Abstract
Objective:
To examine low birthweight and preterm birth of second children born to home-visited first-time mothers.
Subjects:
Women were previously recruited for a randomized controlled trial of the home visiting model disseminated as Nurse-Family Partnership. 512 of these women had second children within 18 years of the first child’s birth, and were included in our sample.
Results:
The intervention was associated with a lower likelihood of low birthweight for second children (odds ratio: 0.51, 95% CI: 0.27, 0.97), an effect apparent only if the first-born had low birthweight and mediated by close birth spacing. These moderation and mediation patterns were similar in the preterm birth outcome.
Conclusion:
A home visiting program provided for first-born children reduced low birthweight for second-born children, if the first-born had low birthweight. This finding implies a broader impact than previously documented, because few studies have included these second children.
Keywords: home visiting, low birthweight, preterm birth, birth spacing
Low birthweight is the leading cause of neonatal mortality and remains a significant health metric in the United States (U.S.) and internationally. The low birthweight rate in the U.S. remained relatively stable between 1990 and 2014, when it was 8.2% and ranked ninth among developed countries. Low birthweight impacts early perinatal mortality, compromises child development, and also places the individual at risk for mortality and morbidity into adulthood, particularly cardio-related death and diabetes.1, 2, 3 The etiology of low birthweight is multifactorial and influenced by the socio-demographic and physical environment as well as individual genetic,4 epigenetic,5 and behavioral factors.6 Preterm birth is one factor that contributes to low birthweight, and is associated with a similar range of long-term risks.7 Intergenerational history, maternal health, pre-pregnancy body mass index, and gestational weight gain are increasingly recognized as important to pregnancy outcomes.8
Prenatal and infancy home visiting programs have been developed to improve pregnancy and early childhood outcomes. While results are encouraging, only a few studies have found significantly positive effects on birthweight6, 7, 8 and preterm birth9, 10, 11 and others did not detect significant findings.12,13 These differences may be due in part to small-to-moderate sample sizes, or variations in home visiting program delivery, such as length of engagement or training of home visitors; longer duration of engagement and professionally-trained home visitors may be helpful for the families with greatest needs.13 In addition, home visiting often does not begin until the latter half of pregnancy and can be sporadic, resulting in later and fewer visits than expected and/or needed to improve birth outcomes.13
Determining whether home visiting during one pregnancy affects the birth outcomes of subsequent pregnancies has received limited attention. Home visiting improves birth spacing,14 maternal health,14 and maternal stress,15 all of which are known to be associated with birth outcomes.16, 17 Consequently, alteration of these factors may result in a reduction of risk for low birthweight and preterm births during subsequent pregnancies. Evaluations of home visiting programs have included number of subsequent pregnancies and spacing between pregnancies as outcomes,18 yet there is little evidence that the home visiting initiated in one pregnancy will impact the birthweight of subsequent children.19 A recent study found a decrease in preterm births for second children and improvement in spacing, but did not examine birthweight.20 For example, because a short inter-pregnancy interval is associated with lower birthweight and preterm birth,16, 21 one might expect to observe improved birth outcomes in later children born to home-visited mothers if these mothers did not have close pregnancy spacing. Low birthweight and preterm delivery of a first child increases the risk of these outcomes in later births.22 The birthweights of subsequent children are highly correlated with birthweights of the first child in samples from Norway23 and Maryland,24 although second births are likely to be heavier than their older sibling25, 26 by an average difference of 138g.27
The purpose of this study was to determine if second children born to home-visited mothers were less likely to have low birthweight (primary outcome) than second children born to comparison group mothers. We examined whether a) intervention effects on low birthweight in subsequent births were explained by home visiting program effects on inter-birth intervals, and b) intervention effects were more pronounced among mothers whose first child was low birthweight. We also examined preterm birth as the secondary outcome. Although both low birthweight and preterm birth have health consequences, low birthweight is considered the more reliable from a measurement perspective, based both on previous studies of birth certificate data28 and the origin of these data (weighing at birth vs. estimates based on fetal size at ultrasound, newborn size, and/or last menstrual period).
Methods
We analyzed data from the Memphis New Mother’s Study (referred to here as the original study), a randomized controlled trial (RCT) of prenatal and infancy/toddler home visiting for women with no previous live births.29 This is one of three trials that led to the program now disseminated as the Nurse-Family Partnership. Pregnant women in this trial were recruited at an obstetric clinic used primarily by Medicaid-insured patients in Memphis, TN in 1990-91. To enroll in the original study, women had to be primiparous and have two of three risk factors: unmarried, unemployed, and not graduated from high school. Women with known chronic conditions associated with poor birth outcomes were excluded.29 Women in the intervention received home visits from nurses to provide support and education with the goals of improving maternal health and first pregnancy outcomes, child health and development, and family economic self-sufficiency. Frequency of visits varied from weekly to monthly over the course of the program, from the initial pregnancy to the child’s second birthday. Both intervention and comparison group women received transportation to prenatal care and early childhood developmental screening/referrals until the child was two years old. One goal of the New Mother’s Study was improving the health of any future children, and a pathway to better outcomes for future children was considered to be improved pregnancy spacing.30
Data were collected at enrollment, two time points during pregnancy (28 and 36 weeks), and after the child was born (6, 12, 24 months; 4, 6, 9, 12, and 18 years). Birth certificate data were obtained on the study children and subsequent children. All data collection was blinded to treatment group assignment. In the original study, 1139 mothers were enrolled. For these analyses, we excluded the groups that were only followed through the first child’s birth (n=396); twins (n=17) for both first and second births, due to the impact on birth outcomes; mothers with no known second births (n=108); and those for whom no information on second births was available (no birth certificates (n=26) and no follow-up interviews (n=80)). The final sample size was 512 triads (mothers and 2 children). Because of potential intervention effects on the decision to have a second child, we consider the current study a comparison study and not a true RCT. The original study was designed to have sufficient power for expected changes in child health care use for injuries and maternal subsequent birth spacing.29 With power of 0.80 and a significance level of 0.05, we estimated that only a large effect (decrease in rates of low birthweight of 65%) would be detected using the available sample size. However, given the lack of studies in this area, we felt it was important to investigate. This power calculation and all analyses reported were two-sided.
