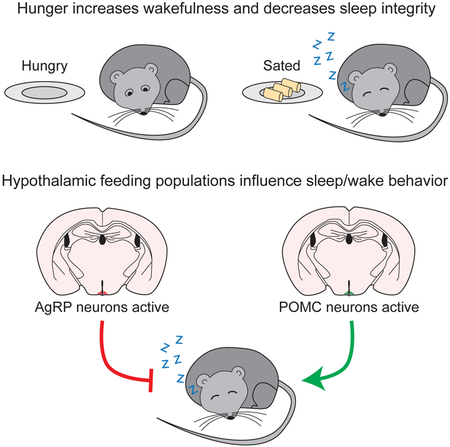
SUMMARY
Eating and sleeping represent two mutually exclusive behaviors that satisfy distinct homeostatic needs. Because an animal cannot eat and sleep at the same time, brain systems that regulate energy homeostasis are likely to influence sleep/wake behavior. Indeed, previous studies indicate that animals adjust sleep cycles around periods of food need and availability. Furthermore, hormones that affect energy homeostasis also affect sleep/wake states: the orexigenic hormone ghrelin promotes wakefulness, while the anorexigenic hormones leptin and insulin increase the duration of slow wave sleep. However, whether neural populations that regulate feeding can influence sleep/wake states is unknown. The hypothalamic arcuate nucleus contains two neuronal populations that exert opposing effects on energy homeostasis: agouti-related protein (AgRP)-expressing neurons detect caloric need and orchestrate food-seeking behavior, while activity in pro-opiomelanocortin (POMC)-expressing neurons induces satiety. We tested the hypotheses that AgRP neurons affect sleep homeostasis by promoting states of wakefulness, while POMC neurons promote states of sleep. Indeed, optogenetic or chemogenetic stimulation of AgRP neurons in mice promoted wakefulness while decreasing the quantity and integrity of sleep. Inhibition of AgRP neurons rescued sleep integrity in food-deprived mice, highlighting the physiological importance of AgRP neuron activity for the suppression of sleep by hunger. Conversely, stimulation of POMC neurons promoted sleep states and decreased sleep fragmentation in food-deprived mice. Interestingly, we also found that sleep deprivation attenuated the effects of AgRP neuron activity on food intake and wakefulness. These results indicate that homeostatic feeding neurons can hierarchically affect behavioral outcomes depending on homeostatic need.
Keywords: Sleep, appetite, homeostasis, agouti-related protein, AgRP, pro-opiomelanocortin, POMC, optogenetics, chemogenetics
Graphical Abstract
eTOC BLURB
Eating and sleeping represent two mutually exclusive behaviors that satisfy distinct homeostatic needs. We tested the hypothesis that hypothalamic arcuate nucleus neurons that regulate feeding also modify sleep behavior depending on homeostatic need. Our results demonstrate that these neurons can bidirectionally affect sleep behavior.
INTRODUCTION
To survive, animals must carefully regulate behavior to satisfy distinct homeostatic needs including the need for food, sleep, water, and optimal body temperature. Eating [1, 2], sleeping [3, 4], drinking [5, 6], and thermoregulation [7, 8] are each regulated by distinct networks of neural systems and circuits in the brain that detect a homeostatic deficiency and orchestrate an appropriate behavioral response. While great progress has been made in mapping these neural networks, the balance between homeostatic systems is poorly understood, as is the ability of one homeostatic system to override another in times of need.
The hypothalamic arcuate nucleus contains two neural populations that are well known to regulate the homeostatic need for food [1, 2]. The activity of arcuate neurons that express agouti-related protein (AgRP), neuropeptide Y (NPY), and γ-amino-butyric acid (GABA) (commonly referred to as “AgRP neurons”) increases during food deprivation [9-12]. These neurons are activated by the orexigenic hormones ghrelin [13-16] and asprosin [17], and are inhibited by the anorexigenic hormones insulin and leptin [18]. Optogenetic or chemogenetic stimulation of AgRP neurons increases food-seeking behavior in mice [19, 20]. In contrast, activity of a separate population of arcuate neurons that express pro-opiomelanocortin (POMC)-derived peptides increases during states of satiety and energy surfeit [10]. POMC neurons are stimulated by insulin and leptin [18, 21], and optogenetic or chemogenetic stimulation of these neurons decreases food intake [20, 22].
Recent studies indicate that increasing activity in AgRP neurons can lead to the prioritization of food-seeking behavior over competitive need states. For example, stimulation of AgRP neurons causes an increase in food intake and a decrease in water-seeking behavior, anxiety-related behavior, and social interactions [23]. AgRP neurons reduce anxiety, fertility, and inflammatory pain through direct, inhibitory projections to the medial nucleus of the amygdala [24], hypothalamic kisspeptin neurons [25], and the lateral parabrachial nucleus [26], respectively. These studies demonstrate that stimulation of AgRP neurons is sufficient to prioritize food-seeking behavior over other behaviors.
Sleep is another evolutionarily conserved behavioral state that is necessary for survival in virtually all animal species [27]. Because eating and sleeping are mutually exclusive behaviors, they must be appropriately balanced to maximize the likelihood of animal survival [28]. Thus, most animals adjust sleep cycles around periods of food need and availability [28]. Food deprivation increases the time spent in wakefulness and decreases the time spent in non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep states [28-31]. Food deprivation also fragments sleep, increasing abrupt transitions from sleep to wakefulness [32, 33]. These latter effects have strong implications for animal health, as fragmented sleep (even without a reduction in total sleep time) causes cognitive and physiological deficits [34].
Interestingly, the peripheral hormones and central neuropeptides that regulate energy balance and food intake can also affect sleep/wake parameters [35, 36]. In general, factors that increase appetite also promote wakefulness and arousal. Administration of the orexigenic hormone ghrelin stimulates wakefulness and reduces NREM sleep and REM sleep [37-39]. Mice deficient for ghrelin exhibit shorter and less stable episodes of wakefulness and do not show an increase in wakefulness upon food deprivation [40]. Central injections of NPY increase food intake and cause a corresponding increase in wakefulness [41, 42]. In contrast, factors that decrease food intake also promote and maintain sleep. Administration of anorexigenic insulin or leptin hormones increases the duration of NREM sleep [43, 44], while mice deficient for leptin show an elevated number of awakening events and more frequent, shorter-lasting sleep episodes [45]. Additionally, central injection of neuropeptide derivatives of the cleaved POMC protein increases sleep [46, 47]. Taken together, these findings suggest a model in which peripheral signals converge on AgRP/POMC neurons to regulate energy homeostasis in a manner that may also influence sleep/wake parameters. However, the effects of AgRP and POMC neural activity on sleep/wake architecture are unknown.
To better understand how activity in homeostatic feeding circuits influences sleep, we first tested the hypothesis that increased AgRP neuron activity prioritizes food-seeking behavior at the expense of sleep duration and integrity. We next examined whether inhibiting AgRP neurons could restore normal sleep parameters in food restricted animals. We also tested the hypothesis that POMC neurons can stabilize sleep by signaling the absence of caloric need. Finally, we tested the hypothesis that increasing sleep need by sleep deprivation would attenuate the effects of increased AgRP activity on sleep. Our results show that activity in hypothalamic arcuate neurons that regulate energy homeostasis influences sleep/wake states, demonstrating a mechanism by which a deficit in one homeostatic system can modulate a separate homeostatic behavior.
