001)There was no significant difference in race, time from CRPC diagnosis to time of metastasis diagnosis or in type of metastasis at diagnosis. The majority of patients (63%) were on continuous ADT with the remaining on intermittent ADT.
Table 1:
Baseline Patient Characteristics at time of metastasis
| None(N=284) | RP +/− XRT(N=176) | XRT alone(N=269) | p value | |
|---|---|---|---|---|
| Age at metastases | <0.0011 | |||
| Median | 80 | 72 | 77 | |
| Q1, Q3 | 73, 86 | 65, 78 | 69, 82 | |
| Race | 0.0522 | |||
| Non-black | 191 (67%) | 133 (76%) | 203 (75%) | |
| Black | 93 (33%) | 43 (24%) | 66 (25%) | |
| Year of metastases | 0.0011 | |||
| Median | 2007 | 2008 | 2009 | |
| Q1, Q3 | 2004, 2011 | 2006, 2012 | 2005, 2011 | |
| PSA at metastases | <0.0011 | |||
| Median | 51.3 | 20.6 | 34.1 | |
| Q1, Q3 | 19.1, 144.0 | 7.3, 70.7 | 12.8, 96.9 | |
| PSADT at metastases | 0.5092 | |||
| Non-calculable | 52 (18%) | 41 (23%) | 49 (18%) | |
| <9 months | 154 (54%) | 89 (51%) | 155 (58%) | |
| ≥9 months | 78 (27%) | 46 (26%) | 65 (24%) | |
| Grade group | 0.0332 | |||
| Unknown | 92 (32%) | 77 (44%) | 83 (31%) | |
| 1 | 33 (12%) | 21 (12%) | 41 (15%) | |
| 2–3 | 56 (20%) | 35 (20%) | 64 (24%) | |
| 4–5 | 103 (36%) | 43 (24%) | 81 (30%) | |
| Months from ADT to CRPC | 0.0011 | |||
| Median | 49.7 | 40.1 | 31.9 | |
| Q1, Q3 | 21.0, 82.7 | 17.9, 76.2 | 17.2, 64.0 | |
| Months from CRPC to metastasis | 0.0541 | |||
| Median | 15.8 | 13.5 | 14.0 | |
| Q1, Q3 | 6.6, 34.8 | 4.4, 26.4 | 6.0, 27.6 | |
| Type of metastasis at diagnosis | 0.1692 | |||
| Unknown | 4 (1%) | 1 (1%) | 1 (0%) | |
| Bone metastasis | 162 (57%) | 82 (47%) | 146 (54%) | |
| Soft tissue metastasis | 21 (7%) | 11 (6%) | 21 (8%) | |
| Soft tissue and bone metastasis | 97 (34%) | 82 (47%) | 101 (38%) | |
| Charlson Comorbidity Index | 0.5312 | |||
| 0 | 47 (17%) | 26 (15%) | 45 (17%) | |
| 1 | 44 (15%) | 28 (16%) | 33 (12%) | |
| 2 | 39 (14%) | 28 (16%) | 29 (11%) | |
| 3+ | 154 (54%) | 94 (53%) | 162 (60%) |
Kruskal Wallis
Chi-Square
Time from mCRPC to Overall Survival (Primary Outcome)
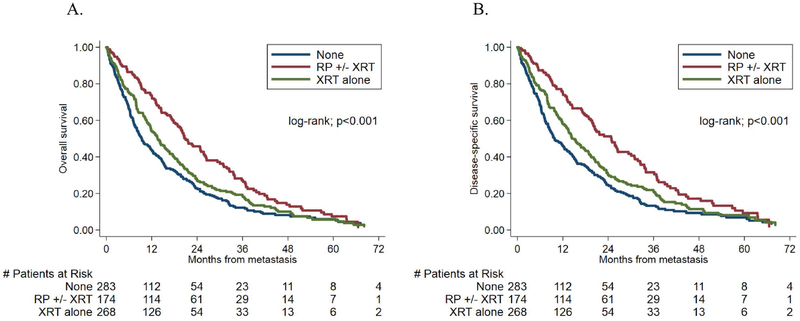
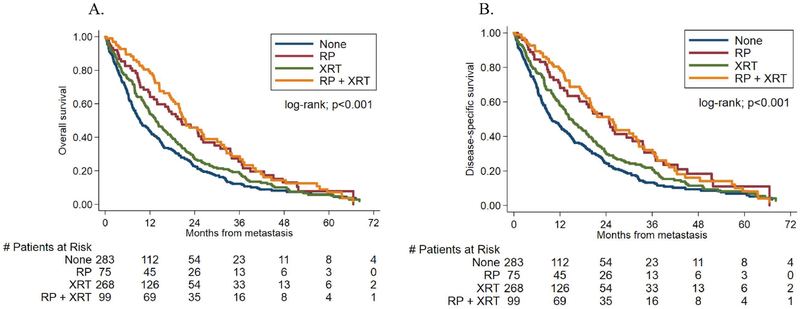
We compared OS rates between patients who had received previous local therapy and those who had not, from the time of metastasis diagnosis. The median follow-up time after metastasis was 14.9 months (IQR- 4.8–27.0) among patients who survived. During follow-up, 588 (81%) patients died. Patients who had received RP ± XRT had a decreased risk of death on univariable analysis (HR, 0.58; 95% confidence interval [CI], 0.47–0.72; p<0.001). Results on multivariable analysis remained statistically significant (HR, 0.71; p=0.005). On univariable analyses, patients who had received prior XRT had a decreased risk of death (HR, 0.83; 95% CI, 0.69–0.99; p=0.043); however, this decreased risk of death was no longer significant on multivariable analysis (HR, 0.89; 95% CI, 0.73–1.07; p=0.219). These results are illustrated in Table 2 and Figure 2a. We then assessed for possible differences in OS in patients who had received RP alone versus those who had received RP + XRT. Compared to patients with RP alone, there was no improvement in OS in patients with prior RP + XRT on univariable (HR, 0.90; 95% CI, 0.64–1.29 p=0.55) or multivariable (HR, 0.88; 95% CI, 0.61–1.26; p=0.47) analysis (Figure 3, Table S1). Hazard ratios for the association between primary treatment and time from metastasis to all-cause mortality are shown in Supporting Table 2 (Table S2).