The primary outcome for the current study was low birthweight (< 2500 grams) of the second child born to mothers in the trial. These data were obtained from birth certificate data, or maternal self-report if birth certificate data were not available (7.4%). The primary independent variable was assignment to the intervention group in the original study. Adequate birth spacing reduces the risk of low birthweight for the second child,16 and improving this spacing was a goal of the original study.29 Therefore, we examined birth spacing as a mediator between the intervention and second child low birthweight. We defined rapid repeat pregnancy as the second birth occurring within 18 months of the first child.16 We also examined if the second birth occurred during the home visiting program time period. This variable was defined as a second birth within 27 months of the first birth, which means at least the first two trimesters of the second pregnancy would be during the intervention, because the intervention continued until the first child was 24 months old. The extended time (additional 9 months beyond the rapid repeat designation) captures the possibility that the intervention could impact low birthweight of the second child through the mother’s interactions with the nurse while she was pregnant with the second child. Both measures were calculated from the two birth dates obtained from birth certificates. These variables are added in separate models (Models 2 and 3 for each outcome; Model 1 is the base model, without these potential mediators; Figure 1).
Figure 1:
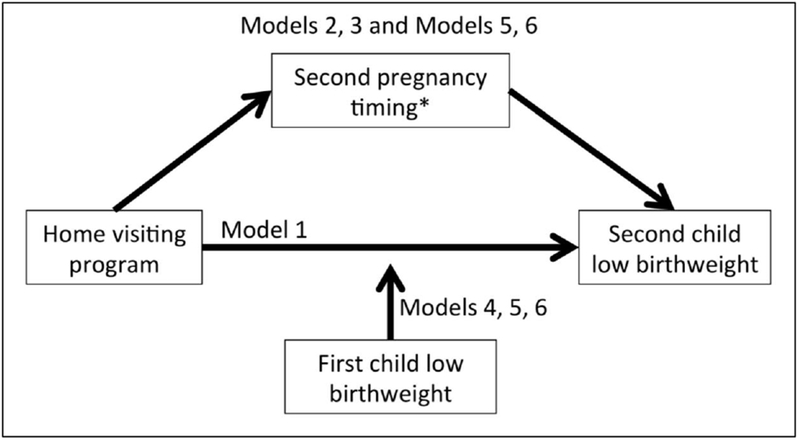
Hypothesized relationships between the intervention, second pregnancy timing, first child birthweight status, and second child birthweight status. For Models 2 and 5, second pregnancy timing is measured as whether the pregnancy began within 18 months of the previous birth. For Models 3 and 6, second pregnancy timing is measured as whether the pregnancy began within the time frame of the home visiting program.
Whether the first child was born with low birthweight was examined as a potential moderator of the association between the intervention and low birthweight of the second child. The first child’s birthweight was dichotomized to low birthweight using the established cutoff of 2500 g. Given the inter-conceptual maternal health focus of the intervention, we hypothesized that the program would reduce low birthweight among second births, especially for those at greatest risk because they delivered a low-birthweight first-born. Although the intervention began before the first child was born, moderation fits conceptually because the intervention did not have a direct effect on the first child’s birthweight.29 The intervention may have begun too late to have a main effect on birthweight, as evidenced by a median of 4 pregnancy visits for the cases of low birthweight in the intervention group and supported by evidence that some causes of low birthweight occur pre-conceptually (e.g., maternal pre-pregnancy weight31) or early in pregnancy (e.g., late entry into prenatal care31) and/or are slow to change (e.g., socioeconomic status32). In addition, much of the intervention occurred after the first child’s birth.29 This possible moderation was examined with a separate set of models: Model 4 for the initial model; Models 5 and 6 include moderation and the birth spacing variables as described above (Figure 1).
Additional covariates were included in our models if they were known contributors to low birthweight or if the treatment and comparison groups were not equivalent at baseline (described below). Before analyzing our data, we chose a conservative, inclusive p-value cutoff, slightly higher than the recommendation of at least .25 (p<.3)33, 34 to ensure that all potential important variables were included. Because the focus of this study was on the potential intervention effect, and many factors measured after enrollment could be impacted by the intervention, we included only variables available at enrollment in the original study prior to intervention. The only exception was the sex of the second child, which was not likely to be impacted by the intervention, and was included due to the well-established differences in birthweight related to sex. Additional covariates included due to known associations with birth outcomes were: maternal smoking during pregnancy (intake interview), weeks of gestation at study enrollment (medical record), maternal height at intake (self-report), pre-pregnancy obesity (obese or not; height and weight at intake from medical records), and maternal psychological resources (intake interview).