RESULTS
Food deprivation increases wakefulness and disrupts sleep integrity
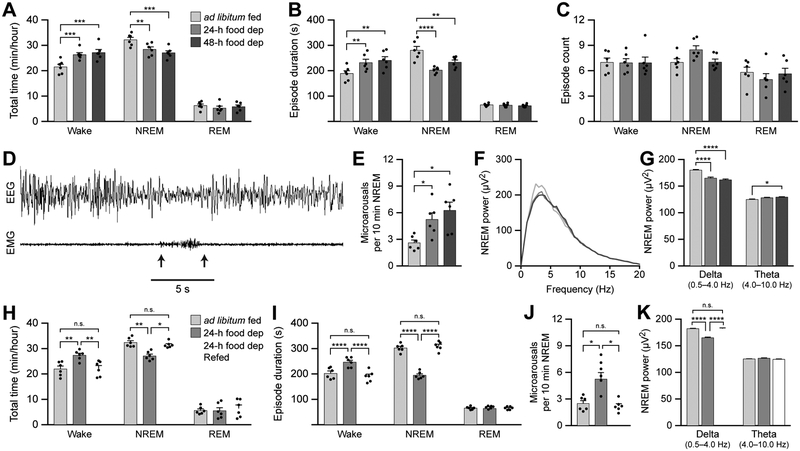
To gain initial insight into the effect of increasing the homeostatic need for food on sleep behavior in mice, we compared sleep/wake architecture during the middle four hours of the inactive period between ad libitum fed mice and mice deprived of food for 24 or 48 h. Food-deprived animals spent more total time awake and less total time in NREM sleep compared with ad libitum fed animals (Figure 1A; see figure legends for p values and Table S1 for detailed statistical analyses). Additionally, food deprived animals exhibited longer wake episodes and shorter NREM sleep episodes (Figure 1B). There were no differences between animals in total REM sleep time or REM sleep episode duration (Figure 1A and 1B). There were also no differences in the number of wake, NREM sleep, or REM sleep episodes between mice (Figure 1C).
Figure 1. Food deprivation increases wakefulness and disrupts sleep integrity.
(A-C) The total time (A), episode duration (B), and episode count (C) of wake, NREM sleep, and REM sleep states in ad libitum fed mice and 24-h and 48-h food deprived mice.
(D) Representative recording of a microarousal event during NREM sleep. Arrows represent the onset and offset of the microarousal.
(E) Frequency of microarousals during NREM sleep in ad libitum fed mice and 24-h and 48-h food deprived mice.
(F) Power spectra of EEG recorded during NREM sleep.
(G) The average EEG power density in the delta (0.5-4 Hz) and theta (4-10 Hz) bands.
(H-I) The total time (H) and episode duration (I) of wake, NREM sleep, and REM sleep states in ad libitum fed mice, 24-h food deprived mice, and 24-h food deprived mice allowed to re-feed for 2 h.
(J) Frequency of microarousals during NREM sleep.
(K) The average EEG power density in the delta (0.5-4 Hz) and theta (4-10 Hz) bands.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. Post hoc comparisons: n.s. = not significant (p>0.05); *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; also see Table S1 for additional statistical information.
To assess sleep integrity, we measured the frequency of microarousal events during NREM sleep. We defined a microarousal as a brief awakening (desynchronized EEG of 4-10 Hz and increased EMG) lasting less than five seconds and immediately returning to sleep (Figure 1D). Food deprivation increased the frequency of microarousals during NREM sleep (Figure 1E). We did not observe microarousal events during REM sleep. We also performed EEG power analyses during NREM sleep and observed decreased delta power (0.5-4 Hz) during food-deprived conditions, indicative of lower drive to sleep (Figures 1F and 1G). There was also an increase in theta power (4-10 Hz) when animals were food deprived for 48 h compared with ad libitum fed conditions (Figure 1G), suggesting disrupted NREM sleep quality and an increased propensity for wakefulness. Taken together, these results show that food deprivation for 24 or 48 h increases the duration of wake states and disrupts NREM sleep integrity.
To determine whether the effects of food deprivation on sleep architecture were reversible upon calorie restoration, we compared sleep/wake architecture during the middle four hours of the inactive period between ad libitum fed mice, mice deprived of food for 24 h, and mice deprived of food for 24 h but allowed to re-feed 2 h prior to sleep/wake recordings. Allowing food deprived animals to re-feed restored the total times in wake and NREM states to those observed in the ad libitum fed group (Figure 1H), and also restored individual wake and NREM episode durations (Figure 1I). Re-feeding also restored the frequency of microarousals and NREM delta power to values in the ad libitum fed group (Figures 1J-K). Therefore, calorie restoration in food-deprived animals can reset sleep/wake architecture to levels observed in ad libitum fed animals.
Stimulation of AgRP neurons can increase wakefulness and disrupt sleep integrity
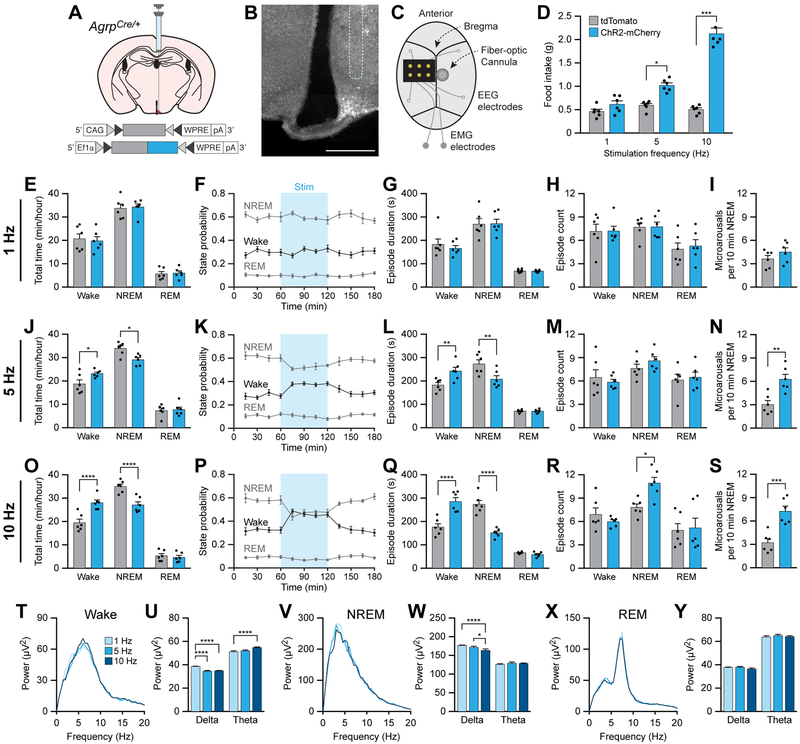
To test the hypothesis that increased AgRP neuron activity prioritizes the motivation to seek food at the expense of sleep states, we optogenetically stimulated orexigenic AgRP neurons to increase appetite in ad libitum fed animals and determined the effects on sleep/wake behavior. To target AgRP neurons, we unilaterally injected AAV carrying a Cre-inducible tdTomato or ChR2-mCherry transgene into AgrpCre/+ knockin mice (Figure 2A and 2B). We also affixed a fiber-optic cannula for blue light illumination and an EEG/EMG implant for polysomnographic recording onto the skull (Figure 2C). To vary the gain of activity in AgRP neurons, we photostimulated AgRP neurons using a previously established protocol to deliver 10 ms light pulses at 1, 5, or 10 Hz for 1 s every 4 s for 1 h [20]. To measure food intake, mice were provided with diluted Vanilla Ensure (450 kcal/L) mounted on weigh scales to precisely measure food intake in 0.1g increments and to prevent inaccurate measurements from partially eaten or fragmented solid chow pellets. In initial experiments, 1 h photostimulation of AgRP neurons at 5 or 10 Hz, but not 1 Hz, was able to increase food intake in ChR2-mCherry-transduced animals relative to tdTomato-transduced animals during the inactive period (Figure 2D).
Figure 2. Optogenetic stimulation of AgRP neurons increases wakefulness and disrupts sleep integrity.
(A) Diagram showing viral injection strategy to unilaterally target AgRP neurons with tdTomato or ChR2-mCherry.
(B) Representative photomicrograph showing AgRP neurons expressing ChR2-mCherry. Dashed line shows approximate location of cannula track. Scale bar, 500 μm.
(C) Diagram showing placement of EEG/EMG implant, EEG electrodes, and fiber-optic cannula on the skull. EMG electrodes were placed within the nuchal musculature.
(D) Optogenetic stimulation of AgRP neurons for 1 h at 5 or 10 Hz increases food intake.
(E-I) Optogenetic stimulation of AgRP neurons for 1 h at 1 Hz does not affect (E) the total time (F) state probability, (G) episode duration, or (H) episode count of wake, NREM sleep, and REM sleep states, nor does it affect (I) the frequency of microarousal events during NREM sleep.
