Abstract
19F NMR spectroscopy is an attractive and growing area of research with broad applications in biochemistry, chemical biology, medicinal chemistry, and materials science. We have explored fast magic angle spinning (MAS) 19F solid-state NMR in assemblies of HIV-1 capsid protein. Tryptophan residues with fluorine substitutions in the 5 position of the indole ring were used as the reporters. The 19F chemical shifts for the five tryptophans are distinct, reflecting differences in their local environment. Spin diffusion and radio frequency driven recoupling experiments were performed at MAS frequencies of 35 kHz and 40–60 kHz, respectively. Fast MAS frequencies of 40–60 kHz are essential for consistently establishing 19F-19F correlations, yielding interatomic distances of the order of 20 Å. To our knowledge, this is the first example of 19F MAS NMR being applied to a very large protein assembly. Our results demonstrate the potential of fast MAS 19F NMR for structural analysis in large biological assemblies.
Keywords: magic angle spinning, 19F NMR, HIV-1 capsid, protein assemblies, fast MAS
Graphical Abstract
Structural characterization of large biological assemblies is a challenge by conventional techniques. We present fast 19F MAS NMR as an attractive alternative method for investigating protein assemblies. We show that high resoution is attained and nanometer distances of the order of 20 Å are detected in 19F-19F correlation spectra. Our results establish the potential of 19F MAS NMR for structural analysis in large protein assemblies.
Protein structure determination by magic-angle spinning (MAS) NMR spectroscopy relies largely on experimental interatomic distance constraints. These are extracted from 13C, 15N and 1H based correlations, which yield distances of up to 6–8 Å.[1–4] While this approach has been successfully employed for deriving structures of a number of proteins,[5–12] for multi-domain proteins and protein assemblies, additional information generally is required to determine the quaternary structure or supramolecular organization. 19F MAS NMR spectroscopy is an attractive alternative way for investigating biological systems. 19F is a spin 1/2 nucleus with a very high gyromagnetic ratio, 100% natural abundance, exhibiting a large chemical shift range (>300 ppm). The strong 19F-19F dipolar couplings make fluorine well suited as a long-range distance probe in the solid state and distances up to 20 Å have been detected.[13–14] Fluorine is absent from any naturally occurring biological molecule, yet it can be readily and selectively incorporated into proteins,[15–17] largely without causing major structural perturbations.[18] 19F NMR, both solution and solid-state, has therefore emerged as an essential method with broad applications in pharmaceutical chemistry (as ~30% of all drugs at present in the clinic contain fluorine),[19] chemical biology,[20] biochemistry,[16, 21–24] and materials science.