Abstract
Background:
Decay products of radioactive materials may attach to ambient fine particles and form radioactive aerosol. Internal ionizing radiation source from inhaled radioactive aerosol may contribute to the fine particulate matter (PM2.5)-inflammation pathway. However, few studies in humans have examined the associations.
Objectives:
To examine the associations between particle radioactivity and biomarkers of oxidative stress and inflammation among participants from the Framingham Offspring and Third Generation cohorts.
Methods:
We included 3,996 participants who were not current smokers and lived within 50 km from our central air pollution monitoring station. We estimated regional mean gross beta radioactivity from monitors in the northeastern U.S. as a surrogate for ambient radioactive particles, and calculated the 1- to 28-day moving averages. We used linear regression models for fibrinogen, tumor necrosis factor α, interleukin-6, and myeloperoxidase which were measured once, and linear mixed effect models for 8-epi-prostaglandin F2α, C-reactive protein, intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), P-selectin, and tumor necrosis factor receptor-2 that were measured up to twice, adjusting for demographics, individual- and area-level socioeconomic positions, time, meteorology, and PM2.5. We also examined whether the associations differed by median age, sex, diabetes status, PM2.5 levels, and black carbon levels.
Results:
The mean age was 54 years and 54% were women. An interquartile range (3×10−3 pCi/m3) higher beta radioactivity level at the 7-day moving average was associated with 5.09% (95% CI: 0.92, 9.43), 2.65% (1.10, 4.22), and 4.71% (95% CI: 3.01, 6.44) higher levels of interleukin-6, MCP-1, and P-selectin, but with 7.01% (95% CI: −11.64, −2.15) and 2.70% (95% CI: −3.97, −1.42) lower levels of 8-epi-prostaglandin F2α and ICAM-1, respectively.
Conclusions:
Regional mean particle radioactivity was positively associated with interleukin-6, MCP-1, and P-selectin, but negatively with ICAM-1 and 8-epi-prostaglandin F2α among our study participants.
Keywords: gross beta radiation, particle radioactivity, epidemiology, environment, inflammation, oxidative stress
Graphical Abstract
Associations between the 7-day moving average of regional mean gross beta radioactivity (per 3×10−3 pCi/m3) and levels of oxidative stress and inflammation biomarkers among participants from the Framingham Offspring and Third Generation cohorts.
1. Introduction
Individuals are constantly exposed to naturally occurring radioactive materials, mainly contributed by decay products of radon (222Rn) and thoron (220Rn) (Amrane et al. 2014). The radioactive gaseous nuclides escape from the soil produce near ground and can attach to ambient particles forming a radioactive airborne particle mixture (radioactive aerosol). Studies have shown that the majority of this radioactivity is connected with suspended fine particles (PM2.5; particulate matter with aerodynamic diameter ≤2.5 μm) (Mohery et al. 2014; Moriizumi et al. 2014; Papastefanou 2009; Röbig et al. 1981). Compared to particles with larger aerodynamic diameter (e.g. PM2.5–10), PM2.5 has larger surface, higher pulmonary deposition efficiency, and can penetrate deeper into the lung. Once inhaled, the radioactive aerosol may reach the alveoli and may be capable of inducing local oxidative stress and inflammation. In the indoor environment, the levels of radioactive aerosol may build up and have potential adverse health effects: higher exposure to indoor radon (222Rn) has been associated with elevated lung cancer risk (Field et al. 2006; IARC 1988). In the outdoor environment, the decay products of radon and thoron (220Rn) contribute to the majority of radioactive exposure (Akan et al. 2014) but at lower levels.
Exposure to ambient PM2.5 has been associated with cardiovascular and respiratory disease, with air pollution-induced oxidative stress and inflammation hypothesized as important underlying mechanisms. However, the contributions of PM2.5 constituents to these mechanisms are unclear. We, therefore, hypothesized that internal ionizing radiation source formed by inhaled ambient radioactive particles may contribute to the PM2.5-inflammation pathway.
Beta particles are high-energy and high-speed electrons or positrons that are emitted during radioactive decay. In the current study, we measured regional gross beta radioactivity levels as a surrogate of particle radioactivity, and examined the associations of regional beta radioactivity with systemic biomarkers of oxidative stress and inflammation among participants from the Framingham Offspring and Third Generation cohorts, an ambulatory community-based sample residing in the Northeast U.S..
2. Material and methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Framingham Heart Study at https://www.framinghamheartstudy.org/researchers/.
2.1. Study sample
We included participants who attended the seventh (1998–2001) and eighth (2005–2008) examinations of the Framingham Offspring cohort and the first (2002–2005) and second (2008–2011) examinations of the Third Generation cohort. The details of study design and selection criteria of both cohorts have been described elsewhere (Kannel et al. 1979; Splansky et al. 2007). For the current study, we included 4,056 participants (6,963 observations) who were not current smokers (1,115 observations from 571 participants were excluded) and lived within 50 km from the Harvard Supersite air pollution monitoring station (Boston, MA, USA). We excluded current smokers because the likely elevated levels of inflammatory biomarkers among current smokers may interfere with our ability to estimate the expected small magnitude of changes in biomarker levels associated with particle radioactivity (Levitzky et al. 2008). We assigned “missing” to biomarker levels that were lower than the minimum detection limit (27 observations for C-reactive protein (CRP), 10 observations for interleukin-6, and 1 observation for tumor necrosis factor receptor-2 (TNFR-2)), or that were extremely high (1 observation with TNFR-2 level at 948,114 pg/ml). In total, we excluded 32 observations with no measurement of any biomarker, and 103 observations with missing data on body mass index, alcohol intake, and cigarette smoking pack-years. We additionally excluded 14 observations with no measurement of PM2.5 on the day before examination visit, because we adjusted for PM2.5 in the primary analyses, leaving 3,996 participants (6,814 observations) eligible for the present analyses. At each examination visit, physical exams were performed following standardized protocols, and we collected data on demographics, medication history, smoking history, and alcohol intake using publicly available questionnaires. All participants provided written informed consent at each examination, and the Institutional Review Boards at Beth Israel Deaconess Medical Center (CCI Protocol#: 2009-P-000224) and Boston University Medical Center approved the study.
