Abstract
Background & Aims:
We performed an integrated analysis to identify microRNAs (miRNAs) and mRNAs with altered expression in liver tumors from 3 mouse models of hepatocellular carcinoma (HCC) and human tumor tissues.
Methods:
We analyzed miRNA and mRNA expression profiles of liver tissues from mice with diethylnitrosamine-induced hepatocarcinogenesis, conditional expression of lymphotoxin alpha and lymphotoxin beta , or inducible expression of a Myc transgene (Tet-O-Myc mice), as well as male C57BL/6 mice (controls). miRNA mimics were expressed and miRNAs and mRNAs were knocked down in human (Huh7, Hep3B, JHH2) hepatoma cell lines; cells were analyzed for viability, proliferation, apoptosis, migration, and invasion. Cells were grown as xenograft tumors in nude mice and analyzed. We combined in-silico target gene prediction with mRNA profiles from all 3 mouse models. We quantified miRNA levels in 146 fresh-frozen tissues from patients (125 HCCs, 17 matched non-tumor tissues, and 4 liver samples from patients without cancer) and published human data sets and tested correlations with patient survival times using Kaplan-Meier curves and the log-rank test. Levels of NUSAP1 mRNA were quantified in 237 HCCs and 5 non-tumor liver samples using the Taqman assay.
Results:
Levels of the microRNA 193a-5p (MIR193A-5p) were reduced in liver tumors from all 3 mouse tumor models and in human HCC samples, compared with non-tumor liver tissues. Expression of a MIR193A-5p mimic in hepatoma cells reduced proliferation, survival, migration, and invasion and their growth as xenograft tumors in nude mice. We found nucleolar and spindle associated protein 1 (NUSAP1) to be a target of MIR193A-5p; HCC cells and tissues with low levels of MIR193A-5p had increased expression of NUSAP1.Increased levels of NUSAP1 in HCC samples correlated with shorter survival times of patients. Knockdown of NUSAP1 in Huh7 cells reduced proliferation, survival, migration, and growth as xenograft tumors in nude mice. Hydrodynamic tail-vein injections of a small hairpin RNA against NUSAP1 reduced growth of AKT1-MYC–induced tumors in mice.
Conclusions:
MIR193A-5p appears to prevent liver tumorigenesis by reducing levels of NUSAP1. Levels of MIR193A-5p are reduced in mouse and human HCC cells and tissues, leading to increased levels of NUSAP1, associated with shorter survival times of patients. Integrated analyses of miRNAs and mRNAs in tumors from mouse models can lead to identification of therapeutic targets in humans.
Keywords: Liver cancer, systems biology, translation, gene regulation
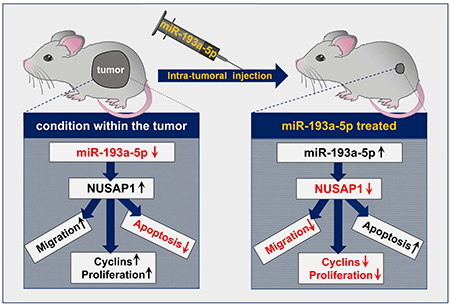
Graphical Abstract
Schematic diagram on the role of miR-193a-5p in reducing liver cancer formation after intra-tumoral injection. ↑ indicates upregulation; ↓ indicates downregulation.
Introduction
Beyond conventional transcriptomics, it became recently evident that micro-RNAs (miRNAs) are frequently dysregulated in human HCC and can act as oncogenes and tumor suppressors.1 However, the identification of novel molecular pathways and specifically miRNA-dependent networks in hepatocarcinogenesis based on ‘omics’ analyses on human tumor material can be hampered by the strong heterogeneity of human HCC. Further potential confounding factors in human HCC samples include gender, age, genetic factors, obesity, alcohol consumption, exposure to carcinogens, intra- and inter-tumor heterogeneity as well as variations in the handling of patients’ material.2 Studying mouse models of HCC development offers the chance to overcome some of these confounding factors by standardizing experimental conditions.3 However, the rate of successful translation from animal model to clinical trials is still very low in cancer.4 In contrast to many other cancers, many different models with fundamentally differing pathogenic traits are used in the context of HCC.3 Some of these models display pathogenic similarities to the human situation [e.g. HBx transgenics 5 or NASH cancer models 6], while other models such as the most abundantly used chemical model of Diethylnitrosamine (DEN) injections 7 do not share the main pathogenic routes with human liver cancer. At present, it is unclear which mouse HCC model is best suited to study molecular mechanisms of significant relevance in human hepatocarcinogenesis.
Here, we combined array based analyses of miRNA and mRNA expression in three prototypic, pathogenically distinct mouse HCC models (1. Diethylnitrosamine as a model of genotoxic hepatocarcinogenesis; 2. transgenic over-expression of lymphotoxin alpha and lymphotoxin beta as an inflammatory HCC model; 3. Tet-O-Myc transgenic mice as an oncogene-driven liver tumor model). We identified a previously unrecognized miR-193a-5p/NUSAP1 dependent pathway which represents a new therapeutic target in human HCC.
Materials and Methods
Genetic mouse liver tumor models
Diethylnitrosamine (DEN) driven liver tumors,8 lymphotoxin alpha and lymphotoxin beta (AlbLTα/β) driven tumors 9 and Myc-driven liver tumors (Tet-O-Myc) 10 were generated as described previously on a C57BL/6 background. In brief, for generation of DEN-driven tumors, male mice were injected intraperitoneally with DEN (Sigma) at a dose of 10 mg per kg body weight at 15 d of age 11 and sacrificed at 9 months of age. For AlbLTα/β driven tumors, tg1223 mice expressing LT-α and -β in a liver-specific manner (control of Albumin promoter) at high level were followed for 12 months for tumor development. 12 For Mycdriven liver tumors (Tet-O-Myc), TRE-MYC mice were crossed to LAP-tTA (liver-specific promoter) mice.13 Animals were maintained on doxycycline (200 mg/kg doxy chow) to suppress MYC expression until 8 weeks of age. Doxycycline was then removed, and mice were followed for evidence of tumor formation.13 In all models, livers were macroscopically dissected and tumor material, non-tumorous liver tissue as well as liver tissue from untreated, sex- and age-matched control mice were immediately snap frozen, followed by histopathological confirmation of the tumor tissue. All animal experiments were performed in accordance to the respective national, federal, and institutional regulations.9, 11, 13
Human patients’ miRNA and mRNA analysis
A total of 146 fresh-frozen tissue samples, including 125 HCCs, 17 non-tumor liver tissues and 4 normal liver tissue samples, were used to analyze miR-193a-5p expression levels by TaqMan® Low Density Array A Human MicroRNA v2.0 (Thermo Fisher Scientific, Carlsbad, California, U.S.). Clinical characteristics of HCC patients for miR-193a-5p expression are embedded in Supplementary Table 1. Total RNA and miRNA extraction was performed using TRIzol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA) according to the manufacturer’s instructions. MiRNAs were quantified by NanoDrop ND-1000 spectrophotometer and the quality was assessed by agarose gel electrophoresis. 600 ng of total RNA were reverse transcribed using Megaplex™ RT Primers Human Pool A (Thermo Fisher Scientific, Carlsbad, California, U.S.) according to manufacturer’s protocol. The array containing the cDNA was centrifuged and then run on ABI-Prism 7900HT system (Thermo Fisher Scientific, Carlsbad, CA).
NUSAP1 mRNA levels were assessed in a large cohort of 237 HCCs and 5 normal liver tissue samples in a previously published series14 using Fluidigm 96.96 Dynamic Arrays and a specific TaqMan predesigned assay (Hs01006195_m1; Life Technologies, Carlsbad, CA). Clinical characteristics of HCC patients for NUSAP1 expression in Affymetrix Array (n = 57) and Fluidigm Array (n = 237) are embedded in Supplementary Table 2 and 3, respectively. Changes in miRNA/mRNA expression levels were determined using a comparative CT method. All patients gave informed consent according to French law and the Paris Saint-Louis Institutional Review Board committee approved this study (Paris Saint-Louis, 2004; INSERM IRB 2010; the French Liver Biobanks Network, AFAQ NF S96–900; and Hepatobio Bank). Low- and high- expression groups from 237 HCC cohorts were defined by the median of NUSAP1 expression levels of the total number of analyzed samples.
Statistical analysis
Data were analyzed using PRISM software (GraphPad Software, Inc., La Jolla, CA) and are expressed as mean. Differences between groups were assessed by an unpaired twosample t-test or Mann-Whitney test and multiple comparisons between more than two groups have been conducted by one-way ANOVA with Newman-Keuls post-hoc analysis. Univariate survival analysis was performed using Kaplan-Meier curve and log-rank test.
Results
Model-independent downregulation of miR-193a-5p in mouse and human liver cancer
We applied three prototypic models of liver tumor formation in mice that depend on distinct pathogenic stimuli: a widely used model of chemical hepatocarcinogenesis induced by single injection of diethylnitrosamine (DEN),8 a model of chronic inflammation-induced hepatocarcinogenesis relying on transgenic liver-specific overexpression of the cytokines lymphotoxin alpha and lymphotoxin beta (AlbLTα/β) 12 and an oncogenic tumor model relying on c-Myc overexpression (Tet-O-Myc) 13 (Supplementary Figure 1A). Whole RNA was isolated from tumors and control tissue (untreated or transgene negative [AlbLTα/β and Tet-O-Myc] matched liver samples) (n = 3–6 for each group) and was subjected to Taqmanbased miRNA microarray analysis, each array comprising 750 miRNAs (Figure 1A). Application of a fold change and significance threshold filter (ddCt = ± 0.90, P<0.05) led to the identification of miRNAs that were up- and downregulated in tumor tissue compared with the respective control tissue in each distinct model (downregulated: DEN: 26, AlbLTα/β: 20, Tet-O-Myc: 50; upregulated: DEN: 7, AlbLTα/β: 11, Tet-O-Myc: 1) (Figure 1A; Supplementary Table 4). Unsupervised hierarchical clustering confirmed a proper segregation of miRNA patterns between tumors and control samples in each distinct tumor model (Supplementary Figure 1B).