Eight maternal intake covariates were not equivalent (p<.3) between intervention and comparison groups at enrollment (first pregnancy; Table 1) and were therefore included in the model, even though they were within the more traditional .05 cutoff. The non-equivalent demographics were race and discretionary household income (self-report; calculated difference between subsistence income needed based on household size and reported household income).29 The number of weeks into pregnancy when prenatal care began was not equal (medical record). The mother’s age at enrollment, both as a continuous variable and dichotomized at 18 years, was not equal. Two maternal health characteristics were not equal: sub-fecundity (no pregnancy in 2 or more years using birth control less than 25% of the time; self-report) and prehypertension or hypertension (systolic pressure ≥120 or diastolic ≥80 mmHg for those 18 years or older; systolic or diastolic ≥90th percentile for those younger than 18 years; medical record). Social support from maternal mother was also unequal.
Table 1.
Demographics of analysis sample, N=512.
| Variable | Comparison group n=355 (% or mean) | Nurse-visited group n=157 (% or mean) | Group difference (P-value) |
|---|---|---|---|
| Mother intake demographics | |||
| African-American | 94.4% | 91.7% | .25 |
| Head of household employed at intake | 54.7% | 51.3% | .49 |
| Household discretionary income | $1,283 | $15 | .06 |
| Mother health habits | |||
| Nicotine exposed | 63.9% | 65.6% | .82 |
| Ever smoked regularly, at intake | 9.6% | 12.1% | .36 |
| Start of prenatal care, weeks | 15.6 weeks | 16.3 weeks | .27 |
| Weeks’ gestation at intake | 16.4 weeks | 16.8 weeks | .54 |
| Mother physical & health characteristics | |||
| Maternal age at intake | 17.6 yrs | 17.9 yrs | .28 |
| 18+ | 45.6% | 51.0% | .14 |
| <18 | 54.4% | 49.0% | |
| Maternal height | 164.3 cm | 163.9 cm | .46 |
| Maternal pre-pregnancy body mass index | 22.8 kg/m2 | 23.1 kg/m2 | .45 |
| Prepregnancy obesity | 9.3% | 7.1% | .41 |
| Subfecunditya | 12.4% | 8.9% | .27 |
| Pre-hypertension at intake | 20.5% | 25.0% | .24 |
| Hypertension (measured) at intake | 12.2% | 10.1% | .55 |
| Mother psychosocial characteristics | |||
| Maternal IQ | 96.0 | 96.4 | .69 |
| Social support from maternal grandmother at intake | 4.02 | 4.12 | .14c |
| Maternal personal resources low at intake | 51.6% | 56.7% | .34 |
| Married at intake | 1.7% | 0.6% | .35 |
| Child characteristics | |||
| Child gender combinations | .70 | ||
| Both first and second children male | 21.1% | 21.7% | |
| Both first and second children female | 22.8% | 26.8% | |
| First child male, second female | 29.6% | 28.7% | |
| First child female, second male | 26.5% | 22.9% |
Defined as no conception for 2 years without birth control or birth control methods used less than 25% of the time.
t-tests for continuous variables (all are sufficiently normally distributed and equal variances, except as noted) and χ2 for categorical variables.
Wilcoxon rank-sum test due to non-normal distribution.
Seven percent of our sample was missing data on one or more variables. The most frequently missing data were on blood pressure (5%) and weeks from last menstrual period (LMP) to first prenatal visit or lab (3%). Therefore, we used multiple imputation with chained equations to manage missing data, using 20 imputed datasets. Twenty was considered sufficient following the guideline of at least one per percentage of missing data.35 We utilized data from later time points when possible to inform our imputation model. For instance, we included blood pressure measurements from follow-up phases of the study in the model, because hypertensive status at one point in time is predictive of hypertensive status later.36
Due to the dichotomous nature of our outcome variable, we utilized logistic regression. To test the two birth spacing measures as mediators, we calculated the Sobel test for each imputed dataset and then combined them using Rubin’s Rules.37 To test for moderation, we included an interaction term between first child’s low birthweight status and the intervention. We present the number-needed-to-treat (NNT) as a measure of the number of mothers who would need to receive home visiting to avoid one second low birthweight child, calculated for each model.38, 39
Preterm birth was analyzed as the secondary outcome, following the models described above for low birthweight and using. preterm birth of the first pregnancy as a moderator in the model. Preterm birth was defined as born before the 37th week of gestation, and term birth, defined as birth from the 37th to 41st weeks of gestation. We retained all covariates in the models as they predict both preterm birth and low birthweight. The sample size for these analyses was 470 due to missing gestational age data (8.2% missing).
Results
A description of the sample is provided in Table 1. At enrollment, the women were primarily young, low-income, and African-American. Of the first children, 11.7% had low birthweight and 9.6% were born premature. Of the second children, 12.8% had low birthweight and 16.0% were born premature. Neither the original treatment arm nor having a low birthweight or preterm birth first child was associated with having a second child (p<.64 and p<.95, respectively).
The results of the six multivariate logistic regression models with the intervention as the primary independent variable and low birthweight as the primary outcome are presented in Table 2. Model A1 includes only the intervention and covariates. Models A2 and A3 add timing of the second pregnancy (within 18 months or within the intervention time frame) as mediators. Models A4 – A6 repeat this progression, with the addition of low birthweight of the first child as a moderator. Model A1 shows that the intervention was associated with a decreased likelihood of the second child having a low birthweight, and that 16 families (95% CI: 9, 142) would need to be treated to avoid one low birthweight second child. Model A2 supports pregnancy as a mediator of this association. The second pregnancy occurring during the home visiting period was not supported as a mediator in Model A3. Model A4 indicates that the first child having low birthweight is associated with an increased odds of the second child having low birthweight. This factor moderates the association between the intervention and second child low birthweight: the intervention is associated with a reduction in the risk for low birthweight for children whose older sibling was also born with low birthweight (p<.03), but not for children whose older sibling was born with a normal or high birthweight (p<.43); see Figure 2). In Model A5, the mediation effect is attenuated compared to Model A2 by the inclusion of the first child being low birthweight in the model. In each of Models A4-A6, the number needed to treat is low for those with a first low birthweight child (3 to 4), but much higher if the first born was not low birthweight (46 to 71).