2.2. Exposure assessment
Gross beta particle radioactivity, which measures beta radioactivity regardless of specific radionuclide source, was used as a surrogate for particle radioactivity in this study. The gross beta activity data was obtained from the U.S. Environmental Protection Agency’s RadNet monitoring network (U.S. Environmental Protection Agency 2017). The RadNet network was established in 1973 and it consists of 135 sampling stations that collect airborne particulate samples. Each stationary air monitoring station is equipped with a Total Suspended Particle high volume air sampler that collects particles on filters that are replaced twice per week. The gross beta radioactivity was then measured in the field following a 5-hour period to permit decay of short-lived radon progenies (e.g., 214Pb, 214Bi) that may be attached to the collected particles. Based on laboratory experiments, conducted at the Kuwait Institute of Scientific Research, we found that much of the beta radiation, after the short-lived radon progenies are decayed and in the absence of artificial radionuclides, is due to the 212Pb. This is a progeny of 220Rn (Thoron) and has a half-life of approximately 10 hr. We also found strong correlation between gross beta and gross alpha activities (adjusted R2=0.98). To determine the quantity of beta radioactivity on the filters, background radioactivity level was subtracted from the gross beta activity measurements.
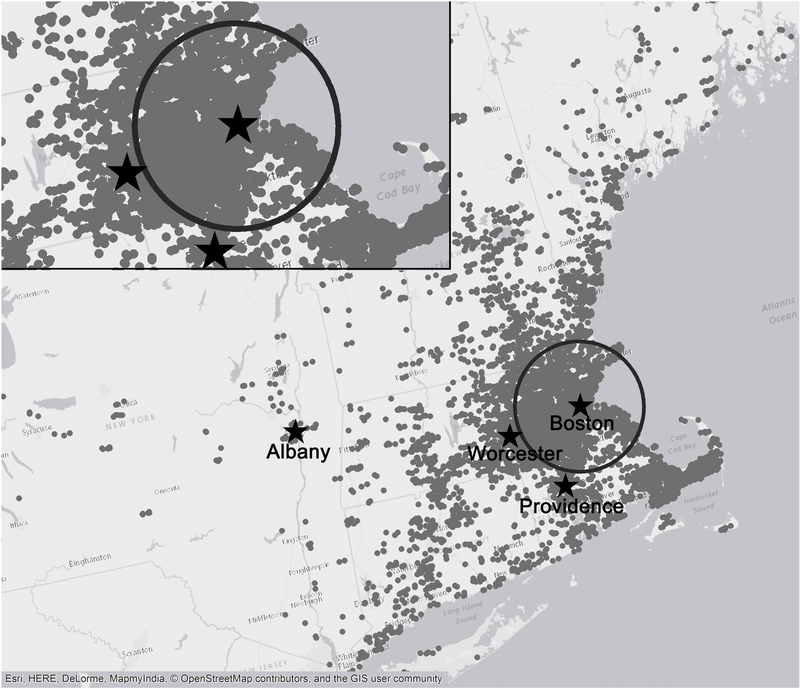
Data from four monitoring sites (Boston, Massachusetts; Worcester, Massachusetts; Providence, Rhode Island; and Albany, New York) were used in the current study. Because the measurements were taken over several days (typically 5 to 7 days), the same mean gross beta radioactivity level was assigned to days within the same sampling period, and we then calculated moving averages based on daily values. Because data collected from each site did not cover the entire study period, we used measurements of gross beta radioactivity from the Albany site, which covered the majority days in the study period from 1998 to 2011, to impute missing levels of gross beta radioactivity in each of the other three sites separately. Briefly, for each site we regressed the available daily measurements on the corresponding data collected at the Albany site to estimate a slope and an intercept that we used to predict the missing gross beta radioactivity levels. The R2 values for the three calibration regressions ranged from 0.62 to 0.71. For the 83 noncontiguous days during the study period that the Albany site had no measurement, levels of gross beta radioactivity were first interpolated based on measured values at the site, and then were used to predict beta radioactivity levels in the other three sites. Last, we averaged the daily gross beta radioactivity levels from all three sites (Boston, Worcester, and Providence) to obtain the regional daily beta radioactivity level for the current study. Detailed assessment methods have been described elsewhere (Nyhan et al. 2018). We have provided a map showing the relative locations of the monitoring sites and the distributions of our study sample (Figure 1).
Figure 1. Map of the study region showing the distribution of the study participants and the locations of monitors.