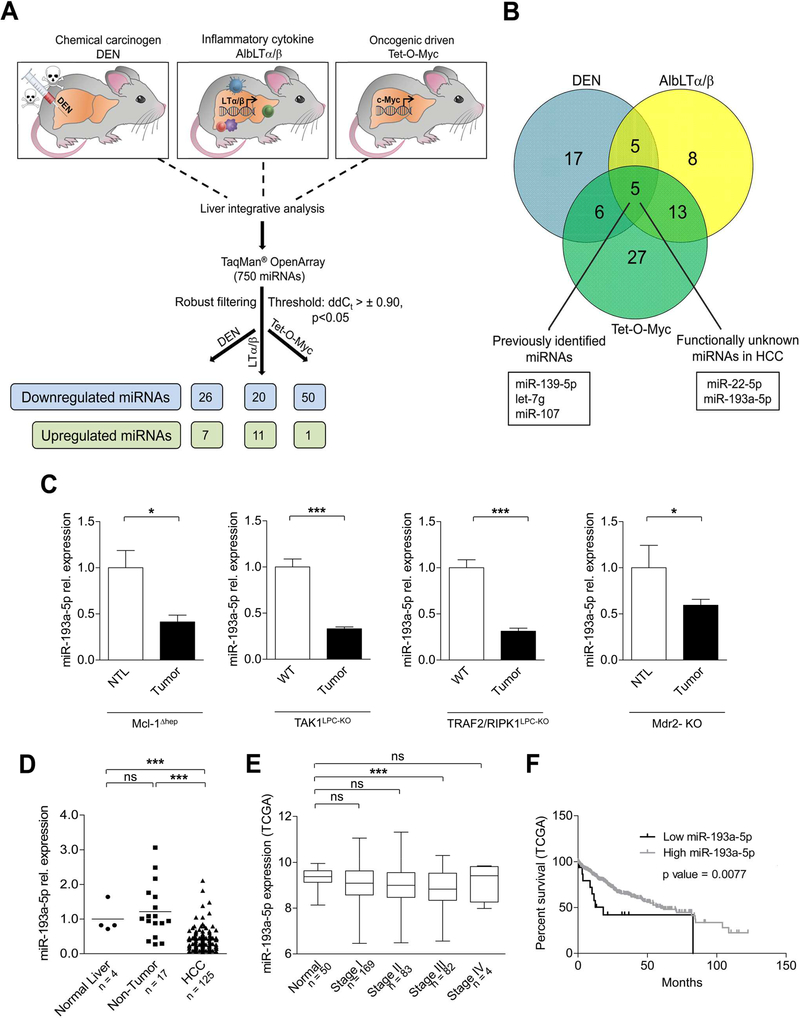
Figure 1.
MicroRNA-193a-5p is downregulated in hepatocellular carcinoma in mice and human. (A) Schematic diagram showing the experimental set-up of 750 miRNA array in three experimental HCC mouse models DEN / Untreated (n = 6 per group), AlbLTα/β / Untreated (n = 3 per group) and Tet-O-Myc/Untreated (n = 4 per group). (B) Venn diagram representing the principle of selecting functionally unknown miR-193a-5p. (C) qRT-PCR analysis of miR-193a-5p in four different genetic HCC mouse tumor models: Mcl-1Δhep (n = 5), TAK1-KO (n = 11), TRAF2-RIPK1-KO (n = 7), Mdr2-KO (n = 7) compared to either nontumoral liver (NTL) or Wild type (WT) mice. (D) qRT-PCR of miR-193a-5p expression in normal liver (n = 4), non-tumor liver (n = 17) and HCC tissues (n = 125) obtained from patients. (E) miR-193a-5p log2 expression in HCC patients with different TNM stages: Stage I (n = 169), Stage II (n = 83), Stage III (n = 82), Stage IV (n = 4) and normal liver (n = 50) obtained from TCGA HCC datasets. (F) Kaplan-Meier curves for the overall survival of HCC patients (n = 372) plotted against time (months) based on miR-193a-5p expression levels (obtained from the TCGA HCC datasets). Results are represented as mean ± SEM. ns nonsignificant, * p<0.05, *** p<0.001 by 1-way ANOVA with Newman-Keuls post-hoc test (D and E); 2- tailed, unpaired t test (C); Log-Rank with Mantel-Cox test (F).
We next tested which of these miRNAs showed a common intra-tumoral regulatory pattern between all the three models. As depicted in Figure 1B, only five miRNAs shared a common intra-tumoral regulation between all three tumor models and were all downregulated compared to control tissue. Of these, miR-139–5p, let-7g and miR-107 were previously shown to be downregulated and functionally relevant in human HCC,15–17 strongly supporting the translational potential of our present comparative experimental approach. In addition, two miRNAs – miR-193*/miR-193a-5p and miR-22*/ miR-22–5p were previously not known for a specific function in human HCC and were not found within the “top” regulated miRNAs in each single model (Supplementary Table 5), prompting us to further study the functional role of one of these latter miRNAs, miR-193a-5p, in hepatocarcinogenesis. We therefore analyzed expression of miR-193a-5p, miR-107, miR-139–5p, miR-22–5p and let-7g in liver tumors, non-tumoral liver tissues (NTL) and untreated control tissue in all three models that we initially used for our array analysis by qRT-PCR and confirmed a significant intra-tumoral downregulation (Supplementary Figure 2). We also confirmed intra-tumoral downregulation of miR-193a-5p in four additional well-established genetic mouse HCC models [Mcl-1Δhep,18 TAK1LPC-KO mice,19, 20 TRAF2/RIPK1LPC-KO mice 21 and Mdr2-KO mice 8 ] (Figure 1C).
We next examined the regulation of miR-193a-5p in several mouse (Hepa 1–6, Hepa1c1c7) and human (Huh7, HepG2) hepatoma cell lines and compared it to mouse and human liver tissue and primary mouse hepatocytes (Supplementary Figure 3A). Similarly to the mouse HCC models, miR-193a-5p was downregulated in mouse und human hepatoma cells. We further tested how these findings in mouse tumors corresponded to the regulation in the human situation and measured miR-193a-5p in HCC tissues in a well-defined cohort of human HCC patients (n = 125), corresponding surrounding, non-tumorous liver tissues (n = 17) and normal liver tissue (n = 4) (Supplementary Table 1). Consistent with our findings in mice, miR-193a-5p was downregulated in human HCC tissues, whereas no changes in nontumor-tissues were found when compared to normal human liver (Figure 1D). This respective downregulation was independent of the underlying etiology of liver disease (Supplementary Figure 3B). We confirmed downregulation of miR-193a-5p (not miR-193a-3p) in an analysis of HCC patients from The Cancer Genome Atlas (TCGA) data set (Supplementary Figure 3C). In these patients miR-193a-5p was significantly downregulated in advanced HCC (stage T3) when compared to normal liver and earlier stages (Figure 1E). In line, patients with lower expression of miR-193–5p had a shorter survival time than those with high expression (Figure 1F). Taken together, our comparative miRNA analysis between prototypic mouse HCC models of distinct pathogenesis could identify miR-193a-5p as a previously unrecognized miRNA that is specifically downregulated in human HCC.
Inhibition of HCC cell proliferation by miR-193a-5p
The model-independent and conserved regulation of miR-193a-5p in HCC suggested that it might have a functional effect on basic biological properties of liver tumor cells. Since sustained cell growth is a distinctive hallmark of cancer,22 we first investigated the effect of miR-193a-5p on cellular proliferation. Of note, transfection of miR-193a-5p mimic into Huh7 HCC cells significantly reduced cell viability compared with control siRNA transfected cells (Figure 2A). miR-193a-5p overexpression also inhibited cellular proliferation assessed by immunostaining for the proliferation marker Ki67 (Figure 2B). We also performed a colony formation assay and observed that Huh7 cells transfected with miR-193a-5p mimic formed significantly fewer colonies than control-transfected cells (Figure 2C). In contrast, transfection with anti-miR-193a-5p enhanced the number of colonies when compared with control cells (Figure 2D).
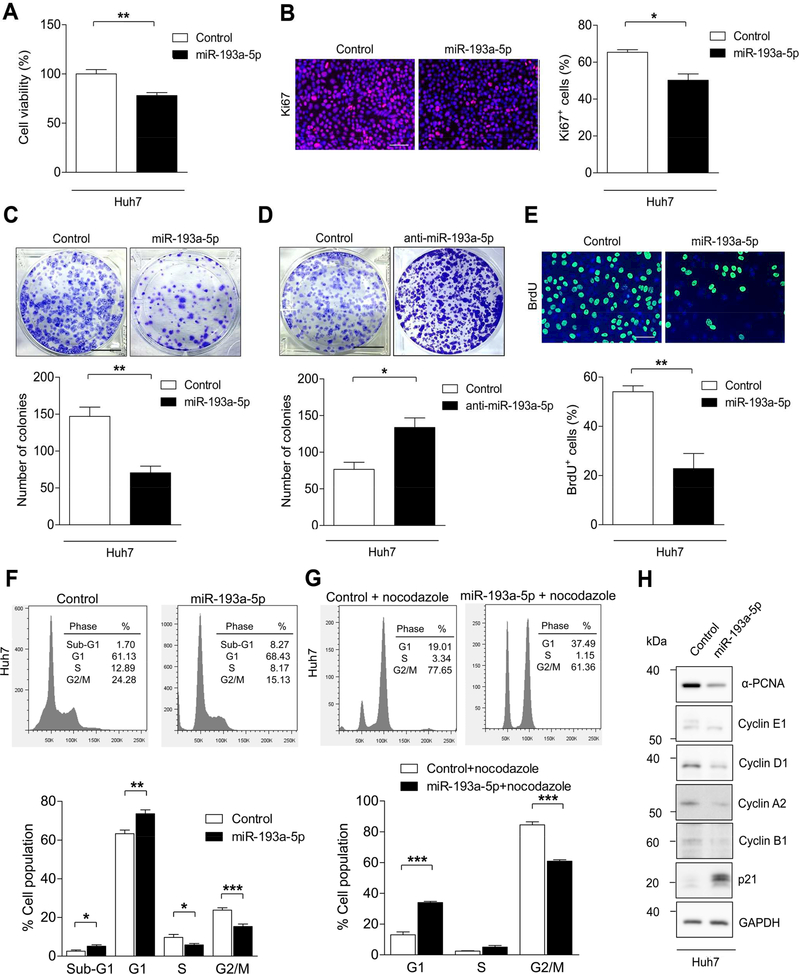
Figure 2.
Overexpression of miR-193a-5p suppresses the progression of cell cycle in vitro. (A) Huh7 cells were transfected with miR-193a-5p mimic and Control siRNA and cell viability was analyzed by CCK-8 assay (n = 4 per group). (B) Representative immunofluorescence images and quantification of Ki67 staining of Huh7 cells transfected with miR-193a-5p mimic or Control siRNA (n = 3 per group) (bar = 50 μm). (C) Colony formation assay was performed after transfection of Huh7 cells with miR-193a-5p mimic or Control siRNA (n = 4 per group) (bar = 1 cm). (D) Colony formation assay was performed after transfection of Huh7 cells with miR-193a-5p Antagomir and Control (miScript Inhibitor Negative Control) (n = 4 per group) (bar = 1 cm). (E) Representative images and quantification of BrdU+ Huh7 cells transfected with miR-193a-5p mimic or Control siRNA (n = 3 per group). (F) Huh7 cells were transfected with miR-193a-5p for 72 h and analyzed with flow cytometry (FACS) by DAPI staining (n = 6 per group). The percentage of cells in sub-G1, G1, S and G2/M phases of the cell cycle are indicated. (G) Huh7 cells were transfected with miR-193a-5p and treated with nocodazole (100 ng/ml) for 24 h before addition of DAPI and analyzed by FACS (n = 6 per group). The percentage of cells in the G1, S and G2/M phases of the cell cycle are indicated. (H) Western Blot showing the protein expression of the indicated proteins in Huh7 cells transfected with either miR-193a-5p mimic or Control siRNA (n = 3 per group). Results are represented as mean ± SEM. * p<0.05, ** p<0.01, *** p<0.001 by 2- tailed, unpaired t test.