Table 2:
Low birthweight of the second child - multivariable logistic regression models estimating intervention effects with covariates examining moderating and mediating relationships.
| Model A1 odds ratio (95% CI) | Model A2 odds ratio (95% CI) | Model A3 odds ratio (95% CI) | Model A4 odds ratio (95% CI) | Model A5 odds ratio (95% CI) | Model A6 odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Intervention | 0.51 (0.27 ,0.97)* | 0.55 (0.29, 1.04) | 0.53 (0.28, 0.996)* | 0.80 (0.40, 1.58) | 0.87 (0.43, 1.75) | 0.81 (0.41, 1.63) |
| First child low birthweight | 4.65 (2.25, 9.62)* | 4.75 (2.26, 9.96)* | 4.63 (2.23, 9.58)* | |||
| 1st child LBW X Intervention | 0.09 (0.01, 0.85)* | 0.08 (0.01, 0.80)* | 0.09 (0.01, 0.87)* | |||
| Timing of 2nd birth | ||||||
| Within 18 months of 1st | 2.60 (1.46, 4.63)* | 2.67 (1.47, 4.86)* | ||||
| Within intervention perioda | 1.23 (0.72, 2.12) | 1.19 (0.68, 2.08) | ||||
| Timing of 2nd birth: mediatorb | p<.049* | p<.17 | p<.058 | p<.20 | ||
| Intervention effect if first child LBWc | p<.03 | p<.02 | p<.03 | |||
| Intervention effect if first child not LBWc | p<.43 | p<.62 | p<.49 | |||
| Number need to treatd | 16 (9, 142) | 18 (9, e) | 17 (9, 374) | |||
| First child LBW | 3 (2, 6) | 3 (2, 6) | 4 (2, 9) | |||
| First child not LBW | 46 (13, e) | 71 (14, e) | 47 (13, e) | |||
Note: Additional covariates (all measured at enrollment, unless indicated; all not significant in all models in Table 2) include: gestational weeks, prepregnancy obesity, prehypertension, second child sex, maternal race, smoking history (ever smoked regularly), psychological resources (dichotomous according to original study sample mean), discretionary household income; weeks from last menstrual period (LMP) to first prenatal visit or lab, mother over 18 years, subfecundity (no pregnancy >=2 yrs/birth control < 25%), prehypertension, social support from grandmother, and maternal height.
birth within 27 month.
Sobel test for mediation.
P-value for full contribution of intervention in each scenario. When the first child was not LBW, this is the p-value for the intervention term; when the first child was LBW, this is a Wald test of the linear combination of the intervention term and the intervention-LBW interaction term.
The number of mothers who would need to receive home visiting to avoid one second low birthweight child, calculated from each model.38, 39
Confidence interval includes infinity.
p<.05
Figure 2:
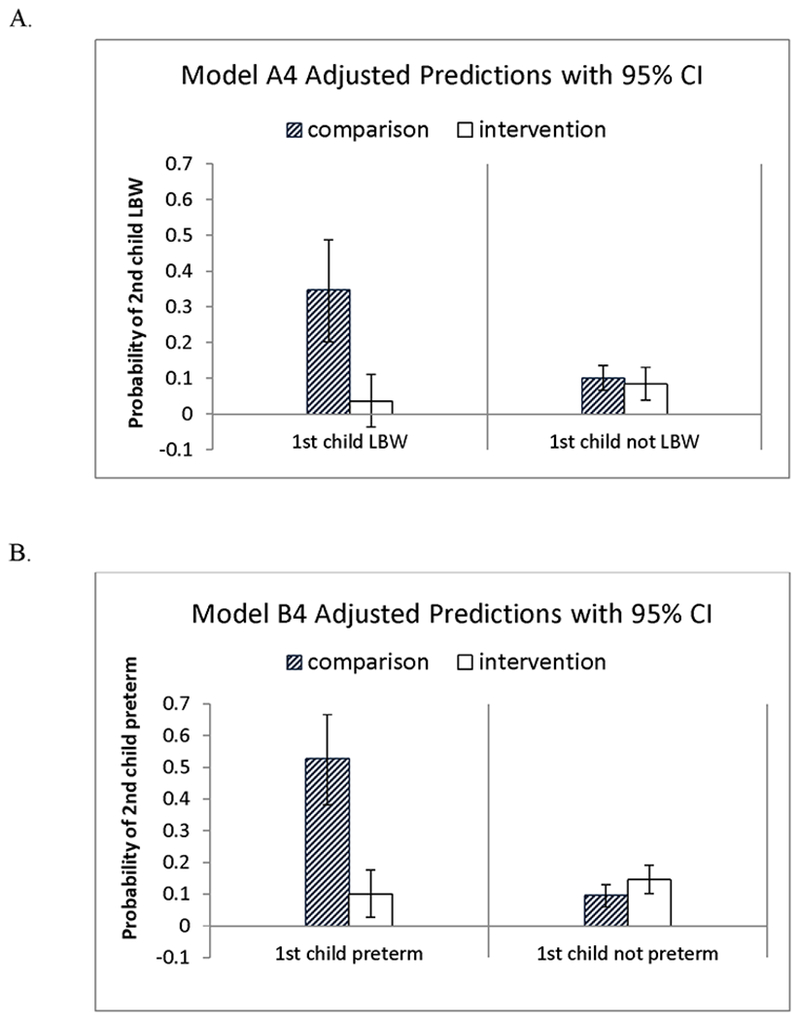
Interactions between the first child being born with low birthweight, or preterm, and the mother being in the original intervention group are illustrated for Models A4 and B4. A. The left pane in the top figure shows that, when the first child has low birthweight (LBW), the intervention effect is substantial; the right pane shows, when the first child does not have low birthweight, there is not detectable intervention effect; 95% confidence intervals are indicated with error bars. B. The bottom figure shows the same information for preterm birth.