[25] 19F NMR has been applied to investigate proteins, lipids, nucleic acids, synthetic small-molecule ligands, as well as their complexes, both in solution[16, 21] and in the solid state.[22–24] 19F MAS NMR of biological systems remains underutilized, due to challenges associated with inherently broad lines due to strong homonuclear 19F-19F and heteronuclear 19F-1H dipolar couplings, particularly at traditionally used MAS frequencies below 25 kHz. Only three recent studies used faster spinning frequencies. In two, resolution enhancements were observed at 35 and 40 kHz,[14, 26] while a report from our group demonstrated that frequencies of 40–60 kHz yielded significant line narrowing for fluorosubstituted tryptophan solids, thus alleviating the need for 1H decoupling.[27]
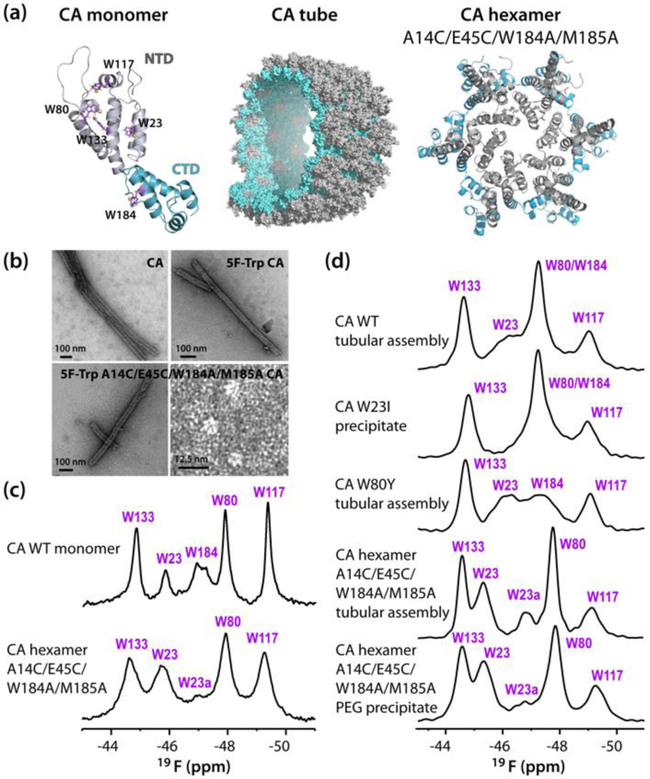
Here, we present a 19F MAS NMR investigation of HIV-1 CA protein tubular assemblies. Such tubes have been studied extensively by cryo-EM[28–29] and MAS NMR spectroscopy before,[1, 30–31] representing an excellent system for exploring the potential of 19F MAS NMR for structural analysis of protein assemblies. HIV-1 CA protein was uniformly labeled with 5F-tryptophan (Trp). Trp residues are excellent sites for 19F substitution as this amino acid is generally rare in protein sequences and simple methodologies exist for introducing modified tryptophans into proteins, using standard E. coli expression systems.[16–17] CA contains five tryptophans, four of which (W23, W80, W117, and W133) are located in the N-terminal domain (NTD) and one (W184) at the C-terminal (CTD) dimerization interface (Fig. 1).
Figure 1.
(a) Structures of the HIV-1 CA monomer (W23, W80, W117, W133 and W184 are shown in purple stick representation with fluorine atoms as yellow spheres), a section of the CA tube and the A14C/E45C/W184A/M185A CA cross-linked hexamer (NTD; grey, CTD; teal) (b) Transmission electron micrographs of U-15N CA tubes, 5-19F-Trp,U-15N tubes, 5-19F-Trp,U-13C,15N A14C/E45C/W184A/M185A tubes and 5F-Trp A14C/E45C/W184A/M185A CA soluble cross-linked hexamers. (c) 19F solution NMR spectra at 14.1 T of 5-19F-Trp,U-15N CA and 5-19F-Trp,U-13C,15N A14C/E45C/W184A/M185A CA cross-linked hexamer. (d) 19F MAS NMR spectra (19.96 T, MAS frequency; 40 kHz) of (top to bottom): 5-19F-Trp,U-13C,15N CA tubes, 5-19F-Trp,U-15N W23I mutant CA tubes, 5-19F-Trp,U-15N W80Y mutant CA tubes, 5-19F-Trp,U-13C,15N A14C/E45C/W184A/M185A mutant CA tubes, and A14C/E45C/W184A/M185A cross-linked CA hexamer precipitated with PEG-4000.
We show that fast MAS frequencies of 40–60 kHz for 19F spectroscopy result in narrow lines for CA assemblies, even in the absence of 1H decoupling. Furthermore, employing proton-driven spin diffusion (PDSD)[32] and/or radio frequency driven recoupling (RFDR),[33] it was possible to observe intramolecular 19F-19F correlations, corresponding to distances of the order of 20 Å. To our knowledge, this is the first example of 19F MAS NMR being applied to a very large protein assembly.
Introduction of 19F into CA Does Not Perturb Assembly or Overall Structure.