To protect the confidentiality of the participants, the residential locations on the map were masked by altering the longitude and latitude by a small random amount. Thus, although these locations are representative of the distribution of participants’ residential locations, none of them represent actual residential locations of the study participants. Circle indicates the 50 km radius from Supersite. Dots: residential locations of the study participants.
2.3. Ambient PM2.5, black carbon (BC), temperature, and relative humidity
We calculated daily PM2.5 and BC concentrations based on hourly measurements from the Harvard Boston Supersite air pollution monitoring station. This monitoring station is located on the rooftop of the Francis A. Countway Library of Medicine (20 m above ground) and 50 m from the nearest street. PM2.5 was measured using a tapered element oscillating microbalance (Model 1400A, Rupprecht & Patashnick Co., Inc.); BC was measured using an aethalometer (Model AE-16, Magee Scientific Corp.). The detailed measurement methods have been described previously (Kang et al. 2010). We obtained temperature and relative humidity data from Boston Logan International Airport Weather Station, located 12 km from the central site.
2.4. Biomarker assessment
The blood samples were collected in the morning after an overnight fast, and were stored at −80ºC until the biomarkers were assayed. The urine samples were collected and stored at −20ºC (for creatinine) or −80ºC (for 8-epi-prostaglandin F2α (8-epi-PGF2α)) until the biomarkers were assayed. Plasma samples were analyzed for myeloperoxidase (MPO), P-selectin, TNF-α, TNFR-2, and fibrinogen, serum samples were assessed for CRP, interleukin-6, intercellular adhesion molecule-1 (ICAM-1), and monocyte chemoattractant protein-1 (MCP-1). CRP was measured in the Offspring cohort examination cycle 7 and Third Generation cohort examination cycle 1 using a Dade Behring BN 100 nephelometer; cycle 8 and Third Generation cohort examination cycle 2 used Roche Diagnostics Latex High Sensitivity Assay. Fibrinogen was measured in duplicate from citrated plasma using the Clauss method (Diagnostica Stago Reagents) in the Offspring cohort examination cycle 7 and Third Generation cohort examination cycle 1. MPO was measured in the Offspring cohort examination cycle 7 and 8-epi-PGF2α were measured in examination cycles 7 and 8, both by enzyme immunoassay kit (OXIS Health Products and Cayman Chemical, respectively). Measured 8-epi-PGF2α was adjusted for urinary creatinine to account for urine dilution. Commercially available ELISA kits (R&D Systems) were used to measure ICAM-1, interleukin-6, MCP-1, P-selectin, TNF-α, and TNFR-2. All intra-assay coefficients of variation were <10%. Details regarding biomarker measurements have been reported elsewhere (Fontes et al. 2013; Keaney et al. 2003; McManus et al. 2013; Pou et al. 2007) and can also be found at the Framingham Heart Study website: https://www.framinghamheartstudy.org/researchers/description-data/noninvasive-biomarker.php.
2.5. Statistical methods
We calculated 1-, 7-, 14-, 21-, and 28-day moving averages of the regional mean beta radioactivity level, PM2.5, and BC prior to examination dates. We defined the 1-day moving average as the average value on the day before the examination. For each moving average, we fitted multivariable linear regression models for MPO, fibrinogen, TNF-α, and interleukin-6, and multivariable linear mixed effects models with subject-specific random intercepts for indexed 8epi-PGF2α, CRP, TNFR-2, ICAM-1, MCP-1, and P-selectin. We loge-transformed the levels of biomarkers so that the residuals approximated a normal distribution. In the statistical models we adjusted for demographic variables and individual- and area-level socioeconomic position variables: centered age and (centered age)2; sex; body mass index; cigarette smoking status (former or never smoker); cigarette pack-years; alcohol intake (drinks/week; standardized to 15 mL alcohol/drink) (Elias et al. 1999); educational level (high school or less, some college, and college graduate); median household income in the participant’s census tract from U.S. Census 2000 data. To account for seasonality and time, we adjusted for a linear term of examination date, day of week of examination date, and sine and cosine day of the year (calculated as sin (2π×(day of year)/365.24) and cos (2π×(day of year)/365.24)). We also adjusted for temperature and relative humidity to account for meteorological conditions. In addition, we adjusted for PM2.5 in the model because the concentration of PM2.5 may affect measured radioactivity and is associated with levels of inflammation (Li et al. 2017). Last, we adjusted for an examination identifier if the biomarker was measured in multiple examinations. Because the percent of missingness in education attainment is very small (N=35; 0.5%), we created a missing indicator for those participants.
For sensitivity analyses, we examined whether associations differed after including current smokers or after replacing PM2.5 with BC in the model. We also examined whether associations varied by median age (53 years), sex, diabetes, and PM2.5 and BC levels above or below the 75th percentile (12 μg/m3 for PM2.5 and 1 μg/m3 for BC (Li et al. 2017)) by adding interaction terms.
Because adjusting for PM2.5, temperature, and relative humidity in the primary analyses did not materially alter our results, we explored the associations among 5,709 eligible participants (9,957 observations) who were non-current smokers and lived in the northeast U.S. We removed PM2.5, temperature, and relative humidity from that model.
Estimates were scaled to a 3×10−3 pCi/m3 increment, which approximated the interquartile range of the regional mean beta radioactivity level, and we reported estimated % difference with 95% confidence intervals (CIs). We focused on describing and highlighting association patterns that we considered consistent across multiple moving averages. Analyses were performed using PROC GLM and PROC MIXED in SAS 9.4 (SAS Institute, Inc., Cary, NC). Figures were plotted using Stata 13 (StataCorp LP, College Station, TX).
