Abstract
Environmental and social factors have profound impacts on immune homeostasis. Our work on environmental enrichment (EE) has revealed a novel anti-obesity and anticancer phenotype associated with enhanced activity of CD8+ cytotoxic T lymphocytes in secondary lymphoid tissues. Here we investigated how an EE modulated thymus and thymocyte development. EE decreased thymus mass and cellularity, decreased the double positive thymocyte population, increased the proportion of CD8+ T cells, reduced the CD4:CD8 ratio, and downregulated CD69 expression in T cells. In a model of multiple sclerosis: experimental autoimmune encephalomyelitis (EAE), EE alleviated symptoms, inhibited spinal cord inflammation through regulation of type 1 T-helper cells mediated by glucocorticoid receptor signaling, and prevented EAE-induced thymic disturbance. Our mechanistic studies demonstrated that hypothalamic BDNF activated a hypothalamic-pituitary-adrenal axis mediating the EE’s thymic effects. Our results indicate that a lifestyle intervention links the nervous, endocrine, and adaptive immune system, allowing the body to adapt to internal and external environments.
Keywords: environmental enrichment, BDNF, thymus, T cell, experimental autoimmune encephalomyelitis
Introduction
The thymus is the primary organ providing the microenvironment for T cell development and plays a vital role in maintaining immune homeostasis (1). Thymocyte selection is responsible for the development of a self-tolerant T cell repertoire capable of responding to foreign antigens. The selective processes require occupancy of the T cell receptor (TCR) by peptide-loaded MHC-encoded molecules (self pMHC). The bone marrow-derived primitive thymocyte precursors expressing neither TCR nor CD4 or CD8 molecules, are called double-negative (DN) cells. Their maturation involves a productive rearrangement of the TCR locus followed by upregulation of CD4 and CD8 molecules. The majority of CD4+CD8+ double-positive (DP) thymocytes fail to express TCR with sufficient avidity to rescue them from a default apoptotic pathway termed “death by neglect” (1, 2). Thymocytes expressing TCRs with high avidity for self over a certain threshold are then eliminated by apoptosis (negative selection) (1, 2). Consequently, only thymocytes bearing TCRs with intermediate avidity for self are rescued, differentiate into CD4+ or CD8+ single-positive (SP) cells, and migrate from the thymus to the periphery, which includes secondary lymphoid tissues (SLT) and other tissues. This process is called positive selection (1).
Extensive evidence has illustrated the crosstalk between the nervous and immune systems via multiple pathways (3). The brain can modulate immune cell development and the various stages of an immune response via signaling to target cells of the immune system. This process occurs primarily through direct sympathetic innervation of primary and secondary lymphoid organs and through hypothalamic-pituitary-adrenal axis (HPA) hormonal outflow. Many signaling molecules and their receptors that were initially thought to be endogenous to nervous and endocrine systems, have been found in the immune system, including neurotransmitters, neuropeptides, and hormones (4). These signaling molecules can arise from different sources within the three systems to act upon multiple targets. This indicates that the body’s major adaptive systems (nervous, endocrine, and immune systems) are interconnected in a complex network thereby allowing the body to respond to a variety of changes in both internal and external environments.
Our recent work demonstrates that environmental enrichment (EE) is a unique eustress model to study how an individual’s physical and social environment can influence physiology and disease progression (5). EE is a housing environment for laboratory animals, which, in contrast to the standard laboratory environment (SE), is socially, physically, and cognitively stimulating. EE has profound impact on brain structure, function, and the progression of neurologic diseases (6). We demonstrated that EE leads to anticancer and anti-obesity phenotypes and have identified one underlying mechanism, the activation of a specific brain-adipocyte axis: the hypothalamic-sympathoneural-adipocyte (HSA) axis. The complex stimuli provided by EE induce hypothalamic brain-derived neurotrophic factor (BDNF) expression and subsequently elevate the sympathetic tone preferentially to the adipose tissue leading to increased energy expenditure, leanness, resistance to diet-induced obesity, a sharp drop of leptin, and inhibition of multiple types of cancer (7, 8). More recently we have shown that EE modulates T cells in the secondary lymphoid tissues (SLT) and in tumor infiltrates, contributing to the anticancer effects (9). Here we investigated the effects of EE on the thymus and thymocyte development and elucidated the mechanisms underlying this modulation. Furthermore, we used experimental autoimmune encephalomyelitis (EAE), an autoimmune model of multiple sclerosis (MS), to assess whether an improved lifestyle such as EE could affect autoimmune disease progression (10).
Materials and Methods
Mice.
All use of animals was approved by, and in accordance with the Ohio State University Institutional Laboratory Animal Care and Use Committee.
EE Protocol.
Male or female 3-week-old C57BL/6 mice (Charles River) were housed in groups in cages of 1.5 m × 1.5 m × 1.0 m (20 mice per cage) supplemented with running wheels, tunnels, igloos, huts, retreats, wood toys, a maze, and nesting material (11). We housed control mice under standard laboratory (SE) cages of 30.5 cm × 17 cm × 15 cm (5 mice per cage). Mice were housed in temperature (22–23 ºC) and humidity controlled rooms with food and water ad libitum. We fed the mice with normal chow diet (NCD, 11% fat, caloric density 3.4 kcal/g, Teklad). Young animals were used because of the high brain plasticity, less risk of fighting in EE, and our previous mechanistic studies (genetic and pharmacological) identifying hypothalamic BDNF as a key brain mediator of EE’s anticancer and anti-obesity phenotypes. All EE experiments were conducted in cages of 1.5 m × 1.5 m × 1.0 m (20 mice per cage) unless specified.
Sacrifice and tissue collection.
All sacrifice started at 10:00 am. The mice of each group were anesthetized at the same time with 2.5% isoflurane followed by decapitation. Truckal blood was collected, set on ice for 30 min, and centrifuged. Corticosterone was measured using a corticosterone ELISA (Enzo). Thymus and spleen were dissected, weighed, and immediately processed for flow cytometry.
Flow cytometry.
We mechanically dissociated thymuses or spleen through a 100 μm strainer to obtain single cell suspensions. Cells were stained with antibodies for 20 minutes on ice in the dark. Conjugated antibodies for CD4-APCH7 (Cat. #56081, GK1.5), NK1.1-PE (Cat. #551114, PK136), CD25-APC (Cat. #557192, PC61), CD8V450. (#560469, 53–6.7), CD44-PE (Cat. #553134, IN7), and CD69-FITC (Cat #553236, H1.2F3) were purchased from BD Biosciences. CD3-PECy7 (Cat. #100220, 17A2) was purchased from Biolegend. Intracellular staining for Foxp3, GATA3, RORγt, and T-bet was completed after washing in Fix/perm and Permeability buffers (eBioscience). Conjugated antibody for Foxp3-PE (BioLegend Cat. #126404) was used to identify Tregs. Conjugated antibody for GATA3-FITC (Miltenyi Cat. #130–100-689) was used to identify Th2 cells. Conjugated antibodies for RORγt-APC (eBioscience Cat. #4291992) and T-bet-PE-Cy7 (eBioscience Cat. #E15138–105) were used to identify Th17 and Th1 cells, respectively. Flow cytometry was performed using a BD LSRII, and results were analyzed using Flowjo.
rAAV Vector Construction and Packaging.
The rAAV plasmid contains a vector expression cassette consisting of the CMV enhancer and chicken β-actin (CBA) promoter, woodchuck post-transcriptional regulatory element (WPRE) and bovine growth hormone poly-A flanked by AAV2 inverted terminal repeats. Transgenes: Human BDNF, destabilized YFP, dominant negative TrkB (TrkB.T1) (8) or microRNA targeting Bdnf (7) was inserted into the multiple cloning sites between the CBA promoter and WPRE sequence. rAAV serotype 1 vectors were packaged, and purified as described elsewhere.
rAAV mediated gene delivery to hypothalamus.