Replacement of all native tryptophans by 5F-Trp has no measurable effect on the in vitro tubular assemblies, given that the morphology and the dimensions of U-15N-CA and 5F-Trp,U-15N CA tubes are indistinguishable. (Fig. 1b). Furthermore, no significant chemical shift perturbations (other than those associated with residues close to the substitution sites) were noted in the 2D 1H-detected MAS (H)NH and (H)CH HETCOR spectra (Fig. S1, Supporting Information). Thus, the introduction of fluorine into CA does not interfere with assembly and does not perturb the overall structure of the tubes.
19F Chemical Shifts Are Sensitive to the Local Environment.
19F solution NMR spectrum of monomeric CA (Fig. 1c) exhibits several resonances of varying intensity. They were assigned by mutagenesis (Fig. S2, Supporting Information). W80, W117 and W133 possess ~unit intensity resonances at −47.9 ppm, −49.4 ppm and −44.9 ppm, while W23 and W184 exhibit several, smaller intensity resonances at −45.9, −46.3, −46.7 ppm and −48.1, −47.2, −47.8 ppm, respectively. This observation is consistent with prior findings that W23 and W184 exists in several conformations in solution.[34] The multiple resonances of W184 arise from monomeric and different dimeric quaternary structures.[34] All 19F chemical shifts are summarized in Table S1 (Supporting Information).
Solid-state 19F NMR spectra of CA tubular assemblies were acquired at MAS frequencies of 30, 40, and 60 kHz (Fig. 1d and S3). As can be appreciated from comparing the spectra at different frequencies (Fig. S3, Supporting Information), for MAS frequencies below 60 kHz, proton decoupling is necessary to obtain sharp resonances. The 19F line widths in the MAS NMR spectrum acquired at ωr = 30 kHz with high-power proton decoupling (0.5–1.3 ppm) are still broader than those in the spectrum recorded at ωr = 60 kHz without decoupling (0.3–1.0 ppm). The spectra collected at ωr = 40 kHz, without decoupling, exhibit line widths of 1.1 ppm. This observation is in agreement with our findings for fluorosubstituted tryptophan solids[27] and clearly illustrates the benefits of fast MAS conditions.
The general chemical shift range for all five 5F-Trp sidechain resonances is similar to that for CA in solution (−44.7 to −49.1 ppm), yet noticeable differences exist. Assemblies of several mutants were prepared, and in particular W23I, W80Y, and A14C/E45C/W184A/M185A samples permitted unambiguous assignments (Fig. 1d). A14C/E45C/W184A/M185A was cross-linked into a hexamer and the spectrum of this hexamer in the tubular assembly was very useful in this regard. The 5F-Trp resonances of W133 and W117 are at −44.7 and −49.1 ppm, respectively. W80 exhibits a relatively narrow resonance at −47.3 ppm, superimposed on the broad resonance of W184. W23 resonates at −46.3 ppm in the WT assembly and is downfield-shifted to −45.3 ppm with the second, minor conformer at −46.8 ppm in the A14C/E45C/W184A/M185A hexamer mutant. All chemical shifts are summarized in Tables 1 and S2 (Supporting Information). The W23 and W184 resonances are broader than the others and of the order of 1.3 ppm, suggesting some degree of conformational heterogeneity. However, in the tubular assembly of the hexamer, the W23 resonance at −45.3 ppm (major conformer) is sharpened up, suggesting that conformational heterogeneity is reduced and a slightly different local environment is present.
Table 1.
MAS NMR experimental 19F chemical shift parameters for the 5F-Trp substituted CA capsid tubular assemblies.
