Abstract
Background & Aims:
Inactivating mutations in myosin VB (MYO5B) cause microvillus inclusion disease (MVID), but the physiological cause of the diarrhea associated with this disease is unclear. We investigated whether loss of MYO5B results in aberrant expression of apical enterocyte transporters.
Methods:
We studied alterations in apical membrane transporters in MYO5B-knockout mice, as well as mice with tamoxifen-inducible, intestine-specific disruption of Myo5b (VilCreERT2;Myo5bflox/flox mice) or those not given tamoxifen (controls). Intestinal tissues were collected from mice and analyzed by immunoelectron microscopy or cultured enteroids were derived. Functions of brush border transporters in intestinal mucosa were measured in Ussing chambers. We obtained duodenal biopsy specimens from individuals with MVID and individuals without MVID (controls) and compared transporter distribution by immunocytochemistry.
Results:
Compared to intestinal tissues from littermate controls, intestinal tissues from MYO5B-knockout mice had decreased apical localization of SLC9A3 (also called NHE3), SLC5A1 (also called SGLT1), AQP7, and sucrase isomaltase and subapical localization of intestinal alkaline phosphatase and CDC42. However, CFTR was present on apical membranes of enterocytes from MYO5B knockout and control mice. Intestinal biopsies from patients with MVID had subapical localization of NHE3, SGLT1, and AQP7, but maintained apical CFTR. Following tamoxifen administration, VilCreERT2;Myo5bflox/flox mice lost apical NHE3, SGLT1, DRA, and AQP7, similar to germline MYO5B KO mice. Intestinal tissues from ViCreERT2;Myo5bflox/flox mice had increased CFTR in crypts and CFTR localized to the apical membranes of enterocytes. Intestinal mucosa from VilCreERT2;Myo5Bbflox/flox mice given tamoxifen did not have an intestinal barrier defect, based on Ussing chamber analysis, but did have decreased SGLT1 activity and increased CFTR activity
Conclusions:
Although trafficking of many apical transporters is regulated by MYO5B, trafficking of CFTR is largely independent of MYO5B. Decreased apical localization of NHE3, SGLT1, DRA, and AQP7 might be responsible for dysfunctional water absorption in enterocytes of patients with MVID. Maintenance of apical CFTR might exacerbate water loss by active secretion of chloride into the intestinal lumen.
Keywords: mouse model, mislocalization, sodium hydrogen exchanger, aquaporin
Introduction
Microvillus inclusion disease (MVID) is a rare congenital disorder that results in unremitting, life-threatening diarrhea in neonates1, 2 Patients with MVID have inactivating mutations in the motor protein Myosin Vb (MYO5B)3-5. Newborns with MVID require life-long total parenteral nutrition (TPN), which carries a high risk for sepsis, or small bowel transplantation2. As a result, the outcome for patients with MVID under the current treatment regimens remains poor. In an effort to understand the molecular mechanisms that underpin MVID, our lab7 and several other groups 8, 9 have generated mouse models with loss of MYO5B that mimic the human disease.
Previous studies have identified the mislocalization of apical proteins, villus atrophy and intracellular inclusions that contain microvilli in the setting of MVID. However, the pathophysiology of MVID still remains unclear due to the complications that arise from prolonged TPN treatment in individuals with MVID. Prolonged TPN treatment is linked to intestinal atrophy and weakening of mechanical and immunological barriers in the intestine. Intestinal alterations that are observed in MVID patients that have been on TPN for months or years may not always solely result from loss of MYO5B10, 11. Thus in vivo models of MVID are necessary to study intestinal abnormalities that arise from loss of MYO5B and are not confounded by TPN treatment. Early studies in patients with MVID showed abnormal glucose absorption and defective Na+ absorption1, 2 suggesting defects in ion transporters that promote net water absorption. In the small intestine, Na+ plays a pivotal role in the absorption of solutes and water across the apical brush border of enterocytes. The sodium hydrogen exchanger isoform 3 (NHE3, SLC9A3) and sodium glucose co-transporter (SGLT1, SLC5A1) are highly expressed in the brush border of the small intestine and play a major role in transepithelial absorption of Na+ and water. In addition to transporters, the aquaporin water channels (AQPs) are another component of the intestinal membrane that transports water and small-uncharged solutes across cellular membranes. The chloride bicarbonate exchanger, Down Regulated in Adenoma (DRA, SLC26A3), is a transporter that is essential for the proper absorption of chloride in the intestine13. Mutations in DRA give rise to congenital chloride diarrhea14. Excessive intestinal fluid secretion by activation of luminal Cl− channels can also contribute to diarrhea. The cystic fibrosis transmembrane conductance regulator (CFTR) mediates Cl− and HCO3− secretion in the intestine. CFTR can play a major role in net fluid secretion during secretory diarrhea through increased functional expression in the brush border of enterocytes15.
While much is known about the role of intestinal ion transporters in the settings of homeostasis and diarrheal disorders, further elucidation of the status of functional ion transport in the absence of MYO5B is needed. To address the mechanism of MVID induced diarrhea, we employed two mouse models of MVID, mouse-derived duodenal enteroids, an electrophysiological approach and human biopsies. Our work demonstrates that germline MYO5B knockout (MYO5B KO) mice and tamoxifen-induced intestinally targeted MYO5B knockout mice (VillinCreERT2;MYO5Bflox/flox) exhibit improper localization of NHE3, SGLT1 and AQP7 in enterocytes. In contrast, CFTR was maintained on the apical membrane in MYO5B KO mice and tamoxifen-induced VillinCreERT2;MYO5Bflox/flox mice.
Previous studies have used the Caco2-BBE colonic cancer cell line to investigate molecular changes that arise from loss of MYO5B15. However, caveats exist with cancer-derived cell models. Herein we used mouse enteroids generated from neonatal MYO5B KO mice to advance our understanding of the role of MYO5B in maintenance of the apical brush border. Enteroids generated from germline MYO5B KO mice also showed apically expressed CFTR and responded to forskolin demonstrating functional CFTR. Electrophysiological studies of adult VillinCreERT2;MYO5Bflox/flox mice after administration of tamoxifen demonstrated that CFTR is the major contributor to short circuit current (Isc) in these mutant mice, while in control mice CFTR has no basal activity under stable conditions. Adult VillinCreERT2;MYO5Bflox/flox mice also showed significantly less SGLT1 activity than control mice. Finally, immunostaining of human biopsies from patients with MVID showed decreased apical expression of NHE3 and SGLT1, but normal CFTR expression. Collectively, these results suggest that loss of transporters that absorb Na+ and thus facilitate water absorption and the maintenance of functional CFTR are the likely drivers of the diarrhea associated with MVID.
Methods
Animals
The Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center approved all experimental procedures and animal care and maintenance. Germline MYO5B knockout mice and VillinCreERT2MYO5Bflox/flox mice were generated as previously reported7. Intestinal tissue was collected from the proximal small intestine (duodenum and jejunum) of germline MYO5B KO mice and control littermates within one week of life. To activate Cre recombinase, 2 mg of tamoxifen was administered via IP injection to 8-12 week old VillinCreERT2;MYO5Bflox/flox and VillinCreERT2 or MYO5Bflox/flox (control) mice. Adult mice were euthanized and samples collected 4 days after tamoxifen administration.
Human tissue
Paraffin-embedded tissues from normal intestine and from the duodenum of Navajo MVID patients were obtained as previously described16. The intestinal tissue was collected from individuals ranging from one day old to one year old.
Immunoelectron Microscopy
Duodenal tissue collected from 2 day old control and MYO5B KO mice were fixed in 4% buffered formaldehyde solution for 3 days. Thawed cryosections (~100 nm thick) were subjected to indirect immunogold labeling with antisera detecting NHE3, STX3, Rab11 and Rab8a as previously described in detail17. Bound primary antibodies were visualized by Nanogold®-Fab' goat anti-rabbit IgG (H+L) (Nanoprobes, 2004, diluted 1:150) followed by silver enhancement with HQ-Silver® (Nanoprobes, 2012).