To test the effects of miR-193a-5p on cell cycle progression, we first performed BrdU staining (a marker of S-Phase entry), which showed that miR-193a-5p mimic transfection caused a significant decrease in BrdU labelling of Huh7 cells as compared with control transfected cells (Figure 2E). We next performed flow cytometry analysis, which revealed that a significantly higher number of Huh7 cells transfected with miR-193a-5p mimic were in G1 phase of the cell cycle compared with control transfected cells (Figure 2F). Synchronization to G2/M phase by nocodazole treatment confirmed that significantly more miR-193a-5p transfected cells were arrested in G1 phase than control-transfected cells (Figure 2G). This went along with decreased expression of cyclins associated with S-Phase (Cyclin E1, Cyclin D1) as well as G2/M phase (Cyclin B1 and Cyclin A2), and upregulation of the cell cycle inhibitor p21 (Figure 2H). Importantly, these data gained in Huh7 hepatoma cells could be confirmed in Hep3B cells (low endogenous miR-193a-5p expression) as well as JHH2 cells (high endogenous miR-193a-5p expression) (Supplementary Figure 4A-F). Collectively, these data show that miR-193a-5p inhibits HCC cell proliferation and colony formation by inhibiting cell cycle progression.
Apoptosis induction and reduced cell migration and invasion through miR-193a-5p expression
Because evasion from cell death represents another hallmark characteristic of tumor cells,22 we next tested if miR-193a-5p mediated G1-arrest and p21 overexpression in hepatoma cells were linked with cell death. Indeed, miR-193a-5p overexpression in Huh7 cells was associated with an increase in TUNEL+ cells and increased cleavage of the apoptosis mediator Caspase-3 (Figure 3A,). Correspondingly, miR-193a-5p expression induced an increase in PI+/Annexin V+ cells in FACS analysis (Figure 3B), providing further evidence that cell cycle arrest of tumor cells induced by miR-193a-5p was associated with apoptotic cell death.
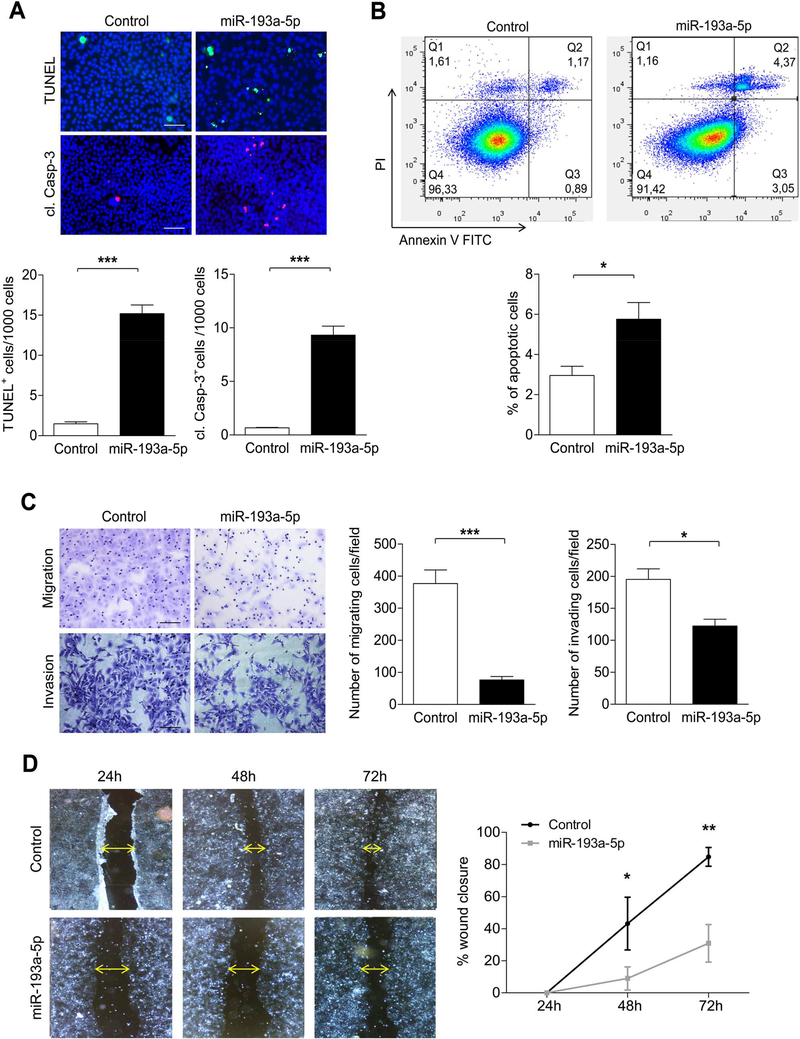
Figure 3.
Overexpression of miR-193a-5p increases apoptosis in vitro. (A) Cell death (TUNEL staining (upper row); cl. Casp-3 staining (lower row)) was analyzed in Huh7 cells transfected with miR-193a-5p and Control siRNA for 72h. (bar = 50 μm). (B) Apoptosis of Huh7 cells transfected with miR-193a-5p was determined by Annexin V/Propidium iodide staining and flow cytometry (n = 3 per group). (C) Transwell migration assay (upper row) or Transwell coated with Matrigel invasion (lower row) assay for Huh7 cells was determined after transfection with miR-193a-5p mimic or Control siRNA for 72 h (n = 3 per group) (bar = 50 μm). (D) Wound-healing assay was performed on Huh7 cells transfected with miR-193a5p mimic and wound closure was monitored over the indicated time post-transfection after treatment with 10 ¼g/ml Mitomycin C for 2 h (n = 3 per group) (bar = 200 μm). Results are represented as mean ± SEM. ns non-significant, * p<0.05, ** p<0.01, *** p<0.001 by 2- tailed, unpaired t test.
Based on the fact that miR-193a-5p was strongly downregulated in more advanced HCC (Figure 1E), we wondered if its functional effects on cell cycle arrest and cell death were associated with alterations in migration and invasion of tumor cells, biological features important for the systemic spreading of tumor cells. In line with this hypothesis, overexpression of miR-193a-5p in HCC cells significantly suppressed their migration and invasion capabilities when compared to the control cells (Figure 3C). To further confirm these findings, we performed a wound healing assay which revealed that the migratory capacity of Huh7 and Hep3B cells transfected with miR-193a-5p mimic or JHH2 cells transfected with miR-193a-5p antagomir were significantly perturbed compared to the control transfected cells (Figure 3D and Supplementary Figure 4G). Collectively, our data show that – in addition to cell cycle arrest and apoptosis induction – miR-193a-5p overexpression inhibits migration and invasion of tumor cells, suggesting that loss of miR-193a-5p increases the malignant potential of liver cancer cells by promoting their proliferation, survival and pro-metastatic features.
NUSAP1 is a direct target of miR-193a-5p and is upregulated in mouse and human HCC
To elucidate the underlying mechanism of the suppressive effects of miR-193a-5p on cell proliferation, cell death and migration, we aimed at identifying a miR-193a-5p-dependent target gene that mediated these respective effects. For this, we first performed an additional mRNA array profiling on the previously described liver tumor and control tissues from DEN treated, AlbLTα/β and Tet-O-Myc overexpressing mice. The numerous genes that were upregulated in this analysis (with the threshold filter, fold change >2, p<0.01) were merged with the predicted target genes of miR-193a-5p obtained from the “miRWalk” algorithm 23 (Supplementary Table 6). Based on the hypothesis of a common regulation of a potential target gene between all three models, we identified four genes as potential targets of miR-193a-5p: Nusap1, Plat, Serpine1 and Syt12 (Figure 4A). We tested their expression in a cohort of 57 human HCC and 5 control liver samples (Supplementary Table 2) and found that only NUSAP1 - a microtubule-associated protein (MAP) controlling cellular proliferation by governing spindle assembly, chromosome segregation and cytokinesis 24 – was significantly upregulated in human HCC (Figure 4B; Supplementary Figure 5A). We confirmed NUSAP1 overexpression in a large dataset of 237 HCC patients (Supplementary Table 3) obtained from the Fluidigm array 14 (Supplementary Figure 5B) and also could show that its overexpression was etiology-independent (Supplementary Figure 5C) and represented an independent negative prognostic factor in this cohort of patients (Supplementary Table 7). Strikingly, patients with higher NUSAP1 expression showed a significantly worse overall survival than patients with lower NUSAP1 expression (Figure 4C). Finally, we confirmed upregulation of NUSAP1 in HCC patients from the TCGA data set and – in concordance with our miR-193a-5p data – could show that high NUSAP1 expression was associated with a shorter survival time (Figure 4D and Supplementary Figure 5D).
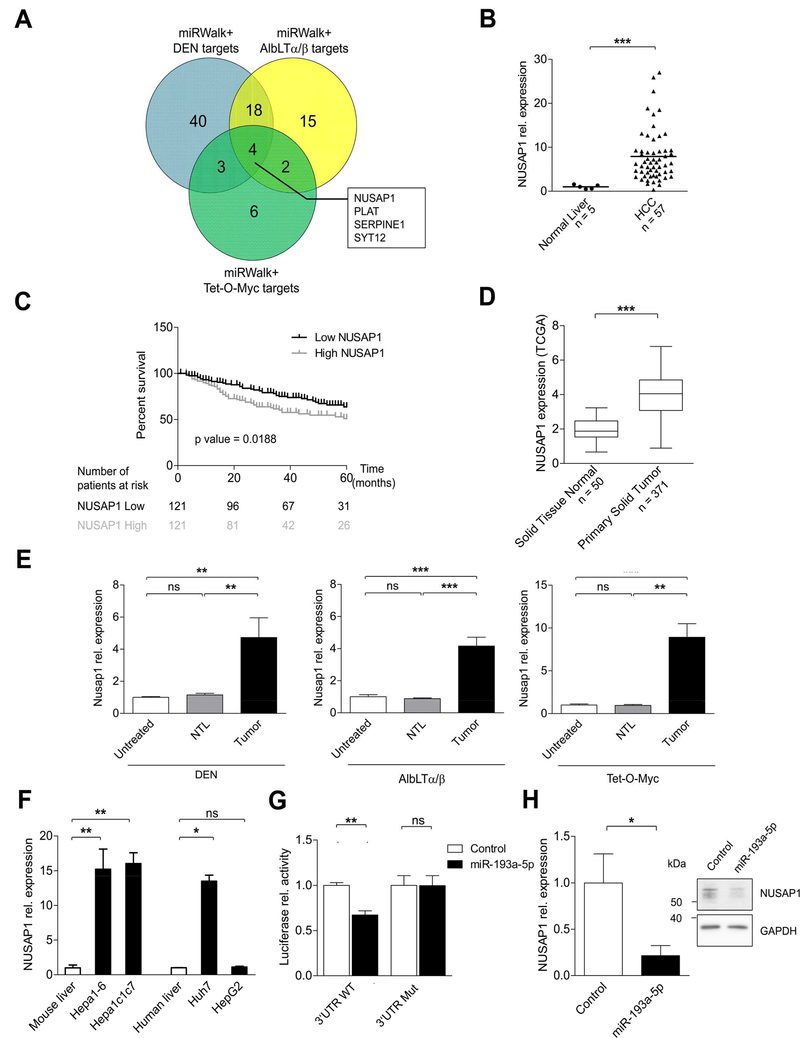
Figure 4.