Male C57BL/6 mice, 6 weeks of age, were purchased from Charles River and randomly assigned to receive AAV1-BDNF, AAV1-TrkB.T1 or AAV1-YFP. We chose to use 6-week-old mice for rAAV experiments because we found stereotaxic surgery targeting the hypothalamus of mice younger than 5 weeks was less accurate and prone to death risk after surgery. Mice were anaesthetized with a single dose of ketamine/xylazine (100mg/kg and 20mg/kg; i.p.) and secured via ear bars and incisor bar on a Kopf stereotaxic frame. A mid-line incision was made through the scalp to reveal the skull and two small holes were drilled into the skull with a dental drill above the injection sites (−0.8AP, ±0.3ML, −5.0DV; mm from bregma). rAAV vectors (1×1010 vg per site) were injected bilaterally into the hypothalamus the at a rate of 0.1μL/min using a 10μL Hamilton syringe attached to Micro4 Micro Syringe Pump Controller (World Precision Instruments Inc., Sarasota, USA). At the end of infusion, the syringe was slowly removed from the brain and the scalp was sutured. Animals were placed back into a clean cage and carefully monitored post-surgery until fully recovered from anesthesia. We monitored body weight every 5–7 days. Mice were maintained on NCD in SE until the end of the study (4 weeks after surgery).
rAAV-MicroRNA Experiment.
We randomly assigned male 6-week-old C57BL/6 mice to receive AAV-miR-Bdnf or AAV-miR-scr targeting a scrambled sequence not interfering any known gene (7). We injected rAAV vectors (1.4 × 1010 vg) bilaterally into the hypothalamus at the stereotaxic coordinates described above. Mice were maintained on NCD in SE for 5 weeks. In a second experiment, mice receiving AAV injection and randomly assigned to live in EE or SE housing for 5 weeks: AAV-miR-Bdnf/SE; AAVmiR-Bdnf/EE; AAV-miR-scr/SE; AAV-miR-scr/EE.
Hypothalamic Microdissection.
Brains were quickly isolated and the hypothalamus was dissected from 2 mm-thick-coronal sections (−0.7~−2.7 mm from bregma, 1.5 mm dorsal to the bottom of the brain, 1 mm bilateral to the midline) under a dissection scope. The PVH, ARC, and VMH/DMH were further dissected from the hypothalamus block section (8) and rapidly frozen at −80 °C.
Propranolol Experiment.
We randomly assigned 20 male C57BL/6 mice, 3 weeks of age, to live in EE or SE supplied with propranolol (Roxane Laboratories, Inc.) in drinking water (0.5 g/L).
Mifepristone administration
Male C57BL/6 mice, 6-week-old, were randomized to receive AAV1-BDNF or AAV1-YFP and housed in SE. Daily intraperitoneal injection of mifepristone (Sigma-Aldrich Co.) or vehicle was initiated one week after AAV injection and continued for two weeks. Mifepristone (30 mg/kg i.p.) was suspended in distilled water containing one drop of Tween® 20 (Calbiochem Co.) per 10 ml.
Adrenalectomy.
Male adrenalectomized mice and sham surgery mice, 4 weeks of age, were purchased from Charles River and randomly assigned to live in EE or SE supplied with 0.9% sodium chloride in drinking water. Tissues were collected and analyzed after 5-week EE.
GRlck-Cre mice EE experiment.
The GRlck-Cre mice were a gift from Dr. Jonathan D. Ashwell of NIH. Breeding and genotyping followed Dr. Ashwell’s protocol by PCR with the following primers Lck (forward: CAGTCAGGAGCTTGAATCCC; reverse: CACTAAAGGGAACAA AAGCTGG), Nr3c (forward: ATGCCTGCTAGGCAAATGAT; reverse: TTCCAGGGCTATAGGAAGCA). Because of the difficulty in obtaining large numbers of age-matched same sex transgenic mice, we tested EE with mid-size bin (73 cm × 41 cm × 46 cm cage, 5 mice per cage) using male WT mice. Our preliminary data showed no significant difference between mid-size and large size bin (1.5 m × 1.5 m × 1.0 m cage, 20 mice per cage) regarding thymus changes (Supplementary Table 1). Male, four-week-old GRlck-Cre mice were randomly assigned to SE or EE (73 cm × 41 cm × 46 cm cage, 5 mice per cage) housing for 5 weeks.
RAG2/GFP mice EE experiment.
The RAG2/GFP mice were a gift from Dr. Janko Nikolich-Zugich of University of Arizona. Breeding and genotyping followed Dr. Nikolich-Zugich’s protocol by PCR with the following primers: forward:
GGGGCAGGAGGTGTCTTAT; reverse: GTTTACGTCGCCGTCCAGT. Male, three-weeks old RAG2/GFP mice were randomly assigned to SE or EE (73 cm × 41 cm × 46 cm cage, 5 mice per cage) housing for 2 weeks.
EAE induction and scoring.
Male C57BL/6 mice or GRlck-Cre mice, 3–4 weeks of age were housed in SE or EE for 4 weeks. All EAE experiments were conducted in large size bin (1.5 m × 1.5 m × 1.0 m cage, 20 mice per cage). The induction of EAE and clinical score evaluation followed published method (12). Briefly, we injected 100 μl of myelin oligodendrocyte glycoprotein (MOG35–55)/CFA emulsion subcutaneously into two different sites on each hind flank. The final concentrations of MOG35–55 (AnaSpec Inc. AS-60130–5) and CFA (Chondrex Inc. 7009) in the emulsion were 1 mg/ml. Mice also received 200 μl of pertussis toxin (2 μg/ml, Sigma-Aldrich) intraperitoneally on the day of immunization and two days later. The mice were weighted and monitored daily. The scoring of EAE symptom was based on 0–10 scale: 0, no clinical signs; 1, partially limp tail; 2, paralyzed tail; 3, hind limb paresis, uncoordinated movement; 4, One hind limb paralyzed; 5, both hind limbs paralyzed; 6, hind limbs paralyzed, weakness in forelimbs; 7, hind limbs paralyzed, one forelimb paralyzed; 8, hind limbs paralyzed, both forelimbs paralyzed; 9, Moribund; 10, death. Tissues were harvested for analysis at sacrifice date specified in Results, respectively.
Purification of infiltrating mononuclear cells (MNCs) in CNS.
Mice were anesthetized and perfused through the left ventricle with ice-cold PBS. Brain and spinal cord were dissected and homogenized prior to filtration through 70 μm cell strainers (Falcon). CNS MNCs were purified using a 37/70% (vol/vol) discontinuous Percoll gradients (GE health care). In vitro stimulation assay of splenocytes. 1 million spleen cells were cultured with MOG35–55 (20ug/mL) for 36 h, followed by stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin cocktail (eBioscience) in the presence of GolgiPlug (BD Bioscience) for 5 h. Cells were intracellular stained with anti-IFNγ (BD Cat. #554411) for Th1, anti-IL4 (BD Cat. #560700) for Th2 and anti-IL17 (BD Cat. #560522) for Th17 cells. The detection of regulatory T (Treg) cells was carried out using a kit from BD Bioscience.
Adoptive transfer of thymocytes.
Male C57BL/6 mice were immunized with MOG as described above and maintained in SE housing. Ten days after MOG immunization, thymocytes, obtained from two-week EE or SE mice, were administered by i.v. injection (3 million thymocytes per mouse).
Histology.