3. Results
The characteristics of the study population are shown in Table 1. The mean age of the study population was 54 (standard deviation: 14) years old, and over half were women. On the day before the examination, the average regional mean gross beta radioactivity was 7.45 (standard deviation: 2.26) ×10−3 pCi/m3, and the average PM2.5 concentration was 9.68 (standard deviation: 5.81) μg/m3. The characteristics of the 1-, 7-, 14-, 21- and 28-day moving averages of regional mean beta radioactivity level are shown in Table 2. As expected, correlations between moving averages were moderate to high. Histograms showing the distribution of the biomarkers are shown in Figure S1, numbers of participants in each examination are shown in Table S1, and the correlations between beta radioactivity, PM2.5, and BC are shown in Table S2.
Table 1.
Characteristics of 6,814 observations (3,996 participants) from the Framingham Offspring and Third Generation cohorts.
| Characteristicsa | Offspring cohort | Third Generation cohort | Overall | ||
|---|---|---|---|---|---|
| Cycle 7 | Cycle 8 | Cycle 1 | Cycle 2 | ||
| Number of participants | 1,844 | 1,560 | 1,817 | 1,593 | 6,8 14 |
| Age, years | 61.6 ± 9.53 | 67.3 ± 9.15 | 40.1 ± 8.86 | 46.5 ± 8.55 | 53.6 ± 14.2 |
| Women | 985 (53.4%) | 844 (54.1%) | 968 (53.3%) | 852 (53.5%) | 3649 (53.6%) |
| Educational attainmentb | |||||
| High school or less | 665 (36.1%) | 554 (35.5%) | 265 (14.6%) | 230 (14.4%) | 1714 (25.2%) |
| College | 562 (30.5%) | 486 (31.2%) | 575 (31.7%) | 481 (30.2%) | 2104 (30.9%) |
| Higher than college | 587 (31.8%) | 520 (33.3%) | 973 (53.6%) | 881 (55.3%) | 2961 (43.5%) |
| Nonsmoker | 793 (43.0%) | 670 (43.0%) | 1254 (69.0%) | 1046 (65.7%) | 3763 (55.2%) |
| Former smoker | 1051 (57.0%) | 890 (57.1%) | 563 (31.0%) | 547 (34.3%) | 3051 (44.8%) |
| Pack years | 14.0 ± 20.5 | 14.0 ± 20.5 | 3.51 ± 8.91 | 4.63 ± 10.2 | 9.02 ± 16.8 |
| Alcohol intake, drink/eek | 4.49 ± 7.02 | 3.98 ± 6.65 | 4.28 ± 6.20 | 4.49 ± 6.13 | 4.32 ± 6.52 |
| BMI, kg/m2 | 28.4 ± 5.33 | 28.6 ± 5.45 | 27.1 ± 5.62 | 28.2 ± 5.75 | 28.1 ± 5.57 |
| MPOc, ng/mL | 40.7 ± 22.6 | - | - | - | 40.7 ± 22.6 |
| 8-epi-PGF2αc, ng/mmol creatinine | 111 ± 63.6 | 106 ± 74.9 | - | - | 109 ± 69.7 |
| CRPc, mg/L | 2.27 ± 2.53 | 1.68 ± 1.79 | 1.11 ± 1.38 | 1.40 ± 1.51 | 1.56 ± 1.82 |
| Fibrinogenc, mg/dL | 372 ± 71.9 | - | 331 ± 66.1 | - | 351 ± 72.0 |
| ICAM-1c, ng/mL | 245 ± 58.0 | 291 ± 91.4 | 240 ± 57.8 | - | 256 ± 70.6 |
| Interleukin-6c, pg/mL | - | 2.01 ± 1.50 | 1.37 ± 0.909 | - | 1.63 ± 1.19 |
| MCP-1c, pg/mL | 312±104 | 374±116 | 331±110 | - | 336±113 |
| P-selectinc, ng/mL | 35.2 ± 13.3 | 39.1 ± 12.6 | 45.4 ± 16.8 | - | 39.7 ± 14.9 |
| TNF-αc, pg/mL | 1.28 ± 0.639 | - | - | - | 1.28 ± 0.639 |
| TNFR-2c, pg/mL | 2082 ± 664 | 2555 ±926 | 2180±524 | - | 2251±720 |
| Gross beta radioactivityd, x10-3 pCi/m3 | 7.97 ± 2.23 | 6.60 ± 1.69 | 8.23 ± 2.45 | 6.80 ± 2.06 | 7.45 ± 2.26 |
| PM2.5d, μg/m3 | 10.6 ± 5.26 | 9.10 ± 5.39 | 10.9 ± 7.10 | 7.83 ± 4.49 | 9.68 ± 5.81 |
Note: BMI, body mass index; MPO, myeloperoxidase; 8-epi-PGF2α, 8-epi-prostaglandin F2α; CRP, C-reactive protein; ICAM-1, intercellular adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor receptor-a; TNFR-2, tumor necrosis factor receptor-2; PM2.5, fine particulate matter.
Mean ± Standard Deviation or N (%).
35 (0.5%) participants had no information on educational attainment.
Geometric mean and standard deviation.
Calculated based on the 1-day moving averages.
Table 2.