The results of the multivariate logistic regression models with preterm birth as the dependent variable are presented in Table 3. Models B1 – B6 are parallel to Models A1 - A6 described above, except that preterm birth was the outcome. In Models B1 – B3, preterm birth of the second child was not associated with the intervention. However, when preterm status of the first birth was included as a moderator, the same pattern was seen: the intervention was associated with a significantly lower rate of preterm birth for second children if the first child was born preterm, but not if the first child was born at full term. The birth of the second child either within 18 months or within the intervention period were both associated with an increased likelihood of preterm birth, although neither of these were supported as mediators.
Table 3:
Preterm birth of the second child - multivariable logistic regression models estimating intervention effects with covariates examining moderating and mediating relationships. Preterm defined at <37 weeks of gestation at birth. Sample size of 470 for all models.
| Model B1 odds ratio (95% CI) | Model B2 odds ratio (95% CI) | Model B3 odds ratio (95% CI) | Model B4 odds ratio (95% CI) | Model B5 odds ratio (95% CI) | Model B6 odds ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Intervention | 1.07 (0.62, 1.85) | 1.15 (0.64, 2.02) | 1.12 (0.64, 1.95) | 1.55 (0.86, 2.81) | 1.79 (0.96, 3.34) | 1.69 (0.92, 3.09) |
| First child preterm | 9.87 (4.32, 22.58)* | 11.03 (4.67, 26.07)* | 10.27 (4.42, 23.85)* | |||
| 1st child preterm X Intervention | 0.06 (0.007, 0.64)* | 0.05 (0.004, 0.49)* | 0.06 (0.006, 0.66)* | |||
| Timing of second birth | ||||||
| Within 18 months of 1st | 3.24 (1.89, 5.56)* | 3.59 (2.02, 6.36)* | ||||
| Within intervention perioda | 2.00 (1.18, 3.36)* | 2.07 (1.20, 3.58)* | ||||
| Timing of 2nd birth: mediatorb | p<.08 | p<.10 | p<.08 | p<.11 | ||
| Intervention effect if first child pretermc | p<.04 | p<.03 | p<.05 | |||
| Intervention effect if first child not pretermc | p<.15 | p<.07 | p<.09 | |||
| Number need to treatd | 114 (13, e) | 44 (11, e) | 54 (11, e) | |||
| First child preterm | 3 (2, 11) | 3 (2, 8) | 4 (2, 57) | |||
| First child not preterm | 19 (8, e) | 15 (7, e) | 16 (8, e) | |||
Note: Additional covariates (all measured at enrollment, unless indicated; all not significant in all models in Table 3) include: gestational weeks, prepregnancy obesity, prehypertension, second child sex, maternal race, smoking history (ever smoked regularly), psychological resources (dichotomous according to original study sample mean), discretionary household income; weeks from last menstrual period (LMP) to first prenatal visit or lab, mother over 18 years, subfecundity (no pregnancy >=2 yrs/birth control < 25%), prehypertension, social support from grandmother, and maternal height.
birth within 27 month.
Sobel test for mediation.
P-value for full contribution of intervention in each scenario. When the first child was not LBW, this is the p-value for the intervention term; when the first child was LBW, this is a Wald test of the linear combination of the intervention term and the intervention-LBW interaction term.
The number of mothers who would need to receive home visiting to avoid one second low birthweight child, calculated from each model.38, 39
Confidence interval includes infinity.
p<.05
Discussion
We report evidence of an intervention effect on reduction of low birthweight for second children born to mothers who received home visiting during their first pregnancy. This effect was mediated by pregnancy spacing and moderated by the first child’s low birthweight status, with intervention effects apparent only among women with low-birthweight first-borns. The intervention effect was not found with preterm birth as the outcome; however, the pattern of intervention on mediating and moderating effects found in low birthweight is similar.
Although birth outcomes have previously been studied in home visiting, the first child generally has been the focus and many studies have been unable to demonstrate intervention effects on birth outcomes in first children. Our findings provide evidence for the importance of a focus on primiparous mothers by home visiting programs, because the advantages of the program are likely to extend to later children. In addition, these findings suggest that targeting mothers of low birthweight or preterm birth children may be important for reducing the likelihood of subsequent low birthweight and preterm delivery. Whether the timing of interventions should be inter-conceptual or during a subsequent pregnancy cannot be determined from the findings reported here. The reason for improvements in birth outcomes for the second, but not first, child may be due to intervention well before conception, or may be due to intervention from the very start of the second pregnancy for those who were engaged in the program at that time. It may also be that the time between the first and second births exposed the mothers to additional stress, or “weathering,” that may accumulate to increase the risk for poor birth outcomes,40 but that the intervention helps to mitigate this effect. This possibility is supported by the higher rates of poor birth outcomes overall for second children in this sample. Additional research is necessary to determine the feasibility of targeting mothers of low birthweight and preterm birth infants, but one study supports the idea: an intervention specifically targeting African-American women with a first very low birthweight child successfully increased pregnancy spacing and reduced poor birth outcomes in a subsequent pregnancy.41
The role of home visits during pregnancy is of particular interest because it may be a time when advice from a home visitor would be best received.42 This advice could well extend into the inter-conceptual and future pregnancy periods. In addition, not all home visiting program models include visits during pregnancy, so exploring the importance of pregnancy visits may shed light on differences in outcomes between program models.