Table 2:
Hazard ratios for the association between primary treatment and time from metastasis to all-cause mortality and PC-specific mortality
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| All-cause mortality | ||||
| Primary treatment | ||||
| none | ref | ref | ||
| radical prostatectomy ± radiation | 0.58 (0.47, 0.72) | <0.001 | 0.71 (0.56, 0.90) | 0.005 |
| radiation alone | 0.83 (0.69, 0.99) | 0.043 | 0.89 (0.73, 1.07) | 0.219 |
| Prostate cancer-specific mortality | ||||
| Primary treatment | ||||
| none | ref | ref | ||
| radical prostatectomy ± radiation | 0.55 (0.44, 0.69) | <0.001 | 0.68 (0.53, 0.87) | 0.002 |
| radiation alone | 0.80 (0.66, 0.96) | 0.019 | 0.87 (0.71, 1.06) | 0.170 |
Multivariable model adjusted for age, race, year, PSA, PSADT, grade group, time from ADT to CRPC, time from CRPC to metastasis, type of metastasis, and treatment center
Fig 2:
Kaplan-Meier curves showing the relationship between primary treatment stratified into three groups and time from metastasis to (A) all-cause mortality (B) prostate cancer-specific mortality
Fig 3:
Kaplan-Meier curves showing the relationship between primary treatment stratified into four groups and time from metastasis to (A) all-cause mortality (B) prostate cancer-specific mortality
Time from mCRPC to Prostate Cancer Specific Survival
We next compared CSS rates between patients who had received previous local therapy and those who had not, from the time of metastasis diagnosis. Of the 588 men who died, 543 died of prostate cancer (92%). Patients who had received RP ± XRT had a decreased risk of CSS on univariable analysis (HR, 0.55; 95% CI, 0.44–0.69; p<0.001). Analogous to the results with OS, results remained statistically significant on multivariable analysis (HR, 0.68; 95% CI, 0.53–0.87; p=0.002). Similar to the OS results, patients who had received prior XRT had a decreased risk of CSS on univariable analyses (HR, 0.80; 95% CI, 0.66–0.96; p=0.019); however, this decreased risk was no longer significant on multivariable analysis (HR, 0.87; 95% CI, 0.71–1.06; p=0.170). Hazard ratios for the association between primary treatment and time from metastasis to prostate cancer-specific mortality are shown in Supporting Table 3 (Table S3). These results are illustrated in Table 2 and Figure 2b. We then assessed for possible differences in CSS in patients who had received RP alone versus those who had received RP + XRT. Compared to patients with RP alone, there was no improvement in CSS in patients with prior RP + XRT on univariable (HR, 0.97; 95% CI, 0.67–1.40; p=0.88) or multivariable (HR,0.97; 95% CI, 0.66–1.42; p=0.88) analysis (Figure 3, Table S1).