In the EAE experiment, one SE mouse and one EE mouse with the most severe symptom on day 20 were sacrificed and the spinal cords were fixed with 4% paraformaldehyde. Paraffin-embedded 5-μm transverse sections of lumbar spinal cord or thymus were processed and stained with H&E by Histology Core of Comprehensive Cancer Center of The Ohio State University. Sections were subjected to citrate-based antigen retrieval following by incubations with antibody against CD4 (Affymetrix ebioscience #14–9766-80, 1:100). The sections were visualized with DAB and counterstained with hematoxylin. The luxol fast blue stain (Abcam #ab150675) was performed to stain myelin sheath. In a short-term EE experiment, thymus was collected after 6-day EE and fixed in 10% formalin. Histology was evaluated by a pathologist of Comparative Pathology & Mouse Phenotyping Shared Resource of The Ohio State University.
Quantitative RT-PCR.
We dissected hypothalamus and isolated total RNA using RNeasy Kit plus RNase-free DNase treatment (Qiagen). We generated first-strand cDNA using TaqMan Reverse Transcription Reagent (Applied Biosystems) and carried out quantitative PCR using StepOnePlus Real-Time PCR System (Applied Biosystems) with the Power SYBR Green PCR Master Mix (Applied Biosystems). Primer sequences are available on request. We calibrated data to endogenous control Hprt1 and quantified the relative gene expression using the 2 −ΔΔCT method.
Statistical analysis.
Data are expressed as mean±s.e.m. Data were determined to be normally or approximately normally distributed according to the Shapiro-Wilk test. Means between two groups were compared with two-tailed Student’s t-tests. For multiple comparisons, two-way ANOVAs with pairwise comparisons were used to determine statistical significance. Microsoft Excel was used to perform Student’s t-tests and IBM SPSS Statistics v24.0.0.0 was used to analyze multiple comparisons.
Results
EE modulates thymocyte development and migration in young mice
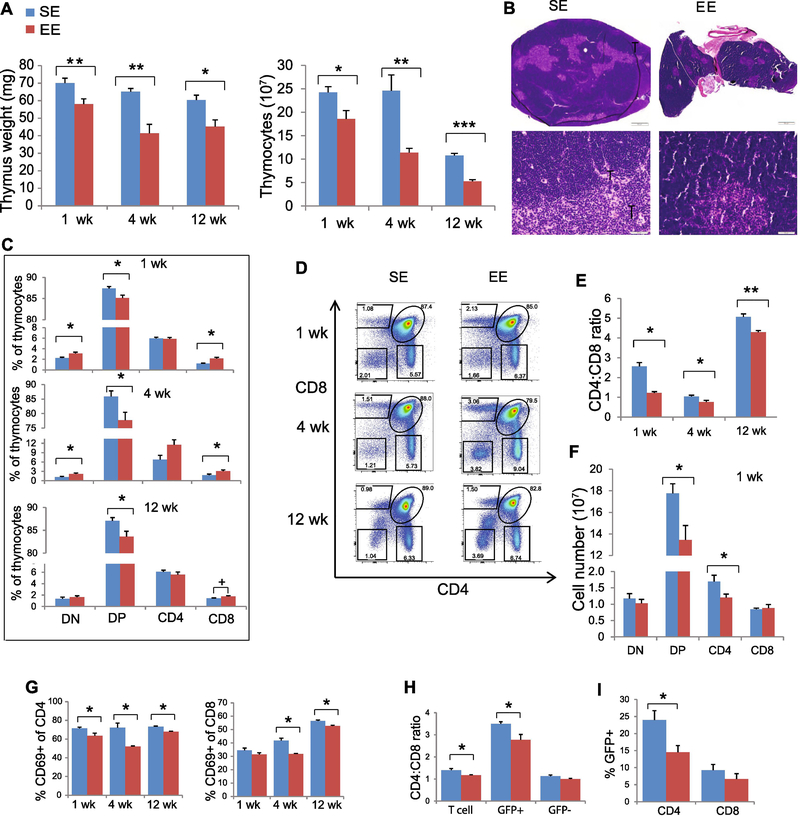
To assess the effects of EE on thymus and thymocyte development, 3-week-old male mice were randomly assigned to live in SE or EE for various durations immediately after weaning. A 1-week exposure to EE was sufficient to induce a significant reduction in thymic mass. The thymic involution persisted at 4 and 12 weeks (Fig. 1A). Pathological examination noted no overt histologic differences in thymuses from mice placed in the EE and SE beyond the smaller size found in the EE. The total number of thymocytes was significantly lower in mice exposed to the EE for 1, 4 or 12 weeks (Fig. 1A). Under both environments, the peripheral cortex was wide and populated by appropriate numbers of viable, small lymphocytes, and distinct from the central medulla. There was no evidence of lymphocytolysis or lymphoid depletion (Fig. 1B). Next, we examined whether EE affected thymocyte development. CD4 and CD8 define the developmental stages of T cells from precursor DN cells to DP and to SP CD4+ T-helper or CD8+ cytotoxic T lymphocytes (CTLs) (13). After 1-week EE, the thymuses of EE mice contained a higher proportion of DN (Fig. 1C). One-week exposure to the EE significantly decreased the proportion of DP cells, and this pattern was sustained at 4 and 12 weeks (Fig. 1C). When gating on TCRβ+ thymocytes, a proportionate increase in CD8+ SP cells and a reduced CD4:CD8 ratio among SP cells were observed (Fig. 1C-E). EE exposure significantly reduced the absolute numbers of DP and SP CD4+ T cells (Fig. 1F).
Figure 1. EE modulates thymocyte development and T cell emigration.
(A) Thymus weight and total thymocyte numbers at 1-, 4- and 12-weeks EE. (B) H&E staining of thymus at 1 week EE. (C) Thymocyte subsets. (D) Representative FACS analyses for CD4 and CD8 expression on thymocytes. (E) The ratio of TCRβ+ CD4+: TCRβ+ CD8+ in thymus. (F) Numbers of thymocyte subsets at 1-week EE. (G) Percentage of CD69+ cells of CD4 or CD8 SP cells. (H) CD4:CD8 ratio in lymph node from Rag2-GFP mice. (I) Percentage of GFP+ cells in CD4 and CD8 T cells in spleen from Rag2-GFP mice. Data represent mean ±SEM; n =10–15 per group for 1- and 4- weeks and 5 per group for 12-week EE (A-F); n=5–10 per group (G-I) * P < 0.05, ** P < 0.01, *** P<0.001, + P=0.08, unpaired t test.