(J-N) Optogenetic stimulation of AgRP neurons for 1 h at 5 Hz (J,K) increases total time in wakefulness and decreases total time in NREM sleep, (L) increases wake and decreases NREM sleep episode duration, and (N) increases the frequency of microarousal events during NREM sleep, but (M) does not affect the number of wake, NREM sleep, or REM sleep episodes.
(O-S) Optogenetic stimulation of AgRP neurons for 1 h at 10 Hz (O,P) increases total time in wakefulness and decreases total time in NREM sleep, (Q) increases wake and decreases NREM sleep episode duration, (R) increases the number of NREM sleep episodes, and (S) increases the frequency of microarousal events during NREM sleep.
(T and U) Power spectra of EEG recorded during wakefulness (T), and (U) the average EEG power density in the delta and theta bands.
(V and W) Power spectra of EEG recorded during NREM sleep (V), and (W) the average EEG power density in the delta and theta bands.
(X and Y) Power spectra of EEG recorded during REM sleep (X), and (Y) the average EEG power density in the delta and theta bands.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests and post hoc comparisons: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001; also see Table S1.
To determine whether stimulation of AgRP neurons is able to increase wakefulness, we photostimulated AgRP neurons at 1, 5, or 10 Hz (1 s every 4 s) for 1 h in the middle of the inactive period and measured sleep/wake architecture. Photostimulation at 1 Hz had no effect on the total duration of sleep/wake states, episode duration, episode count, or frequency of microarousals during NREM sleep (Figures 2E-2I). However, the effects of photostimulation of AgRP neurons at 5 Hz or 10 Hz mimicked sleep/wake architecture in food-deprived conditions (Figures 2J-2S). Stimulation caused an increase in the total time awake and length of wake episodes with a decrease in the total time in NREM sleep and length of NREM sleep episodes (Figures 2J-L and 2O-Q). REM sleep was unaffected. There was no effect of 5 Hz photostimulation on the number of sleep or wake state episodes (Figure 2M), but 10 Hz photostimulation caused an increase in the number of NREM sleep episodes (Figure 2R). Similar to food deprivation, both 5 Hz and 10 Hz photostimulation of AgRP neurons increased the frequency of microarousal events during NREM sleep (Figures 2N and 2S).
To measure the integrity of wake, NREM sleep, and REM sleep states, we analyzed the EEG power spectra during photostimulation. Photostimulation of AgRP neurons at 5 or 10 Hz significantly decreased delta and increased theta EEG power during wakefulness relative to stimulation at 1 Hz (Figure 2T and 2U). Photostimulation at 5 or 10 Hz also decreased delta power during NREM sleep relative to photostimulation at 1 Hz (Figure 2V and 2W). There was no difference in EEG power spectra during REM sleep (Figure 2X and 2Y). Therefore, optogenetic photostimulation of AgRP neurons increases parameters of wakefulness while decreasing the quantity and integrity of NREM sleep.
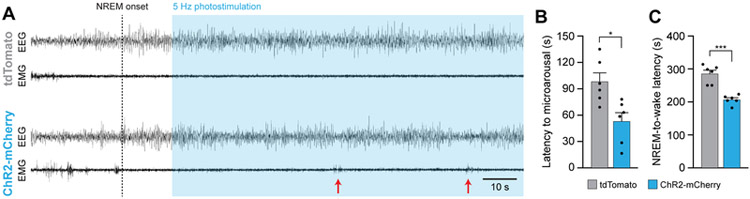
To further study the acute effects of AgRP neuron activation during sleep states, we performed an acute photostimulation protocol, stimulating AgRP neurons at 5 Hz (1 s on, 3 s off) starting 15 s after the onset of NREM sleep. Acute photostimulation caused a decrease in the latency to the first microarousal event (Figures 3A and 3B) and reduced the latency to a full transition to wakefulness (Figure 3C), further suggesting that photostimulation of AgRP neurons decreases the stability of NREM sleep.
Figure 3. Acute optogenetic stimulation of AgRP neurons increases microarousals during NREM sleep and decreases the NREM sleep -to-wake latency.
(A) Representative EEG/EMG traces from an AgrpCre/+ animal transduced with tdTomato (top) or ChR2-mCherry (bottom). Vertical dashed line shows the onset of NREM sleep. Blue shading depicts period of 5 Hz photostimulation for 1 s every 3 s. Red arrows show microarousal events.
(B) Latency to the first microarousal event following the onset of photostimulation.
(C) Latency to the first full transition from NREM sleep to wakefulness following the onset of photostimulation.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests: *p<0.05, ***p<0.001; also see Table S1.
To independently verify the effects of increasing AgRP neural activity on sleep/wake behavior, we examined how chemogenetic stimulation of AgRP neurons affected sleep. We unilaterally injected AAV carrying a Cre-inducible tdTomato or hM3Dq-mCherry transgene into AgrpCre/+ knockin mice (Figure 4A and 4B). In initial experiments, intraperitoneal (i.p.) administration of clozapine-n-oxide (CNO, 0.3 mg/kg) caused a reliable increase in food intake in hM3Dq-mCherry-transduced animals relative to tdTomato-transduced control animals in the hour post-injection (Figure 4C). To determine the effects of chemogenetic stimulation of AgRP neurons on sleep/wake architecture, we injected CNO and immediately measured sleep/wake parameters during the middle 4 h of the inactive period. Similar to food deprivation or optogenetic stimulation of AgRP neurons, chemogenetic stimulation of AgRP neurons caused an increase in the total time awake and length of wake episodes and a decrease in the total time in NREM sleep and duration of NREM sleep episodes (Figures 4D and 4E). There was no difference between hM3Dq-mCherry and tdTomato-transduced animals in the number of episode counts (Figure 4F) or in any measures of REM sleep (Figures 4D-F). Chemogenetic stimulation also disrupted the integrity of NREM sleep, increasing the frequency of microarousal events (Figure 4G) and decreasing delta EEG power during NREM sleep (Figures 4H and 4I). Taken together, these data demonstrate that stimulation of AgRP neurons is able to increase parameters of wakefulness and disrupt integrity of NREM sleep states, mimicking the effects of food deprivation on sleep.
Figure 4. Chemogenetic stimulation of AgRP neurons increases wakefulness and disrupts sleep integrity.
(A) Diagram showing viral injection strategy to unilaterally target AgRP neurons with tdTomato or hM3Dq-mCherry.
(B) Representative photomicrograph showing AgRP neurons expressing hM3Dq-mCherry. Scale bar, 500 μm.
(C) Chemogenetic stimulation of AgRP neurons for 1 h increases food intake.
(D-F) Chemogenetic stimulation of AgRP neurons (D) increases wakefulness and decreases NREM sleep, (E) increases wake and decreases NREM sleep episode duration, and (F) does not affect the number of wake, NREM sleep, or REM sleep episode counts.
(G) Chemogenetic stimulation of AgRP neurons increases the frequency of microarousals during NREM sleep.
