Summary
Immune check-point inhibition is dramatically improving patient outcomes in diverse cancers, many of which responded poorly to traditional cytotoxic agents. Drivers of heterogeneous response to immune check-point therapy are poorly characterized. Cachectic cancer patients exhibit elevated pembrolizumab clearance and poor response highlighting the immense therapeutic challenge posed by cancer cachexia.
Body
In this issue of Clinical Cancer Research, Turner and colleagues (1) reveal how patient protein metabolism dramatically impacts the therapeutic efficacy of pembrolizumab, a humanized IgG4 monoclonal antibody and immune-check point inhibitor that binds directly to programmed death 1 (PD1). Unlike small molecule xenobiotics, the catabolism and clearance of pembrolizumab interfaces with a complex and highly orchestrated host protein intermediary metabolism which displays high rates of dysfunction in cancer patients.
In two large clinical trials, the investigators report a seemingly paradoxical association between rapid baseline plasma clearance (CL0) of pembrolizumab and poor overall survival (OS), despite a lack of association between plasma exposure and OS at doses between 2 mg/kg and 10 mg/kg. They also note dose-proportional pembrolizumab pharmacokinetics yet a large within-dose variability in OS. These findings are consistent with previous reports of significant percentages of patients failing to respond to immune check-point therapy despite ample expression of relevant targets. Yet this current study goes a crucial step further in identifying features of poorly responding patients. Despite having roughly 5-fold higher systemic exposure, high CL0 patients receiving 10 mg/kg pembrolizumab had similar poor outcomes as high CL0 patients receiving 2 mg/kg pembrolizumab. At both doses, patients with low CL0 exhibited a remarkable 15-month advantage in OS compared to patients with high CL0. The relationship between CL0 and survival was observed in both advanced melanoma (n= 211) and advanced previously–treated non-small cell lung cancer (NSCLC, n=537) and persisted even after correcting for established baseline clinical risk factors. In both populations, on-study reductions in patient body weight and serum albumin were associated with reduced survival in high CL0 patients leading the authors to hypothesize that underlying disease involvement and cancer cachexia may be confounding the exposure-response relationship to pembrolizumab. In other words, high therapeutic antibody clearance in the presence of elevated patient catabolism appears to be a marker of refractory disease rather than a cause for therapy failure; as a result, cachectic cancer patients appear refractory to pembrolizumab.
Cachexia, or involuntary and often extreme loss of bodyweight, has been a well-recognized feature of advanced malignancy for millennia, but only recently has a consensus definition emerged for cachexia in the context of cancer (2). Inherent to this definition, and distinct from simple starvation, is the fact that cachectic wasting cannot be reversed with appropriate nutritional support. Malnutrition is a frequent result of cancer progression and therapy and undoubtedly contributes to cachectic decline. It is currently unclear when generally malnourished cancer patients transition to cachexia that is refractory to aggressive nutritional support. However, both malnutrition and cancer cachexia are associated with a pro-catabolic state that remains poorly understood by those providing cancer therapy and is the subject of robust ongoing research efforts. Turner and colleagues point out that this pro-catabolic state, often characterized by hypoalbuminemia, could explain elevated pembrolizumab CL0. In fact, this is a standard assumption made by pharmacologists studying antibody therapies – that dysregulated protein breakdown mechanisms driving skeletal muscle decay likely overlaps with those same processes clearing exogenous and endogenous serum proteins (3). However, the authors’ analyses reveal that while delivering increased pembrolizumab to correct for this increased CL does in fact increase drug exposure, the increased exposure does not translate to improved outcomes. The data suggest the pro-catabolic patient displays primary resistance to pembrolizumab (Fig. 1).
Figure 1.
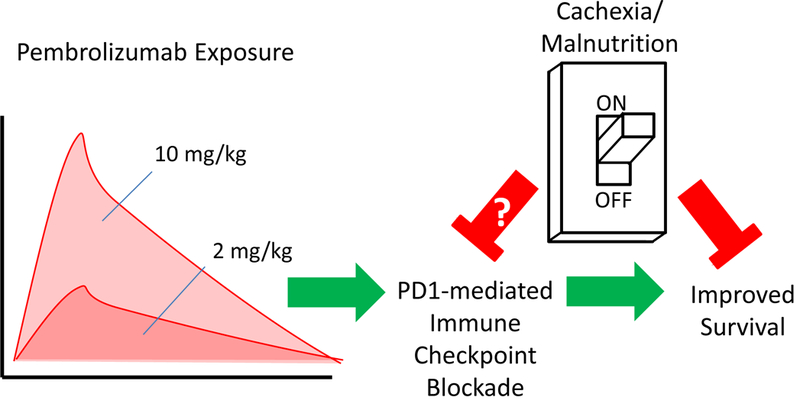
Cachexia/malnutrition blocks the beneficial effects of pembrolizumab independent of pembrolizumab exposure.