| Field strength/ MAS frequency |
Residue | δiso (ppm) (±0.1) |
δσ (ppm) (±1.0) |
η (±0.3) |
|---|---|---|---|---|
| 19.96 T/15 kHz | W23 | −46.3 | 44.4 | 0.4 |
| W80/W184 | −47.3 | 46.1 | 0.0 | |
| W117 | −49.1 | 44.6 | 0.0 | |
| W133 | −44.7 | 45.3 | 0.3 | |
| 11.74 T/4 kHz | W23 | −46.1 | 44.6 (±1.6) | 0.6 |
| W80/W184 | −47.3 | 46.7 | 0.4 | |
| W117 | −49.1 | 44.1 | 0.4 | |
| W133 | −45.0 | 44.8 | 0.7 |
The conformational heterogeneity for W184 at the CTD-CTD dimer interface comes as no surprise, since different sidechain conformers have been observed by X-ray (PDB 3NTE, 4XFX, 4XFY)[35–36] and NMR.[34] The side chain of W23 is buried in the NTD core and is essential for proper core assembly.[37] Solution NMR revealed multiple amide resonances for W23,[34] and its side chain was shown to be displaced by more than 3 Å in complexes of CA with potent antiviral capsid inhibitors, derivatives of benzodiazepines and benzoimidazoles,[38] indicative of side chain conformational variability.
While isotropic chemical shifts are a key NMR observable, orientation-dependent chemical shift anisotropy (CSA) tensors are an even richer source for exploring local electronic and geometric structure. CSA parameters are also important for inter-fluorine distance measurements by spin exchange experiments.[14] Furthermore, our long-term goal is to combine experiment and quantum chemical calculations of 19F CSA parameters in conjunction with 19F-19F distance restraints for structure elucidation of large protein assemblies. While we have recently demonstrated the proof of principle on model crystalline tryptophans,[27] to apply this approach broadly it is necessary to establish a database of experimental 19F CSA parameters for a range of proteins and protein assemblies. As the first step in this endeavor, we have determined experimental 19F CSA parameters for HIV-1 capsid assemblies, a system that is well established and extensively studied in our laboratories.
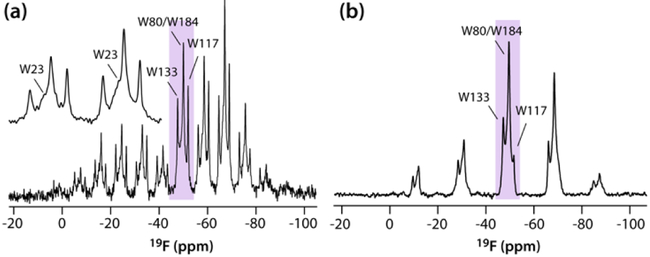
19F CSA tensors for the WT CA tubular assemblies were recorded in two sets of experiments: (i) at 11.74 T with a MAS frequency of 4 kHz and high-power 1H decoupling, (ii) at 19.96 T with a MAS frequency of 15 kHz without 1H decoupling. Good agreement for the 19F CSA tensor parameters is obtained by both approaches (Fig. 2; Table 1), with uncertainties of ±1.0 ppm and ±0.3 for the reduced anisotropy and the asymmetry parameters, respectively. We note that even at slow MAS frequencies, accurate CSA parameters can be obtained, even in the absence of 1H decoupling, since no contribution from 19F-19F homonuclear dipolar interactions are present. Interestingly, no correlation between the magnitude of the reduced anisotropy and the isotropic chemical shift is observed.
Figure 2.
19F MAS NMR spectra of 5-19F-Trp,U-15N CA tubes (11.74 T; MAS frequency 4 kHz (a), and 19.96 T; MAS frequency 15 kHz (b)). The inset in (a) shows the W23 resonance.
Observation of Interatomic 19F-19F Correlations in Fast MAS Spectra.