Short-circuit current (Isc) measurement in Ussing Chambers
Mucosal-submucosal preparations were obtained from murine jejunum and terminal ileum. The intestinal segments were opened along the mesenteric border, and the tunica muscularis was stripped with fine forceps under a stereomicroscope in ice-cold Krebs buffer. Two preparations were prepared from each segment and then mounted in sliders with an aperture = 0.1 cm2 (Physiologic Instruments, San Diego, CA). Removal of muscle layer was confirmed by histological examination of paraffin sections.
Luminal and serosal surfaces of tissue were bathed with 4 ml Krebs-Ringer solution, maintained at 37°C using a water-recircul ating heating system. The bathing solution contained 117 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2 mM NaH2PO4, 25 mM NaHCO3, 11 Mm glucose. To prevent prostaglandin synthesis the cyclooxygenase inhibitor, indomethacin (10 μM), was routinely added into the serosal bath. The solution was bubbled with a gas mixture of 95% O2-5% CO2 to maintain the pH at 7.4.
The tissues were short-circuited by a voltage clamp (Physiologic Instruments) at zero potential difference with compensation for solution resistance. Short-circuit current (Isc) was continuously recorded by the DataQ system (Physiologic Instruments). To determine tissue conductance (Gt, mS/cm2) clamped voltage was changed automatically at ±3 mV for 20 ms every 2 sec. Transmucosal resistance (Rt, Ω•cm2) was calculated as inverse of Gt. An increase of Isc indicates luminal-to-serosal current flow, e.g., anion secretion or cation absorption. The tissues were stabilized for 30 min before the effects of drugs were investigated. A potent CFTR inhibitor (R)-BPO-27 fully blocks Cl− current in Ussing chambered murine jejunum at 1 μM19. CFTR activity was determined using a concentration of BPO-27 racemate that should completely abolish CFTR-dependent currents (10 μM; MedChem Express, Monmouth Junction, NJ). SGLT1 activity was represented by phlorizin (0.1 mM)-sensitive Isc. This concentration of phlorizin blocks SGLT1-mediated 3-O-methylglucose transport up to 30 mM in rat jejunum20, and we confirmed that Isc increase by luminal glucose (10 mM) was fully reversed by 0.1 mM of phlorizin in murine jejunum (data not shown). DMSO < 0.3% in the bathing solution did not affect the Isc or Gt.
Results
Germline MYO5B Loss Leads to Loss of Apical Ion Transporters
Despite recent work on the generation of mouse models that recapitulate the MVID phenotype, the underlying cause of the severe diarrhea remains unknown. To characterize the intestinal defects that give rise to persistent diarrhea in neonates with MVID, we examined key apical markers and ion transporters in the proximal small intestine of control and MYO5B-deficient neonatal mice. The proximal small intestine was chosen since the largest epithelial defects following loss of MYO5B manifest in this intestinal region7. To identify enterocyte morphology, the plasma membrane and actin cytoskeleton linker phosphorylated ezrin (P-ERM immunostaining) was examined (Supplemental Figure 1A). In control duodenum, antibodies against P-ERM immunostained the microvilli on the apical surface of enterocytes as expected. In MYO5B KO duodenum, P-ERM staining showed the presence of intracellular P-ERM positive inclusions along the length of the villi and diminished apical brush border staining consistent with the reduced length of microvilli previously reported7.
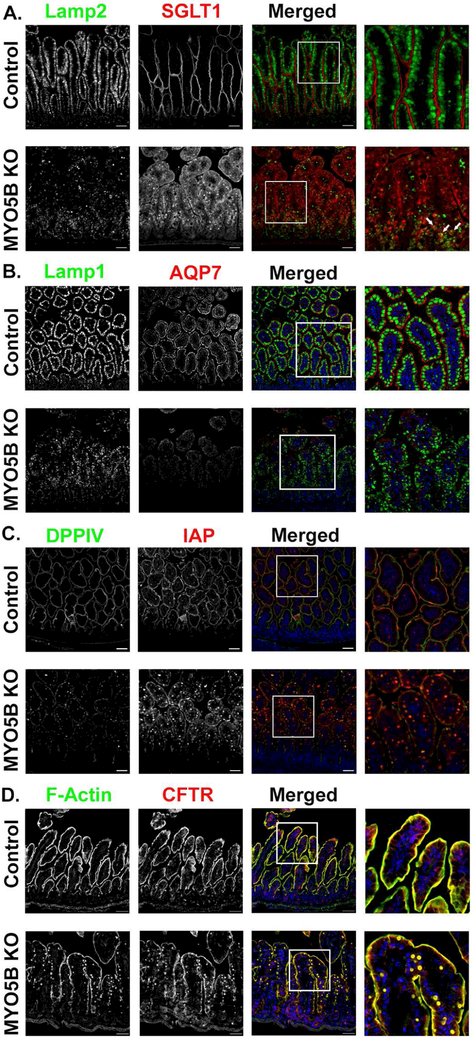
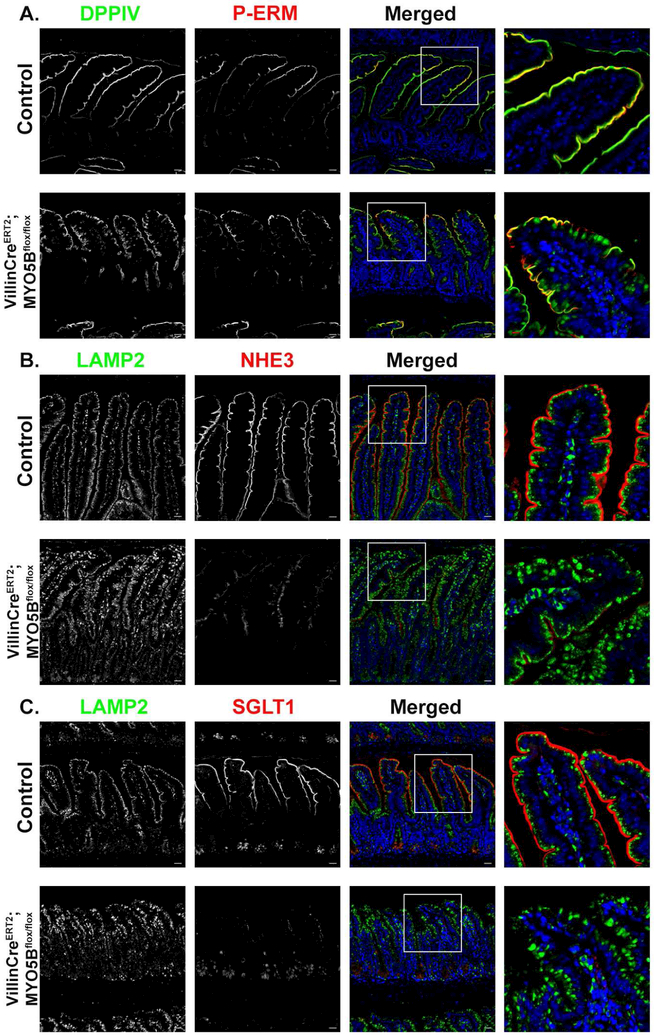
The primary function of the small intestine is the digestion and absorption of nutrients and water. While absorption under homeostatic conditions is well understood, little is known in regards to the movement of water and nutrients in the setting of MVID. To address the role of ion transport in MVID-associated diarrhea, neonatal murine proximal small intestine was examined for several ion transporters that facilitate nutrient and water absorption (NHE3, SGLT1, AQP7). NHE3 plays a major role in the transepithelial absorption of Na+ and water in the small and large intestine21. Immunostaining of NHE3 in control mice showed robust expression in the apical brush border of enterocytes with no subapical staining (Supplemental Figure 1B). Control mice also exhibited proper apical localization of the surface marker DPPIV. In contrast, MYO5B KO enterocytes showed diffuse subapical expression of NHE3 as well as NHE3 positive inclusions that were also positive for DPPIV as previously reported7 (Supplemental Figure 1B). In addition to the dual NHE3 and DPPIV positive inclusions, DPPIV was also mislocalized subapically in MYO5B KO mice. SGLT1, the sodium-glucose co-transporter that drives water and glucose absorption, labeled the apical surface of control enterocytes. SGLT1 immunostaining of MYO5B KO mouse duodenum showed a large amount of intracellular SGLT1. Additionally, we observed an overall decrease in expression of apical SGLT1 (Figure 1A). To determine whether SGLT1 was trafficked to lysosomes, tissue was co-immunostained with the lysosome marker, Lamp2. Control mice showed large Lamp2 positive lysosomes aligned below the apical membrane, in accordance with previously published reports22 (Figure 1A). In the MYO5B KO mice, Lamp2 positive lysosomes appeared less distinct and dispersed throughout the enterocyte cytoplasm, with some lysosomes surrounding inclusions containing SGLT1 (Figure 1A). Aquaporin 7 (AQP7), a water-selective membrane channel, localized to the apical surfaces of enterocyte microvilli of control duodenum. In contrast, MYO5B KO mice appeared to lose apical expression of AQP7 in duodenal enterocytes and instead showed exclusively cytoplasmic expression of AQP7 (Figure 1B). Similar to Lamp2 immunostaining, we observed a disorganized pattern of Lamp1 in MYO5B KO mice (Figure 1B). Collectively, the decreased apical expression of apical transporters that regulate sodium and water absorption may all individually contribute to the severe diarrhea in MYO5B KO mice.