(H-I) Chemogenetic stimulation of AgRP neurons decreases the delta power spectra of EEG recorded during NREM sleep.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests and post hoc comparisons: *p<0.05, ***p<0.001, ****p<0.0001; also see Table S1
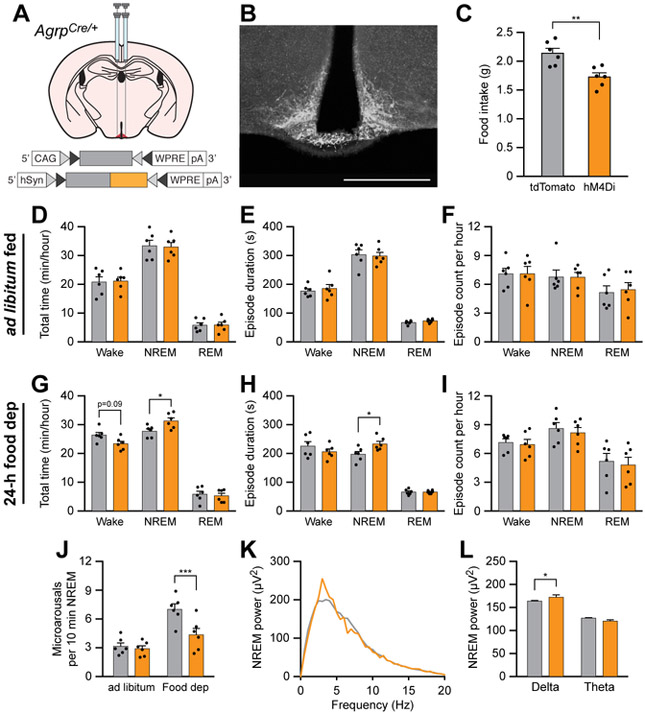
Chemogenetic inhibition of AgRP neurons rescues sleep integrity in food-deprived animals
Because stimulation of AgRP neurons caused increases in wakefulness and decreases in NREM sleep integrity similar to that in food deprived animals, we hypothesized that inhibition of AgRP neurons could rescue disrupted sleep/wake parameters following food deprivation. To inhibit AgRP neurons, we bilaterally transduced these neurons with the chemogenetic inhibitor hM4Di (Figure 5A and 5B). As expected, i.p. administration of CNO (0.3 mg/kg) decreased cumulative food intake in hM4Di-mCherry-transduced animals relative to tdTomato-transduced animals over a 4-h period in fed mice (Figure 5C), similar to previous experiments [19]. To determine the effects of chemogenetic inhibition of AgRP neurons on sleep/wake behavior, we administered CNO and measured sleep/wake architecture over the middle 4 h of the inactive period. When animals were fed ad libitum, AgRP neuron inhibition did not alter total time, episode duration, or episode count in wakefulness, NREM sleep, or REM sleep (Figures 5D-F). However, when animals were food deprived for 24 h, chemogenetic inhibition of AgRP neurons increased the total duration of NREM sleep and the duration of NREM sleep episodes (Figures 5G-I). Inhibition of AgRP neurons in food-deprived animals also reduced the frequency of microarousals during NREM sleep (Figure 5J) and increased delta power during NREM sleep (Figure 5K and 5L). Therefore, inhibiting AgRP neurons rescued disruptions to NREM sleep caused by food deprivation, indicating the physiological importance of AgRP neuron activity for the suppression of sleep by hunger.
Figure 5. Chemogenetic inhibition of AgRP neurons rescues sleep integrity in food-deprived animals.
(A) Diagram showing viral injection strategy to bilaterally target AgRP neurons with tdTomato or hM4Di-mCherry.
(B) Representative photomicrograph showing AgRP neurons expressing hM4Di-mCherry. Scale bar, 500 μm.
(C) Chemogenetic inhibition of AgRP neurons for 4 h decreases food intake in ad libitum fed mice.
(D-F) Chemogenetic inhibition of AgRP neurons for 1 h in ad libitum fed mice does not affect (D) the total time, (E) episode duration, or (F) episode count of wake, NREM sleep, and REM sleep states.
(G-I) Chemogenetic inhibition of AgRP neurons for 1 h in 24-h food deprived mice (G) decreases wakefulness and increases NREM sleep, (H) increases NREM sleep episode duration, and (I) does not affect the number of wake, NREM sleep, or REM sleep episodes.
(J) Chemogenetic inhibition of AgRP neurons for 1 h decreases the frequency of microarousals in 24-h food deprived mice.
(K and L) Chemogenetic inhibition of AgRP neurons increases the delta power spectra of EEG recorded during NREM sleep of 24-h food deprived mice.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests and post hoc comparisons: *p<0.05, ***p<0.001; also see Table S1.
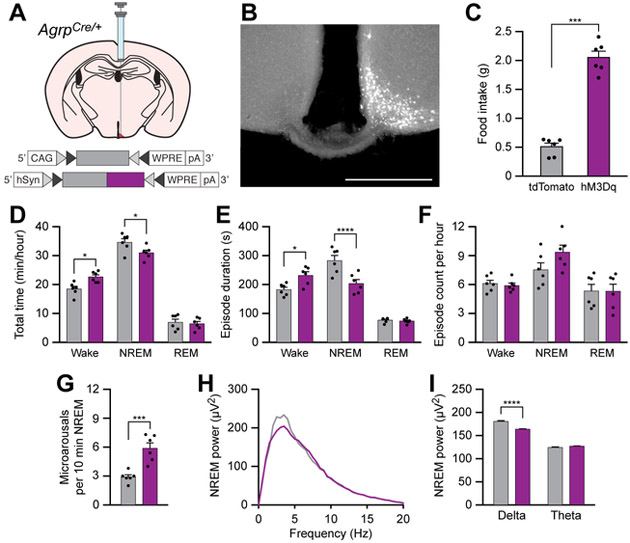
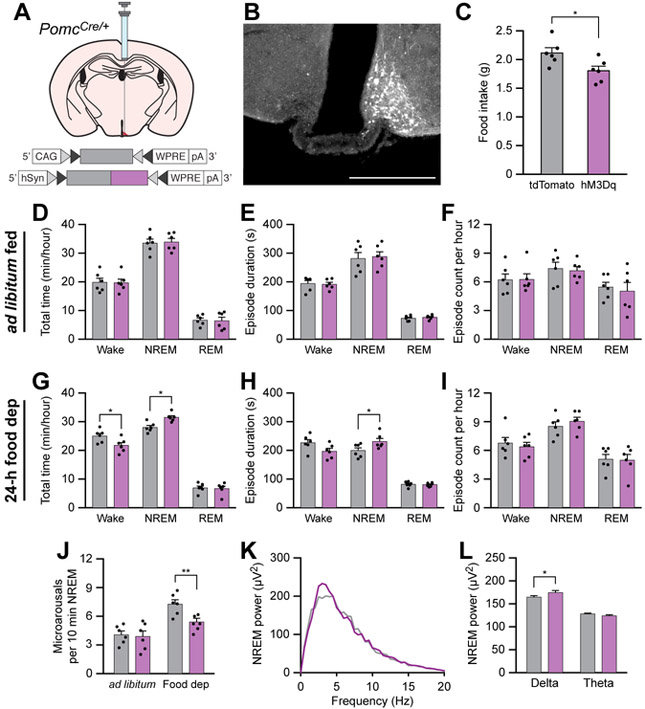
Chemogenetic stimulation of POMC neurons rescues sleep integrity in food-deprived animals
Because AgRP neurons directly inhibit POMC neurons [48], and because anorexigenic factors have been shown to promote sleep states, we hypothesized that stimulation of anorexigenic POMC neurons could stabilize or promote sleep. To stimulate POMC neurons, we unilaterally transduced these neurons with the chemogenetic actuator hM3Dq (Figures 6A and 6B). As expected, i.p. administration of CNO (0.3 mg/kg) decreased cumulative food intake in hM3Dq-mCherry-transduced animals relative to tdTomato-transduced animals over a 4-h period during the inactive cycle in fed mice (Figure 6C), similar to previous experiments [22]. To determine the effects of chemogenetic stimulation of POMC neurons on sleep/wake behavior, we administered CNO and measured sleep/wake architecture over the middle 4 h of the inactive period. There was no effect of CNO administration on total time, episode duration, or episode count of wakefulness, NREM sleep, or REM sleep in ad libitum fed animals (Figures 6D-F). However, chemogenetic stimulation of POMC neurons decreased the total time awake, and increased the total time in NREM sleep and duration of NREM sleep episodes in 24-h food deprived animals (Figure 6G-I). Furthermore, chemogenetic stimulation of POMC neurons in 24-h food deprived animals decreased the frequency of microarousals during NREM sleep (Figure 6J) and increased delta EEG power during NREM sleep (Figures 6K and 6L). Taken together, these results indicate that stimulation of POMC neurons can stabilize NREM sleep in 24-h food deprived animals but has no effect on animals fed ad libitum.
Figure 6. Chemogenetic stimulation of POMC neurons rescues sleep integrity in food-deprived animals.
(A) Diagram showing viral injection strategy to unilaterally target POMC neurons with tdTomato or hM3Dq-mCherry.