Expression of the surface protein CD69 retains SP thymocytes within the thymus, and SP cells downregulate CD69 prior to emigration to the SLT (14). EE reduced the CD69+ population among CD4+ SP cells as early as the 1-week time point. In contrast, the reduction of CD69+ among CD8+ SP cells was observed only after 4 weeks of EE (Fig. 1G). These data suggested that EE might influence the egression of SP T cells. We have previously reported that EE reduces total T cell numbers and CD4:CD8 ratio in the SLT (9). To assess whether the thymic changes contributed to the change of CD4:CD8 ratio in SLT, we obtained RAG2-GFP mice in which GFP is expressed in T cells that have recently undergone TCR rearrangement, and thus enable detection of recent thymic emigrants in SLT (15). RAG2-GFP mice were housed in SE or EE for 2 weeks. In SLT, we observed a significantly reduced CD4:CD8 ratio only among GFP+ T cells in EE (SE = 3.50±0.09, EE = 2.77±0.25, P = 0.039) but not among GFP- T cells (SE = 1.13±0.05, EE = 1.00±0.03, P = 0.17) (Fig. 1H). This reduced CD4:CD8 ratio was caused by decreased GFP+ CD4+ T cells (Fig. 1I). These observations indicate that the T cell changes in SLT under the EE were derived from modulation of the thymic population. Females are more likely than males to be diagnosed with certain autoimmune diseases. Therefore, we tested whether EE’s thymic modulations were sex-dependent. EE led to a similar thymocyte phenotype in female mice (fig. S1). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1 (S1P1) (16). EE mice showed upregulation of S1P1 expression in both CD4 and CD8 SP cells (fig. S1G). The hypothalamus is composed of a number of discrete nuclei including the arcuate (ARC), paraventricular (PVH), ventromedial (VMH), dorsomedial (DMH), and lateral hypothalamic area (LHA). Of these, the ARC nucleus in particular is a critical locus for food intake regulation as it integrates signals from the brainstem and the periphery, and the PVH nucleus plays an important role in the HPA axis through the release of corticotropin-releasing hormone (CRH) (17). We have demonstrated that EE upregulates BDNF expression in the ARC and VMH/DMH, and subsequently activates the HSA axis leading to leanness and resistance to diet-induced obesity (7, 8). To investigate whether BDNF serves as a brain mediator of the EE’s thymic modulation, we microdissected the PVH, ARC and VMH/DMH after exposing mice to the EE for 2 weeks. Quantitative RT-PCR (qRT-PCR) showed that EE significantly upregulated Bdnf expression in the VMH/ARC nuclei and Crh expression in the PVH nucleus (fig. S2A). Consistent with upregulation of Crh expression, we observed a higher level of serum corticosterone (CORT) in EE mice after one-week EE exposure (fig. S2B).
Hypothalamic overexpression of BDNF reproduces EE-induced thymic phenotypes
To test whether hypothalamic BDNF also mediates the EE-induced thymic phenotype, a recombinant adeno-associated viral (rAAV) vector was used to overexpress BDNF in the hypothalamus. Mice were stereotactically injected with rAAV-BDNF or rAAV-YFP as control to the hypothalamus and housed in SE. Immunohistochemistry confirmed the transgene expression largely within ARC, VMH, and DMH (fig. S3). Both thymus mass and total thymocyte number were significantly decreased in BDNF-injected mice, mimicking the phenotype observed in EE. Hypothalamic BDNF overexpression also reproduced EE’s effects on thymocyte populations characterized by a proportional decrease in DP population, a proportional increase in the SP CD8+ cells, a reduction in the CD4:CD8 ratio when gated on TCRβ, and a downregulation of CD69 expression in CD4+ SP cells (Fig. 2A). Hypothalamic BDNF overexpression was confirmed by qRTPCR (Fig. 2A). Consistent with our previous reports(18), Crh expression was upregulated in BDNF overexpressing mice (Fig. 2A).
Figure 2. Hypothalamic BDNF mediates EE-induced thymic phenotypes.
(A) Hypothalamic overexpression of BDNF mimics EE-induced thymic phenotypes. Thymic parameters, representative FACS result for CD4 and CD8 expression on thymocytes, and hypothalamic Bdnf and Crh expression. (B) TrkB.T1 inhibition of hypothalamic BDNF signaling reverses thymic phenotypes associated with EE. (C) Knockdown hypothalamic Bdnf by miR-Bdnf blocks EE’s thymic modulations. Data represent mean ±SEM; n=5 per group (A); n =5–7 per group (B-C). * P < 0.05, ** P < 0.01, *** P < 0.001, unpaired t test.
Hypothalamic BDNF knockdown reverses EE-induced thymic phenotypes
To assess whether depletion of hypothalamic BDNF could reverse EE’s effects on the thymus, we injected mice with a microRNA (miR) targeting Bdnf or a scrambled (scr) sequence targeting no known gene (7). rAAV-miR-Bdnf knocked down hypothalamic Bdnf mRNA over 80% (fig. S4G) and decreased Crh expression approximately 40% (fig. S4G). Hypothalamic Bdnf knockdown resulted in a reversal of the thymus phenotype compared to BDNF overexpressing mice or EE mice. In miR-Bdnf mice, both thymus mass and total thymocyte number were increased (fig. S4A, B). The proportion of DP cells was increased while SP populations were reduced (fig. S4C, D). When gated on TCRβ+ cells, the CD4:CD8 ratio was increased (fig. S4E), and CD69 expression was increased in CD4+ T cells (fig. S4F).
The TrkB receptor is encoded by the Ntrk2 gene and is a ligand-specific receptor for BDNF(19). To further assess whether EE’s effect on thymus is dependent on hypothalamic BDNF signaling, we used a dominant-negative truncated form of the high-affinity BDNF receptor (TrkB.T1) to inhibit BDNF signaling specifically in hypothalamus (8). Hypothalamic expression of TrkB.T1 reversed the thymic phenotypes associated with EE or BDNF overexpression (Fig. 2B).
To determine whether EE’s thymic effects require hypothalamic BDNF, we randomly assigned the mice to receive AAV-miR-scr or AAV-miR-Bdnf, and split each vector-injected group to live in SE or EE. Hypothalamic Bdnf knockdown blocked the effect of EE on the thymus (Fig. 2C).
β-adrenergic signaling is not critical for EE-induced thymic phenotypes
Many adaptations induced by EE require intact sympathetic signaling including adipose remodeling, anticancer effects, and SLT immune phenotypes as evidenced by the complete pharmacologic blockade with propranolol (7–9). Interestingly, in repeated experiments, the same treatment of propranolol failed to prevent the EE-induced thymic phenotypes (fig. S5).
HPA signaling is required for EE-induced thymic phenotypes
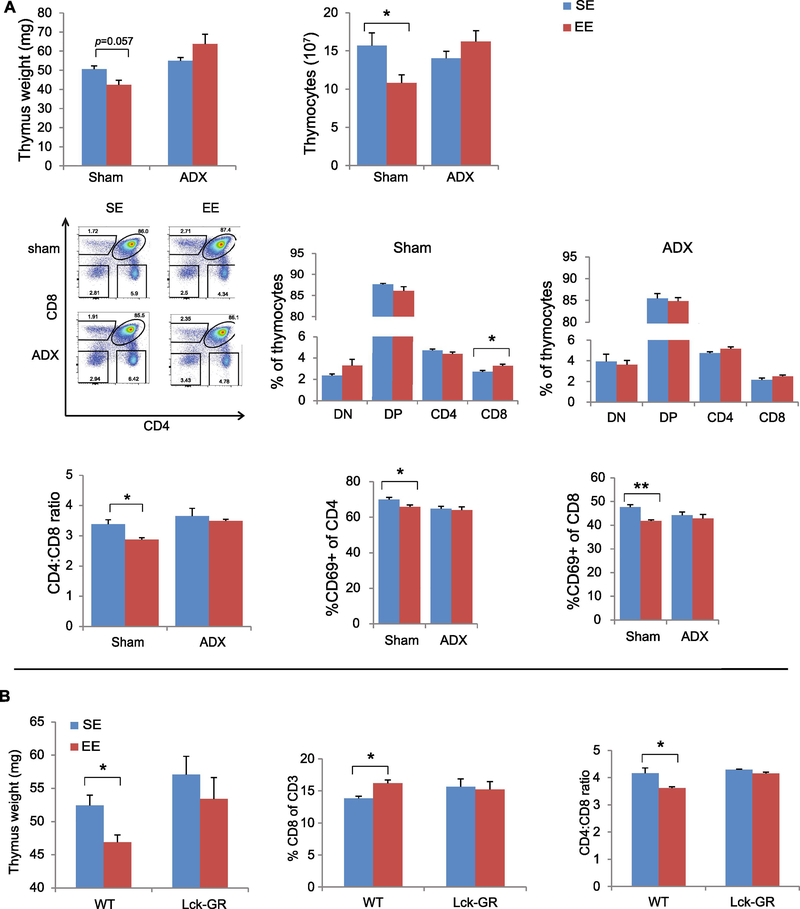
Activation of the HPA axis is an adaptive response to acute and chronic stress, and our data suggest that EE mildly activates the HPA axis (7). In the HPA axis, hypothalamic CRH stimulates secretion of aderenocorticotrophin horomone (ACTH) in the anterior pituitary, which stimulates glucocorticosteroid (GC) production in the adrenal glands. After one week of EE, serum corticosterone level was increased in EE compared to those in SE (fig. S2B). We used two paradigms to evaluate the role of the HPA axis in EE-induced thymic phenotypes. First, we randomly assigned mice that had undergone an adrenalectomy (ADX) or sham surgery to live in SE or EE for five weeks. The majority of the thymic modulations associated with EE were observed in sham surgery mice. In contrast, ADX completely eliminated the EE-induced effects on thymus including thymus mass, thymus cellularity, thymocyte maturation and selection, and CD69 expression on SP cells (Fig. 3A), indicating an essential role of adrenal gland-derived GC.