Figure 1: MYO5B KO mice exhibit loss of SGLT1, apical alkaline phosphatase and AQP7 but maintain apical CFTR expression.
(A) Lamp2 positive lysosomes (green) were present below the brush border labeled by SGLT1 (red) in control mice. MYO5B KO mice showed disorganized Lamp2 lysosomes as well as decreased apical SGLT1 and increased intracellular SGLT1. Arrows indicate several SGLT1 positive inclusions surrounded by Lamp2 lysosomes. (B) AQP7, is normally expressed in the brush border of duodenal enterocytes. Apical AQP7 (red) is absent in MYO5B KO mice. Lamp1 lysosomes (green) were aligned below the apical membrane in control mice. MYO5B KO mice had dispersedLamp1 lysosomes. (C) Immunofluorescence staining for IAP (red) in control mice showed clear apical expression. MYO5B KO mice exhibited subapical accumulation of IAP below the apical membrane. DPPIV (green) was present in numerous intracellular inclusions in MYO5B KO mice. (D) CFTR (red) and F-actin (green) are normally expressed in the brush border of enterocytes. Following loss of MYO5B F-actin positive inclusions were observed. CFTR was present in F-actin positive inclusions in MYO5B KO mice. However, CFTR was still present on the apical membrane of MYO5B KO mice. n=4-6 mice per group, scale bars=50 μm.
Given the mis-localization of key intestinal ion transporters, other apical membrane markers were also examined by immunostaining. The apical brush border enzyme, intestinal alkaline phosphatase (IAP), is mislocalized in epithelial cells of patients with MVID and in an inducible mouse model of MVID9, 23 As expected, control mice displayed exclusive apical staining for IAP, confirmed by colocalization with the apical marker DPPIV (Figure 1C). In contrast, MYO5B KO mice had decreased apical IAP expression with large amounts of cytoplasmic IAP, including IAP and DPPIV dual positive inclusions. Previous investigations have shown that apical Rho GTPase and CDC42 are lost from the apical cortex in patients with MVID24. Consistent with these studies, control mice exhibited apical CDC42 staining in enterocytes, while MYO5B KO mice had no apical CDC42 expression (Supplemental Figure 2A). Dual staining with P-ERM clearly demonstrated that loss of CDC42 is not correlated with the presence of inclusions (Supplemental Figure 2A). Another component of the brush border, sucrase isomaltase (SI) was investigated to determine its localization following loss of MYO5B (Supplemental Figure 2B). As expected, SI was present in the brush border of enterocytes of control mice. However, MYO5B KO mice showed no apical brush border expression of SI and altered Lamp1 localization.
Abnormal Subapical Vesicle Accumulation in Enterocytes of MYO5B KO Mice
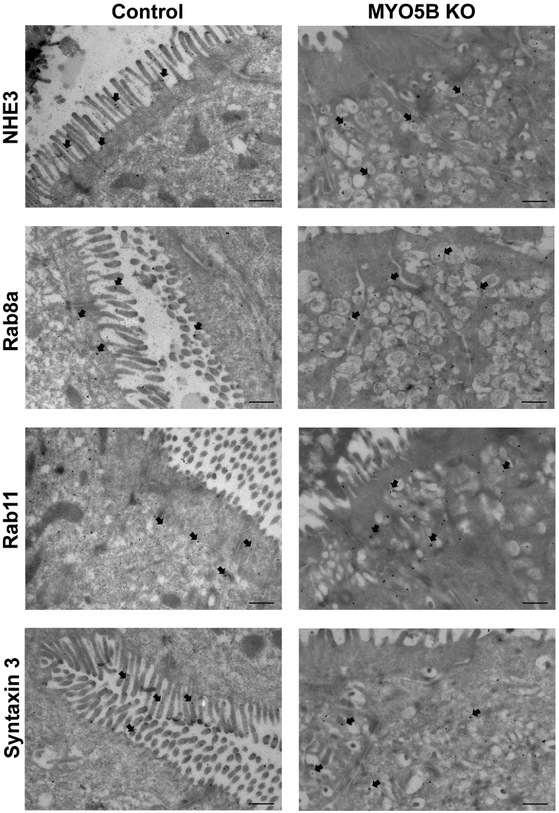
Transmission electron microscopy (TEM) immunogold labeling was performed on control and MYO5B KO duodenal tissue to examine in greater detail the subcellular structures associated with the localization of NHE3 and cellular trafficking proteins Syntaxin 3, Rab8a and Rab11 following loss of MYO5B (Figure 2). In contrast to control enterocytes, MYO5B KO mice showed an accumulation of subapical vesicles/tubules in duodenal enterocytes similar to the electron microscopy pattern in the duodenum from patients with MVID17, 25 Furthermore, NHE3 displayed a subapical distribution in these accumulated vesicles/tubules that contrasts with the NHE3 localization to the brush border microvilli found in control mouse tissue. Syntaxin 3 (STX3), an apical t-SNARE protein, participates with MYO5B, Rab8a and Rab11a in apical cargo trafficking17, 25. As expected in control mice, immunogold labeling for these apical trafficking proteins demonstrated STX3 predominately localized to the brush border; while Rab8a and Rab11 were found in the subapical cytoplasm. In MYO5B KO mice immunogold labeling of STX3, Rab8a, and Rab11 showed localization throughout the cytoplasm as well as in association with the distinct accumulated subapical vesicles/tubules that form in enterocytes after the loss of MYO5B.
Figure 2: Localization of NHE3, Rab8a, Rab11 and STX3 by immunogold Transmission Electron Microscopy.
NHE3 immunogold labeled microvilli of control mice but was additionally present in subapical vesicles in MYO5B KO mice. Rab8a and Rab11 showed normal subapical distribution in control mice and in MYO5B KO mice a certain accumulation in the aberrant subapical vesicle clusters. STX3 was found on the brush border of control mice but was preferentially located in subapical vesicles in MYO5B KO mice. n=3-4 mice per group, scale bars=500 nm.
CFTR is Maintained at the Apical Membrane Following Loss of MYO5B
While defects in absorptive ion transporters are important factors in diarrhea, active secretion of Cl− can also contribute to diarrhea. CFTR is the major cyclic adenosine monophosphate (cAMP)-regulated Cl− channel activated in secretary diarrhea26. Unlike the absorptive ion transporters, which were all decreased in MYO5B KO mice, both control and MYO5B KO mice showed the presence of apical CFTR along the brush border of enterocytes throughout the villi (Figure 1D). Although MYO5B KO mice also showed CFTR positive inclusions that were also positive for F-actin, the staining pattern of CFTR in MYO5B KO mice showed no diffuse subapical staining as was seen for SGLT1 and NHE3. To determine whether Cl− was being secreted into the intestinal lumen of MYO5B KO mice, the luminal contents of each segment of the intestine were collected and analyzed for Cl− ions. In the duodenum, MYO5B KO mice had higher levels of luminal Cl− than littermate controls (control 8.13 ± 1.39, MYO5B KO 18.22 ± 4.24, P=0.0066) (Supplemental Figure 3A). The jejunum, ileum and colon showed comparable levels of luminal Cl− between control and MYO5B KO mice (Supplemental Figure 3B-D).