NUSAP1, a direct target of miR-193a-5p, is upregulated in mouse and human HCC tissues. (A) Venn diagram showing the overlap between predicted targets of miR-193a-5p and genes (threshold: fold change > 2, p value < 0.01) obtained from mRNA-array of DEN, AlbLTα/β and Tet-O-Myc mouse HCC models. (B) Relative expression of NUSAP1 in human HCC (n = 57) compared to normal liver (n = 5) from Affymetrix array. (C) Kaplan-Meier curves for the overall survival of HCC patients (n = 242) plotted against time (months) based on NUSAP1 expression levels. (D) Expression levels of NUSAP1 in 371 HCC patients and solid normal tissue (n = 50) are shown (primary data obtained from TCGALIHC datasets). (E) Relative expression of Nusap1 was measured in non-tumoral liver (NTL) and HCC tumors of mice from DEN, AlbLTα/β and Tet-O-Myc in comparison with untreated mice. (F) Relative expression of NUSAP1 in mouse (Hepa1–6 and Hepa1c1c7) and human (Huh7 and HepG2) HCC cell lines was quantified by qRT-PCR (n = 3 per group). (G) Relative luciferase activity was quantified in Huh7 cells co-transfected with reporter constructs containing either WT or Mutant NUSAP1 together with miR-193a-5p mimic or control (n = 3 per group). (H) Relative expression of NUSAP1 in Huh7 cells transfected with miR-193a-5p mimic was quantified by qRT-PCR and Western Blot (n = 3 per group). Results are represented as mean ± SEM. ns non-significant, * p<0.05, ** p<0.01, *** p<0.001 by 2- tailed, unpaired t test (B, D, G and H); Log-Rank with Mantel-Cox test (C); 1-way ANOVA with Newman-Keuls post-hoc test (E and F).
Next, we confirmed that – similarly to miR-193a-5p downregulation – NUSAP1 mRNA was upregulated strictly in the tumor tissue but not in the non-tumoral liver tissue (NTL) of DEN, AlbLTα/β and Tet-O-Myc HCC (Figure 4E). High expression of NUSAP1 was observed in both mouse (Hepa1–6 and Hepa1c1c7) and one human HCC cell line (Huh7) (Figure 4F), but not in HepG2 HCC cells. Finally, to confirm the direct binding of miR-193a-5p to NUSAP1, we cloned the WT NUSAP1 3’UTR as well as a mutated NUSAP1 3’UTR (Supplementary Figure 5E) downstream of a luciferase reporter. Upon co-transfection of these constructs together with miR-193–5p into Huh7 and Hepa 1–6 cells, miR-193a-5p reduced the luciferase activity of the WT but not the mutated constructs (Figure 4G and Supplementary Figure 5F). Moreover, we confirmed reduced mRNA and protein expression of NUSAP1 upon overexpression of miR-193a-5p in Huh7 cells (Figure 4H). Taken together, our combinatory systematic approach of conventional in silico target gene prediction and a systematic mRNA profiling in three prototypic mouse tumor models revealed that NUSAP1 expression is controlled by miR-193a-5p in HCC cells and human HCC and that high NUSAP1 levels were associated with a shorter survival time of human HCC patients.
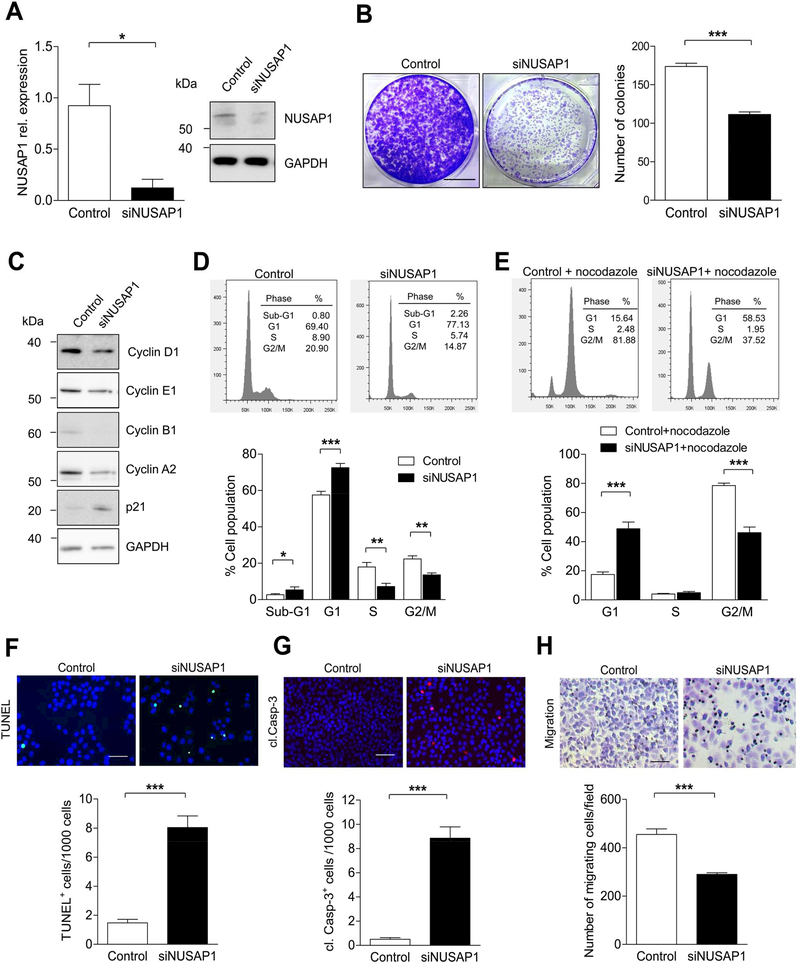
Impaired cellular proliferation, survival and migration upon NUSAP1 silencing
To further assess whether NUSAP1 knockdown could mimic the functional effect of miR-193a-5p overexpression, we silenced NUSAP1 expression in Huh-7 using RNA interference. Knockdown of NUSAP1 by siRNA efficiently inhibited the mRNA and protein expression of NUSAP1 (Figure 5A). As shown in Supplementary Figure 6A, NUSAP1 knockdown led to a significant suppression of cell viability compared with control cells. In line, a colony formation assay demonstrated a significant reduction in numbers of colonies in NUSAP1 knockdown cells compared with control cells (Figure 5B). Similar to miR-193a-5p overexpression, knockdown of NUSAP1 also led to reduced expression of Cyclin A2 and Cyclin B1 (Figure 5C and Supplementary Figure 6B), cyclins involved in G2/M phase, supporting NUSAP1’s putative function in controlling spindle formation and mitosis. 24, 25 Moreover, we confirmed in hepatocytes that NUSAP1 is expressed during interphase, metaphase/anaphase and telophase of mitosis and that downregulation of NUSAP1 led to aberrant metaphase spindle appearance and inhibition of mitosis as demonstrated by α-Tubulin and p-H3 staining, respectively (Supplementary Figure 6C-E). Interestingly, NUSAP1 knockdown also resulted in increased G1-arrest of cells compared with control (Figure 5D and E), which went along with reduced numbers of S-phase+ cells upon NUSAP1-knockdown as shown by BrdU assay (Supplementary Figure 7A). Again, similar to miR-193a-5p overexpression, cell cycle inhibition upon NUSAP1-knockodown went along with increased numbers in TUNEL+, cleaved caspase-3+ (Figure 5F and G) and PI/Annexin V+ cells (Supplementary Figure 7B) as well as a reduced migration capacity (Figure 5H; Supplementary Figure 7C). It was previously shown that aberrations in G2/M phase can be functionally linked with G1 arrest and apoptosis through alterations in the spindle assembly checkpoint (SAC) 26. In line with this hypothesis, we found components of the SAC machinery transcriptionally dysregulated in cells with NUSAP1 downregulation (Supplementary Figure 7D), suggesting a molecular link between G2/M phase aberrations and G1 arrest/apoptosis in cells with NUSAP1-downregulation. Importantly, these molecular data gained in Huh7 cells could be confirmed in Hep3B cells with high endogenous NUSAP1 expression (Supplementary Figure 8A-H). Moreover, loosening the threshold filter for the transcriptional analysis of putative miR-193a-5p target genes (see Figure 4A) from >2 down to >1.5 increased the number of target genes to 99, of which most genes were related to cell cycle regulation and more specifically to G2/M phase transition and cytokinesis (Supplementary Figure 9A-C). Collectively, our data suggest that NUSAP1 represents a dominant functional mediator of miR-193a-5p, but it probably collaborates with other miR-193a-5p-targets in cell-cycle regulation.
Figure 5.
NUSAP1 knockdown suppresses the cell growth and increases apoptotic cell death in Huh7 cells. (A) qRT-PCR and Western blot analyses of NUSAP1 in Huh7 cells transfected with siRNA against NUSAP1 or Control siRNA (n = 3 per group). (B) Quantification of colonies from Huh7 cells transfected with NUSAP1 or Control siRNA for 72 h (n = 3 per group) (bar = 1 cm). (C) Levels of cell-cycle proteins (Cyclin D1, Cyclin E1, Cyclin B1, Cyclin A2, p21) were detected in cell lysates of NUSAP1 siRNA transfected Huh7 cells by Western blot with GAPDH as a loading control (n = 3 per group). (D) Huh7 cells were transfected with siNUSAP1 for 72 h and analyzed with flow cytometry by DAPI staining (n = 6 per group). The percentage of cells in the Sub-G1, G1, S and G2/M phases of the cell cycle are indicated. (E) Huh7 cells were transfected with siNUSAP1 and treated with nocodazole (100 ng/ml) for 24 h before addition of DAPI and analyzed by FACS (n = 6 per group). The percentage of cells in the G1, S and G2/M phases of the cell cycle are indicated. (F and G) Representative images and quantification of TUNEL (F) and cl. Casp-3 (G) stainings in Huh7 cells transfected with NUSAP1 siRNA for 72 h (n = 3 per group) (bar = 50 μm). (H) Transwell migration assay for Huh7 cells was determined after transfection with NUSAP1 siRNA or Control (n = 3 per group) (bar = 50 μm). Results are represented as mean ± SEM. ns non-significant, * p<0.05, *** p<0.001, by 2- tailed, unpaired t test.