Figure 3. The HPA axis is required for EE-induced thymic phenotypes.
(A) Thymic phenotypes in sham surgery mice and ADX mice after 5-weeks EE. (B) Thymocyte-specific GR knockout (Lck-GR mice) prevents EE-induced thymic phenotypes. Data represent mean ±SEM; n =5–9 per group. * P < 0.05, ** P < 0.01,two-way ANOVAs in (A); unpaired t test in (B).
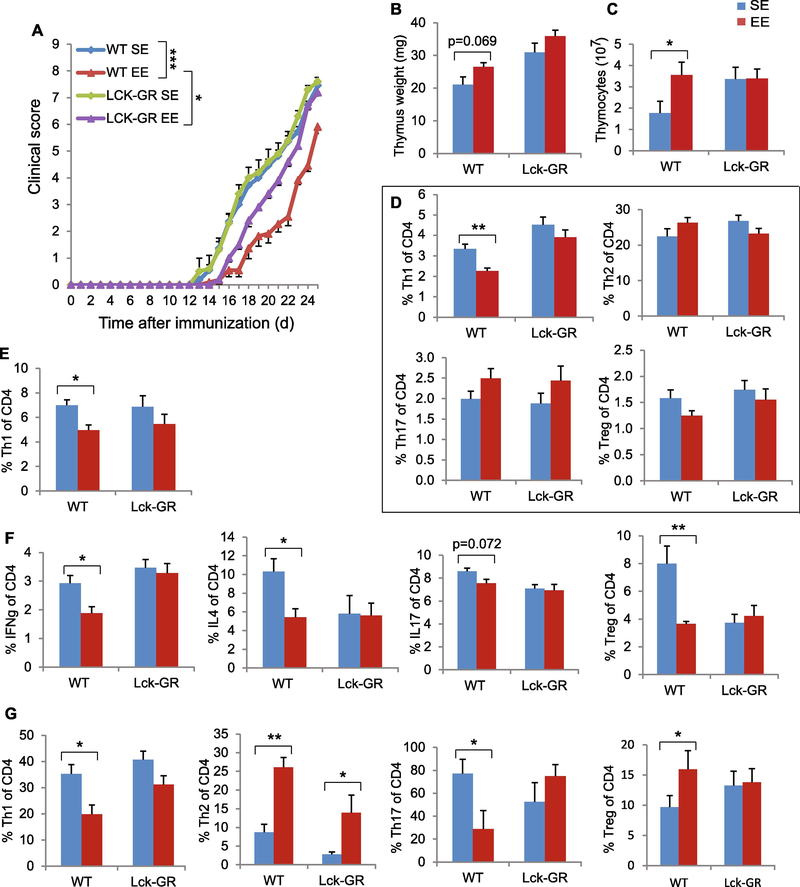
To investigate whether thymocytes were directly regulated by EE-induced HPA activation, we used a conditional knockout mouse strain LCKCreGRlox (GRlck-Cre) (20), in which the glucocorticoid receptor (GR) gene is specifically deleted in the thymocytes prior to selection and therefore their DP and SP cells are unable to respond to GC (20). After a 5-week EE, none of the EE-induced thymic changes in wild type mice were observed in the GRlck-Cre mice (Fig. 3B), suggesting the EE’s thymic modulation via thymocyte GR signaling.
Mifepristone attenuates hypothalamic BDNF’s effect on thymic phenotypes
To further assess whether the HPA axis mediates hypothalamic BDNF-induced thymic modulation, we used mifepristone, an antagonist of cortisol receptor to block GC’s function and to pharmacologically inhibit the HPA axis. Mice injected with rAAV-BDNF or rAAV-YFP were split to receive mifepristone or vehicle and housed in SE. Consistent with published data (21), mifepristone administration to rAAV-YFP mice led to lower thymus weight and thymocyte number compared to rAAV-YFP mice receiving vehicle (fig. S6A, B). However, the BDNF-induced reduction of thymus weight and thymocyte count was attenuated by mifepristone (fig. S6A, B). Moreover, mifepristone treatment completely precluded hypothalamic BDNF-elicited thymocyte phenotypic changes (fig. S6C-G).
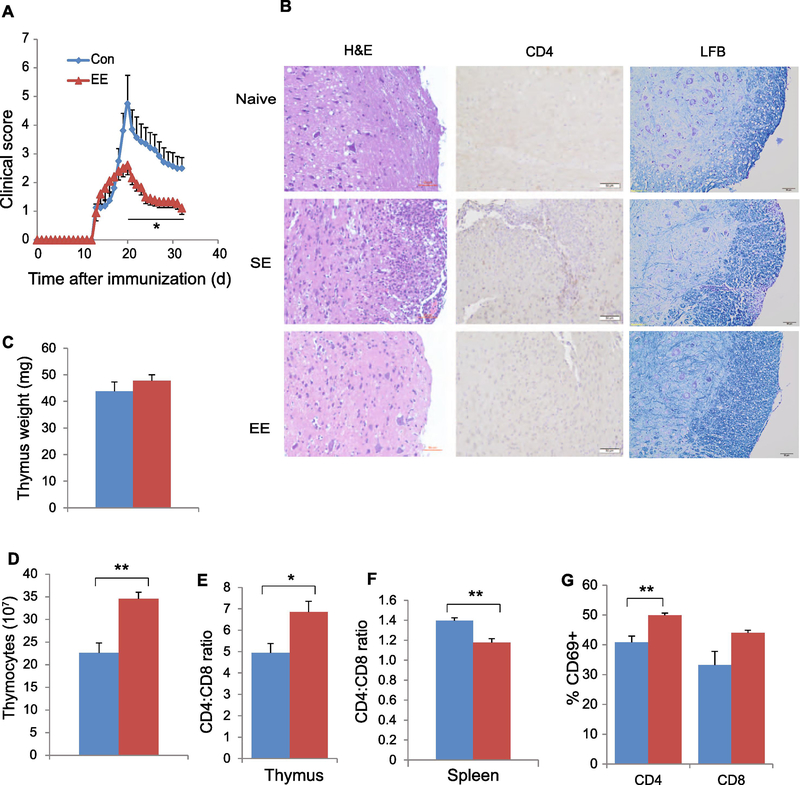
EE ameliorates EAE
EAE is a commonly used model of MS. It is characterized by mononuclear cell infiltration into the CNS and degeneration of the myelin sheath, and CD4+ T cells are critically involved in EAE pathogenesis (22). EE attenuated the development of EAE with a significantly lower clinic score (Fig. 4A). Histological analysis of lumbar spinal cord sections showed that naïve mice had no inflammatory foci or CD4+ T cell infiltration. EAE mice living in SE exhibited abundant mononuclear cell infiltration including CD4+ T cell in spinal cord. In contrast, EAE mice living in EE showed a substantial reduction of immune cell infiltration and attenuated demyelination (Fig. 4B). The mice were sacrificed on day 34 after immunization when the recovery of symptom was plateaued and the signature immune changes associated with EE were examined (Fig. 4C-G). Interestingly, instead of reduced thymus cellularity, a decreased CD4:CD8 ratio, and a decreased proportion of CD69- expressing SP cells associated with EE in naïve mice, EE increased total thymocyte numbers (Fig. 4D), elevated CD4:CD8 ratio (Fig. 4E) and CD69 expression on SP cells in the thymus of the EAE disease model (Fig. 4G). This could reflect the attenuation of EAE-induced thymic changes. For example, EAE mice living in SE showed decreased CD69 expression in CD4+ cells (40% CD69+ of CD4 cells, Fig. 4G) compared to normal mice in SE (60~70%, Fig. 1G). Living in EE attenuated this EAE-induced drop of CD69 expression in thymus (Fig. 4G).