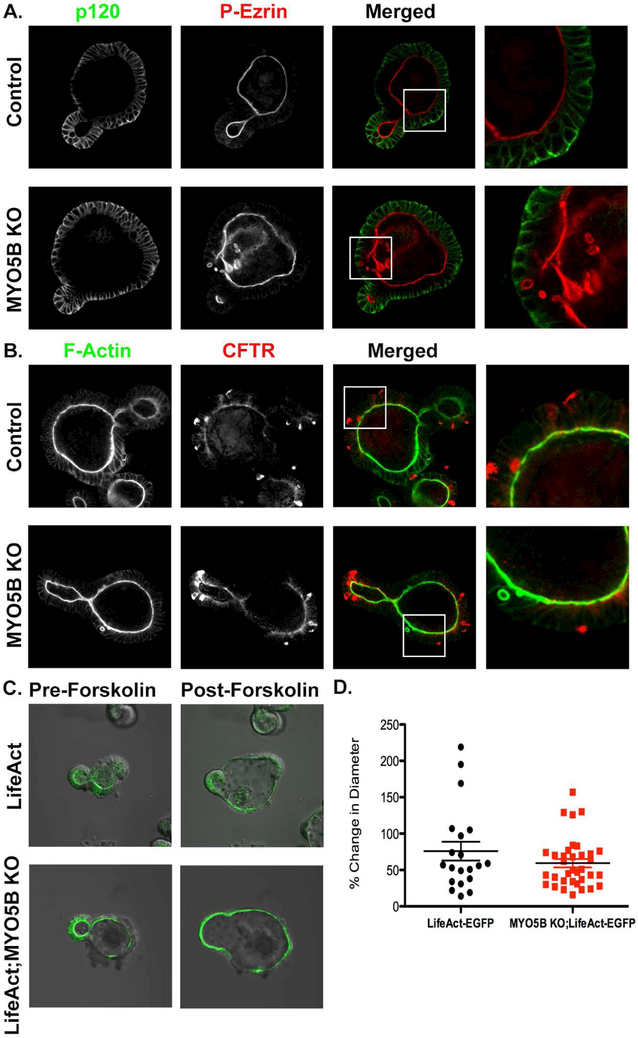
To elucidate further enterocyte abnormalities that arise from loss of MYO5B, an enteroid culture system was utilized (Figure 3). Enteroids were generated from the crypts of control and MYO5B KO duodenum. Gross morphology of enteroids revealed no differences between control and MYO5B KO derived enteroids (Supplemental Figure 4A). However, immunostaining of enteroids from MYO5B KO mice revealed intracellular abnormalities similar to MYO5B KO tissue. Enteroids generated from MYO5B KO mice had characteristic F-actin and phospho-ERM-positive inclusions (Figure 3A and B). Staining for CFTR showed similar apical expression between control and MYO5B KO enteroids (Figure 3B). To determine whether CFTR was functional in control and MYO5B KO enteroids, we generated germline MYO5B KO mice that express LifeAct-eGFP, which labels F-actin in live cells27. LifeAct and LifeAct;MYO5B KO mouse derived enteroids showed robust GFP labeling of the brush border lining the lumen of enteroids. Further, Lifeact;MYO5B KO-derived enteroids showed GFP-positive inclusions (Supplemental Figure 4B). A forskolin swelling assay was used to determine whether CFTR was functional following loss of MYO5B28. Forskolin increases intracellular cAMP, thereby activating CFTR and inducing apical fluid secretion into the enteroid lumen, resulting in swelling28, 29 LifeAct and LifeAct;MYO5B KO mouse derived enteroids were differentiated by withdrawal of Wnt conditioned media and treated with 5 μM forskolin. Differentiation of enteroids was determined by maturation or thickening of the brush border as identified by phalloidin (F-actin) staining and LifeAct fluorescence. Live imaging and quantification showed similar enteroid swelling of LifeAct and LifeAct;MYO5B KO-derived enteroids (Figure 3C and D). These findings suggest that loss of MYO5B results in inclusions in vitro, but does not interfere with the presence or functional capacity of CFTR on the apical membrane. Collectively, these results indicate that the decreased expression pattern of apical transporters that promote water absorption (NHE3, SGLT1 and AQP7) in MYO5B KO mice and the maintenance of apical CFTR and secretion of Cl− into the intestinal lumen could both contribute to the severe diarrhea in MYO5B KO mice.
Figure 3: MYO5B KO derived enteroids display intracellular inclusions and have functional CFTR.
(A) Differentiated enteroids from control and MYO5B KO mice immunostained for the basolateral marker p120 (green) and apical protein P-ERM (red). MYO5B KO enteroids have P-ERM intracellular inclusions. (B) CFTR (red) immunostaining showed comparable apical CFTR in control and MYO5B KO enteroids. Whole mount CFTR immunostaining of enteroids showed some expression in cells that may be Paneth cells which have been reported to take up primary and secondary antibodies18. (C) A forskolin swelling assay showed that lifeact and lifeact;MYO5B KO derived enteroids swelled to similar degrees in response to 5 μM forskolin. (D) Quantification of percentage increase in enteroid diameter measured in lifeact and lifeact;MYO5B KO enteroids. n=3 mice per group, forskolin swelling assay was repeated in at least three separate experiments. Scale bars=50 μm.
Human MVID Results in Loss of Apical Transporters and Maintenance of CFTR
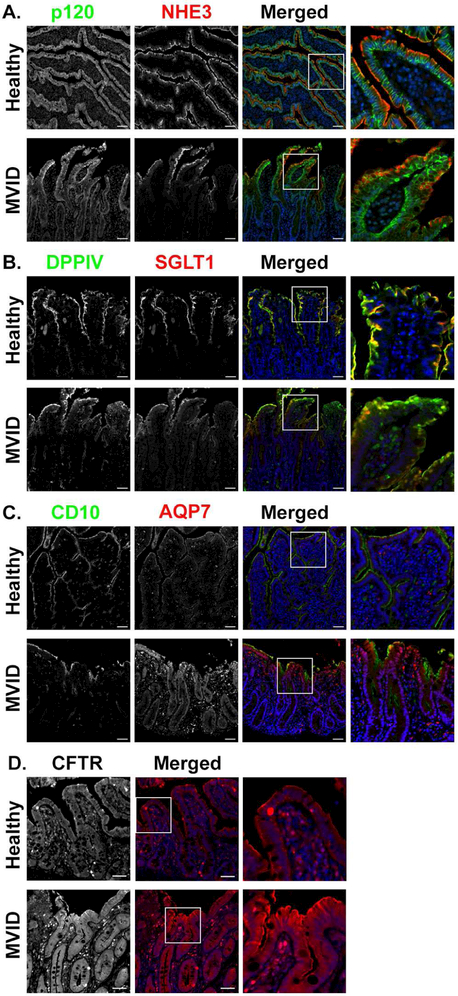
Since the MYO5B mouse model revealed robust changes in apical proteins, we sought to determine if patients with MVID also exhibited similar mislocalization of key ion transporters. Duodenal biopsies were collected from healthy individuals and from patients with a Navajo-specific mutation that causes MVID (MYO5B-P660L). In the duodenal biopsies from healthy individuals, NHE3 was localized to the apical brush border of villus enterocytes (Figure 4A). Patients with MVID showed decreased apical NHE3 in the brush borders of enterocytes and diffuse subapical NHE3 especially in enterocytes at the tips of the villi. SGLT1 and DPPIV staining of healthy tissue showed completely apical expression, while MVID biopsies showed cytoplasmic SGLT1 and DPPIV (Figure 4B). AQP7 immunofluorescence in healthy patient duodenal biopsies showed localization in the brush border just below CD10 immunostaining. CD10 is a membrane associated neutral peptidase that is normally expressed in the brush border of the small intestine30. In intestinal biopsies from patients with MVID, AQP7 was completely lost from the brush border and CD10 showed diffuse subapical staining (Figure 4C). In contrast, CFTR immunostaining showed apical expression in MVID biopsies similar to control tissue (Figure 4D). These findings closely correspond to findings in the MYO5B KO mice, suggesting that this mouse model of MVID closely recapitulates the human disease.