Because the program was individualized and based on the mother’s goals, however, there was no attempt made to anticipate which mothers would choose to have subsequent pregnancies. The multiple components of the intervention are designed to work synergistically to produce outcomes, making it difficult to determine which aspect of the intervention was responsible for reduction in the subsequent child rates of low birthweight. It is important to note that we hypothesized in the original study that mothers in the intervention group would have greater spacing between children than their counterparts in the comparison group. This hypothesis was supported43 and has now been found to be linked with low birthweight in the second infant. The finding that the intervention effect on low birthweight in the subsequent pregnancy was only present for those mothers whose first born was low birthweight was not expected, and illustrates the advantage of study designs including siblings44 in intervention trials where environments are dynamic and expected to change. While the current analysis suggests that avoiding rapid repeat birth partially accounts for the effect of the intervention on subsequent low birthweight, it is entirely possible that the program effect observed here may also be explained by other processes. First, those mothers most vulnerable to delivery of a low birthweight infant may have improved their general health and environment as a result of the intervention. We know that the intervention improved the home environment29 and possibly health, as suggested by a reduction in mortality in mothers and children in the intervention group;45 differential effects by low birthweight have not yet been tested. Second, mothers who had given birth to a previous low birthweight infant may have recognized their vulnerability, utilized what they learned during the intervention in the first pregnancy and improved their self-care before and during the second pregnancy. Third, mothers who gave birth to low birthweight babies during their first pregnancy may have focused on the health and development of that infant, thus delaying a subsequent pregnancy during a period of greater risk. Our sample size and lack of data surrounding second pregnancies prohibited us from examining these potential explanations.
The impact of covariates on low birthweight is consistent with the literature. Despite the low rates of smoking in this population, the impact of smoking on low birthweight was detected as expected. Race was significant in models that included a second birth within 18 months. Given the small number (n=33) of white women within the sample, we were unable to examine race-treatment interaction effects. Although we included household income in the model, it was not a significant predictor of low birthweight, likely due to the relative homogeneity of this very low-income sample. Since neighborhood socioeconomic status has been posited to be associated with low birthweight and preterm birth, we tested a model that included neighborhood as a covariate and subsequently removed it because it added nothing to the model. This is consistent with work of Phillips et al. who found that among U.S. Black women, neighborhood SES was not associated with preterm birth.46
Given the association of low birthweight and preterm deliveries on long term outcomes of mothers47 and their offspring,48 reducing subsequent low birthweight and preterm infants is a national and international priority. Although findings here are promising in terms of the effect of home visitation on low birthweight of subsequent children, they do not provide a clear path on the timing or frequency of home visiting. If the effect of home visiting is primarily derived from an intervention during the first pregnancy and infancy period, there may be little reason to enroll pregnant women who have previously given birth to a low birthweight infant, except for protection during future pregnancies. Future studies that include biomarkers preceding the birth of the second child could potentially elucidate the mechanisms of how the intervention impacts low birthweight.
Limitations
In this analysis, time between first and second pregnancies and low birthweight of first child were the only explanatory variables included in the model that were measured after intake. We did not examine meditational effects of other environmental, biological, and behavioral factors associated with low birthweight and that have been reported previously as having been affected by the intervention. For example, other mediators of treatment such as emergence of hypertension or smoking were not included. These could be examined in future studies. The original study began in 1990, which means that most of the second children were born in the 1990s. However, the rates of low birthweight and preterm birth have not changed substantially since this time, and the factors studied here, and the relationships between them, are not expected to change over this time period. The intervention itself is now widely disseminated, with efforts to ensure fidelity to the original model. In addition, few datasets are available with second child birth outcomes from home visiting randomized trials.
Because the original study was powered for other outcomes, and not all participants had a second child, our sample size was limited, which limited our power to detect intervention effects. For instance, although rapid repeat pregnancy was not supported as a mediator with low birthweight of the first child included (i.e., Model 5A), this may be due to the decrease in power resulting from additional terms in the model. It is unlikely that there is a meaningful difference between the mediation findings in Model 2 (p<.049 vs. p<.058). Because of the limited power of this analysis (due to moderate sample size and relatively low prevalence of the outcome), we interpret this to suggest mediation in both cases, although further work is required to confirm this.
Conclusion
Home visiting primiparous women during pregnancy and/or postnatally has the potential to improve the birth outcomes of later children. This finding contributes additional evidence to the positive impacts of home visiting for families served by the Nurse-Family Partnership program, which was derived from a series of trials, including the New Mother’s study. In addition, a focus on first-time mothers may be justified, because they are more likely to receive benefits that extend beyond the initial pregnancy. These findings may also contribute to our understanding of how spending on social services (including home visiting) can improve population-level health outcomes,49, 50 even when impacts on first child birth outcomes may have been limited.
Acknowledgements
YM was supported by the University of Rochester CTSA award number TL1 TR002000 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of potential conflicts of interest
No authors have competing interests regarding the findings reported.
Contributor Information
Margaret L. Holland, Yale School of Nursing, 400 West Campus Drive, Orange, CT 06518, USA, 203-737-4929, margaret.holland@yale.edu.
Susan W. Groth, University of Rochester, School of Nursing, 255 Crittenden Ave, Rochester, NY 14642, USA, 585-275-8895, susan_groth@urmc.rochester.edu.