Figure 4. EE ameliorates EAE symptoms associated with immune modulations.
(A) The EAE clinical score of SE and EE mice. (B) Representative H&E staining (left), CD4 immunohistochemistry (middle), and Luxol Fast Blue (LFB) staining (right) of spinal cords from naïve mouse, EAE SE, and EAE EE mice. (C) Thymus mass. (D) Total thymocyte numbers. (E) The CD4:CD8 ratio in thymus. (F) The CD4:CD8 ratio in spleen. (G) The percentage of CD69+ cells of CD4+ or CD8+ SP cells in thymus. Data represent mean ±SEM; n =8–10 per group. * P < 0.05, ** P < 0.01 unpaired t test. Scale bar: 50 μm.
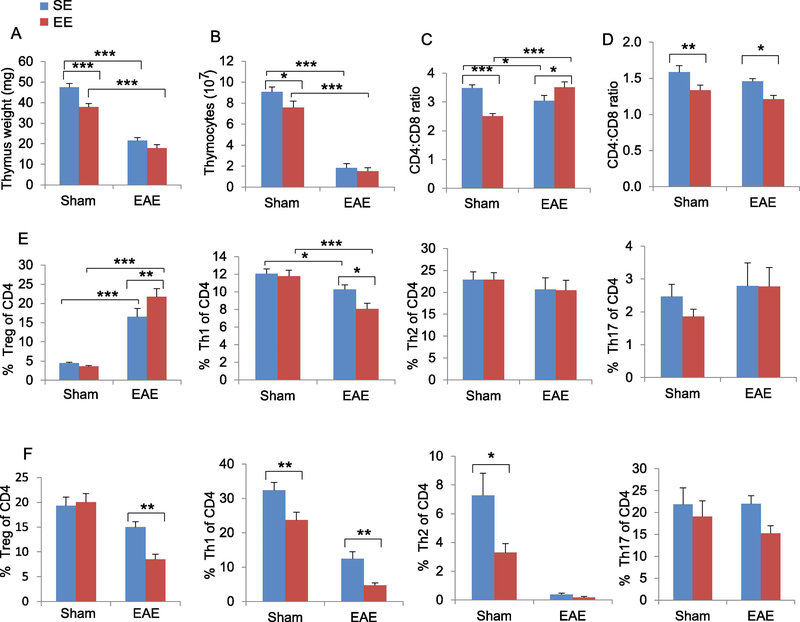
To further investigate how EE-induced thymic phenotypes influence the progression of EAE in early stage, we repeated the EAE experiment but sacrificed mice (10 days post-immunization) prior to the occurrence of clinical symptoms. Myelin oligodendrocyte glycoprotein (MOG) immunization to induce EAE resulted in a robust reduction of thymus mass and cellularity (SE-EAE compared to SE-Sham, Fig. 5A, B). However, EE attenuated this EAE-associated change (EE-EAE compared to EE-Sham, Fig. 5A, B). MOG immunization under EE conditions did not decrease the thymus mass or cellularity (EE-EAE compared to SE-EAE, Fig. 5A, B). Furthermore, in contrast to mice receiving sham immunization under an EE condition, mice receiving MOG immunization under EE condition had reversal of their CD4:CD8 ratio in thymus (Fig. 5C) while maintaining their reduction in spleen (Fig. 5D). CD4 cell subpopulations: Treg, Th1, Th2 and Th17 cells are the main pathogenic T cells in EAE (10, 23–25). Thus we profiled the subpopulations of CD4 cells in the thymus (Fig. 5E) and spleen (Fig. 5F) to identify the key differential populations mediating the inhibition of EAE disease by EE. In the absence of MOG immunization, EE had no significant effects on CD4 subpopulations in the thymus. In contrast, EE increased Treg percentage and reduced Th1 percentage in the thymus of EAE mice (Fig. 5E). In spleen, EE reduced both Treg and Th1 cell percentages in EAE mice (Fig. 5F).
Figure 5. EE affects immune responses in the early stage of EAE.
(A) Thymus mass. (B) Total thymocyte numbers. (C) The CD4:CD8 ratio in thymus. (D) The CD4:CD8 ratio in spleen. (E) The percentage of Treg, Th1, Th2 and Th17 in thymus. (F) The percentage of Treg, Th1, Th2 and Th17 in spleen. Data represent mean ±SEM; n =9–10 per group. * P < 0.05, ** P < 0.01, *** P < 0.001, two-way ANOVAs.
EE’s thymic modulation contributes to protection against EAE
To further assess the link between the EE’s thymic modulation and the protection against EAE, we repeated the EAE experiment using GRlck-Cre mice that failed to respond to EE-induced thymic regulation (Fig. 3B). Male wild type (WT) mice and GRlck-Cre mice were housed in SE or EE for 4 weeks followed by MOG immunization and remained in their corresponding housing till sacrifice 27 days after MOG immunization around the peak of EAE progression. Consistent with previous results (Fig. 4A), EE improved clinical outcome in WT mice (Fig. 6A). In contrast, GRlck-Cre mice attenuated the EE-associated protection and showed worse clinic scores compared to WT-EE mice (Fig. 6A). The EE-associated changes in thymus mass and cellularity observed in WT mice were absent in GRlck-Cre mice (Fig. 6B, C). Coinciding with the changes of CD4 cell subpopulations found in the early stage of EAE (Fig. 5E, F), WT-EE mice exhibited reduced Th1 in thymus and spleen, which was abrogated in GRlck-Cre mice (Fig. 6D, E). We then performed in vitro stimulation assay on spleen cells. The reduced levels of IFN-γ, IL4 and IL17 levels as well as Treg cells were only observed in WT-EE mice but not in GRlckCre mice (Fig. 6F). Moreover, we profiled the CD4 subpopulations in the CNS. In WT mice, EE led to a reduction of pathogenic Th1 and Th17 cell populations, whereas increased Th2 and Treg cell populations compared to SE mice (Fig. 6G). The majority of these changes were absent in GRlck-Cre mice (Fig. 6G).
Figure 6. Thymocyte-specific GR knockout prevents thymic modulation and attenuates EAE protection associated with EE.
(A) The EAE clinical score of WT and GRlck-Cre in SE or EE housing. (B) Thymus mass. (C) Total thymocyte numbers. (D) The percentage of Th1, Th2, Th17 and Treg in thymus (E) The percentage of Th1 in spleen. (F) in vitro stimulation of spleen cells harvested from EAE mice. (G) The percentage of Th1, Th2, Th17 and Treg cells in brain and spinal cord. Data represent mean ±SEM; n =10–11 per group. * P < 0.05, ** P < 0.01, unpaired t test in (A), two-way ANOVAs in (B-G).