Figure 4: MVID small intestine shows loss of apical transporters but maintenance of CFTR.
(A) Immunostaining for NHE3 (red) showed apical expression in enterocytes from healthy individuals. In contrast, MVID patient tissue showed diffuse subapical expression of NHE3. The basolateral membrane, as marked by p120 (green), appeared similar in control and MVID tissue. (B) SGLT1 (red) and DPPIV (green) immunofluorescence was observed in the brush border in healthy tissue. In MVID intestinal tissue SGLT1 and DPPIV was largely cytoplasmic. (C) The brush border protein CD10 (green) was present on the apical membrane of enterocytes in healthy tissue. In MVID tissue, CD10 showed diffuse subapical staining. AQP7 (red) immunostaining showed AQP7 present in the brush border of healthy biopsies. In contrast, apical AQP7 was decreased in MVID and instead was located in the subapical domain. (D) Immunostaining for CFTR (red) showed CFTR present on the apical membrane of healthy individuals. In MVID biopsies CFTR immunofluorescence appeared to be increased and to remain on the apical membrane. n=3 per group. 3 biopsies were analyzed from healthy individuals aged 1 day to 6 weeks, 2 of the 3 individuals received TPN. From MVID patients, the biopsies were from individuals ranging from 6 weeks to one year, all biopsies (3) from MVID patients received TPN, scale bars=50 μm. The entire biopsy was assessed for representative images, approximately 4 fields of view.
Loss of MYO5B in Adult Mice Induces Loss of Apical Transporters
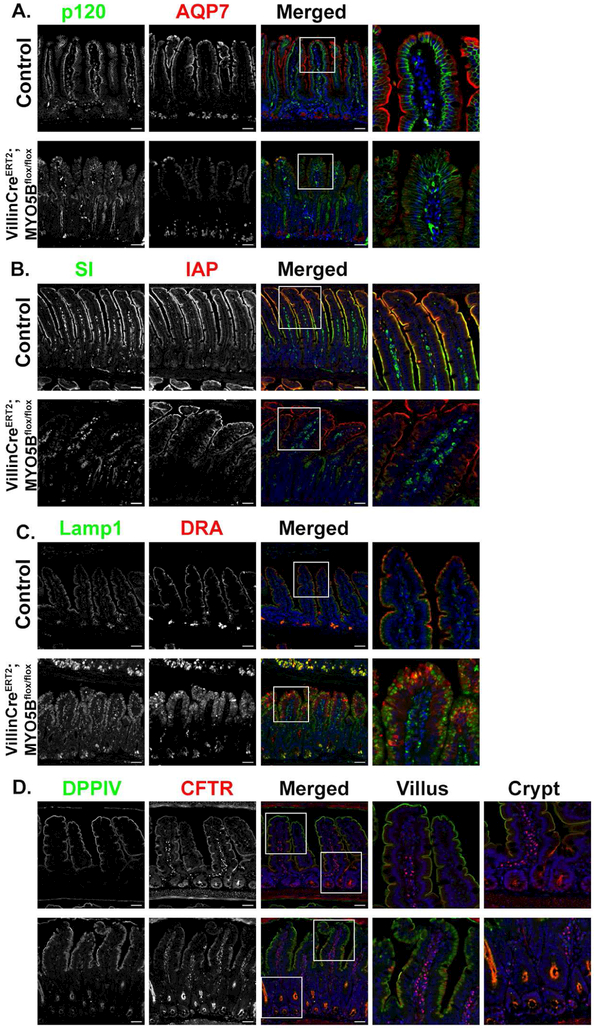
To evaluate the possibility that the observed MYO5B KO intestinal phenotypes were the result of the inclusions that are present in the neonatal mice, we used a tamoxifen inducible VillinCreERT2MYO5Bflox/flox mouse model. This mouse model effectively deletes MYO5B in the intestine leading to severe diarrhea within 3–4 days in adult mice7. At 4 days post-induction, VillinCreERT2;MYO5Bflox/flox mice exhibited loss of transporters that promote water absorption similar to MYO5B KO mice. As shown in Figure 5A P-ERM-positive inclusions were rarely present in adult VillinCreERT2;MYO5Bflox/flox mice following loss of MYO5B; however, DPPIV showed diffuse cytoplasmic localization. NHE3 immunostaining demonstrated loss of apical NHE3 in VillinCreERT2;MYO5Bflox/flox mice (Figure 5B). Examination of SGLT1 also showed a dramatic loss of apical expression in the brush borders of enterocytes after tamoxifen induced MYO5B loss (Figure 5). Lamp2 positive lysosomes also displayed a different staining pattern than control mice. Lamp2 lysosomes were dispersed from the normal alignment below the apical membrane in VillinCreERT2;MYO5Bflox/flox mouse enterocytes (Figure 5B and 5C). In addition to the decreased apical expression of NHE3 and SGLT1, VillinCreERT2;MYO5Bflox/flox mice showed complete loss of apical expression of AQP7 (Figure 6A). SI staining was abundant along the brush border of villus enterocytes in control mice, but was lost from the brush border in VillinCreERT2;MYO5Bflox/flox mice (Figure 6B). IAP also showed robust apical staining in control mice; however, VillinCreERT2;MYO5Bflox/flox mice showed subapical expression of IAP in enterocytes (Figure 6B). Immunostaining of the chloride transporter DRA showed abundant apical staining predominantly along the brush border of the villus enterocytes (and not crypt region) in control mice. However, VillinCreERT2;MYO5Bflox/flox mice showed loss of expression of DRA in the brush-border of enterocytes (Figure 6C). CFTR was observed in the brush border of enterocytes in the villi of control mice. Interestingly, CFTR was more highly expressed in the crypt and midway up the villi of tamoxifen-induced VillinCreERT2;MYO5Bflox/flox mice compared to control mice (Figure 6D).
Figure 5: Adult inducible intestinally targeted MYO5B KO mice show loss of apical transporters without inclusion formation.
(A) P-Ezrin (P-ERM) (red) and DPPIV (green) immunofluorescence staining showed apical expression in tamoxifen treated control mice. Induced VillinCreERT2MYO5Bflox/flox mice showed internalization of DPPIV but rare P-ERM positive inclusions. (B) Proximal small intestine from control and VillinCreERT2MYO5Bflox/flox were immunostained for NHE3 (red) and Lamp2 (green). NHE3 in induced VillinCreERT2MYO5Bflox/flox mice showed significant loss of apical NHE3 and increased subapical staining of NHE3 as well as enlarged Lamp2 lysosomes compared to control mice. (C) SGLT1 (red) was found along the length of the villi in control mice. Apical SGLT1 was almost completely lost in VillinCreERT2MYO5Bflox/flox mice. n=4-6 mice per group, scale bars=50 μm.
Figure 6: Loss of MYO5B in adult mice leads to decreased apical expression of SI, AQP7 and DRA, but preservation of CFTR.
(A) Immunostaining for p120 (green) and AQP7 (red) in control mice showed proper apical localization of AQP7. Tamoxifen-treated VillinCreERT2MYO5Bflox/flox mice lost apical expression of AQP7. (B) In control mice IAP (red) and SI (green) were both expressed on the apical membrane of of enterocytes. In VillinCreERT2MYO5Bflox/flox tamoxifen-treated mice SI was greatly reduced on the apical membrane and most SI appeared to be intracellular. IAP remained at the tips of the villi in VillinCreERT2MYO5Bflox/flox but also showed a subapical staining pattern through much of the villi (C) Immunostaining for DRA showed loss of apical DRA in tamoxifen-treated VillinCreERT2;MYO5Bflox/flox mice. (D) CFTR (red) and DPPIV (green) immunostaining in control mice demonstrated apical expression. In VillinCreERT2MYO5Bflox/flox CFTR was present below the apical membrane in the villi, however CFTR was also present on the apical membrane and appeared to be highly expressed in the crypts. n=4-6 mice per group, scale bars=50 μm.