(B) Representative photomicrograph showing POMC neurons expressing hM3Dq-mCherry. Scale bar, 500 μm.
(C) Chemogenetic stimulation of POMC neurons for 4 h decreases food intake in ad libitum fed mice.
(D-F) Chemogenetic stimulation of POMC neurons for 1 h in ad libitum fed mice does not affect (D) the total time, (E) episode duration, or (F) episode count of wake, NREM sleep, and REM sleep states.
(G-I) Chemogenetic stimulation of POMC neurons for 1 h in 24-h food deprived mice (G) decreases wakefulness and increases NREM sleep, (H) increases NREM sleep episode duration, and (I) does not affect the number of wake, NREM sleep, or REM sleep episodes.
(J) Chemogenetic stimulation of POMC neurons for 1 h decreases the frequency of microarousals in 24-h food deprived mice.
(K and L) Chemogenetic stimulation of POMC neurons increases the delta power spectra of EEG recorded during NREM sleep of 24-h food deprived mice.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests and post hoc comparisons: *p<0.05, ***p<0.001; also see Table S1.
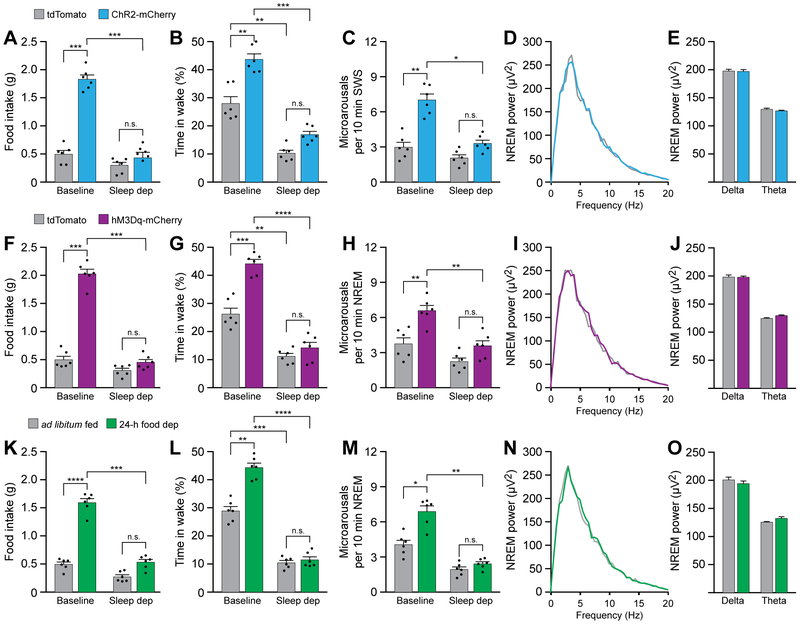
Sleep deprivation attenuates AgRP neuron-mediated promotion of feeding and wakefulness
Because increasing the motivation for food disrupted sleep homeostasis and decreased NREM sleep, we hypothesized the converse effect, that increasing sleep pressure by sleep depriving animals would attenuate the effects of optogenetic or chemogenetic stimulation of AgRP neurons. To test the effects of sleep deprivation on optogenetic stimulation of AgRP neurons, we photostimulated ChR2-mCherry- and tdTomato-transduced animals in baseline conditions and in animals sleep deprived by gentle handling for 6 hours during the inactive period. This method of sleep deprivation has been shown previously not to increase plasma corticosterone or adrenocorticotropic hormone levels, demonstrating that this procedure does not increase stress in animals [49]. Indeed, sleep deprivation attenuated AgRP neuron-mediated increases in food intake (Figure 7A), time in wakefulness (Figure 7B), and frequency of microarousals during NREM sleep (Figure 7C). Furthermore, sleep deprivation blocked AgRP neuron-mediated changes to delta EEG power during NREM sleep (Figures 7D and 7E). Sleep deprivation similarly attenuated the effects of chemogenetic stimulation of AgRP neurons (Figures 7F-J). We also studied the effects of sleep deprivation on naturally-induced motivation to eat by sleep depriving animals after 24-h food deprivation. Similar to the effects of sleep deprivation on AgRP neuron stimulation, sleep deprivation attenuated the effects of fasting-induced increases on feeding and wakefulness (Figures 7K-O). Taken together, these results suggest that increased sleep pressure caused by sleep deprivation can prevent AgRP-mediated and fasting-mediated increases in food-seeking behavior and wakefulness. Therefore, energy homeostasis and sleep homeostasis can bidirectionally influence animal behavior based on competitive homeostatic needs.
Figure 7. Sleep deprivation attenuates AgRP neuron-mediated promotion of feeding and wakefulness.
(A-E) 6 h sleep deprivation attenuates the effects of optogenetic stimulation of AgRP neurons on feeding and sleep/wake architecture. (A) 6 h sleep deprivation blocks AgRP neuron-mediated increases in feeding. (B) 6 h sleep deprivation blocks increases in wakefulness and (C) the frequency of microarousals in NREM sleep. (D and E) 6 h sleep deprivation also blocks AgRP neuron-mediated changes in power density during NREM sleep.
(F-J) 6 h sleep deprivation attenuates the effects of chemogenetic stimulation of AgRP neurons on feeding and sleep/wake architecture. (F) 6 h sleep deprivation blocks AgRP neuron-mediated increases in feeding. (G) 6 h sleep deprivation blocks increases in wakefulness and (H) the frequency of microarousals in NREM sleep. (I and J) 6 h sleep deprivation also blocks AgRP neuron-mediated changes in power density during NREM sleep.
(K-O) 6 h sleep deprivation attenuates the effects of food deprivation on feeding and sleep/wake architecture. (F) 6 h sleep deprivation blocks 24-h food deprivation-mediated increases in feeding. (G) 6 h sleep deprivation blocks increases in wakefulness and (H) the frequency of microarousals in NREM sleep. (I and J) 6 h sleep deprivation also blocks 24-h food deprivation-mediated changes in power density during NREM sleep.
Data represent the mean ± standard error of the mean (SEM). Dots represent individual experimental animals. T-tests and post hoc comparisons: n.s. = not significant (p>0.05), **p<0.01, ***p<0.001, ****p<0.0001; also see Table S1.
DISCUSSION
Activity in arcuate nucleus neurons affects sleep/wake behavior
Our results support the hypothesis that increasing the activity of AgRP neurons promotes wakefulness and disrupts sleep integrity. We found that optogenetic and chemogenetic stimulation of AgRP neurons reduced total time in NREM sleep and reduced NREM sleep episode durations (Figure 2J-L, Figure 2 O-Q, and Figure 4D-E). We also found that stimulation of AgRP neurons increased the frequency of microarousal events during NREM sleep (Figure 2N, Figure 2S, Figure 3A-B, and Figure 4G) and decreased delta EEG power during NREM sleep (Figure 2T-U and Figure 4H-I). Our results also support the hypothesis that inhibiting AgRP neurons could rescue disruptions to sleep integrity caused by food deprivation (Figure 5). Finally, our results support the hypothesis that activity in POMC neurons is able to decrease disruption of sleep integrity following food deprivation (Figure 6). Taken together, we conclude that AgRP and POMC neurons in the arcuate nucleus can prioritize food intake behavior over sleep behavior depending on homeostatic need, demonstrating an interaction between energy and sleep homeostatic systems.
Interestingly, we did not observe effects of manipulating AgRP or POMC neural activity on REM sleep. Arousal thresholds are highest during REM sleep, and our results suggest that increasing activity in AgRP neurons during this state is not able to cause REM-to-wake transitions. Therefore, REM periods may exhibit a higher interoceptive sensory threshold in addition to a higher external sensory threshold relative to NREM sleep. There may be evolutionary benefit to maintaining REM states over NREM sleep states because they are relatively infrequent and necessary for cognitive processes such as learning and memory [50].