Characteristics of the 1- to 28-day moving averages of gross beta radioactivity level.
| Day moving average, ×10−3 pCi/m3 | Mean ± SD | Interquartile Range | Pearson Correlation Coefficients |
|||
|---|---|---|---|---|---|---|
| 7-day | 14-day | 21-day | 28-day | |||
| 1-day | 7.45 ± 2.26 | 2.93 | 0.82 | 0.68 | 0.61 | 0.58 |
| 7-day | 7.46 ± 2.04 | 2.63 | 0.88 | 0.78 | 0.73 | |
| 14-day | 7.48 ± 1.81 | 2.42 | 0.94 | 0.87 | ||
| 21-day | 7.48 ± 1.65 | 2.30 | 0.96 | |||
| 28-day | 7.48 ± 1.56 | 2.23 | ||||
Note: SD, standard deviation.
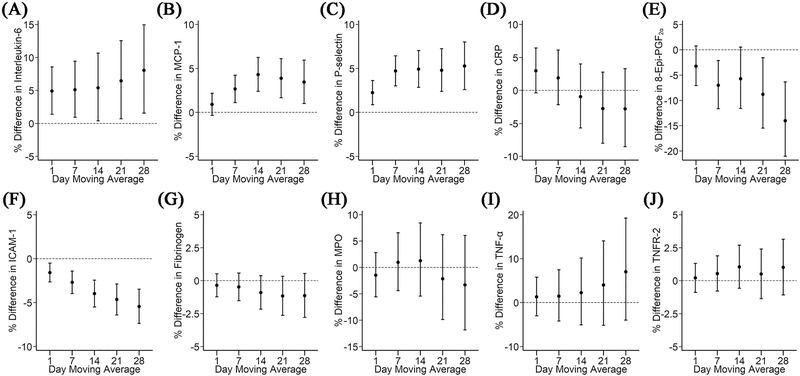
The results from our main analyses are shown in Figure 2. Exposure to higher levels of regional mean beta radioactivity was associated with higher levels of interleukin-6, MCP-1, and P-selectin across multiple moving averages. For example, a 3×10−3 pCi/m3 increase in the 7-day moving average was associated with 5.09% (95% CI: 0.92, 9.43), 2.65% (95% CI: 1.10, 4.22), and 4.71% (95% CI: 3.01, 6.44) higher levels of interleukin-6, MCP-1, and P-selectin, respectively (Figures 2A, 2B, and 2C). The magnitude of the associations were similar between 7-day and 28-day moving averages. The 1-day moving average of regional mean particle beta radioactivity was associated with 2.97% (95% CI: −0.38, 6.44) higher CRP levels; however, the associations were attenuated for 7- to 28-day moving averages (Figure 2D). We unexpectedly found negative associations of regional mean particle beta radioactivity level with indexed 8-epi-PGF2α and ICAM-1(Figures 2E and 2F): a 3×10−3 pCi/m3 increase in the 7-day moving average of regional mean beta radioactivity level was associated with 7.01% (95% CI: −11.64, −2.15) and 2.70% (95% CI: −3.97, −1.42) lower levels of indexed 8-epi-PGF2α and ICAM-1. Longer moving averages of regional mean beta radioactivity level appeared to be negatively associated with fibrinogen (Figure 2G), however, the 95% CIs were wide and overlapped the null. The associations for MPO, TNF-α, and TNFR-2 were generally null. Detailed results are provided in Table S3.
Figure 2. Associations of 1- to 28-day moving averages of gross beta radioactivity with (A) interleukin-6, (B) MCP-1, (C) P-selectin, (D) CRP, (E) 8-epi-PGF2α, (F) ICAM-1, (G) fibrinogen, (H) MPO, (I) TNF-α, and (J) TNFR-2 from participants of the Framingham Offspring and Third Generation cohorts.
Models were adjusted for centered age and (centered age)2; sex; body mass index; cigarette smoking status; cigarette pack-years; alcohol intake; educational level; median household income in the participant’s census tract from U.S. Census 2000 data; linear term of examination date, day of week of examination date, and sine and cosine term of the examination date; temperature and relative humidity; and PM2.5. We added an examination identifier for 8-epi-PGF2α, CRP, fibrinogen, ICAM-1, interleukin-6, MCP-1, Pselectin, and TNFR-2. Results were scaled by 3×10−3 pCi/m3. Error bars indicate the 95% confidence intervals.
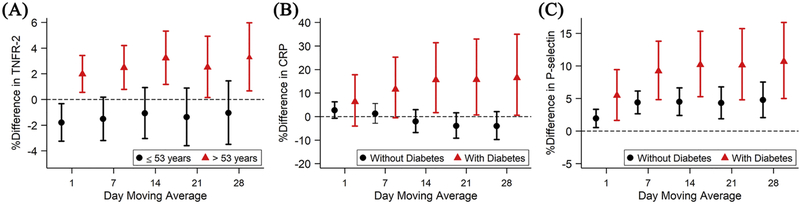
Including current smokers or replacing PM2.5 with BC did not alter our conclusion. Stronger associations between beta radioactivity and circulating ICAM-1 were observed for those who were older than 53 years old (median age). Although the regional mean beta radioactivity was not associated with TNFR-2 in our main analyses, the associations were positive among participants who were older (Figure 3A; Figure S2). The positive associations of regional mean beta radioactivity with P-selectin were stronger among men, while the negative associations with ICAM-1 were stronger among women (Figure S3). Furthermore, stronger positive associations were observed at longer moving averages with CRP and P-selectin among those who had type 2 diabetes than those without (Figures 3B and 3C; Figure S4). Last, we examined whether the associations differed by PM2.5 levels and BC levels. The associations of longer moving averages of regional mean beta radioactivity with indexed 8-epi-PGF2α, CRP, and fibrinogen were negative among those who were exposed to PM2.5 above the upper quartile (12 μg/m3), and the negative associations with ICAM-1 were stronger among those who were exposed to BC above the upper quartile (1 μg/m3).