Table 3:
Hazard ratios for the association between primary treatment and time from metastasis to all-cause mortality and PC-specific mortality adjusted for Charlson comorbidity index
| Multivariable | ||
|---|---|---|
| HR (95% CI) | p-value | |
| All-cause mortality | ||
| Primary treatment | ||
| none | ref | |
| radical prostatectomy ± radiation | 0.70 (0.55, 0.88) | 0.003 |
| radiation alone | 0.88 (0.72, 1.07) | 0.185 |
| Prostate cancer-specific mortality | ||
| Primary treatment | ||
| none | ref | |
| radical prostatectomy ± radiation | 0.66 (0.52, 0.85) | 0.001 |
| radiation alone | 0.86 (0.70, 1.05) | 0.142 |
Multivariable model adjusted for age, race, year, PSA, PSADT, grade group, time from ADT to CRPC, time from CRPC to metastasis, type of metastasis, treatment center, and Charlson comorbidity index
Comorbidity and Overall and Prostate Cancer Specific Survival
To account for the possibility that differences in health status accounted for this difference in survival, we further adjusted for CCI in our models to assess differences in time from development of metastases to OS and CSS (Table 3). After adjusting clinical and pathological characteristics along with CCI, results were nearly identical to those without adjusting for CCI in that patients with prior local therapy with RP ± XRT had a decreased risk of OS (HR, 0.70; 95% CI, 0.55–0.88; p=0.003) while this was not seen in patients who received prior XRT alone (HR, 0.88; 95% CI, 0.72–1.07, p=0.185). Similarly, adjusting for CCI in addition to clinical and pathological characteristics did not affect the association between prior local therapy with RP ± XRT and lower CSS (HR, 0.66; 95% CI, 0.52–0.85; p=0.001) nor the null association between prior XRT alone and CSS (HR, 0.86; 95% CI, 0.7–1.05; p=0.142).
Discussion:
The role of RP in multimodal treatment for advanced prostate cancer has gained increasing interest. Various retrospective studies have shown that following the development of metastases, men who had previously undergone local therapy had improved survival and in men with metastatic prostate cancer at diagnosis, definitive local therapy was associated with improved survival.(6, 7, 10) Six prospective randomized controlled trials evaluating the role of therapy to the primary tumor in the metastatic setting are currently ongoing. However, these previous studies and trials have not included men with mCRPC, the most advanced form of prostate cancer. On multivariable analysis, controlling for patient and disease related factors, including comorbidity, we found that men with mCRPC who had undergone prior local therapy when the disease was presumed to be localized, particularly with RP, had improved OSS and CSS. The current data support continued research to evaluate the potential benefit of removal of the primary tumor.