The discovery that AgRP neurons can antagonize NREM sleep drive supports previous studies showing that increasing the gain of AgRP neural activity biases animals towards food-seeking behavior at the expense of competitive need states, such as thirst [23], anxiety-related behavior [23, 24], social interactions[23], fertility [25], and inflammatory pain [26]. We surmise that these neurons engage downstream populations that rearrange an animal’s behavioral output to facilitate the search for food at the expense of other activities. Furthermore, because stimulation of POMC neurons decreased the effects of food deprivation on sleep integrity, we suggest that these neurons signal caloric sufficiency, allowing an animal to seek other homeostatic needs.
AgRP/POMC neurons are likely not primary effectors of sleep/wake behavior
Although stimulation of AgRP neurons promoted wakefulness and decreased NREM sleep, we do not suggest that these neurons are primary effectors of sleep/wake states or sleep homeostasis. Stimulation of neurons that directly regulate sleep/wake states, such as hypocretin-expressing neurons of the lateral hypothalamus or locus coeruleus neurons of the midbrain, not only causes sleep-to-wake state transitions, but phasic bursts of activity in these neurons are also predictive of sleep/wake transitions [3, 4, 51]. We did not measure functional activity in AgRP neurons in our study, but we do not hypothesize that acute increases in AgRP or POMC neural activity correlate with microarousal events or transitions from NREM sleep to wakefulness. Instead, we suggest that AgRP neurons directly or indirectly increase the gain of activity in other populations that directly gate wake states, decreasing delta EEG power during NREM sleep and promoting theta EEG power during wake. Future studies using in vivo imaging or electrophysiological recordings of neurons that directly control sleep/wake cycles during hunger or AgRP/POMC neuron stimulation will be important to substantiate this hypothesis. Similarly, activity recordings of AgRP and POMC neurons, as well as neurons that regulate other homeostatic systems such as thirst, will be essential to assess how activity in these neurons fluctuates as animals enter sleep and transition into different sleep stages. Because AgRP neurons receive top-down inhibitory input [52] and because sleep deprivation reduces the effects of AgRP neuron-mediated promotion of feeding and wakefulness (Figure 7), neural populations that promote sleep might inhibit AgRP neurons in order to maintain sleep. Therefore, food deprived animals exhibiting relatively high AgRP neuron activity could show reduced activity in these neurons upon entering NREM sleep. Alternatively, neural populations that promote sleep might act on populations downstream of AgRP signaling.
Our results also beg the question of which AgRP and POMC neuron projections might mediate their effects on sleep. Both AgRP and POMC neurons project to several regions that promote wakefulness and arousal including the bed nucleus of the stria terminalis [53], lateral hypothalamus [54-56], and parabrachial nucleus [57]. However, AgRP neurons release the inhibitory neurotransmitter GABA, as well as AgRP and NPY, neuropeptides that act on Gi-coupled receptors. Therefore, it is unlikely that a monosynaptic connection between AgRP neurons and one or more of these populations suppresses NREM sleep during hunger. Instead, AgRP neurons could elicit wake-promoting effects via inhibition of local inhibitory neurons or via multiple synapses to activate wake-promoting circuits and/or inhibit NREM-promoting circuits. Alternatively, AgRP neurons send projections to the ventrolateral periaqueductal gray (vlPAG) [58], a region that has recently been shown to consolidate NREM sleep [59]. Therefore, it is possible that vlPAG neurons mediate at least part of AgRP neuron-mediated effects on NREM sleep reduction. POMC neurons comprise a heterogeneous population releasing both excitatory and inhibitory neurotransmitters as well as melanocyte-stimulating hormones that target a diverse family of melanocortin receptors. POMC neurons, therefore, also have the potential to affect sleep-wake dynamics at several downstream targets, both by activating populations that promote NREM sleep integrity and by inhibiting wake-promoting populations. Determining the role of individual AgRP and POMC neuron projections on sleep/wake dynamics will be essential toward mapping how feeding circuits interact with primary regulators of sleep and wakefulness.
Distinguishing between the motivation to seek food versus caloric deficits
Previous studies have shown that food deprivation increases the duration of wake episodes and fragments sleep [28-33]. Food deprivation increases the motivation to seek food, but also causes alterations in circulating hormones, blood glucose imbalances, vitamin and nutrient deficits, metabolic utilization of alternative fuel sources, and many other phenomena that could negatively impact sleep architecture. Some previous studies have attempted to identify the causes of increased wakefulness by modifying food deprivation regimens, or by comparing sleep in animals with different body weights. For example, when food deprived, lean rats exhibit a dramatic decrease in sleep, while obese rats show no change in sleep duration [60]. This difference most likely occurs because large rats possessing large peripheral energy stores do not experience the same hormonal and nutritional alterations as lean rats. In our experiments, we isolated the effect of directly modulating activity in AgRP or POMC neurons on sleep/wake architecture. Interestingly, AgRP neuron stimulation was able to elicit an increase in wakefulness and a decrease in NREM sleep time and integrity similar to the effects of food deprivation, even in the absence of energy state abnormalities. Therefore, it is possible that the obese rats discussed above did not experience sleep deficits because they had sufficient circulating levels of leptin and other nutritional cues such that AgRP and POMC neural activity was not significantly changed.
Food deprivation and AgRP neuron activation increases the frequency of microarousal events
We found that food deprivation and AgRP neuron activation increased the frequency of microarousal events during NREM sleep (Figure 1D and 1E, Figure 2N and 2S, Figure 4G). Rolls et al. (2011) demonstrated that increasing the frequency of microarousal events during sleep caused corresponding cognitive deficits [34], suggesting that hunger-induced increases in microarousals may negatively impact animal cognition and behavioral performance. Indeed, food deprivation has been shown to affect cognitive performance in rats [61, 62]. Our results showing that modulating AgRP or POMC neurons alters the frequency of microarousal events during NREM sleep in mice may therefore have consequences for animal cognition as well as for sleep/wake architecture and NREM sleep integrity.
Animal behaviors are prioritized depending on homeostatic need
Similar to how food deprivation caused changes in sleep/wake behavior, we found that sleep deprivation attenuated the effects of AgRP neuron-mediated increases in food intake and increases in wakefulness (Figure 7). Therefore, energy homeostasis and sleep homeostasis can each exert a higher priority on animal behavior depending on homeostatic need. Canonical neural populations and circuits that regulate energy balance and appetite [1, 2] are separate from those that regulate sleep homeostasis and sleep state transitions [3, 4]. Homeostatic systems for thirst [5, 6] and body temperature [7, 8] also seem to have their own dedicated neural systems and networks. Therefore, fascinating areas for future investigation include measuring the effect that these separate populations exert over each other during changing survival needs, and determining how the brain ultimately integrates several distinct homeostatic cues into a single, focused behavioral choice.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Matthew Carter (mc10@williams.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments were approved by the Institutional Animal Care and Use Committee at Williams College and were performed in accordance with the guidelines described in the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. We used male AgrpCre/+ mice [63] (Jackson Labs, catalogue #012899) and male PomcCre/+ mice [64] (Jackson Labs, catalogue #005965) bred on a C57Bl/6 background. All mice were 7-9 weeks old at the time of surgery and no more than 16-20 weeks old at the cessation of experiments. Mice were housed in individual cages with a 12h/12h light/dark cycle at 22 °C.
METHOD DETAILS
Virus Preparation.
Cre-inducible recombinant AAV1 vectors carrying either ChR2-mCherry (#AV-1-20297P) or tdTomato (#AV-1-ALL864) transgenes were obtained from the Vector Core of the University of Pennsylvania. Cre-inducible AAV8 vectors containing either hM3Dq-mCherry (#44361) or hM4Di-mCherry (#44362) were obtained from Addgene. Viral aliquots were stored at −80 °C before stereotaxic injection.
Stereotaxic surgery.
Mice were anaesthetized with 4% isoflurane and placed on a stereotaxic frame (David Kopf Instruments). Once on the frame and throughout the remainder of surgical procedures, mice received 1-2% isoflurane trans-nasally. After the skull was exposed and leveled in the horizontal plane, AAV was stereotaxically injected unilaterally or bilaterally, as described in the text, into the arcuate nucleus [anteroposterior (AP), −1.4 mm; mediolateral (ML), 0.45 mm; dorsoventral (DV), −5.9 mm]. A total of 0.5 μl of virus was injected at a rate of 0.1 μl/min and was allowed 8-10 min to diffuse before the injection needle was removed.