Figure 3.
Associations of 1- to 28-day moving averages of gross beta radioactivity with (A) TNFR-2 by median age, and of (B) CRP and (C) P-selectin by diabetes status. Models were adjusted for centered age and (centered age)2; sex; body mass index; cigarette smoking status; cigarette pack-years; alcohol intake; educational level; median household income in the participant’s census tract from U.S. Census 2000 data; linear term of examination date, day of week of examination date, and sine and cosine term of the examination date; temperature and relative humidity; and PM2.5; and an exam identifier. Results were scaled by 3×10−3 pCi/m3. Error bars indicate the 95% confidence intervals.
Extending the analyses to all 5,709 eligible participants in the northeast U.S. led to attenuated associations for CRP, interleukin-6, MCP-1, and P-selectin. However, the positive associations of particle radioactivity with P-selectin and negative associations with ICAM-1 and fibrinogen remained.
4. Discussion
Among participants from the Framingham Offspring and Third Generation cohorts, we observed that exposure to higher levels of regional mean beta radioactivity was associated with higher circulating levels of interleukin-6 and P-selectin, but lower levels of urinary 8-epi-PGF2α and serum ICAM-1 after adjusting for ambient PM2.5 level. We further observed that the associations may differ by age, diabetes status, PM2.5 level, or BC level. Although we observed consistent positive associations with MCP-1 in the primary analyses, the association pattern was not observed after we extended analyses to a broader geographic region. Our study is among the first studies to examine the associations of exposure to ambient particle beta radioactivity with oxidative stress and inflammatory biomarkers.
We observed moderate associations of regional mean particle beta radioactivity with inflammatory biomarkers after adjusting for PM2.5 concentrations; however, the underlying mechanisms are unclear. Radiation therapy administered at low dose, but much higher than ambient levels, has been reported to be associated with heightened immune response and systemic inflammation (Frey et al. 2015; Hekim et al. 2015). The current U.S. Environmental Protection Agency (EPA) recommendation on indoor radon level is <4 pCi/L (i.e., 4,000 pCi/m3) (U.S. Environmental Protection Agency 2016) and the current WHO recommendation is <2.7 pCi/L (World Health Organization 2009). However, the ambient particle beta radioactivity levels cannot be directly compared to indoor radon levels, and we measured particle beta radioactivity as a surrogate of the overall ambient particle radioactivity. According to the USEPA’s RadNet database (https://iaspub.epa.gov/enviro/erams_query_v2.simple_query), between January, 2000 and August, 2018, the overall average level of gross beta activity in the U.S. was 0.01 pCi/m3, the average gross beta activity in Massachusetts between October, 2004 and August, 2018 was 6.86×10−3 pCi/m3 (range: 0.219×10−3 pCi/m3 to 35.2×10−3 pCi/m3). Outside of the U.S., a study conducted in Malaga, Spain, found an average of 15.3×10−3 pCi/m3 for gross beta activity between 1992 and 1995 (Dueñas et al. 1999); meanwhile another study conducted in Tehran, Iran, observed an average of 6.02×10−3 pCi/m3 for gross beta activity between 2001 and 2004 (Arkian et al. 2008).
We unexpectedly observed negative associations of the regional mean beta radioactivity with indexed 8-epi-PGF2α and ICAM-1; however, the reasons for the negative associations are unclear. Because the associations between radiation exposure and inflammatory response are complicated and the exact biological mechanisms are unclear, further mechanistic research will be required to explain the observed negative associations.
Among participants of the Framingham Heart Study, we examined the associations between short-term exposure to ambient air pollution with biomarkers of oxidative stress and inflammation in our previous work (Li et al. 2017; Li et al. 2016). In those analyses, we observed positive associations of PM2.5 and BC with levels of MPO, 8-epi-PGF2α, CRP, and TNFR-2, and negative associations between PM2.5 and fibrinogen. In the current study, although we accounted for PM2.5 in the analyses, we still observed negative associations between particle radioactivity and levels of 8-epi-PGF2α. Consistent with our previous study in which we examined the associations between ambient air pollution and inflammatory biomarkers, we observed stronger associations of radioactive particles with circulating levels of CRP and P-selectin among participants with type 2 diabetes than those without. The susceptibility may be attributable to chronic inflammation-induced endothelial dysfunction among these participants. We also found positive associations between regional mean beta radioactivity level and TNFR-2 only among participants who were older than the median age (53 years old), suggesting increased susceptibility among older individuals.