There is a growing biological rationale for local management of systemic disease. Despite systemic chemohormonal therapy, aggressive prostate cancer often remains within the primary tumor site.(11, 12) Cancer cells that leave the primary tumor can seed metastases in distant organs via circulating tumor cells.(13) In a bidirectional process, these circulating cells can also colonize their tumors of origin.(14) This interaction of metastatic and primary tumor cells promotes disease progression, androgen independence, and development of metastasis.(15) Retrospective reviews have expanded on these pre-clinical models. In the metastatic setting, analysis of Surveillance Epidemiology and End Results (SEER) data and secondary analysis of results from several trials on metastatic patients have shown a correlation with improved survival outcome in men who underwent prior primary therapy before a diagnosis of metastatic disease.(6, 16) Retrospective analysis of the SEER database and Munich cancer registry on RP in men after a diagnosis of metastatic prostate cancer have similarly shown associations with improved survival.(17, 18) Although these studies have provided a rationale for further studying the impact of local therapy in advanced prostate cancer, the inherent patient and tumor selection bias associated with survivorship outcomes in observational studies limits the ability to make meaningful recommendations based on them. (19, 20) Recent studies have examined the feasibility of RP in men with metastatic hormone sensitive prostate cancer with all showing complication rates comparable to those reported in a series of RP in high-risk localized disease.(21–24) Similar results have also been shown in those with castration resistant metastatic disease. (25)
The finding of improved OS in men newly diagnosed with mCRPC who had undergone prior RP is consistent with previous reports in men newly diagnosed with metastatic castration-sensitive disease. Interestingly, we found that this associated benefit was similar in men who had received prior RP alone and prior RP ± XRT. The impact of previous XRT in men with metastatic prostate cancer remains controversial. Though two studies showed that among men with metastatic prostate cancer, those who previously received XRT had improved survival relative to no treatment(7, 18), one study found these men actually had worse survival compared to those who received either RP or no prior local therapy.(6) Moreover, in one of the studies where XRT was associated with improved survival, a propensity score-matched analysis showed that this benefit was still less than that seen after RP.(10) One possible explanation for the improved survival after metastasis in men receiving RP compared with men receiving XRT is tumor selection bias. Patient’s receiving XRT may have done so because of unresectability by RP or large tumor burden in the primary. Alternatively, younger, healthier patients may opt for surgery at time of initial diagnosis and in turn live longer than those undergoing XRT. Other authors have suggested that tumor self-seeding of the irradiated prostate leads to increased metastatic potential of the cancer cells. However, these results remain speculative, and three ongoing clinical trials (STAMPEDE, NCT00268476; PEACE-1, NCT01957436; and HORRAD, NTR271) investigating the role of local XRT in metastatic disease are ongoing with early results from STAMPEDE showing improved survival in the cohort who had XRT.(26)
Our findings are consistent with the growing literature showing an association between improved survival and previous local therapy in men with advanced prostate cancer, specifically mCRPC. Although the theory of circulating tumor cells provides a biologic rationale for our findings, alternative explanations are plausible. Our multivariable model attempted to adjust for various patient and tumor specific factors including comorbidity; however, it is possible that healthier men selectively underwent previous local therapy. Although we attempted to control for comorbidity with the CCI, this tool primarily focuses on disease presence and not severity. Therefore, it is unable to capture the spectrum of severity within a given comorbid condition. However, previous studies have shown a differential prognostic impact of comorbidity, with concurrent comorbidities having a low impact in patients with advanced cancer and highly morbid cancer, such as mCRPC.(27) Alternatively, our cohort was men newly diagnosed with mCRPC (i.e. they had all been diagnosed with non-metastatic CRPC prior). It is possible that men who received local therapy, who were diagnosed in later years, were followed more closely and had their metastases detected earlier. Indeed, the PSA levels at mCRPC diagnosis were lower as was the time from ADT to CRPC and CRPC to metastases (though this may also reflect more aggressive disease). Though we controlled for these factors, it remains possible that a lead time bias exists, which may have influenced these results. As newer, more metastatic sensitive, imaging modalities develop, the heterogeneity of the M0 CRPC population will become better elucidated. An examination of our findings within this context will help clarify these results.
Despite the strengths afforded by our study, including a VA cohort from multiple centers and the large number of African American and non-African American patients, it has several limitations. First, this was a retrospective study that relied on procurement of information via interpretation of patient medical records. Although our inclusion of only VA patients is a strength for evaluating patients who have equal access, it does make our results potentially difficult to extrapolate to other patient populations. Importantly, because these results were outside of a clinical trial setting, treatment with various anticancer therapies was not standardized. However, most patients in our study were diagnosed in in an era that pre-dated the modern use of agents, such as abiraterone and enzalutamide. Given the modest improvements in survival associated with these pre-modern chemotherapy agents, it is unlikely that differences in chemotherapy use would entirely explain these results. Also, though we controlled for comorbidities, patient and clinical factors, it is possible residual confounding explains our results. Finally, as noted above, imaging for metastases was not uniform and we cannot rule out lead time bias.
Multimodal treatment including local radiation or surgery for advanced prostate cancer has gained increasing interest. No prior study or trial has evaluated the potential impact in patients with progressive M0 CRPC. Independent of patient and disease related factors, including comorbidity, we found that men with M0 CRPC who progressed to mCRPC and who had undergone prior RP had improved OS and CSS when compared to men with no prior local therapy. These data suggest the need for prospective evaluation to determine the potential impact of removal of the primary tumor even in the most advanced stages of prostate cancer.
Supplementary Material
Acknowledgment:
William J Aronson was supported by a National Institutes of Health research grant (grant number: R01 CA231219).