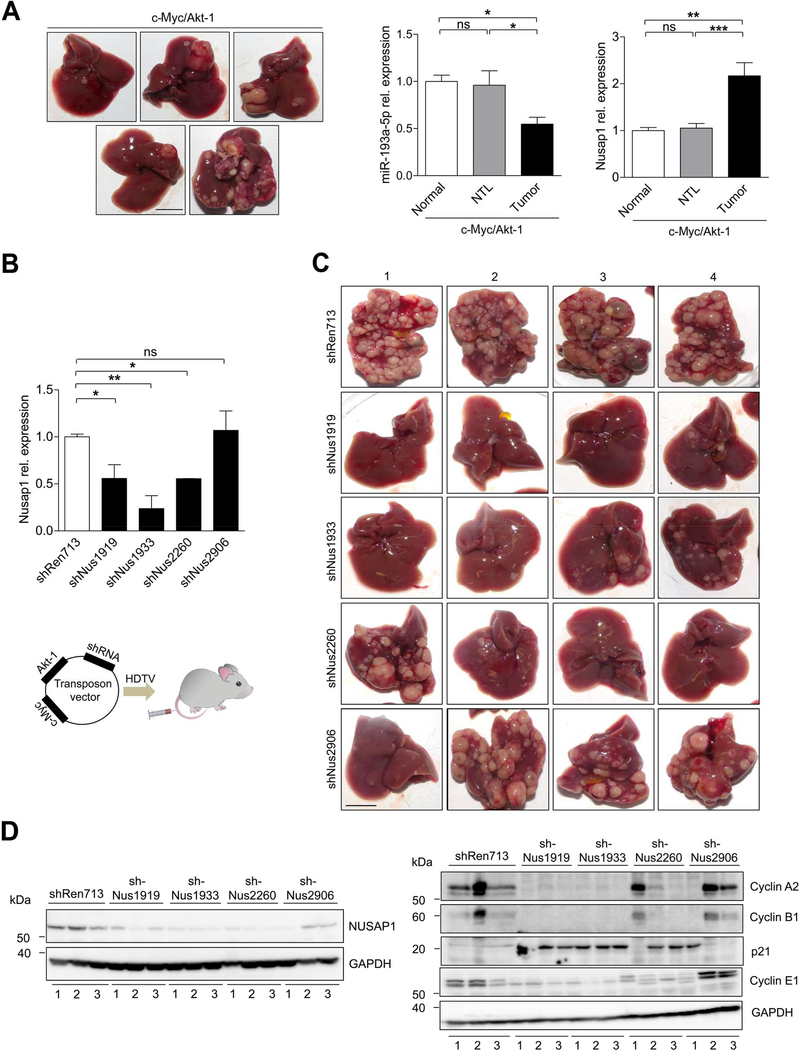
Figure 7.
Knockdown of NUSAP1 limits liver cancer development in a transposon- based endogenous mouse HCC model (A) Representative images of mouse livers that developed c-myc/Akt-1 driven liver cancer 8 weeks after hydrodynamic injection (n = 5) (bar = 1 cm). qRT-PCR showing the relative expression of miR-193a-5p and NUSAP1 in normal livers, non-tumoral liver (NTL) and liver tumors isolated from c-Myc/Akt-1 injected mice. (B) Top panel: The efficiency of each shRNA used for HDTV experiments was determined by qRT-PCR in retroviral transfected liver cancer cells isolated from c-Myc/Akt-1 driven tumors of p19−/− mice. Lower panel: Schematic diagram illustrating the HDTV injection vector containing either shREN713 (Control) or the varying sequences of shNUSAP1 along with cMyc and Akt-1. (C) Representative images of tumor burden 7 weeks after delivery of transposon vectors containing either shREN713 as a control or the respective sequences bearing shNUSAP1 (shNUS1919, shNUS1933, shNUS2260, shNUS2906) (n = 4 per group) (bar = 1 cm). (D) Western Blot of NUSAP1 and cyclins (CyclinE1, CyclinA2, CyclinB1 and p21) with protein extracts from c-Myc/Akt-1/shRNA HDTV-injected livers (n = 3 per group) corresponding to the respective liver number (1–3) as indicated in Figure 7C. Results are represented as mean ± SEM. ns: non-significant, * p<0.05, ** p<0.01, *** p<0.001 by 1-way ANOVA with Newman-Keuls post-hoc test (A and B).
To provide further functional evidence that NUSAP1 was an important mediator of miR-193a-5p’s effects on the malignant properties of HCC cells, we wanted to test if silencing NUSAP1 would compensate for the effects of miR-193a-5p downregulation. For this, we transfected anti-miR-193a-5p for 48h followed by NUSAP1 siRNA transfection for 24h. As hypothesized, this resulted in increased viability and proliferation shown by Western Blot, colony assay, Ki67 assay and BrdU assay (Supplementary Figure 10A-E) when compared to si-NUSAP1-only-transfected cells. Similarly, the effect of miR-193a-5p inhibition on cell cycle, migration could be partly reversed by NUSAP1 silencing (Supplementary Figure 10F). Collectively, these data provide further evidence that NUSAP1 represents an important functional mediator of the pro-carcinogenic effects of miR-193a-5p downregulation in mouse and human HCC.
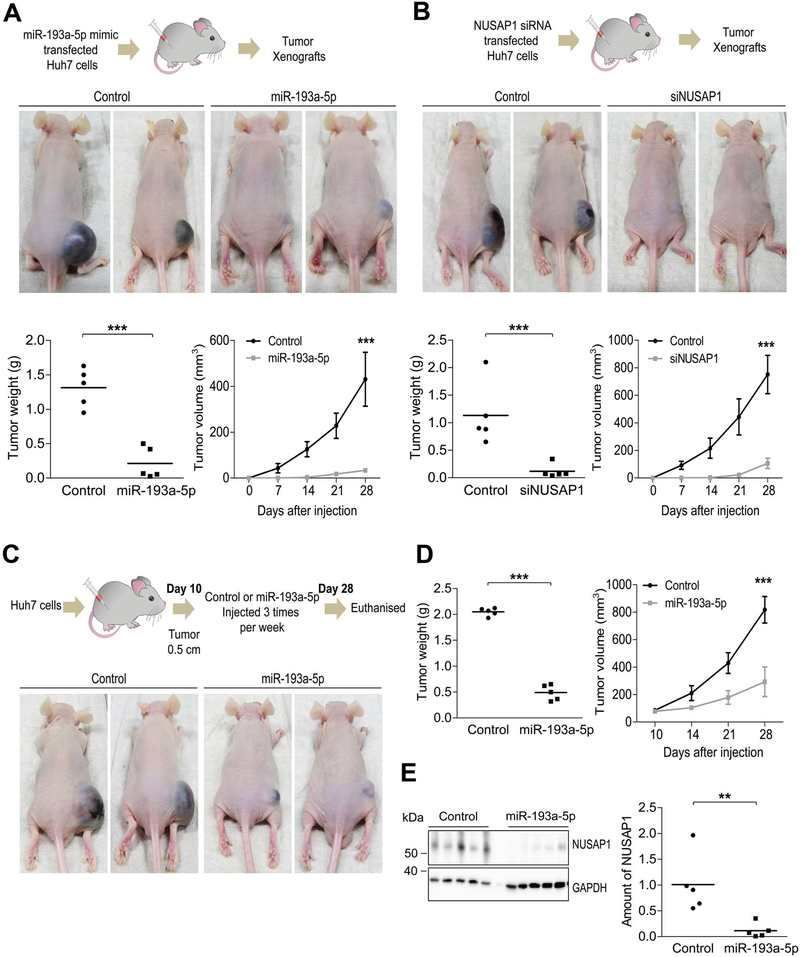
Attenuation of hepatocarcinogenesis upon delivery of miR-193a-5p mimic and NUSAP1 siRNA in vivo
Based on the striking effects of miR-193a-5p and its target NUSAP1 on basic biological features of liver cancer cells, we finally aimed at assessing if modulation of their expression might influence liver tumor growth in a xenograft tumor model in vivo. To test this, we first subcutaneously injected Huh-7 cells and Hepa 1–6 cells transfected with miR-193a-5p mimic or control miRNA into nude mice. Given the anticipated effect of miR-193a-5p transfection on cell viability, equal amounts of living cells overexpressing miR-193a-5p were injected 72 hours after transfection (Figure 6A; Supplementary Figure 11A-C). As shown in Figure 6A and Supplementary Figure 11D and E, a significant decrease in tumor weight and volume were observed 28 days after injection of miR-193a-5p transfected cells compared to controls. In line with these findings, knockdown of NUSAP1 in Huh7 cells using siRNA and subcutaneous injection of equal amounts of living cells into nude mice (Figure 6B; Supplementary Figure 11F-H) also led to a significant decrease in tumor weight and volume (Figure 6B; Supplementary Figure 11I). To assess the therapeutic potential of targeting miR-193a-5p in established HCC, we subcutaneously injected untreated Huh-7 HCC cells into the left flank of nude mice. 10 days after tumor initiation, we performed intra-tumoral injections of miR-193a-5p mimic or control RNA three times per week for 28 days (Figure 6C; Supplementary Figure 12A). Strikingly, miR-193a-5p injections but not control injections successfully inhibited further tumor growth and resulted in a significant decrease in tumor weight at 4 weeks after beginning of intratumoral injections (Figure 6C and D; Supplementary Figure 12B). Moreover, Western blot analysis of tumors on protein extracts confirmed downregulation of NUSAP1-expression in miR-193a-5p-injected tumors compared with controls (Figure 6E).
Figure 6.
Overexpression of miR-193a-5p and knockdown of NUSAP1 reduce tumor growth in vivo. (A) Huh7 cells transfected with either miR-193a-5p mimic or control siRNA were injected subcutaneously to the right flank of nude mice to obtain tumor xenografts. Tumor weight was measured at 28th day after injection when the mice were sacrificed (n = 5 per group). Tumor volume was measured every 7 days. (B) Huh7 cells transfected with either NUSAP1 siRNA or Control siRNA was injected subcutaneously to the right flank of nude mice to obtain tumor xenografts. Tumor weight was measured at 28th day after injection when the mice were sacrificed (n = 5 per group). Tumor volume was measured every 7 days. (C) Huh7 cells were injected when tumor growth reached 0.5 cm, Control siRNA or miR-193a-5p mimic was injected intra-tumorally three times per week for additional 2 weeks. (D) Tumor weight was measured after 28th day of injection when the mice were sacrificed. Tumor volume was also calculated using a digital caliper (n = 5 per group). (E) Amounts of NUSAP1 protein from intra-tumoral parts were measured by Western blot (n = 5 per group). Results are represented as mean ± SEM. ** p<0.01, *** p<0.001 by 2- tailed, unpaired t test.
Finally, we wanted to confirm these findings in an endogenous HCC tumor model. For this, we applied a model of hydrodynamic tail vein (HDTV) injection of oncogenes (c-Myc/Akt-1), because this respective model had previously shown a strong translational potential from mouse to men.18, 27 In c-Myc/Akt-1 driven HCC, NUSAP1 was significantly higher expressed than in normal liver (Figure 7A and Supplementary Figure 12C). We then generated 4 independent shRNAs against NUSAP1, which showed a varying efficiency in NUSAP1-downregulation, and cloned them into the oncogene vector (Figure 7B and Supplementary Figure 12D). Correlating with the efficiency of NUSAP1 downregulation by the distinct shRNA constructs, HDTV induced carcinogenesis was significantly reduced (Figure 7C and Supplementary Figure 12E). Finally, in line with our previous functional analyses on NUSAP1, we could demonstrate a concordant regulation of NUSAP1-related cell cycle genes in protein lysates from these HDTV-treated livers (Figure 7D). Collectively, our results suggest that miR-193a-5p suppresses liver tumor growth in vivo by downregulating NUSAP1 expression. Targeting this pathway by overexpressing miR-193a-5p or inhibition of NUSAP1 might represent a promising therapeutic strategy in human HCC.