In vitro induction of Cre recombinase using 4-hydroxytamoxifen (4-OHT) in VillinCreERT2;MYO5Bflox/flox mouse derived enteroids resulted in the formation of inclusions that were P-ERM positive, but did not result in any gross morphological changes in the cultured enteroids (Supplemental Figure 5A&B). Immunostaining of enteroids treated with vehicle (ETOH) showed that normally enteroids express sucrase isomaltase (SI) and NHE3 on the apical membrane (Supplemental Figure 5C). However, in vitro induced loss of MYO5B with 4-OHT treatment resulted in decreased apical expression of SI and NHE3 as well as intracellular accumulation of SI and NHE3 (Supplemental Figure 5A&B). We observed a similar mislocalization of other brush border components, DPPIV and intestinal alkaline phosphatase, following 4-OHT treatment of VillinCreERT2;MYO5Bflox/flox derived enteroids (Supplemental Figure 6A). However, stimulation of CFTR through administration of forskolin resulted in similar degrees of swelling between ETOH or 4-OHT treated VillinCreERT2;MYO5Bflox/flox enteroids (Supplemental Figure 6B&C). These data closely mirror our in vivo findings and emphasize the advantages of enteroids as an experimental tool.
Increased Contribution of CFTR in VillinCreERT2;MYO5Bflox/flox Mice
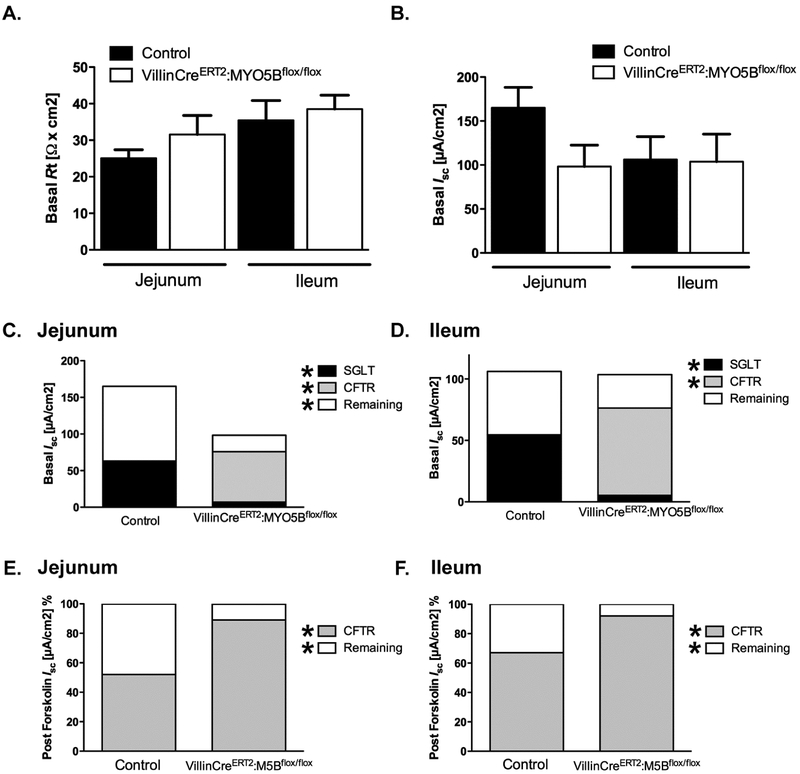
Measurement of Cl− in the intestinal luminal contents showed similar levels between control mice and VillinCreERT2;MYO5Bflox/flox mice following tamoxifen induced MYO5B loss (Supplemental Figure 7). These results suggest that, following tamoxifen treatment, VillinCreERT2;MYO5Bflox/flox mice may maintain the ability to secrete Cl− into the intestinal lumen via CFTR or a calcium dependent Cl− channel. While the Cl− measurement of luminal contents showed no statistical difference in VillinCreERT2;MYO5Bflox/flox mice treated with tamoxifen, the true degree of Cl− secretion or inability to absorb intestinal chloride may not be fully represented in the samples due to the profound diarrhea that is present following loss of MYO5B. The watery content of the collected luminal samples in the tamoxifen treated VillinCreERT2;MYO5Bflox/flox mice may result in a greater degree of dilution compared to control mice. Thus, to better delineate the functional capacity of ion transport Ussing chambers were used to measure epithelial membrane properties in intestinal mucosa of tamoxifen treated VillinCreERT2;MYO5Bflox/flox and control mice. Tissue resistance measurements showed that the jejunum and ileum mucosa of tamoxifen-treated VillinCreERT2;MYO5Bflox/flox mice did not have a barrier defect (Figure 7A&B). These results ruled out the possibility that induced VillinCreERT2;MYO5Bflox/flox mice have a “leaky” gut and suggested that the diarrhea following loss of MYO5B was not the result of loss of water through junction defects. Measurement of Isc demonstrated that, in the jejunum and ileum of tamoxifen-induced VillinCreERT2;MYO5Bflox/flox mice, CFTR was the greatest contributor to electrogenic ion transport under stable conditions (Figure 7C&D). In contrast, in control mice, SGLT1 contributed a larger proportion of the Isc. Furthermore, induced VillinCreERT2;MYO5Bflox/flox mice had significantly less SGLT1 activity than control mice (Figure 7C&D). These findings closely coincide with immunostaining suggesting that tamoxifen-treated VillinCreERT2;MYO5Bflox/flox mice had loss of apical SGLT1, but maintained functionally active CFTR. Although the remaining Isc in control tissues could not be fully characterized, this positive Isc might represent the summation of sodium- or proton-coupled nutrient absorptive currents31 that were decreased in induced VillinCreERT2;MYO5Bflox/flox mice, contributing to malabsorption. Finally, induced VillinCreERT2;MYO5Bflox/flox mice exhibited a greater dependence on CFTR for alterations in Isc than control mice after forskolin stimulation (Figure 7E and F). Collectively, these data support the concept that MVID diarrhea manifests through a loss of apical proteins that drive water absorption coupled to the preservation of functional CFTR to further promote water loss.
Figure 7: Short circuit current (Isc) demonstrated loss of SGLT1 function and maintenance of functional CFTR.
(A and B) Basal electrical resistance (Rt) (A) and Isc (B) measurements in the jejunum and ileum of tamoxifen treated control and tamoxifen induced VillinCreERT2;MYO5Bflox/flox mice from jejunum. Total values of Rt or Isc demonstrated no difference between groups. (C and D) Based on inhibitor effects (SGLT1 inhibition by phlorizin and CFTR inhibition by BPO-27) basal Isc in jejunum (C) and ileum (D) resulted in significantly lower SGLT1 activity and higher CFTR activity in VillinCreERT2;MYO5Bflox/flox mice than control littermates. Contribution of CFTR to forskolin (10 μM) stimulated Isc in jejunum (E) and ileum (F) of control and VillinCreERT2;MYO5Bflox/flox mice. VillinCreERT2;MYO5Bflox/flox mice had significantly increased CFTR dependence compared to control littermates. n=4-5 mice per group, data expressed as mean ± SEM. Significant differences are indicated by * in key, *P<0.05, unpaired t-test.
Discussion
Our group previously reported the development and characterization of three mouse models of MYO5B deletion. These genetic models of MVID included germline, constitutive intestinally targeted, and inducible intestinally targeted deletion of MYO5B7. This previous investigation reported that the diarrheal pathology that develops from MYO5B loss is not the result of microvillus inclusions, but postulated that diarrhea resulted from deficits in transporter presentation at the apical membrane in duodenal enterocytes. The results presented here seek to further our understanding of MVID by providing a comprehensive analysis of transporter deficits that result from loss of functional MYO5B using two of our previously reported mouse models. Germline and inducible intestinally targeted MYO5B KO mice both displayed prominent loss of the apical transporters NHE3, SGLT1 and AQP7. While our neonatal mice (control and MYO5B KO) did not express DRA, likely due to the developmental stage at which they were analyzed, we found that adult VillinCreERT2;MYO5Bflox/flox mice treated with tamoxifen lost apical expression of DRA in enterocytes. Biopsies from patients with MVID showed similar losses in expression of apical membrane proteins. Our lab has reported decreased apical expression of SGLT1 in MVID enterocytes16. Caco2 cells with MYO5B knockdown also showed decreased levels of NHE3 and MVID patient tissue demonstrated decreased NHE3 expression in the brush border of enterocytes7, 15. Moreover, Vogel and colleagues published work showing that MYO5B promotes delivery of NHE3 to the apical membrane25. Our findings in the germline and induced VillinCreERT2MYO5Bflox/flox mice and in MVID tissue corroborate these previous studies and show that, in two in vivo models of MYO5B deletion, NHE3 and SGLT1 expression is decreased at the apical brush border of enterocytes.