There are several limitations to our study. First, several sources may introduce non-differential measurement error to our exposure assessment. Because beta radioactivity levels were not available at all monitors on all days during the study period, we utilized a regression model to estimate the regional mean beta radioactivity level. However, the R2 value of the model ranged from 0.62 to 0.71 for the monitoring sites, and there were moderate correlations across sites (r=0.40 to 0.63). Additionally, beta radioactivity levels at each site was assessed over 5 to 7 days, which limited our ability to examine the associations within shorter time frames. However, the beta radioactivity appeared to be stable over short periods of time. We also lack measures of absorbance dose, the indoor radiation exposures, and participants’ radiation therapy history. However, participants in the Framingham Heart Study were generally healthy and the percentage of participants who received radiation therapy was expected to be small. Overall, because the beta radioactivity levels were measured independent of biomarker measurements, any measurement error is unlikely to be associated with participants’ biomarker levels. Second, participants in the Framingham Heart Study were mostly middle-age individuals of European ancestry. Our results may not be generalizable to populations with different age distributions or of different ethnicity/ancestry. Third, we cannot rule out residual confounding and unmeasured confounding, and we cannot establish temporality. Thus, our findings preclude inference for causality.
Our study also has several strengths. First, the Framingham Heart Study is a well-characterized large community-based cohort. Participants were examined by physicians and nurses with standardized protocols. Second, the levels of ambient air pollution in the study region were relatively low, which reduced potential confounding from PM2.5. Third, the assessment of beta radioactivity level and biomarker assessment were independent. Participants in the Framingham Heart Study scheduled their examination visits months in advance, which reduced potential differential exposure measurement error.
5. Conclusions
Our findings suggested positive associations between exposure to ambient particle radioactivity and several oxidative stress and inflammatory biomarkers after accounting for ambient PM2.5 concentrations, but not all. At the moment, we have very little information on the potential adverse health effects of ambient particle radioactivity as well as the underlying biological mechanism. Thus, our findings have limited public health implications until these associations are confirmed or refuted in future controlled animal studies and human epidemiological studies.
Supplementary Material
Highlights.
Decay products of ambient radioactive materials can attached to ambient particles
Inhaled radioactive particles may form an ionizing radiation source in the lung
Few studies studied the associations of particle radioactivity with inflammation
Particle radioactivity was associated with inflammation adjusting for air pollution
Associations differed by age, sex, diabetes status and ambient air pollution levels
Acknowledgements:
We thank the participants of the Framingham Offspring and Third Generation cohorts.
Sources of funding: This publication was made possible by USEPA grant (RD-835872–01) through the Harvard University USEPA sponsored Air, Climate & Environment (ACE) Centre. The contents of the study are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University (Contract No. N01-HC-25195 and HHSN268201500001I). This work was also supported by the NHLBI grants (Grant No. 1RO1 HL64753; R01 HL076784; 1 R01 AG028321; 1R01HL128914; 2R01 HL092577; and 1P50HL120163), and NIEHS grants (Grant No. P01 ES09825 and P30 ES000002).
Abbreviations:
- 8-epi-PGF2α
8-epi-prostaglandin F2α
- BC
Black carbon
- CRP
C-reactive protein
- ICAM-1
Intercellular adhesion molecule 1
- MCP-1
Monocyte chemoattractant protein 1
- PM2.5
Fine particulate matter
- TNF-α
Tumor necrosis factor α
- TNFR-2
Tumor necrosis factor receptor 2
Footnotes
Disclosures: The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akan Z; Baskurt B; Asliyuksek H; Kam E; Yilmaz A; Yuksel MB; Biyik R; Esen R; Koca D Environmental Radioactivity and High Incidence Rates of Stomach and Esophagus Cancer in the Van Lake Region: A Causal Relationship? Asian Pac J Cancer Prev 2014;15:375–380. [DOI] [PubMed] [Google Scholar]
- Amrane M; Oufni L; Misdaq MA Attached and unattached fractions of short-lived radon decay products in outdoor environments: effect on the human respiratory system. Radiat Prot Dosimetry 2014;162:400–409. [DOI] [PubMed] [Google Scholar]
- Arkian F; Salahinejad M; Bidokhti AA; Meshkatee A Analysis of gross alpha, gross beta activities and beryllium-7 concentrations in surface air: their variations and statistical prediction model. Environmental Monitoring and Assessment 2008;140:325–330. [DOI] [PubMed] [Google Scholar]
- Dueñas C; Fernández MC; Liger E; Carretero J Gross alpha, gross beta activities and 7Be concentrations in surface air: analysis of their variations and prediction model. Atmospheric Environment 1999;33:3705–3715. [Google Scholar]
- Elias PK; Elias MF; D’Agostino RB; Silbershatz H; Wolf PA Alcohol Consumption and Cognitive Performance in the Framingham Heart Study. Am J Epidemiol 1999;150:580–589. [DOI] [PubMed] [Google Scholar]