Discussion
Our present approach revealed a previously unrecognized function of miR-193a-5p in mouse liver tumor promotion that is highly conserved in human HCC. Moreover, by combining conventional in-silico target gene prediction with a comprehensive mRNA transcriptomics analysis from these mouse liver tumors, we identified NUSAP1 as an important mediator of miR-193a-5p’s function in controlling tumorigenesis. In view of previous experimental studies, miR-193a-5p is not the most abundant miRNA in normal or transformed liver cells, when compared e.g. with miR-199-a/b-3p, the third most abundant miRNA in human liver cells which is constantly downregulated in human HCC and suppresses tumor growth through PAK4.28 miR-193a-5p is also not the one with the strongest up- or downregulation in a single tumor model. Instead, by choosing a combinatory algorithm with three HCC models of differing pathogenesis, we enriched the chance to identify those miRNAs with a concordant regulation between independent mouse models. Thereby, we increased the likelihood that this mechanism would be fundamental enough to be conserved not only between mouse models but also between mouse and human hepatocarcinogenesis. The control of cancer cell proliferation and survival by cyclins and CDK represents an integral mechanism in the context of tumor promotion. Of note, miR-193a-3p, another member of the miR-193 family, effectively inhibits proliferation in a wide array of cancer cell types, 29 supporting our present findings in HCC cell lines and human patients.
The prediction of miRNA targets remains challenging and is associated with false negative and false positive results.30 By integrating data from conventional in silico target gene prediction analysis and an unbiased mRNA profiling approach, we identified NUSAP1 as a target regulated by mRNA degradation which mediates miR-193a-5p’s effects on tumor cell proliferation in vitro and in vivo. While this combinatory approach increased the chance to detect a functionally significant target compared to a sole in-silico prediction, it is likely that other, “imperfect” miR-193a-5p targets exist in liver tumor cells that are potentially regulated by translational downregulation and thus could not be detected in our experimental approach. Of note, previously suggested targets of miR-193a-5p like ERBB2,31 mTOR, 32 WT1 33, 34 and or SMARCCB1 35 were not significantly influenced in our array in liver cells, suggesting cell-type specificity of miR-193a-5p regulatory circuits. NUSAP1 is a microtubule-associated protein (MAP) that controls cellular proliferation by governing spindle assembly, chromosome segregation and cytokinesis.24 Moreover, it bundles microtubules, links them to chromosomes and regulates chromosome oscillation.36 Normally, levels of NUSAP1 protein are tightly regulated during the cell cycle by the anaphase-promoting complex/cyclosome.37, 38 Moreover, NUSAP1 is upregulated in several cancers, like prostate cancer39, 40 or pancreatic cancer.41 While in malignant prostate cells, NUSAP1 expression is upregulated by loss of RB1 via the RB1/E2F1 axis, we show here in liver cancer cells that NUSAP1 is regulated through a miRNA-dependent mechanism, suggesting that NUSAP1 is regulated by distinct oncogenic circuits. Finally, the anticarcinogenic effects of NUSAP1-siRNA treatment in our xenograft tumor model underlined the potential of this protein as a therapeutic target in HCC and potentially other cancers.
Several studies demonstrated the feasibility of restoring tumor suppressive miRNAs and targeting oncogenic miRNAs for cancer therapy using in vivo model systems.42 As such, a synthetic miR-34a mimic is currently in clinical trial for the treatment of patients with primary liver cancer or with liver metastases.43 At present, the standard therapy of patients with advanced HCC is based on systemic administration of multi-tyrosine kinase inhibitors 44 that are associated with considerable side effects but only limited response rates. In this context, modulation of miR-193a-5p expression, e.g. through a direct intra-tumoral application of miR-193a-5p mimics, could offer an effective anti-cancer strategy with potentially additive effects to standard therapy. In this line, it is interesting to note that addition of miR-193a-5p mimics could enhance the cytotoxic effect of Sorafenib treatment in hepatoma cells (Supplementary Figure 13). Finally, applying our multiple mouse model strategy to other model combinations and using different high-throughput readouts might help to unmask previously unrecognized molecular circuits driving the initial steps of carcinogenesis, thereby improving the translational potential of mouse liver cancer studies and bridge the huge gap between basic science and clinical application.
Supplementary Materials and Methods
HCC xenograft model.
We used 6–8 weeks old Balb/c nude mice from Charles River laboratories for the HCC xenograft model. For this, Huh7 cells were transfected with 100 nM siRNA negative control or siNUSAP1 or miR-193a-5p with Lipofectamine 2000 (Invitrogen) for 72 h. 1*106 cells post-transfection were suspended in 50 μl PBS and injected subcutaneously in the right flank of Balb/c nude mice (5 mice per group). Tumor growth was measured with a digital caliper every 7 days for a total period of 28 days. Tumor volumes were calculated by the equation volume (mm3) = (length × width2)/2. Mice were killed after 30 days, tumors were collected and total RNA was prepared from the tumor tissues for qRT-PCR analysis. For post-implantation therapeutic experiments, nude mice (5 mice per group) received subcutaneous injection of 1*106 Huh7 or Hepa 1–6 cells into the right flank in a volume of 50 ¼l PBS. Once the tumors reached 0,5 cm, 50 μg of synthetic miR-193a-5p or Control (All Stars Negative Control) (Qiagen) complexed with Lipofectamine 2000 (Invitrogen) were delivered intratumorally in 3 days interval. Mice were killed after 30 days, tumors were collected and total RNA was prepared from the tumor tissues for qRT-PCR analysis. Micrographs of stained H/E cover slides were taken with an original magnification of x50, using an Axio Observer Z1 equipped with a Axio Cam MR and a XLmulti S1 DARK LS incubator (Zeiss, Germany). The data were processed with the ZEN pro.2012 software (Zeiss, Germany). All animal studies were ethically approved by the Federal Ministry for Nature, Environment and Consumers’ Protection of the state of North Rhine-Westphalia and were performed in accordance to the respective national, federal and institutional regulations.
Endogenous c-myc and Akt-1 mouse HCC model.
shRNA sequences were generated using the SplashRNA online tool and cloned via EcoRI and XhoI into a doxycyclin inducible retroviral expression vector including a mirE based miRNA cassette. To test the efficacy of the distinct sh-RNA sequences, Phoenix ECO or HEK293 cells were treated with chloroquine (25 μM) and transfected with retroviral vectors including doxycycline inducible shRNA expression cassettes via a standard CaPO3 method. Viral supernatant was collected 2 days later and filtered with a 0.45 μm filter. Then, Myc and Akt expressing, p19−/− murine liver tumor target cells were treated with polybrene (10 ¼g/ml) and infected with viral supernatant. Successfully infected cells were selected via puromycin (6 μg/ml) and treated with doxycycline (15 ¼g/ml) for 5 days. This method was performed to test the knockdown efficiency of shRNAs by isolating total RNA using Qiazol (Qiagen, Hilden, Germany) followed by chloroform purification and purification via RNeasy columns (Qiagen, Hilden, Germany). cDNA synthesis has been performed with PrimeScript RT Master Mix (Takara) and qPCR was conducted with SYBR Premix ExTaq (Takara) in a 7300 real-time PCR machine (Applied Biosystems). Relative mRNA expression was calculated with the 2 -ΔΔCT method. The list of shRNA sequences used for this experiment are listed in Supplementary Table 8.
Then the most efficient shRNA sequences including the 3’ mirE cassette were subcloned via XhoI and MluI into a transposon vector encoding for Myc, Akt (CaMIA) and the 5’ mirE cassette cut with XhoI and AscI. For hydrodynamic tail vein injection (HDTV) 4–6 week old mice were fixed in a restrainer and plasmid solution (25 ¼g transposon vector, 5 μg SB13 transposase in 10% v/w body weight) was injected into the tail vein within 5 seconds. All animal experiments were approved and conducted according to the local authority (Regierungspräsidium Tübingen, Germany). Mice were housed under specific pathogen free conditions and fed with normal diet.
miRNA profiling on the TaqMan OpenArray microRNA system
The TaqMan OpenArray Rodent MicroRNA Panel (Thermo Fisher Scientific, Carlsbad, California, U.S.; cat.no. 4461105) 27 was used to simultaneously profile the expression of 750 well-characterized miRNAs (miRBase v15) and 6 controls in the mouse tumor and control liver tissue samples, according to the manufacturer’s instructions. In brief, for each sample 100ng of total RNA (that included the miRNA fraction) was reverse transcribed using Megaplex RT primers in a set of two predefined pools (Pool A and Pool B), each pool contained 381 stem-looped RT primers (375 miRNA targets, 5 positive controls, 1 negative control). The recommended RT thermal cycling conditions were used: i.e. [16°C, 2 min; 42°C, 1 min; 50°C, 1 sec for 40 cycles]; 85°C, 5 min; 4°C hold. The cDNA product s (2.5¼l) then underwent unbiased PCR preamplification using Megaplex PreAmp Primers in a set of two pools (Pool A and Pool B) of gene-specific forward and reverse primers. Thermal cycling conditions were: 95°C 10 min; 55°C, 2 min; 72°C, 2 min; [95°C, 15 sec, 60°C, 4 min for 12 cycles], 99.9°C, 10 min; 4°C hold. The pream plified cDNA products (4μL) were subsequently 40x diluted in 0.1X TE buffer pH 8.0, and subjected to real-time PCR amplification and analysis using the TaqMan OpenArray Rodent MicroRNA Panel in 3072-well microfluidic (33nL) OpenArray plates that contained dried TaqMan primers and probes for miRNAs and controls. OpenArray Digital PCR Software Software (v1.0, Applied Biosystems) was used to analyze and review the amplification plots and threshold cycle (Ct) values obtained. Default analysis settings were used, except that for baseline estimations data from cycles 2–10 were used, and that for some samples the minimum signal value was set at 100 instead of the default value of 300. Data was then exported, and further analyzed in R using the Bioconductor packages HTqPCR 28 and limma 29. In HTqPCR, the minimum and maximum allowable Ct values were set at 8 and 31, respectively. The qPCR data was normalized by the ΔCt method, using for each sample as internal reference the mean Ct value of 5 endogenous small non-coding RNA controls (U87, Y1, U6, snoRNA135, snoRNA202). Next, only those miRNAs that were reliably expressed (i.e. Ct<31) in all samples were included for further analysis. This stringent filtering was applied to limit the screening to a set of robustly expressed miRNAs and to increase statistical power, and resulted in the removal of 620 miRNAs. The remaining 130 miRNAs were included in the statistical analysis. Differentially expressed miRNAs were identified by applying the ΔΔCt method 30 using linear models and moderated F- and t-statistics as implemented in limma 29. These moderated statistics have the same interpretation as the ordinary F- and t-statistics, except that the standard errors have been moderated across miRNAs, effectively borrowing information from the ensemble of miRNAs to aid with inference about each individual miRNA. miRNAs that satisfied the criterion of P < 0.05 and ddCt ± 0.9 were significantly regulated. Heatmaps of the results of a hierarchical, unsupervised bi-clustering analysis of samples (columns) and miRNA expression (rows) are presented. Each column indeed represents an individual mouse, and each row represents a microRNA. Briefly, for each sample normalized, ΔCt values were used as input (ΔCt value = Ct miRNA –mean Ct of the 5 endogenous small non-coding RNA controls). Next, the ΔCt values of each miRNA were centered across samples, and subjected to hierarchical bi-clustering analysis using the Euclidean distance and the complete linkage clustering method.
mRNA profiling on Affymetrix Mouse Gene 1.1 ST arrays.