Ussing chamber studies were used to determine the functional impact of loss of MYO5B on intestinal apical transporters. Importantly these experiments demonstrated that VillinCreERT2MYO5Bflox/flox mice do not have an intestinal barrier defect or “leaky gut.” These data are significant in light of the complications that arise in analyzing human MVID. Since the majority of neonates with MVID present with severe diarrhea shortly after birth, in most instances total parenteral nutrition (TPN) is immediately implemented with suspension of enteral feeding. As a result, defects in the intestinal epithelium of MVID patients may not necessarily represent the direct effects of inactivating mutations of MYO5B, but instead may reflect the impact of prolonged TPN exposure and a lack of luminal nutrition in the context of MYO5B loss10. Thus, changes in barrier integrity that have been previously reported may be the result of protracted TPN, since tamoxifen treated VillinCreERT2MYO5Bflox/flox mice did not manifest any changes in tissue resistance compared to control mice. Schneeberger et al. reported enlarged intercellular spaces between enterocytes based on TEM in their inducible intestinally targeted MYO5B knockout mouse model9. In our previous publication, we found no large intercellular spaces between enterocytes in our VillinCreERT2MYO5Bflox/flox mice7. This discrepancy may be due to differences in mouse models and mouse facilities.
Analysis of inducible intestinally targeted MYO5B KO mice provides novel insights into intestinal epithelial alterations that are the direct result of inactivation of MYO5B and independent of TPN. We observed that induced VillinCreERT2MYO5Bflox/flox mice had significantly reduced SGLTI-mediated Isc when measured in Ussing chambers. These results strongly support our immunostaining findings that SGLT1 expression is decreased on the apical membrane of neonatal germline MYO5B KO mice and induced VillinCreERT2MYO5Bflox/flox mice.
Previous investigations in Caco2 cells with over-expression of CFTR had suggested that MYO5B may be a regulator of apical trafficking of CFTR25. In our analysis of apical transporters, we observed maintenance of apical CFTR in germline MYO5B KO mice and up-regulation of CFTR in the crypts of induced VillinCreERT2MYO5Bflox/flox mice. Likewise, biopsies from patients with MVID also showed maintenance of CFTR on the enterocyte apical membrane. Analysis of functional ion transport using Ussing chambers showed that CFTR contributes to basal and stimulated Isc to a greater degree in tamoxifen treated VillinCreERT2MYO5Bflox/flox mice than in control mice. Moreover, analysis of luminal contents in both germline and induced VillinCreERT2MYO5Bflox/flox mice showed Cl− present at similar concentrations in control and KO mice. Forskolin swelling experiments in germline MYO5B KO-derived enteroids that expressed a fluorescent F-actin reporter also supported the finding that CFTR was present and functional on the apical membrane of enterocytes. Thus, the findings with CFTR over-expression in cultured immortalized cells may not fully recapitulate the trafficking of the endogenous transporter in enterocytes.
An early study of jejunal tissue from MVID patients reported Cl− secretion in MVID tissue that was close to the maximal rate of Cl− secretion in normal tissue32. More recent studies have reported preservation of CFTR in the brush border of Caco2 cells with MYO5B knockdown and on the apical membrane of enterocytes in MVID tissue15, 23. Furthermore, knockdown of MYO5B in T84 cells did not affect CFTR ion transport, suggesting that CFTR trafficking to the apical membrane is independent of MYO5B15. Trafficking of CFTR to the brush border of enterocytes is dependent on Myosin 1a33. Even in the absence of MYO5B, the myosin motors, Myosin 1a and Myosin 6, are postulated to deliver CFTR to the brush border33. We speculate that this results in maintenance of CFTR in the brush border despite loss of MYO5B largely contributing to the severe diarrhea in MVID. The proper trafficking of CFTR once endocytosed is likely partially dependent on MYO5B since inclusions in the germline MYO5B KO mice contain CFTR. Despite the presence of inclusions containing CFTR, we observed maintenance of CFTR on the apical membrane indicating that proper delivery of CFTR to the brush border is not MYO5B dependent, which supports the findings that Myosin 1a is responsible for CFTR movement into the brush border33. Therefore, we postulate that while CFTR is trafficked independent of MYO5B, the proper trafficking of other transporters involved in water absorption (SGLT1, NHE3, AQP7 and DRA) are MYO5B dependent. Immunostaining of germline, induced VillinCreERT2MYO5Bflox/flox mice and MVID patient tissue strongly implicate different trafficking patterns of apical membrane proteins and enzymes.
Our results coincide with these previous reports, but differ from data published by Schneeberger et al 9 Schneeberger and colleagues reported that administration of 4-OHT to enteroids derived from an inducible intestinally targeted MYO5B deletion mouse given had diminished swelling in response to forskolin9. We found that induction of MYO5B deletion with 4-OHT did not result in decreased swelling of our VillinCreERT2;MYO5Bflox/flox derived enteroids. The discrepancy between our findings and the ones reported by Schneeberger et al. may result from the different mouse models used to derive the enteroids as well as the conditions used to grow the enteroids. Activation of Cre recombinase in the enteroids generated by Schneeberger et al. resulted in fewer budding structures and more cell death9. This phenomenon differs from our germline mutation-derived enteroids and our adult VillinCreERT2;MYO5Bflox/flox derived enteroids in which we observed no difference in enteroid size, budding structures or increased cell death. As a result, we speculate that increased apoptosis in the in vitro activated enteroid model may decrease the number of viable enterocytes containing CFTR and may yield lower CFTR activity. Our in vitro forskolin swelling data is strongly supported by the presence of Cl− in the intestinal lumen of MYO5B KO mice and immunostaining for CFTR in neonatal germline MYO5B KO mice and in enteroids from these mice. Moreover, our Ussing chamber data in adult induced VillinCreERT2MYO5Bflox/flox mice also support active apical CFTR, as does the immunostaining in this mouse model.
While differences exist between our forskolin induced enteroid swelling data and that reported by Schneeberger and colleagues, we did observe similar accumulation of intracellular alkaline phosphatase (Supplemental Figure 6A) in our 4-OHT treated VillinCreERT2;MYO5Bflox/flox enteroids. Additionally, we also demonstrated the presence of P-ERM positive intracellular inclusions in 4-OHTtreated VillinCreERT2;MYO5Bflox/flox enteroids. The presence of inclusions corroborates Schneeberger’s TEM results showing subapical microvilli-lined inclusions in their 4-OHT-treated enteroids. These results suggest that, while induced deletion of MYO5B in adult VillinCreERT2;MYO5Bflox/flox mice elicits few inclusions7, the enteroids prepared from these mice may recapitulate a more neonatal phenotype leading to increased inclusion formation after MYO5B deletion in vitro.
Mutated NHE3 has been associated with severe chronic diarrhea known as congenital sodium diarrhea, a phenotype similar to MVID34, 35, while mutated DRA has been associated with congenital chloride diarrhea. Abnormal CFTR intestinal secretory pathways have been implicated in other forms of intractable secretory diarrheas presenting in newborns35. To the best of our knowledge our results are likely specific to MVID diarrhea and may not be broadly applicable to other congenital diarrheas. Unlike individuals with congenital chloride diarrhea, patients with MVID require lifelong TPN and do not respond to current treatment regimens for other diarrhea diseases36. We postulate that MVID is unique in causing altered trafficking of more than one transporter, since we see loss of apical NHE3, SGLT1, AQP7 and DRA. Our study also demonstrates a functional deficit in SGLT1 that occurs following loss of MYO5B. Moreover, we report that synchronous with loss of apical transporters, there is maintenance of CFTR and functional chloride secretion. Therefore, we suspect that MVID poses a unique challenge to physicians since it requires treatment that can both promote water absorption despite loss of transporters that drive hydration and simultaneously decrease CFTR mediated chloride secretion.