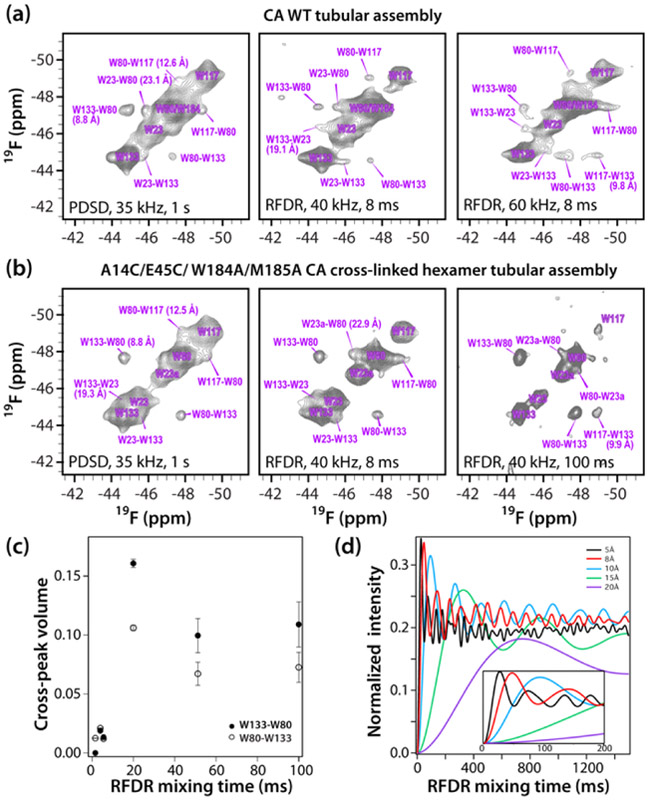
Intra- and intermolecular 19F-19F distances in 5F-Trp labeled CA assemblies are expected to range between 8.8 and 42.5 Å. Based on the X-ray structures of wild type CA[36] and A14C/E45C/W184A/M185A CA cross-linked hexamers[39] (Table S2), five pairs of Trp residues are separated by distances that are within a potentially accessible detection range in dipolar-based experiments: W80–133 (8.8 Å), W117-W133 (9.8 Å), W80–117 (12.6 Å), W23-W133 (19.1 Å), and W23-W80 (23.1 Å). For fluorine distance measurements by MAS NMR, several methods are available: RFDR[33], CODEX,[40] and, more recently, PDSD[32] and CORD[41]. In a recent study at 14.1 T which employed PDSD and CORD mixing at a MAS frequency of 25 kHz, correlations were observed for distances as long as 16 Å for long CORD mixing times of 306 ms.[14]
Here, for CA assemblies, we established that ideally MAS frequencies exceeding 35 kHz are required to ensure sufficiently narrow lines in the absence of 1H decoupling (see Fig. 1), an important consideration when using probes without separate 19F and 1H channels. MAS frequencies of 35, 40, and 60 kHz, and two mixing schemes - PDSD and RFDR, were used for detection of 19F-19F correlations in the fluorinated CA assemblies. These two sequences were selected on the basis of the following criteria: i) efficient polarization transfer over the full spectral width can be achieved; ii) no or minimal contributions from other anisotropic interactions are apparent; iii) insensitivity to experimental imperfections and off-resonance effects has been ascertained; and iv) RF powers compatible with the hardware can be applied.
PDSD spectra of fluorinated wild type CA and cross-linked hexamer A14C/E45C/W184A/M185A CA assemblies, recorded at a MAS frequency of 35 kHz, using a mixing time of 1 s (Fig. 3A,B) contain cross peaks between W80-W133 resonances, W80-W117 resonances and W23-W133 resonances. The associated 5F positions are separated by 8.8 Å, 12.6 Å, and 19.1 Å, respectively. In addition, a W23-W80 cross peak is also observed, corresponding to a F-F distance of 23.1 Å. Since PDSD is no longer an efficient mixing scheme for 40 and 60 kHz spinning frequencies at any practically attainable condition,[41] we employed RFDR mixing at these higher MAS frequencies. At both spinning frequencies, correlations between W80-W133 and W80-W117 resonances are observed with an RFDR mixing time of 8 ms (Fig. 3). At 40 kHz, an additional cross peak between W80-W23 is present. At 60 kHz, the W133-W117 correlation appears,while the equivalent W117-W133 cross peak is not seen. It is known that RFDR and other recoupling sequences may give rise to asymmetric cross peak patterns, even when the magnetization is equal at the beginning of the recoupling period.[42] This can be caused by multiple factors, such as different relaxation rates for individual sites during mixing.
Figure 3.