The mouse tumor and control liver tissue samples were also subjected to expression profiling by microarray. To this end, purified total RNA (100ng per sample) was labelled with the Whole-Transcript Sense Target Assay (Affymetrix, Santa Clara, CA, U.S.; P/N 900652) and hybridized to whole-genome Affymetrix Mouse Gene 1.1 ST arrays (Affymetrix). Quality control and data analysis pipeline have been described in detail previously. 31 Briefly, normalized expression estimates of probe sets were computed by the robust multiarray analysis (RMA) algorithm 32 as implemented in the Bioconductor library AffyPLM. Probe sets were redefined using current genome information according to Dai et al. 33 based on annotations provided by the Entrez Gene database, which resulted in the profiling of 21,266 unique genes (custom CDF v18). Next, the dataset was filtered to only include probe sets (genes) that were reliably expressed in at least 3 samples using the universal expression code (UPC) approach (UPC score > 0.50) . 34 This resulted in the inclusion of 9,737 of the 21,266 probe sets present on the array. Differentially expressed probe sets (genes) were identified by using linear models (limma) and an intensity-based moderated t-statistic. 29, 35 Probesets that satisfied the criterion of P < 0.05 were considered to be regulated.
Accession numbers.
The currently reported miRNA and mRNA profiling data have been submitted to the Gene Expression Omnibus (super-series accession number GSE102418).
TCGA-LIHC data analysis.
The raw data (count data) for the microRNA precursor and isoform expression data sets as well as the messenger RNA data set were downloaded using the Bioconductor package TCGAbiolinks.36 The microRNA datasets 37 consisted of 401 samples that were annotated regarding ‘tumor stage’ (50 normal, 174 stage i, 87 stage ii, 85 stage iii, 5 stage iv; for 24 samples tumor stage was not reported); the mRNA dataset consisted of one sample less (one less sample for stage i [= 173]). Next, nonspecific filtering of the count tables was carried out to increase detection power, 38 based on the requirement that a feature should have an expression level greater than 10 counts at least 50 libraries across all samples. Differences in library size were adjusted by the upper-quartile normalization method,39 implemented in the Bioconductor package edge R 40. Counts were then log-transformed (log2CPM; Counts per million) and the observed mean-variance trend was converted into precision weights by the voom function 41 in the Bioconductor package limma 29. Differentially expressed genes were identified by using linear models and moderated F- and t-statistics 29. Clinical data including the survival time of the HCC patients was also retrieved from TCGA data portal cBioPortal (http://www.cbioportal.org/index.do). Survival cut off value was analyzed using the tool Cutoff Finder 42 (http://molpath.charite.de/cutoff/) and used the cut-off value to separate the patients’ survival on ‘low’ and ‘high’ expression. The percent survival was calculated by GraphPad Prism Software.
Cell culture.
Liver cancer cell lines Hepa1–6, Huh7, Hep3B and HepG2 were obtained from the American type culture collection (Manassas, VA, USA). Hepa1c1c7 cell line was a kind gift from Dr. Irina Lehmann (Helmholtz Centre for Environmental Research GmbH-UFZ, Leipzig Germany). All cells were cultured in DMEM medium (Pan Biotech, Germany) containing 10 % fetal bovine serum (Invitrogen, CA, USA) and 100 unit/ml of penicillin/streptomycin (Invitrogen). JHH2 cell line was a kind gift from Prof. Dr. med Jessica Zucman-Rossi. Cells were maintained in William’s medium (Pan Biotech, Germany) containing 10 % fetal bovine serum (Invitrogen, CA, USA) and 100 unit/ml of penicillin/streptomycin (Invitrogen).
Transfection and treatments.
Miscript miRNA mimics, siRNAs, miRNA antagomiRs and negative control were purchased from Qiagen. The sequences are indicated in Supplementary Table 8. siRNA or miRNA mimics and All Stars negative control (Control siRNA) (1027280) were used at final concentrations of 50 nM. miR-193a-5p inhibitor and miScript Inhibitor Negative control were used at final concentrations of 100nM. All RNAs were transfected using Lipofectamine 2000 (Invitrogen) for 72 h according to manufacturer’s instructions. Cells were treated with 100ng/ml of nocodazole (Sigma) for 24 h to synchronize the cells for cell cycle analysis. Sorafenib tyoslate was kindly provided by Dr. Christian Liedtke. Sorafenib was dissolved in DMSO for in vitro studies. Cells were transfected with miR-193a-5p mimic or Control for 24 h followed by treatment of cells with Sorafenib for additional 48 h.
Bioinformatic analysis.
The 3’ UTR of NUSAP1 was obtained from the ENSEMBL database (http://www.ensembl.org/index.html). microRNA target prediction was performed using the miRWalk target prediction tool (http://zmf.umm.uniheidelberg.de/apps/zmf/mirwalk2/). The seed sequence of miR-193a-5p in 3’UTR of Nusap1 (mouse) was obtained from RNAhybrid algorithm (https://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid). Venn diagram was obtained from the online source (http://genevenn.sourceforge.net/).
Dual luciferase assay.
PCR-amplified NUSAP1 3’UTR region was cloned into the psiCheck-2 vector (Promega, Madison, USA). Primer sequences are listed in Supplementary Table 8. Huh7 cells were co-transfected with NUSAP1-psiCheck-2 vector and miR-193a-5p mimic. After 72 h, luciferase activity was measured using dual luciferase assay kit (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity. To mutate a predicted miR-193a-5p binding site in the 3´UTR of NUSAP1 mRNA, psiCheck-2-TBC1D2–3´UTR was used as a template plasmid for Phusion site-directed mutagenesis kit (Finnzymes). The 5´phosphorylated primers used were listed in Supplementary Table 8.
Cell viability assay.
Cells were seeded in a 24-well plate and following transfection for 72 h, cells were subjected to CCK-8 assay (Dojindo Laboratories, Japan) for 1 h at 37 °C. Absorbance was measured in 540 nm by Biotek Cytation3 imaging reader.
BrdU assay.
Cells were seeded on coverslips in a 24-well plate and pulsed with 6 μg/ml BrdU (B5002, Sigma, USA). After 2 h, the cells were fixed and stained with anti-BrdU antibody (Millipore, USA) as described.
Colony formation assay.
The transfected cells were seeded in 6 - well plate at a density of 100–250 cells per well and incubated for 2 weeks. The cells were fixed and stained in a dye solution containing 0.1 % crystal violet and 20 % methanol. The number of colonies more than 50 cells was counted.
Apoptosis assays.
Transfected cells on coverslips were fixed and subjected to TUNEL staining using the InSitu Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Apoptosis was determined by FACS flow cytometry as previously described. Briefly, the cells collected after trypsinization were washed with PBS and resuspended with binding buffer. Cells were then stained with 5 μl Annexin V- Alexa 647 (Molecular Probes) and 1 μl of PI according to the manufacturer’s instructions. The cells were acquired by the FACS flow cytometry (BD Biosciences) and analyzed with FlowJo 7.5 software (Tree Star, Ashland, OR, USA).
Cell cycle analysis.
Transfected cells were harvested with Trypsin after 72 h and fixed in 1 % PFA for 15 min at room temperature. After having washed in PBS, fixed cells were permeabilized in 0.1 % Triton X-100 for 20 mins, stained with DAPI (BD Biosciences, Heidelberg, Germany) and analysed on a FACS Canto (BD Biosciences). Data were analyzed using FlowJo 7.5 software (Tree Star, Ashland, OR, USA).
Wound healing, cell migration and invasion assay.
The effects on migratory function of miR-193a-5p overexpression and NUSAP1 knockdown were determined by evaluating cellular migration after scratching of a confluent monolayer of cells. Cells were seeded into 6-well plates and transfected with either siRNA, miRNA mimic, miRNA antagomir or control. After 48 h, the cells were starved for additional 24 h before the pre-treatment of the cells with 10 μg/ml Mitomycin C (M0503, Sigma Aldrich) for 3 h. Mitomycin C is a cell cycle antagonist being used to measure migration without the influence of cell proliferation. The cells were washed three times followed by the scratch drawn using a 200-μl pipette. Migration was photographed at the indicated time points after scratching using an inverted microscope (Leica) and the percent wound closure was calculated for five randomly chosen fields.
Migration assay was also confirmed by performing by using Transwell insert chambers (24- well plate, 8.0 μm pore size, Corning, USA). 1*105 transfected cells suspended in serum-free medium were placed in the upper chamber 48 h after transfection while the lower chamber was filled with 700 μl of DMEM with 10 % FBS as chemo-attractant. After incubation for 24 h at 37 °C, the non-migrating cells in the upper chamber were removed by a cotton swab and lower surface of the chamber was fixed and stained with 0.1 % crystal violet. For invasion assay, the cells were seeded in insert chambers pre-coated with a layer of diluted basement membrane matrix Matrigel (Corning) and the same procedure was followed as above. Migrating or invading cells were scored by counting at least 5 fields per membrane under a light microscope.
Immunofluorescence.
Cells were transfected on coverslips as mentioned above and after fixing the cells, cells were blocked with normal goat serum diluted in 0.1 % Triton X-100. The cells were washed three times with PBS and incubated overnight at 4 °C with either of the following primary antibodies: cleaved caspase-3 (9661, Cell signaling, MA, USA), Ki67 (NCL-Ki67p, Leica Microsystems), α-Tubulin (T6074, Sigma Aldrich), phospho-H3 (9706, Cell signaling) or NUSAP1 (120241–1-AP, Proteintech) and treated with secondary antibodies anti-rabbit A555 (A31572, Invitrogen, CA, USA) and anti-mouse A488 (A21202, Invitrogen, CA. Image acquisition was performed at a magnification of 20 x with a Zeiss Axio Imager.Z1 microscope, Axiocam MRm and HRc cameras using Axiovision 4.8 software (Carl Zeiss, Inc., Oberkochen, Germany).
Western Blot.
Protein lysates were obtained by homogenization transfected cells in NP-40 lysis buffer. They were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to PVDF membrane and analyzed by immunoblotting with the following antibodies: Cyclin A2 (sc-596, Santa Cruz Biotechnology), Cyclin E1 (sc-481, Santa Cruz Biotechnology), Cyclin D1(33–3500, ThermoFischer Scientific), Cyclin B1 (sc-4138, Santa Cruz Biotechnology), p21 (RUO-556431, BD Pharmingen), α-PCNA (13–3900, Zymed), NUSAP1 (120241–1-AP, Proteintech), cmyc (ab32072, Abcam), Akt-1 (2967, Cell Signaling) and anti-GAPDH (MCA 4739, ABD Serotec).