For polysomnographic recording, a custom-made EEG/EMG implant (modified from Pinnacle technology, #8402) was placed on the surface of the skull. EEG signals were recorded from electrodes on the frontal (AP, −2 mm; ML ± 2.5 mm) and temporal (AP, 3mm; ML, ± 2.5 mm) cortices. EMG signals were recorded from two electrodes inserted in the neck musculature to record postural tone. For optogenetic experiments, mice also received unilateral surgical implantation of a 5.6 mm mono fiber-optic cannula (Doric Lenses) above the arcuate nucleus (AP, −1.4 mm; ML, 0.45 mm; DV 5.6 mm). The EEG/EMG implant and cannula were fixed onto the skull with C&B Metabond (Parkell) and dental acrylic. All mice were provided with at least 14 days to recover from surgery prior to the start of experimental procedures.
Food intake measurements.
For feeding assays, mice were individually housed in food/liquid intake measurement cages with attached water bottles mounted on scales (Omnitech Electronics). Mice were provided with a liquid diet of Vanilla Ensure (Abbott Nutrition) diluted in a 1:2 ratio with water. This liquid diet had a caloric density of 450 kcal/L. Mice were allowed to habituate for a minimum of 72 hours. Bottles containing liquid diet were washed and disinfected daily and fully replenished in the first few hours of the light cycle. Because the mice tended to increase their food intake in response to the replenishment of the liquid diet, food intake trials were performed at least 3 h after exchanging fresh food bottles. All food intake measurements were obtained during the middle 4 hours of the inactive cycle (4-8 h after light onset).
Polysomnographic recording.
EEG and EMG signals derived from the surgically implanted electrodes were amplified and digitized at 256 Hz (Pinnacle). The signals were digitally filtered and spectrally analyzed by fast Fourier transformation, and polysomnographic recordings were scored using sleep analysis software (Sirenia Sleep Pro, Pinnacle). All scoring was performed manually based on the visual signature of the EEG and EMG waveforms, as well as the power spectra of 5-s epochs.
We defined wakefulness as desynchronized low-amplitude EEG and heightened tonic EMG activity with phasic bursts. We defined NREM sleep as synchronized, high-amplitude, low-frequency (0.5-4 Hz) EEG and highly reduced EMG activity compared with wakefulness with no phasic bursts. We defined REM sleep as having a pronounced theta rhythm (4-10 Hz) and a flat EMG. All sleep scoring was performed by investigators blind to the viral transgene delivered to the animal. Spectral analysis of EEG was performed by fast Fourier transform and binned in 0.5 Hz resolution from 0-25 Hz. Microarousal events were omitted from power analysis of NREM sleep.
Sleep deprivation.
We sleep deprived animals for 6 h starting at the onset of the light (inactive) period using gentle handling procedures. If an animal remained motionless for a few seconds, we gently agitated the animal with a soft brush. Occasionally, we noticed an animal entering NREM sleep during online EEG/EMG analysis, but these periods lasted < 1-3 s before we gently agitated the animal with the brush.
Optogenetic photostimulation.
Mice were attached to fiber optic cables (1.5 m long, 200 μm diameter; Doric Lenses) coated with opaque heat-shrink tubing and allowed to acclimate for at least 5 d prior to experimental sessions. Photostimulation was produced by a 473 nm blue-light laser (LaserGlow) driven by a Master-8 Pulse Stimulator (A.M.P.I.), which was programmed to deliver 10 ms light pulses at 1, 5, or 10 Hz for 1 s every 4 s for 60 min. For acute photostimulation experiments, each stimulation epoch was applied at 10 Hz starting 15 s after a stable NREM sleep sleep event, as detected by real-time online EEG/EMG analysis.
Chemogenetics.
For chemogenetic experiments, hM3Dq-mCherry-, hM4Di-mCherry-, and tdTomato-transduced mice received an intraperitoneal injection of clozapine-N-oxide (CNO; Sigma; 0.3 mg/kg) dissolved in 0.9% saline. All animals received CNO administration to avoid potential off-target effects of CNO [65, 66]. CNO was administered 10 min before sleep recordings started, during the middle 4 h of the inactive period. The mice were allowed at least 48 h to recover between trials.
Histology.
To confirm viral expression at the end of behavioral procedures, mice were anaesthetized with an intraperitoneal injection of 2,2,2 tribromoethanol (Sigma, #48402) dissolved in Tert-amyl alcohol and sterile 0.9% saline. Mice were then transcardially perfused with cold 0.01M phosphate buffered saline (PBS), pH 7.4, followed by 4% paraformaldehyde in PBS. The brains were extracted, allowed to postfix overnight in 4% paraformaldehyde at 4°C, and cryoprotected in 30% sucrose dissolved in PBS for an additional 24 h at 4°C. Each brain was sectioned at 30 μm on a microtome (Leica Microsystems) and collected in cold PBS.
Brain sections were mounted in PBS onto SuperFrost Plus glass slides (VWR, #48311-703), coverslipped with Dapi Fluoromount-G (Southern Biotech, #0100-20), and stored in the dark at 4 °C before microscopy and imaging. Slides were imaged using an Eclipse 80i epifluorescent microscope (Nikon), and images were captured using a RETIGNA 2000R digital camera. The resulting images were minimally processed using Photoshop CS5 (Adobe Systems) to enhance the brightness and contrast for optimal representation of the data.
QUANTIFICATION AND STATISTICAL ANALYSIS
All behavioral experiments included an n = 6 for each cohort of animals with at least 5 trials for each animal. Data were analyzed using Prism 7.0 (GraphPad Software). Statistical tests included two-way ANOVA with repeated measures, one-way ANOVA with repeated measures, and unpaired two-tailed t-test as described in Table S1. Graphs were exported to Illustrator CS5 (Adobe) for preparation of figures.
Supplementary Material
Table S1: Supplemental Statistical Analysis. Related to all figures.
HIGHLIGHTS.
Food deprivation increases wakefulness and disrupts sleep
Stimulation of AgRP neurons increases wakefulness and disrupts sleep.
Inhibition of AgRP neurons rescues sleep integrity in food-deprived animals.
Stimulation of POMC neurons rescues sleep integrity in food-deprived animals.