2D 19F-19F correlation spectra (19.96 T) of (a) 5-19F-Trp CA tubes and (b) 5-19F-Trp A14C/E45C/W184A/M185A CA cross-linked hexamer tubes. MAS frequencies and mixing times in the 19F-19F RFDR spectra are listed. The first contour level was set to 5 x noise rmsd. No 1H decoupling was applied. (c) Experimental 19F-19F RFDR buildup of the W80-W133 and W133-W80 cross-peak volumes in the 5-19F-Trp A14C/E45C/W184A/M185A CA cross-linked hexamer tubes (MAS frequency; 40 kHz). (d) Simulated 19F-19F RFDR cross-peak buildup curves for 19F-19F distances of 5–20 Å (simulation parameters are provided in the Supporting Information). The inset is an expansion for up to 200 ms.
19F-19F RFDR buildup curves were recorded for tubular assemblies of cross-linked A14C/E45C/W184A/M185A hexamer at a MAS frequency of 40 kHz (Fig. 3c). This assembly was used since W184 is not present and the resonance of W80 is no longer overlapped. As can be appreciated, monitoring the W80-W133 cross peak intensity, the polarization buildup has not reached maximum intensity, even at a mixing time as long as 100 ms. It should be noted, that for the two assemblies under investigation, the shortest intermolecular 19F-19F distance is 21.7 Å between W80-W117; therefore, it is highly unlikely that intermolecular contacts will compromise the measurements. In other proteins and protein assemblies, intermolecular 19F-19F distances may be short enough to contribute to RFDR polarization transfers. Isotopic dilution could then be used to distinguish between intra- and intermolecular correlations.
The simulated RFDR buildup curves for the MAS frequencies of 20, 40, and 60 kHz and 19F-19F distances ranging from 5 to 20 Å are shown in Fig. 3d and S5 (Supporting Information). We note that quantitative comparisons with the experimental buildup rates are not possible as the SIMPSON simulations do not include relaxation. Nevertheless, qualitative agreement between the experimental and calculated buildup curves is very good and it appears that in order to observe correlations corresponding to 15–20 Å distances long mixing times of the order of hundreds of milliseconds are necessary.
The results presented in this study highlight the potential of 19F MAS NMR spectroscopy for analyzing structural properties and interactions of HIV-1 capsid. To our knowledge, this is the first such investigation on a large protein assembly. The chemical shift dispersion is high, with separation of at least three of the five resonances (W80, W117, and W133). For two tryptophans, W23 and W184, multiple resonances are present, indicating conformational heterogeneity. This holds true also in solution, not only in the solid-state spectra.
One important outcome of the current investigation is that at fast MAS conditions (frequencies of 40–60 kHz), the lines are sufficiently narrow, obviating the need for decoupling. At these fast MAS conditions, nanometer-range interfluorine distance restraints can be extracted from PDSD and RFDR experiments.
Taken together, our results open up exciting and far-reaching possibilities for widespread use of 19F fast MAS, including characterization of proteins and protein assemblies, without requiring specialized probes capable of simultaneous 19F and 1H radiofrequency irradiation. We envision that ultrafast MAS (>100 kHz) conditions will further benefit resolution and enable 19F MAS NMR on a wide range of large biological systems.
Supplementary Material
Acknowledgements
We thank Mike Delk (University of Pittsburgh) for solution NMR technical support, and Guangjin Hou (University of Delaware, currently at the Institute of Chemical Physics, Dalian, China) for help with several MAS NMR. This work was supported by the National Science Foundation (NSF Grant CHE-1708773 to AMG and TP) and by the National Institutes of Health (NIGMS and NIAID, Grant P50 GM082251, Technology Development Project on MAS NMR). We acknowledge the National Science Foundation (NSF grant CHE-0959496) for the acquisition of the 850 MHz NMR spectrometer at the University of Delaware and the National Institutes of Health (NIH Grants P30GM103519 and P30GM110758) for the support of core instrumentation infrastructure at the University of Delaware.
Footnotes
Experimental Section
Detailed experimental methods can be found in the Supporting Information.