Quantitative real-time PCR.
For mRNA:
Total RNA was extracted from cell lines and frozen tumor samples using Trizol reagent (Invitrogen, CA, USA) and cDNA synthesis was done using using first strand cDNA synthesis kit (Roche Diagnostics, IN, USA). Quantitative PCR was was performed using SYBR Select Master Mix (Thermofischer Scientific, Waltham, MA, USA) and gene specific primers (Eurofins MWG Operon, Ebersberg, Germany) on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). mRNA expression data were normalized to GAPDH. Primer sequences were listed in Supplementary Table 8.
For mRNA:
Total RNA from cells and tumor samples along with miRNA was isolated with Trizol reagent by Directzol™ RNA Miniprep (Zymo Research, Irvine, CA). RNA was reverse transcribed using miScript Reverse Transcription kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. A quantitative PCR was performed using miScript SYBR Green PCR kit (Qiagen, Germany) and miScript Primer assays (Qiagen, Germany) for miR-1224 with RNU6 as an internal control. miRNAs was detected on ABI 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA). The relative expression values were normalized to the internal control by using 2-ΔΔCt. Primer sequences were listed in Supplementary Table 8.
Immunohistochemistry.
Paraformaldehyde (4 %) fixed and paraffin embedded liver tissues were cut in sections (2 μm) and stained with H/E [hemalum (Dako) and eosin (Sigma-Aldrich)] or various primary and secondary antibodies.
Study approval.
Animal studies were ethically approved by the Federal Ministry for Nature, Environment and Consumers’ Protection of the state of North Rhine-Westphalia and were performed in accordance to the respective national, federal and institutional regulations. Human study was approved by Paris Saint-Louis Institutional Review Board committee (Paris Saint-Louis, 2004; INSERM IRB 2010; the French Liver Biobanks Network, AFAQ NF S96–900; and Hepatobio Bank). All patients gave informed consent prior to their participation in the study according to French law.
Supplementary Material
Acknowledgments
The authors would like to express their gratitude to Karina Kreggenwinkel and Larissa Tenten for their great help. The authors would also like to express their gratitude to Sabrine Klotz for her help in c-myc/Akt-1 mouse experiments and to Sandra Schneider for her support in art work.
Grant Support
Research in the lab of T.L. is supported by a Mildred-Scheel Endowed Professorship from the German Cancer Aid (Deutsche Krebshilfe), the German-Research-Foundation (DFG) (LU 1360/3–1 and SFB-TRR57 / P06), the Interdisciplinary-Centre-for-Clinical-Research (IZKF) Aachen-Germany and the Ernst-Jung-Foundation Hamburg. Moreover, this work was supported by project grants from the German Research Foundation (DFG RO 4317/4–1) and a START grant from the medical faculty RWTH Aachen to C.R. The J.Z-R group is supported by the Ligue Nationale contre le Cancer (Equipe Labellisée), Labex OncoImmunology (investissement d’avenir), Coup d’Elan de la Fondation Bettencourt-Shueller, the SIRIC CARPEM and Fondation Mérieux. M.H. was supported by the SFB179 and 209, an ERC consolidator grant (HepatoMetaboPath) and the European Union’s Horizon 2020 research and innovation program under grant agreement No 667273. A.G. was supported by NIH (R01CA170447) and (U19CA179512).
Abbreviations used in this paper
- NUSAP1
nucleolar- and spindle-associated protein
- HCC
hepatocellular carcinoma; miRNA, microRNA
- DEN
Dietylnitrosamine
- AlbLTα/β
Lymphotoxin-α/β
- NTL
non-tumoral liver
- qRT-PCR
quantitative real-time reverse transcription polymerase chain reaction
- GEO
Gene expression omnibus
- BrdU
Bromodeoxyuridine
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- FACS
Fluorescence-activated cell sorting
- PI
Propidium iodide
Footnotes
Conflict of Interest
The authors disclose no conflicts of interest.
Transcript profiling
The currently reported miRNA and mRNA profiling data have been submitted to the Gene Expression Omnibus (super-series accession number GSE102418).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang L, Yue Y, Wang X, et al. Function and clinical potential of microRNAs in hepatocellular carcinoma. Oncol Lett 2015;10:3345–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu LC, Hsu CH, Hsu C, et al. Tumor Heterogeneity in Hepatocellular Carcinoma: Facing the Challenges. Liver Cancer 2016;5:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vucur M, Roderburg C, Bettermann K, et al. Mouse models of hepatocarcinogenesis: what can we learn for the prevention of human hepatocellular carcinoma? Oncotarget 2010;1:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Ress AL, Wagle R, Pichler M. Multi-omics in prognosis of hepatocellular carcinoma. Ann Transl Med 2015;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014;26:549–64. [DOI] [PubMed] [Google Scholar]
- 7.Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokinedriven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005;121:977–90. [DOI] [PubMed] [Google Scholar]
- 8.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461–6. [DOI] [PubMed] [Google Scholar]
- 9.Heikenwalder M, Zeller N, Seeger H, et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 2005;307:1107–10. [DOI] [PubMed] [Google Scholar]
- 10.Goga A, Yang D, Tward AD, et al. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med 2007;13:820–7. [DOI] [PubMed] [Google Scholar]
- 11.Finkin S, Yuan D, Stein I, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol 2015;16:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haybaeck J, Zeller N, Wolf MJ, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 2009;16:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairo S, Armengol C, De Reynies A, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell 2008;14:471–84. [DOI] [PubMed] [Google Scholar]
- 14.Nault JC, De Reynies A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176–87. [DOI] [PubMed] [Google Scholar]
- 15.Qiu G, Lin Y, Zhang H, et al. miR-139–5p inhibits epithelial-mesenchymal transition, migration and invasion of hepatocellular carcinoma cells by targeting ZEB1 and ZEB2. Biochem Biophys Res Commun 2015;463:315–21. [DOI] [PubMed] [Google Scholar]
- 16.Lan FF, Wang H, Chen YC, et al. Hsa-let-7g inhibits proliferation of hepatocellular carcinoma cells by downregulation of c-Myc and upregulation of p16(INK4A). Int J Cancer 2011;128:319–31. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chen F, Zhao M, et al. MiR-107 suppresses proliferation of hepatoma cells through targeting HMGA2 mRNA 3’UTR. Biochem Biophys Res Commun 2016;480:455–460. [DOI] [PubMed] [Google Scholar]
- 18.Xin B, Yamamoto M, Fujii K, et al. Critical role of Myc activation in mouse hepatocarcinogenesis induced by the activation of AKT and RAS pathways. Oncogene 2017;36:5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettermann K, Vucur M, Haybaeck J, et al. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell 2010;17:481–96. [DOI] [PubMed] [Google Scholar]
- 20.Vucur M, Reisinger F, Gautheron J, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep 2013;4:776–90. [DOI] [PubMed] [Google Scholar]
- 21.Schneider AT, Gautheron J, Feoktistova M, et al. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer Cell 2017;31:94–109. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 23.Dweep H, Sticht C, Pandey P, et al. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 2011;44:839–47. [DOI] [PubMed] [Google Scholar]
- 24.Raemaekers T, Ribbeck K, Beaudouin J, et al. NuSAP, a novel microtubuleassociated protein involved in mitotic spindle organization. J Cell Biol 2003;162:1017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanden Bosch A, Raemaekers T, Denayer S, et al. NuSAP is essential for chromatin-induced spindle formation during early embryogenesis. J Cell Sci 2010;123:3244–55. [DOI] [PubMed] [Google Scholar]
- 26.Rossio V, Galati E, Piatti S. Adapt or die: how eukaryotic cells respond to prolonged activation of the spindle assembly checkpoint. Biochem Soc Trans 2010;38:1645–9. [DOI] [PubMed] [Google Scholar]
- 27.Rudalska R, Dauch D, Longerich T, et al. In vivo RNAi screening identifies a mechanism of sorafenib resistance in liver cancer. Nat Med 2014;20:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011;19:232–43. [DOI] [PubMed] [Google Scholar]
- 29.Hydbring P, Wang Y, Fassl A, et al. Cell-Cycle-Targeting MicroRNAs as Therapeutic Tools against Refractory Cancers. Cancer Cell 2017;31:576–590 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh NK. miRNAs target databases: developmental methods and target identification techniques with functional annotations. Cell Mol Life Sci 2017;74:2239–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CH, Tsai CH, Yeh CT, et al. MiR-193a-5p/ERBB2 act as concurrent chemoradiation therapy response indicator of esophageal squamous cell carcinoma. Oncotarget 2016;7:39680–39693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang C, Shen F, Du J, et al. Upregulation of CASC2 sensitized glioma to temozolomide cytotoxicity through autophagy inhibition by sponging miR-193a-5p and regulating mTOR expression. Biomed Pharmacother 2018;97:844–850. [DOI] [PubMed] [Google Scholar]
- 33.Xie F, Hosany S, Zhong S, et al. MicroRNA-193a inhibits breast cancer proliferation and metastasis by downregulating WT1. PLoS One 2017;12:e0185565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Gao S, Wang C, et al. Pathologically decreased expression of miR-193a contributes to metastasis by targeting WT1-E-cadherin axis in non-small cell lung cancers. J Exp Clin Cancer Res 2016;35:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malgulwar PB, Pathak P, Singh M, et al. Downregulation of SMARCB1/INI1 expression in pediatric chordomas correlates with upregulation of miR-671–5p and miR-193a-5p expressions. Brain Tumor Pathol 2017;34:155–159. [DOI] [PubMed] [Google Scholar]
- 36.Li C, Xue C, Yang Q, et al. NuSAP governs chromosome oscillation by facilitating the Kid-generated polar ejection force. Nat Commun 2016;7:10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Zhou Y, Sun L, et al. NuSAP is degraded by APC/C-Cdh1 and its overexpression results in mitotic arrest dependent of its microtubules’ affinity. Cell Signal 2007;19:2046–55. [DOI] [PubMed] [Google Scholar]
- 38.Song L, Rape M. Regulated degradation of spindle assembly factors by the anaphase-promoting complex. Mol Cell 2010;38:369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulzar ZG, McKenney JK, Brooks JD. Increased expression of NuSAP in recurrent prostate cancer is mediated by E2F1. Oncogene 2013;32:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon CA, Gulzar ZG, Brooks JD. NUSAP1 expression is upregulated by loss of RB1 in prostate cancer cells. Prostate 2015;75:517–26. [DOI] [PubMed] [Google Scholar]
- 41.Kokkinakis DM, Liu X, Neuner RD. Modulation of cell cycle and gene expression in pancreatic tumor cell lines by methionine deprivation (methionine stress): implications to the therapy of pancreatic adenocarcinoma. Mol Cancer Ther 2005;4:1338–48. [DOI] [PubMed] [Google Scholar]
- 42.Rothschild SI. microRNA therapies in cancer. Mol Cell Ther 2014;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li XJ, Ren ZJ, Tang JH. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis 2014;5:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, Abou-Alfa GK. The role of tyrosine kinase inhibitors in hepatocellular carcinoma. Clin Adv Hematol Oncol 2014;12:36–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.