Joyce A. Smith, University of Rochester, School of Nursing, 255 Crittenden Ave, Rochester, NY 14642, USA, 585-275-3405, joycea_smith@urmc.rochester.edu.
Ying Meng, University of Rochester, School of Nursing, 255 Crittenden Ave, Rochester, NY 14642, USA, ying_meng@urmc.rochester.edu.
Harriet Kitzman, University of Rochester, School of Nursing, 255 Crittenden Ave, Rochester, NY 14642, USA, 585-275-8874, harriet_kitzman@urmc.rochester.edu .
References
- 1.Class QA, Rickert ME, Lichtenstein P, D’Onofrio BM. Birth Weight, Physical Morbidity, and Mortality: A Population-based Sibling-Comparison Study. Am J Epidemiol 2014, 179(5): 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RC, Schoeni RF. Early-life origins of adult disease: national longitudinal population-based study of the United States. Am J Public Health 2011, 101(12): 2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin Reprod Med 2009, 27(05): 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tekola-Ayele F, Workalemahu T, Amare AT. High burden of birthweight-lowering genetic variants in Africans and Asians. BMC Med 2018, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King K, Murphy S, Hoyo C. Epigenetic regulation of Newborns’ imprinted genes related to gestational growth: patterning by parental race/ethnicity and maternal socioeconomic status. J Epidemiol Commun H 2015, 69(7): 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrman RE, Butler AS. Preterm birth: Causes, consequences, and prevention. National Academy Press: Washington, DC, 2007. [PubMed] [Google Scholar]
- 7.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonat M 2016, 21(2): 68–73. [DOI] [PubMed] [Google Scholar]
- 8.Heerwagen MJR, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol-Reg I 2010, 299(3): R711–R722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee E, Mitchell-Herzfeld SD, Lowenfels AA, Greene R, Dorabawila V, DuMont KA. Reducing low birth weight through home visitation: a randomized controlled trial. Am J Prev Med 2009, 36(2): 154–160. [DOI] [PubMed] [Google Scholar]
- 10.Roman L, Raffo JE, Zhu Q, Meghea CI. A statewide Medicaid enhanced prenatal care program: impact on birth outcomes. JAMA Pediatr 2014, 168(3): 220–227. [DOI] [PubMed] [Google Scholar]
- 11.Shah MK, Austin KR. Do home visiting services received during pregnancy improve birth outcomes? Findings from Virginia PRAMS 2007–2008. Public Health Nurs 2014, 31(5): 405–413. [DOI] [PubMed] [Google Scholar]
- 12.Robling M, Bekkers MJ, Bell K, Butler CC, Cannings-John R, Channon S, et al. Effectiveness of a nurse-led intensive home-visitation programme for first-time teenage mothers (Building Blocks): a pragmatic randomised controlled trial. Lancet 2016, 387(10014): 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peacock S, Konrad S, Watson E, Nickel D, Muhajarine N. Effectiveness of home visiting programs on child outcomes: a systematic review. BMC Public Health 2013, 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Administration On Children & Families. Home Visiting Evidence of Effectiveness: Maternal Outcomes. 2017. [cited 2017 April 15] Available from: https://homvee.acf.hhs.gov/Outcome/2/Maternal-Health/1/1
- 15.Howard KS, Brooks-Gunn J. The Role of Home-Visiting Programs in Preventing Child Abuse and Neglect. The Future of Children 2009, 19(2): 119–146. [DOI] [PubMed] [Google Scholar]
- 16.Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 2006, 295(15): 1809–1823. [DOI] [PubMed] [Google Scholar]
- 17.Holland ML, Kitzman H, Veazie P. The Effects of Stress on Birth Weight in Low-Income, Unmarried Black Women. Women Health Iss 2009, 19(6): 390–397. [DOI] [PubMed] [Google Scholar]
- 18.Administration On Children & Families. Home Visiting Evidence of Effectiveness: Outcomes. 2016. [cited 2016 May 9] Available from: http://homvee.acf.hhs.gov/outcomes.aspx
- 19.Olds DL, Robinson J, Pettitt L, Luckey DW, Holmberg J, Ng RK, et al. Effects of home visits by paraprofessionals and by nurses: age 4 follow-up results of a randomized trial. Pediatrics 2004, 114(6): 1560–1568. [DOI] [PubMed] [Google Scholar]
- 20.Goyal NK, Folger AT, Hall ES, Greenberg JM, Van Ginkel JB, Ammerman RT. Home visiting for first-time mothers and subsequent pregnancy spacing. J Perinatol 2017, 37(2): 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawlings JS, Rawlings VB, Read JA. Prevalence of low birth weight and preterm delivery in relation to the interval between pregnancies among white and black women. N Engl J Med 1995, 332(2): 69–74. [DOI] [PubMed] [Google Scholar]
- 22.Tucker CM, Berrien K, Menard MK, Herring AH, Daniels J, Rowley DL, et al. Predicting Preterm Birth Among Women Screened by North Carolina’s Pregnancy Medical Home Program. Matern Child Health J 2015, 19(11): 2438–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaty TH, Skjaerven R, Breazeale DR, Liang KY. Analyzing sibship correlations in birth weight using large sibships from Norway. Genet Epidemiol 1997, 14(4): 423–433. [DOI] [PubMed] [Google Scholar]
- 24.Beaty TH, Yang P, Munoz A, Khoury MJ. Effect of maternal and infant covariates on sibship correlation in birth weight. Genet Epidemiol 1988, 5(4): 241–253. [DOI] [PubMed] [Google Scholar]