References
- [1].Quinn CM, Polenova T, Q. Rev. Biophys 2017, 50, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Loquet A, El Mammeri N, Stanek J, Berbon M, Bardiaux B, Pintacuda G, Habenstein B, Methods 2018, 138–139, 26–38. [DOI] [PubMed] [Google Scholar]
- [3].Aucoin D, Camenares D, Zhao X, Jung J, Sato T, Smith SO, J. Magn. Reson. 2009, 197, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Linser R, Bardiaux B, Higman V, Fink U, Reif B, J. Am. Chem. Soc 2011, 133, 5905–5912. [DOI] [PubMed] [Google Scholar]
- [5].Retel JS, Nieuwkoop AJ, Hiller M, Higman VA, Barbet-Massin E, Stanek J, Andreas LB, Franks WT, van Rossum BJ, Vinothkumar KR, Handel L, de Palma GG, Bardiaux B, Pintacuda G, Emsley L, Kuhlbrandt W, Oschkinat H, Nat. Commun 2017, 8, 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colvin MT, Silvers R, Ni QZ, Can TV, Sergeyev I, Rosay M, Donovan KJ, Michael B, Wall J, Linse S, Griffin RG, J. Am. Chem. Soc 2016, 138, 9663–9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, Kim JK, Barclay AM, Kendall A, Wan W, Stubbs G, Schwieters CD, Lee VM, George JM, Rienstra CM, Nat. Struct. Mol. Biol 2016, 23, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi C, Fricke P, Lin L, Chevelkov V, Wegstroth M, Giller K, Becker S, Thanbichler M, Lange A, Sci. Adv 2015, 1, e1501087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yan S, Guo C, Hou G, Zhang H, Lu X, Williams JC, Polenova T, Proc. Natl. Acad. Sci. USA 2015, 112, 14611–14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].He L, Bardiaux B, Ahmed M, Spehr J, Konig R, Lunsdorf H, Rand U, Luhrs T, Ritter C, Proc. Natl. Acad. Sci. USA 2016, 113, E272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang S, Munro RA, Shi L, Kawamura I, Okitsu T, Wada A, Kim SY, Jung KH, Brown LS, Ladizhansky V, Nat. Methods 2013, 10, 1007–1012. [DOI] [PubMed] [Google Scholar]
- [12].Zech SG, Wand AJ, McDermott AE, J. Am. Chem. Soc 2005, 127, 8618–8626. [DOI] [PubMed] [Google Scholar]
- [13].Gilchrist ML, Monde K, Tomita Y, Iwashita T, Nakanishi K, McDermott AE, J. Magn. Reson. 2001, 152, 1–6. [DOI] [PubMed] [Google Scholar]
- [14].Roos M, Wang T, Shcherbakov AA, Hong M, J. Phys. Chem. B 2018, 122, 2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gakh YG, Gakh AA, Gronenborn AM, Magn. Reson. Chem. 2000, 38, 551–558. [Google Scholar]
- [16].Sharaf NG, Gronenborn AM, in Isotope Labeling of Biomolecules - Labeling Methods, Vol. 565 (Ed.: Kelman Z), Elsevier Academic Press Inc, San Diego, 2015, pp. 67–95. [Google Scholar]
- [17].Crowley PB, Kyne C, Monteith WB, Chem. Commun 2012, 48, 10681–10683. [DOI] [PubMed] [Google Scholar]
- [18].Campos-Olivas R, Aziz R, Helms GL, Evans JNS, Gronenborn AM, FEBS Lett. 2002, 517, 55–60. [DOI] [PubMed] [Google Scholar]
- [19].Asada MN, Nemoto T, Mimura H, J. Pharm. Sci 2016, 105, 1233–1238. [DOI] [PubMed] [Google Scholar]
- [20].Zhang F, Zhou Q, Yang G, An L, Li F, Wang J, Chem. Commun 2018, 54, 3879–3882. [DOI] [PubMed] [Google Scholar]
- [21].Kitevski-LeBlanc JL, Prosser RS, Prog. Nucl. Magn. Reson. Spectrosc. 2012, 62, 1–33. [DOI] [PubMed] [Google Scholar]