- Field RW; Krewski D; Lubin JH; Zielinski JM; Alavanja M; Catalan VS; Klotz JB; Letourneau EG; Lynch CF; Lyon JL; Sandler DP; Schoenberg JB; Steck DJ; Stolwijk JA; Weinberg C; Wilcox HB An Overview of the North American Residential Radon and Lung Cancer Case-control Studies. J Toxicol Environ Health A 2006;69:599–631. [DOI] [PubMed] [Google Scholar]
- Fontes JD; Yamamoto JF; Larson MG; Wang N; Dallmeier D; Rienstra M; Schnabel RB; Vasan RS; Keaney JF Jr.; Benjamin EJ Clinical Correlates of Change in Inflammatory Biomarkers: The Framingham Heart Study. Atherosclerosis 2013;228:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey B; Hehlgans S; Rodel F; Gaipl US Modulation of Inflammation by Low and High Doses of Ionizing Radiation: Implications for Benign and Malign Diseases. Cancer Lett 2015;368:230–237. [DOI] [PubMed] [Google Scholar]
- Hekim N; Cetin Z; Nikitaki Z; Cort A; Saygili EI Radiation Triggering Immune Response and Inflammation. Cancer letters 2015;368:156–163. [DOI] [PubMed] [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risk to Humans: Man-made Fibres and Radon. International Agency for Research on Cancer, Lyon; 1988. Available from: https://monographs.iarc.fr/iarc-monographs-on-the-evaluation-of-carcinogenic-risks-to-humans-78/ [Google Scholar]
- Kang CM; Koutrakis P; Suh HH Hourly Measurements of Fine Particulate Sulfate and Carbon Aerosols at the Harvard-U.S. Environmental Protection Agency Supersite in Boston. J Air Waste Manag Assoc 2010;60:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB; Feinleib M; McNamara PM; Garrison RJ; Castelli WP An Investigation of Coronary Heart Disease in Families. The Framingham Offspring Study. Am J Epidemiol 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- Keaney JF Jr.; Larson MG; Vasan RS; Wilson PW; Lipinska I; Corey D; Massaro JM; Sutherland P; Vita JA; Benjamin EJ; Framingham S Obesity and Systemic Oxidative Stress: Clinical Correlates of Oxidative Stress in the Framingham Study. Arterioscler Thromb Vasc Biol 2003;23:434–439. [DOI] [PubMed] [Google Scholar]
- Levitzky YS; Guo CY; Rong J; Larson MG; Walter RE; Keaney JF Jr.; Sutherland PA; Vasan A; Lipinska I; Evans JC; Benjamin EJ Relation of Smoking Status to a Panel of Inflammatory Markers: The Framingham Offspring. Atherosclerosis 2008;201:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W; Dorans KS; Wilker EH; Rice MB; Ljungman PL; Schwartz JD; Coull BA; Koutrakis P; Gold DR; Keaney JF Jr.; Vasan RS; Benjamin EJ; Mittleman MA Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler Thromb Vasc Biol 2017;37:1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W; Wilker EH; Dorans KS; Rice MB; Schwartz J; Coull BA; Koutrakis P; Gold DR; Keaney JF Jr.; Lin H; Vasan RS; Benjamin EJ; Mittleman MA Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study. J Am Heart Assoc 2016;5:e002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus DD; Beaulieu LM; Mick E; Tanriverdi K; Larson MG; Keaney JF Jr.; Benjamin EJ; Freedman JE Relationship Among Circulating Inflammatory Proteins, Platelet Gene Expression, and Cardiovascular Risk. Arterioscler Thromb Vasc Biol 2013;33:2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohery M; Abdallah AM; Al-Amoudi ZM; Baz SS Activity size distribution of some natural radionuclides. Radiat Prot Dosimetry 2014;158:435–441. [DOI] [PubMed] [Google Scholar]
- Moriizumi J; Yamada S; Xu Y; Matsuki S; Hirao S; Yamazawa H Indoor/outdoor radon decay products associated aerosol particle-size distributions and their relation to total number concentrations. Radiat Prot Dosimetry 2014;160:196–201. [DOI] [PubMed] [Google Scholar]
- Nyhan MM; Coull BA; Blomberg AJ; Vieira CLZ; Garshick E; Aba A; Vokonas P; Gold DR; Schwartz J; Koutrakis P Associations Between Ambient Particle Radioactivity and Blood Pressure: The NAS (Normative Aging Study). Journal of the American Heart Association 2018;7:e008245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastefanou C Radon Decay Product Aerosols in Ambient Air. Aerosol and Air Quality Research 2009;9:385–393. [Google Scholar]
- Pou KM; Massaro JM; Hoffmann U; Vasan RS; Maurovich-Horvat P; Larson MG; Keaney JF Jr.; Meigs JB; Lipinska I; Kathiresan S; Murabito JM; O’Donnell CJ; Benjamin EJ; Fox CS Visceral and Subcutaneous Adipose Tissue Volumes are Cross-sectionally Related to Markers of Inflammation and Oxidative Stress: the Framingham Heart Study. Circulation 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- Röbig G; Becker KH; Hessin A; Porstendörfer J; Scheibel HG A cascade impactor calibration for measurements of activity size distributions in the atmosphere. Journal of Aerosol Science 1981;12:172. [Google Scholar]
- Splansky GL; Corey D; Yang Q; Atwood LD; Cupples LA; Benjamin EJ; D’Agostino RB Sr.; Fox CS; Larson MG; Murabito JM; O’Donnell CJ; Vasan RS; Wolf PA; Levy D The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: Design, Recruitment, and Initial Examination. Am J Epidemiol 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. A Citizen’s Guide to Radon: The Guide to Protecting Yourself and Your Family from Radon. U.S. Environmental Protection Agency; 2016. Available from: https://www.epa.gov/radon/citizens-guide-radon-guide-protectingyourself-and-your-family-radon [Google Scholar]
- U.S. Environmental Protection Agency. RadNet Monitoring network. U.S. Environmental Protection Agency; 2017. Available from: https://www.epa.gov/radnet [Google Scholar]
- World Health Organization. WHO Handbook on Indoor Radon - A Public Health Perspective. World Health Organization; 2009. Available from: https://www.ncbi.nlm.nih.gov/books/NBK143216/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.