Moreover, we performed an adoptive transfer experiment to investigate the contribution of thymic modulation to EE-induced alleviation of EAE. Male WT mice received adoptively transferred thymocytes from SE mice or EE mice on the day 10 post MOG immunization. The mice that received thymocytes from EE mice exhibited protection against EAE (fig. S7).
Discussion
The thymus is the primary organ for thymocyte differentiation and maturation into functional T cells. Unlike other major organs, the thymus is highly dynamic, capable of undergoing multiple rounds of dramatic involution followed by rapid restoration (26). Chronic thymic involution is primarily related with age, while acute thymic involution can be induced by multiple factors such as various physiological and psychological stressors, infection, pregnancy, cancer, and cancer treatment (26). Several studies including ours have shown EE’s immunomodulatory effects are mostly on SLTs or in the CNS (7, 9, 27). In this study, we demonstrated that living in an EE led to a significant thymic involution in young mice with as little as 1-week of exposure to EE. EE regulated thymocyte maturation and selection at several stages. The main features include reducing thymocyte numbers, lowering the DP cells and CD4:CD8 SP ratio, and downregulating CD69 expression in SP cells, possibly to facilitate emigration of SP cells, particularly CD4+ T cells. The EE-induced thymic phenotypes were sex-independent in young mice. Our mechanistic studies identified hypothalamic BDNF as a key upstream brain mediator of the EE’s effects on thymus (fig. S8). Overexpressing BDNF in the hypothalamus reproduced the EE-induced thymic phenotypic changes. Conversely, knockdown of hypothalamic BDNF expression reversed the thymic features induced by EE. Furthermore, inhibition of BDNF signaling by a dominant-negative TrkB receptor specifically in the hypothalamus also reversed the EE-induced thymic phenotypic changes suggesting an important role of hypothalamic BDNF as an upstream mediator. Hypothalamic BDNF upregulated Crh expression and thereby activated the HPA axis (18). The adrenal GC was essential to the EE’s thymic modulation as evidenced by the complete blockade of EE-induced thymic changes in ADX mice. Furthermore, the elevated GC likely acted directly on thymocytes since thymocyte-specific deletion of GR diminished the effects of EE on the thymus. Moreover, mifepristone attenuated the thymic changes in hypothalamic BDNF overexpressing mice. These results indicate an essential role for GC in T cell development and function in EE housing. This is consistent with the literature, which reports that enhanced GR signaling in T cells affects thymocyte development characterized by lower DP cells and higher CD8 percentage (28). We have demonstrated that an intact sympathetic nervous system (SNS) is required for EE-induced anticancer and anti-obesity effects and that the SNS is also involved in EE’s modulation of SLTs (7–9). However, propranolol failed to attenuate EE’s thymic phenotypes at the dose capable of complete blockade of EE’s metabolic and cancer effects. Based on these findings, we propose one of the mechanisms underlying the EE-induced thymic phenotypes: hypothalamic BDNF activates the HPA axis and the ensuring adrenal GC acts on thymocyte GR to modulate thymocyte development without the requirement of the SNS (fig. S8). Our findings are consistent with prior work that suggests immature thymocytes are more susceptible to GC-induced apoptosis than mature thymocytes or peripheral T cells.
Endogenous GC is required for a robust adaptive immune response because of its promotion of selection for T cells that have sufficient affinity for self. Indeed, the absence of thymocyte GC signaling results in an immunocompromised state (20). In a review article (5), we proposed that EE is a eustress/hormesis model, adopting classical stress literature, which notes that departures from homeostasis could be “eustressful” or “distressful” and that health effects would vary accordingly. Eustress and hormesis relate to milder and/or briefer challenges, which induce a positive or healthy adaptive response. More recently, the concepts of allostasis and allostatic load have been introduced to describe adaptive responses to external challenges and the maladaptive consequences, respectively (29). EE provides dynamic social interactions, enhanced physical activity, and cognitive stimulation. This enriched living condition leads to a mild (within the physiological range) but significant increase in serum GC in C57BL/6 mice. Here we demonstrated that activation of the HPA axis was required for the EE-induced immune regulations, and the elevated GC level in EE was not associated with an immunosuppressive state as evidenced by enhanced anticancer immunity (7, 9). It is reported that GCs inhibit naïve CD8+ T cells but have little impact on activated CD8+ T cells and do not inhibit the antitumor activity (30). Of note, EE on one hand enhances antitumor activity of natural killer cells and tumor infiltrating lymphocytes (7, 9, 31), and on the other hand inhibits autoimmune responses in EAE (Fig. 4, 5, 6). It is interesting to study the EE’s effects on other T-cell-mediated events such as graft-versus-host disease and cancer immunotherapy with adoptive cell transfer (32). Of note, some EE effects may depend on mouse strains. For example, BALB/c mice exposing to EE exhibit greater spleen mass and significantly reduced corticosterone production (33). Further characterization of EE’s thymic effects in different strains is warranted. Here we assessed the EE’s impact on an autoimmune disease model. MS is an inflammatory and degenerative disease of the CNS whose pathogenesis is thought to involve autoimmunity. Stressful life events have been suggested as potential triggers of disease exacerbation (34). Animal research using an EAE model suggests that decreased HPA function plays a role in increased susceptibility and severity of the disease. Animal and clinical studies have shown that hyper- and hypo-activity of the HPA axis are associated with more severe courses of MS (34). Our data demonstrated that EE lessened the maximal disability, reduced the cumulative clinical scores, and accelerated recovery (Fig. 4). This finding is consistent with a previous report of EE that assessed neural progenitor cell mobilization in the EAE model but did not examine immune modulation (35). Here, we showed that the EE-induced thymic phenotypic changes were associated with an improved functional outcome in EAE. Of note, EE reduced thymus size and cellularity as well as CD69 expression in CD4+ SP cells in normal mice (Fig. 1), however, these features were reversed in the EAE model after spontaneous recovery (Fig. 4). To further investigate whether EE protected mice against EAE-induced thymus involution and acceleration of CD4 T cell egression, we examined immune changes in the early stage of EAE prior to the occurrence of clinical symptoms. Induction of EAE led to substantial reduction of thymus mass and cellularity in SE mice whereas EE attenuated this EAE-induced thymus involution. Moreover, EE shifted the balance of Treg, Th1 and Th17 subsets under the condition of EAE disease. This alteration of CD4 subpopulations was not observed in the absence of immune challenge. Of interest, EE mice showed lower Th1 proportions in both of thymus and spleen, suggesting possible contribution of Th1 modulation to the EE’s protection against EAE in the early stage of EAE. Furthermore, we employed two approaches to assess the contribution of thymic modulation to EE-induced protection against EAE. In WT mice, EE led to lower Th1 proportions in the thymus, spleen and CNS compared to mice living in SE. These results are consistent with the prior study that Th1, Th2, and Th17 cells have distinct GC sensitivity and Th1 cells are sensitive to GC-induced apoptosis and cytokine suppression (36). In addition, thymocyte-specific deletion of GR (GRlck-Cre mice) prevented the EE-induced thymic modulation, and also attenuated the EE-associated improvement of EAE clinical score as well as abolished the suppression of Th1, which suggests that thymic modulation and thymocyte GR signaling are required for EE to confer protection against EAE. The thymocyte adoptive transfer data further linked thymic modulation to the EEinduced EAE resistance.
A recent publication reports that EE leads to a unique T helper effector cell phenotype upon differentiation in vitro featured by reduced production of IFN-γ although no in vivo impact was observed (37). In contrast, our in vivo data in naïve or EAE conditions demonstrated significant impact of EE on thymocyte differentiation and T cell repertoire. This discrepancy is likely due to differences in the EE setting and study design.