ACKNOWLEDGEMENTS
This research is supported by National Science Foundation Grant 1652060 and National Institutes of Health Grant DK105510 from the National Institute of Digestive and Diabetes and Kidney Diseases (NIDDK) to M.E.C. We thank A. Alhadeff, O. Barnhill, A. Carter, R. Essner, and K. Odenigbo for constructive comments and feedback on the manuscript.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Andermann ML, and Lowell BB (2017). Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95, 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternson SM, and Eiselt AK (2017). Three Pillars for the Neural Control of Appetite. Annual review of physiology 79, 401–423. [DOI] [PubMed] [Google Scholar]
- 3.Saper CB, and Fuller PM (2017). Wake-sleep circuitry: an overview. Curr Opin Neurobiol 44, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber F, and Dan Y (2016). Circuit-based interrogation of sleep control. Nature 538, 51–59. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman CA, Leib DE, and Knight ZA (2017). Neural circuits underlying thirst and fluid homeostasis. Nat Rev Neurosci 18, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gizowski C, and Bourque CW (2018). The neural basis of homeostatic and anticipatory thirst. Nature reviews. Nephrology 14, 11–25. [DOI] [PubMed] [Google Scholar]
- 7.Tan CL, and Knight ZA (2018). Regulation of Body Temperature by the Nervous System. Neuron 98, 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SF (2016). Central control of body temperature. F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi KA, and Cone RD (2005). Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Lin YC, Kuo TW, and Knight ZA (2015). Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, and Sternson SM (2015). Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, and Andermann ML (2015). Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler LR, Chen Y, Ahn JS, Lin YC, Essner RA, and Knight ZA (2017). Dynamics of Gut-Brain Communication Underlying Hunger. Neuron 96, 461–475 e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, and Wakabayashi I (2001). Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 50, 2438–2443. [DOI] [PubMed] [Google Scholar]
- 15.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, and Wakabayashi I (2000). Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141, 4797–4800. [DOI] [PubMed] [Google Scholar]
- 16.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. (2003). The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661. [DOI] [PubMed] [Google Scholar]
- 17.Duerrschmid C, He Y, Wang C, Li C, Bournat JC, Romere C, Saha PK, Lee ME, Phillips KJ, Jain M, et al. (2017). Asprosin is a centrally acting orexigenic hormone. Nat Med 23, 1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, and Barsh GS (2005). PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115, 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, and Lowell BB (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121, 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aponte Y, Atasoy D, and Sternson SM (2011). AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience 14, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, and Low MJ (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484. [DOI] [PubMed] [Google Scholar]
- 22.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, and Luo M (2013). Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33, 3624–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Bruning JC, and Krashes MJ (2016). Hunger-Driven Motivational State Competition. Neuron 92, 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla SL, Qiu J, Soden ME, Sanz E, Nestor CC, Barker FD, Quintana A, Zweifel LS, Ronnekleiv OK, Kelly MJ, et al. (2016). Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat Neurosci 19, 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Ronnekleiv OK, Kelly MJ, and Palmiter RD (2017). AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc Natl Acad Sci U S A 114, 2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, and Betley JN (2018). A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell 173, 140–152 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel JM (2008). Do all animals sleep? Trends Neurosci 31, 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danguir JN, S.; Gerard H (1979). Relations between feeding and sleep patterns in the rat. Journal of Comparative and Physiological Psychology 93, 820–830. [Google Scholar]
- 29.Jacobs BL, and McGinty DJ (1971). Effects of food deprivation on sleep and wakefulness in the rat. Exp Neurol 30, 212–222. [DOI] [PubMed] [Google Scholar]
- 30.Guesdon B, Minet-Ringet J, Tome DG, and Even PC (2005). Restriction-refeeding of calories and protein induces changes to slow wave and paradoxical sleep that parallel changes in body lipid and protein levels in rats. Behav Brain Res 164, 156–164. [DOI] [PubMed] [Google Scholar]
- 31.Alvarenga TA, Andersen ML, Papale LA, Antunes IB, and Tufik S (2005). Influence of long-term food restriction on sleep pattern in male rats. Brain Res 1057, 49–56. [DOI] [PubMed] [Google Scholar]
- 32.Borbely AA (1977). Sleep in the rat during food deprivation and subsequent restitution of food. Brain Res 124, 457–471. [DOI] [PubMed] [Google Scholar]
- 33.Dewasmes G, Duchamp C, and Minaire Y (1989). Sleep changes in fasting rats. Physiol Behav 46, 179–184. [DOI] [PubMed] [Google Scholar]
- 34.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, and de Lecea L (2011). Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A 108, 13305–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Garcia F, and Drucker-Colin R (1999). Endogenous and exogenous factors on sleep-wake cycle regulation. Progress in neurobiology 58, 297–314. [DOI] [PubMed] [Google Scholar]
- 36.Richter C, Woods IG, and Schier AF (2014). Neuropeptidergic control of sleep and wakefulness. Annu Rev Neurosci 37, 503–531. [DOI] [PubMed] [Google Scholar]
- 37.Szentirmai E (2012). Central but not systemic administration of ghrelin induces wakefulness in mice. PLoS One 7, e41172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szentirmai E, Hajdu I, Obal F Jr., and Krueger JM (2006). Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res 1088, 131–140. [DOI] [PubMed] [Google Scholar]
- 39.Szentirmai E, Kapas L, and Krueger JM (2007). Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol 292, R575–585. [DOI] [PubMed] [Google Scholar]
- 40.Esposito M, Pellinen J, Kapas L, and Szentirmai E (2012). Impaired wake-promoting mechanisms in ghrelin receptor-deficient mice. Eur J Neurosci 35, 233–243. [DOI] [PubMed] [Google Scholar]
- 41.Dyzma M, Boudjeltia KZ, Faraut B, and Kerkhofs M (2010). Neuropeptide Y and sleep. Sleep medicine reviews 14, 161–165. [DOI] [PubMed] [Google Scholar]
- 42.Szentirmai E, and Krueger JM (2006). Central administration of neuropeptide Y induces wakefulness in rats. Am J Physiol Regul Integr Comp Physiol 291, R473–480. [DOI] [PubMed] [Google Scholar]
- 43.Danguir J, and Nicolaidis S (1984). Chronic intracerebroventricular infusion of insulin causes selective increase of slow wave sleep in rats. Brain Res 306, 97–103. [DOI] [PubMed] [Google Scholar]
- 44.Sinton CM, Fitch TE, and Gershenfeld HK (1999). The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res 8, 197–203. [DOI] [PubMed] [Google Scholar]
- 45.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, and Turek FW (2006). Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol 290, R894–903. [DOI] [PubMed] [Google Scholar]
- 46.Chastrette N, and Cespuglio R (1985). Influence of proopiomelanocortin-derived peptides on the sleep-waking cycle of the rat. Neurosci Lett 62, 365–370. [DOI] [PubMed] [Google Scholar]
- 47.Chastrette N, Cespuglio R, Lin YL, and Jouvet M (1990). Proopiomelanocortin (POMC)-derived peptides and sleep in the rat. Part 2--Aminergic regulatory processes. Neuropeptides 15, 75–88. [DOI] [PubMed] [Google Scholar]
- 48.Atasoy D, Betley JN, Su HH, and Sternson SM (2012). Deconstruction of a neural circuit for hunger. Nature 488, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, and Tobler I (2006). Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem 85, 263–271. [DOI] [PubMed] [Google Scholar]
- 50.Boyce R, Glasgow SD, Williams S, and Adamantidis A (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. [DOI] [PubMed] [Google Scholar]
- 51.Adamantidis A, Carter MC, and de Lecea L (2010). Optogenetic deconstruction of sleep-wake circuitry in the brain. Front Mol Neurosci 2, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS, Madara JC, Campbell JN, Kroeger D, Scammell TE, et al. (2016). Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci 19, 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kodani S, Soya S, and Sakurai T (2017). Excitation of GABAergic Neurons in the Bed Nucleus of the Stria Terminalis Triggers Immediate Transition from Non-Rapid Eye Movement Sleep to Wakefulness in Mice. J Neurosci 37, 7164–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, and Elmquist JK (1999). Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786. [DOI] [PubMed] [Google Scholar]
- 55.Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, et al. (1998). Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402, 442–459. [PubMed] [Google Scholar]
- 56.Louis GW, Leinninger GM, Rhodes CJ, and Myers MG Jr. (2010). Direct innervation and modulation of orexin neurons by lateral hypothalamic LepRb neurons. J Neurosci 30, 11278–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, Wellman A, Arrigoni E, Fuller PM, et al. (2017). A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron 96, 1153–1167 e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Betley JN, Cao ZF, Ritola KD, and Sternson SM (2013). Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell 155, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber F, Hoang Do JP, Chung S, Beier KT, Bikov M, Saffari Doost M, and Dan Y (2018). Regulation of REM and Non-REM Sleep by Periaqueductal GABAergic Neurons. Nature communications 9, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danguir J, and Nicolaidis S (1979). Dependence of sleep on nutrients' availability. Physiol Behav 22, 735–740. [DOI] [PubMed] [Google Scholar]
- 61.Rajab E, Alqanbar B, Naiser MJ, Abdulla HA, Al-Momen MM, and Kamal A (2014). Sex differences in learning and memory following short-term dietary restriction in the rat. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience 36, 74–80. [DOI] [PubMed] [Google Scholar]
- 62.Yanai S, Okaichi Y, and Okaichi H (2004). Long-term dietary restriction causes negative effects on cognitive functions in rats. Neurobiol Aging 25, 325–332. [DOI] [PubMed] [Google Scholar]
- 63.Tong Q, Ye CP, Jones JE, Elmquist JK, and Lowell BB (2008). Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11, 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr., Elmquist JK, et al. (2004). Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991. [DOI] [PubMed] [Google Scholar]
- 65.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, et al. (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahler SV, and Aston-Jones G (2018). CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology 43, 934–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Supplemental Statistical Analysis. Related to all figures.