- 25.Bacci S, Bartolucci F, Chiavarini M, Minelli L, Pieroni L. Differences in birthweight outcomes: a longitudinal study based on siblings. Int J Environ Res Public Health 2014, 11(6): 6472–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkle SN, Albert PS, Mendola P, Sjaarda LA, Yeung E, Boghossian NS, et al. The association between parity and birthweight in a longitudinal consecutive pregnancy cohort. Paediatr Perinat Epidemiol 2014, 28(2): 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox MA, Chang AM, Johnson IR. The effects of parity on birthweight using successive pregnancies. Acta Obstet Gynecol Scand 1996, 75(5): 459–453. [DOI] [PubMed] [Google Scholar]
- 28.Northam S, Knapp TR. The Reliability and Validity of Birth Certificates. J Obstet Gynecol Neonatal Nurs 2006, 35(1): 3–12. [DOI] [PubMed] [Google Scholar]
- 29.Kitzman H, Olds DL, Henderson CR, Hanks C, Cole R, Tatelbaum R, et al. Effect of prenatal and infancy home visitation by nurses on pregnancy outcomes, childhood injuries, and repeated childbearing trial: A randomized controlled trial. JAMA 1997, 278(8): 644–652. [PubMed] [Google Scholar]
- 30.Olds DL. Home Visitation Services for Disadvantaged Mothers, NR01-01691-05. National Institute of Nursing Research, National Institutes of Health; 1987. [Google Scholar]
- 31.Shah PS, on behalf of Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta Obstet Gynecol Scand 2010, 89(7): 862–875. [DOI] [PubMed] [Google Scholar]
- 32.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic Disparities in Adverse Birth Outcomes: A Systematic Review. Am J Prev Med 2010, 39(3): 263–272. [DOI] [PubMed] [Google Scholar]
- 33.Hosmer DW Jr, Lemeshow S, Sturdivant RX. Applied logistic regression, vol. 398 John Wiley & Sons, 2013. [Google Scholar]
- 34.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989, 129(1): 125–137. [DOI] [PubMed] [Google Scholar]
- 35.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011, 30(4): 377–399. [DOI] [PubMed] [Google Scholar]
- 36.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014, 311(5): 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin DB, Schenker N. Multiple Imputation for Interval Estimation from Simple Random Samples with Ignorable Nonresponse. J Amer Statist Assoc 1986, 81(394): 366–374. [Google Scholar]
- 38.Bender R nnt_adj.sas. http://www.rbsd.de/softw.html; 2012. Accessed: July 29, 2018
- 39.Bender R, Vervölgyi V. Die Berechnung adjustierter NNTs in randomisierten kontrollierten Studien In: Ortseifen C, Ramroth H, Weires M. Minkenberg R, editor. KSFE 2011 – “Voneinander lernen”, 15. Konferenz der SAS-Anwender in Forschung und Entwicklung; 2011; Ruprechts-Karls-Universität Heidelberg; 2011. [Google Scholar]
- 40.Wallace ME, Harville EW. Allostatic Load and Birth Outcomes Among White and Black Women in New Orleans. Matern Child Health J 2013, 17(6): 1025–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunlop AL, Dubin C, Raynor BD, Bugg GW, Jr., Schmotzer B, Brann AW, Jr. Interpregnancy primary care and social support for African-American women at risk for recurrent very-low-birthweight delivery: a pilot evaluation. Matern Child Health J 2008, 12(4): 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Che S- R, Barrett ES, Velez M, Conn K, Heinert S, Qiu X. Using the Health Belief Model to Illustrate Factors That Influence Risk Assessment during Pregnancy and Implications for Prenatal Education about Endocrine Disruptors. Policy Futures in Education 2014, 12(7): 961–974. [Google Scholar]
- 43.Olds DL, Kitzman H, Cole R, Robinson J, Sidora K, Luckey DW, et al. Effects of nurse home-visiting on maternal life course and child development: age 6 follow-up results of a randomized trial. Pediatrics 2004, 114(6): 1550–1559. [DOI] [PubMed] [Google Scholar]
- 44.Donovan SJ, Susser E. Commentary: advent of sibling designs. Int J Epidemiol 2011, 40(2): 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olds DL, Kitzman H, Knudtson MD, Anson E, Smith JA, Cole R. Effect of Home Visiting by Nurses on Maternal and Child Mortality Results of a 2-Decade Follow-up of a Randomized Clinical Trial. JAMA Pediatrics 2014, 168(9): 800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips GS, Wise LA, Rich-Edwards JW, Stampfer MJ, Rosenberg L. Neighborhood Socioeconomic Status in Relation to Preterm Birth in a U.S. Cohort of Black Women. J Urban Health 2013, 90(2): 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rich-Edwards JW, Klungsoyr K, Wilcox AJ, Skjaerven R. Duration of pregnancy, even at term, predicts long-term risk of coronary heart disease and stroke mortality in women: a population-based study. Am J Obstetr Gynecol, 213(4): 518, e511–518.e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker DJP. In utero programming of chronic disease. Clin Sci 1998, 95(2): 115–128. [PubMed] [Google Scholar]
- 49.Bradley EH, Elkins BR, Herrin J, Elbel B. Health and social services expenditures: associations with health outcomes. BMJ Quality & Safety 2011, 20(10): 826–831. [DOI] [PubMed] [Google Scholar]
- 50.Bradley EH, Canavan M, Rogan E, Talbert-Slagle K, Ndumele C, Taylor L, et al. Variation In Health Outcomes: The Role Of Spending On Social Services, Public Health, And Health Care, 2000–09. Health Aff 2016, 35(5): 760–768. [DOI] [PubMed] [Google Scholar]