Collectively, these data support the notion that EE could train or fine-tune the immune system to be more adaptive when facing a challenge. It is intriguing to investigate whether EE elicits unknown signaling in addition to the HPA axis in the context of EAE. Furthermore, it is likely that multiple mechanisms underlie the EE’s neuroprotection, neural repair, and behavioral rehabilitation in pathological conditions including CNS insult and neurodegenerative diseases (6). The CNS-specific autoimmune T cells have been identified as important players of brain plasticity (38). Future investigations are required to identify the T cell subpopulations contributing to EE’s protection against EAE.
Age-related thymic involution, defined as a progressive regression in thymus size and a diminishment of thymic structure, is evolutionarily and conservatively maintained in vertebrates (39). Thymic involution is associated with immunosenescence, a degeneration of the immune system concomitant with greater susceptibility to infections and increased propensity for autoimmune diseases and cancers in aged population (40). Evidence indicates that early life programming of the thymus is crucial and has long-term effects on T cell development (41). However most of these studies were nutritional (42). Here we described an EE-induced thymic involution without immunosenescence in early adult life when the thymic development is very sensitive to early events (42), whose long-term impact warrants future investigation.
Conclusion.
Our data demonstrate that an EE providing physical, social, and cognitive stimuli regulates thymus mass, thymocyte maturation and selection, CD4/CD8-lineage choice, and T cell export through a central mechanism. Hypothalamic BDNF as a key mediator activates the HPA axis to release GCs that interact on thymocyte GR. The EE-induced thymic modulations contribute to an improved functional outcome in EAE model providing preclinical evidence for a non-pharmacological “environmental” or “life style” intervention in MS.
Supplementary Material
Highlights.
Environmental enrichment (EE) modulates thymocyte maturation and selection in mice
Hypothalamic BDNF mediates the EE-induced thymic phenotypes
Adrenalectomy and thymocyte glucocorticoid receptor knockout block EE’s thymic effects
EE protects mice against autoimmune EAE via regulation of type 1 T helper cells
Adoptive transfer and thymocyte GR knockout link EE thymic modulation to EAE outcome
Acknowledgement
We thank Dr. Jonathan D. Ashwell of NIH for providing GRlck-Cre mice and Dr. Janko Nikolich-Zugich of University of Arizona for providing RAG2/GFP mice. This work was supported by NIH grants AG041250, CA166590, CA178227, CA163640 to L. Cao and NIH grants CA163205, CA210087, CA95426, CA068458 to M.A. Caligiuri.
Footnotes
Competing interests: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. [DOI] [PubMed] [Google Scholar]
- 2.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nature reviews Immunology. 2014;14(6):377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maroder M, Bellavia D, Vacca A, Felli MP, Screpanti I. The thymus at the crossroad of neuroimmune interactions. Ann N Y Acad Sci. 2000;917:741–7. [DOI] [PubMed] [Google Scholar]
- 4.Blalock JE. Shared ligands and receptors as a molecular mechanism for communication between the immune and neuroendocrine systems. Ann N Y Acad Sci. 1994;741:292–8. [DOI] [PubMed] [Google Scholar]
- 5.Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;35:33145. [DOI] [PubMed] [Google Scholar]
- 6.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. [DOI] [PubMed] [Google Scholar]
- 7.Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao R, Bergin SM, Huang W, Slater AM, Liu X, Judd RT, et al. Environmental and Genetic Activation of Hypothalamic BDNF Modulates T-cell Immunity to Exert an Anticancer Phenotype. Cancer Immunol Res. 2016;4(6):488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clinical and experimental immunology. 2010;162(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slater AM, Cao L. A Protocol for Housing Mice in an Enriched Environment. J Vis Exp. 2015(100):e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG3555) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014(86). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nature reviews Immunology. 2008;8(10):788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–4. [DOI] [PubMed] [Google Scholar]
- 15.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5(4):418–25. [DOI] [PubMed] [Google Scholar]
- 16.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–60. [DOI] [PubMed] [Google Scholar]
- 17.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–7. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15(4):447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhya VK, Raju R, Verma R, Advani J, Sharma R, Radhakrishnan A, et al. A network map of BDNF/TRKB and BDNF/p75NTR signaling system. J Cell Commun Signal. 2013;7(4):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittelstadt PR, Monteiro JP, Ashwell JD. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest. 2012;122(7):2384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraml J, Kolinska J, Sinkora J, Zakostelecka M, Kadlecova L, Hirsova D, et al. Glucocorticoid agonistic and antagonistic effects of mifepristone and onapristone on thymocyte subset composition and CD26/dipeptidyl peptidase IV activity in infant male rats. J Steroid Biochem Mol Biol. 2003;87(1):85–96. [DOI] [PubMed] [Google Scholar]
- 22.Goverman J Autoimmune T cell responses in the central nervous system. Nature reviews Immunology. 2009;9(6):393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Domingues HS, Mues M, Lassmann H, Wekerle H, Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5(11):e15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. Journal of immunology (Baltimore, Md : 1950). 2005;175(5):302532. [DOI] [PubMed] [Google Scholar]
- 25.Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, et al. Myelin basic proteinspecific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. The Journal of experimental medicine. 1997;186(2):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol. 2012;42(5):1073–9. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Gelyana E, Rajsombath M, Yang T, Li S, Selkoe D. Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid beta-Protein Oligomers. J Neurosci. 2016;36(35):9041–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–45. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28(6):517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garofalo S, D’Alessandro G, Chece G, Brau F, Maggi L, Rosa A, et al. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight JM, Lyness JM, Sahler OJ, Liesveld JL, Moynihan JA. Psychosocial factors and hematopoietic stem cell transplantation: potential biobehavioral pathways. Psychoneuroendocrinology. 2013;38(11):2383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurfein BT, Davidenko O, Premenko-Lanier M, Milush JM, Acree M, Dallman MF, et al. Environmental enrichment alters splenic immune cell composition and enhances secondary influenza vaccine responses in mice. Mol Med. 2014;20:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heesen C, Gold SM, Huitinga I, Reul JM. Stress and hypothalamic-pituitary-adrenal axis function in experimental autoimmune encephalomyelitis and multiple sclerosis - a review. Psychoneuroendocrinology. 2007;32(6):604–18. [DOI] [PubMed] [Google Scholar]
- 35.Magalon K, Cantarella C, Monti G, Cayre M, Durbec P. Enriched environment promotes adult neural progenitor cell mobilization in mouse demyelination models. Eur J Neurosci. 2007;25(3):761–71. [DOI] [PubMed] [Google Scholar]
- 36.Banuelos J, Lu NZ. A gradient of glucocorticoid sensitivity among helper T cell cytokines. Cytokine Growth Factor Rev. 2016;31:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattazzi L, Piras G, Brod S, Smith K, Ono M, D’Acquisto F. Impact of Enriched Environment on Murine T Cell Differentiation and Gene Expression Profile. Front Immunol. 2016;7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–75. [DOI] [PubMed] [Google Scholar]
- 39.Shanley DP, Aw D, Manley NR, Palmer DB. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30(7):374–81. [DOI] [PubMed] [Google Scholar]
- 40.Prelog M Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5(2):136–9. [DOI] [PubMed] [Google Scholar]
- 41.Gui J, Mustachio LM, Su DM, Craig RW. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012;3(3):28090. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen JH, Tarry-Adkins JL, Heppolette CA, Palmer DB, Ozanne SE. Early-life nutrition influences thymic growth in male mice that may be related to the regulation of longevity. Clin Sci (Lond). 2010;118(6):429–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.