- [22].Koch K, Afonin S, Ieronimo M, Berditsch M, Ulrich AS, in Solid State NMR, Vol. 306 (Ed.: Chan JCC), 2012, pp. 89–118. [DOI] [PubMed] [Google Scholar]
- [23].Williams JK, Tietze D, Lee M, Wang J, Hong M, J. Am. Chem. Soc 2016, 138, 8143–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hellmich UA, Pfleger N, Glaubitz C, Photochem. Photobiol 2009, 85, 535–539. [DOI] [PubMed] [Google Scholar]
- [25].Nartowski KP, Malhotra D, Hawarden LE, Sibik J, Iuga D, Zeitler JA, Fábián L, Khimyak YZ, Angew. Chem 2016, 128, 9050–9054. [DOI] [PubMed] [Google Scholar]
- [26].Shcherbakov AA, Hong M, Biomol J. NMR 2018. [Google Scholar]
- [27].Lu M, Sarkar S, Wang M, Kraus J, Fritz M, Quinn CM, Bai S, Holmes ST, Dybowski C, Yap GPA, Struppe J, Sergeyev IV, Maas W, Gronenborn AM, Polenova T, J. Phys. Chem. B 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang M, Quinn CM, Perilla JR, Zhang H, Shirra R Jr., Hou G, Byeon IJ, Suiter CL, Ablan S, Urano E, Nitz TJ, Aiken C, Freed EO, Zhang P, Schulten K, Gronenborn AM, Polenova T, Nat. Commun 2017, 8, 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang P, Meng X, Zhao G, Methods Mol. Biol 2013, 955, 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Quinn CM, Lu M, Suiter CL, Hou G, Zhang H, Polenova T, Prog. Nucl. Magn. Reson. Spectrosc 2015, 86–87, 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bayro MJ, Chen B, Yau WM, Tycko R, J. Mol. Biol 2014, 426, 1109–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bloembergen N, Physica 1949, 15, 386–426. [Google Scholar]
- [33].Bennett AE, Rienstra CM, Griffiths JM, Zhen W, Lansbury PTJ, Griffin RG, J. Chem. Phys 1998, 108, 9463–9479. [Google Scholar]
- [34].Byeon IJL, Hou GJ, Han Y, Suiter CL, Ahn J, Jung J, Byeon CH, Gronenborn AM, Polenova T, J. Am. Chem. Soc 2012, 134, 6455–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Du S, Betts L, Yang R, Shi H, Concel J, Ahn J, Aiken C, Zhang P, Yeh JI, J. Mol. Biol 2011, 406, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gres AT, Kirby KA, KewalRamani VN, Tanner JJ, Pornillos O, Sarafianos SG, Science 2015, 349, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tang S, Murakami T, Agresta BE, Campbell S, Freed EO, Levin JG, J. Virol 2001, 75, 9357–9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lemke CT, Titolo S, von Schwedler U, Goudreau N, Mercier JF, Wardrop E, Faucher AM, Coulombe R, Banik SS, Fader L, Gagnon A, Kawai SH, Rancourt J, Tremblay M, Yoakim C, Simoneau B, Archambault J, Sundquist WI, Mason SW, J. Virol 2012, 86, 6643–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pornillos O, Ganser-Pornillos BK, Banumathi S, Hua Y, Yeager M, J. Mol. Biol 2010, 401, 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].deAzevedo ER, Hu WG, Bonagamba TJ, Schmidt-Rohr K, J. Am. Chem. Soc 1999, 121, 8411–8412. [Google Scholar]
- [41].Hou G, Yan S, Trebosc J, Amoureux JP, Polenova T, J. Magn. Reson. 2013, 232, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huang K-Y, Siemer AB, McDermott AE, J. Magn. Reson. 2011, 208, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.