Footnotes
Conflicts of interest: The authors declare no relationships that may pose as a conflict of interest.
References
- [1].Mohler JL. Concept and viability of androgen annihilation for advanced prostate cancer. Cancer. 2014; 120: 2628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kyriakopoulos CE, Chen YH, Carducci MA et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol 2018; 36: 1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fizazi K, Tran N, Fein L et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017; 377: 352–60. [DOI] [PubMed] [Google Scholar]
- [4].Bayne CE, Williams SB, Cooperberg MR et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. Eur Urol. 2016; 69: 775–87. [DOI] [PubMed] [Google Scholar]
- [5].Swanson GP, Riggs M, Earle J. Failure after primary radiation or surgery for prostate cancer: differences in response to androgen ablation. J Urol. 2004; 172: 525–8. [DOI] [PubMed] [Google Scholar]
- [6].Thompson IM, Tangen C, Basler J, Crawford ED. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol. 2002; 168: 1008–12. [DOI] [PubMed] [Google Scholar]
- [7].Shao YH, Kim S, Moore DF et al. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. Eur Urol 2014; 65: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Halabi S, Vogelzang NJ, Ou SS, Small EJ. The impact of prior radical prostatectomy in men with metastatic castration recurrent prostate cancer: a pooled analysis of 9 Cancer and Leukemia Group B Trials. J Urol. 2007; 177: 531–4. [DOI] [PubMed] [Google Scholar]
- [9].Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011; 29: 3695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Antwi S, Everson TM. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol. 2014; 38: 435–41. [DOI] [PubMed] [Google Scholar]
- [11].Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008; 180: 565–70; discussion 70. [DOI] [PubMed] [Google Scholar]
- [12].Tzelepi V, Efstathiou E, Wen S et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol. 2011; 29: 2574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006; 25: 521–9. [DOI] [PubMed] [Google Scholar]
- [14].Kim MY, Oskarsson T, Acharyya S et al. Tumor self-seeding by circulating cancer cells. Cell. 2009; 139: 1315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Varkaris A, Katsiampoura AD, Araujo JC, Gallick GE, Corn PG. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 2014; 33: 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eisenberger MA, Blumenstein BA, Crawford ED et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998; 339: 1036–42. [DOI] [PubMed] [Google Scholar]
- [17].Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol. 2014; 66: 602–3. [DOI] [PubMed] [Google Scholar]
- [18].Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014; 65: 1058–66. [DOI] [PubMed] [Google Scholar]
- [19].Williams SB, Huo J, Chamie K et al. Discerning the survival advantage among patients with prostate cancer who undergo radical prostatectomy or radiotherapy: The limitations of cancer registry data. Cancer. 2017; 123: 1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008; 112: 2456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015; 193: 832–8. [DOI] [PubMed] [Google Scholar]
- [22].Gandaglia G, Fossati N, Stabile A et al. Radical Prostatectomy in Men with Oligometastatic Prostate Cancer: Results of a Single-institution Series with Long-term Follow-up. Eur Urol. 2016. [DOI] [PubMed] [Google Scholar]
- [23].Sooriakumaran P, Karnes J, Stief C et al. A Multi-institutional Analysis of Perioperative Outcomes in 106 Men Who Underwent Radical Prostatectomy for Distant Metastatic Prostate Cancer at Presentation. Eur Urol. 2016; 69: 788–94. [DOI] [PubMed] [Google Scholar]
- [24].Moschini M, Morlacco A, Kwon E, Rangel LJ, Karnes RJ. Treatment of M1a/M1b prostate cancer with or without radical prostatectomy at diagnosis. Prostate Cancer Prostatic Dis. 2017; 20: 117–21. [DOI] [PubMed] [Google Scholar]
- [25].Reichard CA, Gregg JR, Achim MF et al. Radical Prostatectomy in Metastatic Castration-resistant Prostate Cancer: Feasibility, Safety, and Quality of Life Outcomes. Eur Urol. 2018. [DOI] [PubMed] [Google Scholar]
- [26].James ND, Sydes MR, Clarke NW et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer--a multi-arm multi-stage randomised controlled trial. Clin Oncol (R Coll Radiol). 2008; 20: 577–81. [DOI] [PubMed] [Google Scholar]
- [27].Read WL, Tierney RM, Page NC et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004; 22: 3099–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.