This is the first study to test functional ion transport deficits in a mouse model of MYO5B loss. Collectively, the data presented here suggest that loss of MYO5B results in altered trafficking of critical transporters to the apical membrane. These trafficking patterns are apparent in the preservation of apical CFTR, decreased expression of apical NHE3 coincident with increased subapical expression, loss of apical SGLT1 and AQP7, and absence of SI in the brush border of MYO5B deficient mice. Therefore, we postulate that MVID-associated diarrhea is due to improper apical expression of primary Na+ transporters, NHE3 and SGLT1 and chloride transporter DRA, which promotes malabsborptive diarrhea, and the maintenance of apical CFTR, which drives secretory diarrhea. Thus, MVID patients may benefit to some extent from inhibition of CFTR driven Cl− secretion. With the advent of several potent CFTR inhibitors37, studies involving CFTR inhibition in in vivo (mouse) and in vitro (mouse and human enteroids) models will likely point to new avenues for MVID therapeutics.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health (NIH) grants R01 DK48370 and R01 DK70856 to J.R.G and a gift from the Christine Volpe Fund and R01 DK077065 to NAA. VGW was supported by T32 DK007673. A.C.E was supported by NIH F32 DK111101. A.R.M. was supported by NIH T32 GM008554. P.K.D. was supported by NIH DK92441, DK81858 and VA BX002011. This work was supported by core resources of the Vanderbilt Digestive Disease Center, (P30 DK058404) the Vanderbilt-Ingram Cancer Center (P30 CA68485), and imaging supported by both the Vanderbilt Cell Imaging Shared Resource and the Vanderbilt Digital Histology Shared Resource.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have declared that no conflict of interests exist.
References:
- 1.Davidson GP, Cutz E, Hamilton JR, et al. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 1978;75:783–90. [PubMed] [Google Scholar]
- 2.Ruemmele FM, Schmitz J, Goulet O. Microvillous inclusion disease (microvillous atrophy). Orphanet J Rare Dis 2006;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson RP, Larson-Thome K, Valenzuela RK, et al. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A 2008;146A:3117–9. [DOI] [PubMed] [Google Scholar]
- 4.Muller T, Hess MW, Schiefermeier N, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 2008;40:1163–5. [DOI] [PubMed] [Google Scholar]
- 5.Ruemmele FM, Muller T, Schiefermeier N, et al. Loss-of-function of MYO5B is the main cause of microvillus inclusion disease: 15 novel mutations and a CaCo-2 RNAi cell model. Hum Mutat 2010;31:544–51. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AD, Schmitz J. Familial microvillous atrophy: a clinicopathological survey of 23 cases. J Pediatr Gastroenterol Nutr 1992;14:380–96. [PubMed] [Google Scholar]
- 7.Weis VG, Knowles BC, Choi E, et al. Loss of MYO5B in mice recapitulates Microvillus Inclusion Disease and reveals an apical trafficking pathway distinct to neonatal duodenum. Cell Mol Gastroenterol Hepatol 2016;2:131–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carton-Garcia F, Overeem AW, Nieto R, et al. Myo5b knockout mice as a model of microvillus inclusion disease. Sci Rep 2015;5:12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneeberger K, Vogel GF, Teunissen H, et al. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci U S A 2015;112:12408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Demehri FR, Xiao W, et al. Interdependency of EGF and GLP-2 Signaling in Attenuating Mucosal Atrophy in a Mouse Model of Parenteral Nutrition. Cell Mol Gastroenterol Hepatol 2017;3:447–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chance WT, Foley-Nelson T, Thomas I, et al. Prevention of parenteral nutrition-induced gut hypoplasia by coinfusion of glucagon-like peptide-2. Am J Physiol 1997;273:G559–63. [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Chen Z, Jiang Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamprecht G, Hsieh CJ, Lissner S, et al. Intestinal anion exchanger down-regulated in adenoma (DRA) is inhibited by intracellular calcium. J Biol Chem 2009;284:19744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoglund P, Haila S, Socha J, et al. Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 1996;14:316–9. [DOI] [PubMed] [Google Scholar]
- 15.Kravtsov DV, Ahsan MK, Kumari V, et al. Identification of intestinal ion transport defects in microvillus inclusion disease. Am J Physiol Gastrointest Liver Physiol 2016;311:G142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles BC, Roland JT, Krishnan M, et al. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest 2014;124:2947–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel GF, Janecke AR, Krainer IM, et al. Abnormal Rab11-Rab8-vesicles cluster in enterocytes of patients with microvillus inclusion disease. Traffic 2017;18:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahe MM, Aihara E, Schumacher MA, et al. Establishment of Gastrointestinal Epithelial Organoids. Curr Protoc Mouse Biol 2013;3:217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cil O, Phuan PW, Gillespie AM, et al. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins. FASEB J 2017;31:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavakkolizadeh A, Berger UV, Shen KR, et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol 2001;280:G209–15. [DOI] [PubMed] [Google Scholar]
- 21.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010;2010:238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox CM, Lu R, Salcin K, et al. The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation. Cell Mol Gastroenterol Hepatol 2018;5:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ameen NA, Salas PJ. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 2000;1:76–83. [DOI] [PubMed] [Google Scholar]
- 24.Michaux G, Massey-Harroche D, Nicolle O, et al. The localisation of the apical Par/Cdc42 polarity module is specifically affected in microvillus inclusion disease. Biol Cell 2016;108:19–28. [DOI] [PubMed] [Google Scholar]
- 25.Vogel GF, Klee KM, Janecke AR, et al. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J Cell Biol 2015;211:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiagarajah JR, Verkman AS. CFTR pharmacology and its role in intestinal fluid secretion. Curr Opin Pharmacol 2003;3:594–9. [DOI] [PubMed] [Google Scholar]
- 27.Riedl J, Flynn KC, Raducanu A, et al. Lifeact mice for studying F-actin dynamics. Nat Methods 2010;7:168–9. [DOI] [PubMed] [Google Scholar]
- 28.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 2013;19:939–45. [DOI] [PubMed] [Google Scholar]
- 29.Foulke-Abel J, In J, Kovbasnjuk O, et al. Human enteroids as an ex-vivo model of host-pathogen interactions in the gastrointestinal tract. Exp Biol Med (Maywood) 2014;239:1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groisman GM, Amar M, Livne E. CD10: a valuable tool for the light microscopic diagnosis of microvillous inclusion disease (familial microvillous atrophy). Am J Surg Pathol 2002;26:902–7. [DOI] [PubMed] [Google Scholar]
- 31.Thwaites DT, Anderson CM. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol 2007;92:603–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhoads JM, Vogler RC, Lacey SR, et al. Microvillus inclusion disease. In vitro jejunal electrolyte transport. Gastroenterology 1991;100:811–7. [DOI] [PubMed] [Google Scholar]
- 33.Kravtsov DV, Caputo C, Collaco A, et al. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 2012;13:1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janecke AR, Heinz-Erian P, Yin J, et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet 2015;24:6614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller T, Rasool I, Heinz-Erian P, et al. Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut 2016;65:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawamura T, Nishiguchi T. Congenital Chloride Diarrhea (CCD): A Case Report of CCD Suspected by Prenatal Ultrasonography and Magnetic Resonance Imaging (MRI). Am J Case Rep 2017;18:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verkman AS, Synder D, Tradtrantip L, et al. CFTR inhibitors. Curr Pharm Des 2013;19:3529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ameen NA, Ardito T, Kashgarian M, et al. A unique subset of rat and human intestinal villus cells express the cystic fibrosis transmembrane conductance regulator. Gastroenterology 1995;108:1016–23. [DOI] [PubMed] [Google Scholar]
- 39.Jakab RL, Collaco AM, Ameen NA. Characterization of CFTR High Expresser cells in the intestine. Am J Physiol Gastrointest Liver Physiol 2013;305:G453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.