In an effort to understand why patients with elevated protein catabolism fail to respond to pembrolizumab, seemingly over a wide range of drug exposure, the authors point to recent work linking metabolic dysfunction and immune suppression demonstrated in cachectic mice (4). The studies show that heightened circulating IL-6 results in the suppression of PPARα-mediated ketogenesis within the liver of cachectic animals, further triggering systemic glucocorticoid release as part of an appropriate biologic response to metabolic stress. Therefore cachectic mice, and likely cachectic humans with elevated circulating IL-6, exhibit some degree of glucocorticoid-mediated immune suppression resulting in limited T-cell chemotaxis and poor response to immune checkpoint inhibition. This hypothesis was nicely validated by recent studies in murine models of pancreatic ductal adenocarcinoma (PDAC) where concurrent IL-6 blockade with PDL-1 targeted therapy resulted in improved overall T-cell activation and anti-tumor response (5). Anti-IL-6 therapy has failed to demonstrate any clinical benefit in cachectic patients, but these studies suggest successful blockade of the IL-6 axis in the cachectic liver could restore homeostatic nutrient utilization and reduce immune-suppressive glucocorticoid release. Importantly, the effects reported in cachectic animals largely parallel the long known association between protein-calorie malnutrition and poor immune response to many types of stimuli (4).
The use of elevated mAb CL0 as an early marker for ongoing malnutrition or cachexia offers an opportunity for intervention that may improve responses to potentially highly effective drugs. There remains intense interest in discovering improved biomarkers of cancer cachexia and malnutrition in order to facilitate early deployment of nutritional support, guide disease management decisions, and more precisely study the efficacy of anti-cachexia interventions. Though balanced for other clinical features at enrollment, Turner and colleagues show that high CL0 of pembrolizumab was predictive of the subsequent development of cancer wasting in advanced NSCLC and melanoma populations. This suggests that rapid clearance of pembrolizumab, and potentially other mAb therapy, could serve as an indicator of a high risk population suffering from malnutrition and sub-clinical cachexia or pre-cachexia. Research is needed to address how these findings may be deployed in an oncology clinic, but in the context of early phase clinical trials, patients with rapid mAb CL warrant close scrutiny based on their cachectic potential.
The study by Turner and colleagues provides evidence that patients experiencing elevated serum protein catabolism are refractory to at least one class of immune check-point inhibitor therapies which are revolutionizing the management of multiple cancers, many of which coincide with high rates of malnutrition and cachexia(2). The strategy to address this issue and improve therapeutic responses is necessarily multidimensional and must include research on appropriate screening, new biomarkers, and novel anti-wasting interventions. The merits of developing specific anti-cachectic therapies have been historically debated based on the notion that effective anti-neoplastic therapy will also reverse cachectic decline whereas the likely impact of effective anti-cachectic intervention on cancer burden and patient survival are unclear. Advocates for treating cachexia have been hopeful that effective anti-cachexia intervention would fortify the cancer patient such that more aggressive and hopefully effective anti-cancer therapy could be pursued to improve both quality and quantity of life. Turner and colleagues have shown that in addition to well characterized reductions in patient tolerance to cytotoxic therapy, malnutrition and/or cancer cachexia is directly associated with pembrolizumab resistance. Furthermore, they highlight similar patterns in data reported for nivolumab, atezolizumab and ipilimumab suggesting a more general relationship between a pro-catabolic state and poor response to immune check-point inhibitors.
When the composite effects of malnutrition and cachexia on anti-cancer therapy are expanded to include primary therapeutic resistance to immune-check point inhibition in addition to increased treatment-related and often dose-limiting toxicities, the need for accurate patient assessment and effective anti-wasting interventions is clarified. It appears increasingly unlikely that the potential benefits of antibody therapy will be fully realized without addressing underlying critical host metabolic dysfunction. After decades of intense study, scientists and clinicians are translating knowledge regarding the highly orchestrated anti-cancer immune response into meaningful and broadly applicable strategies to improve outcomes with durable responses and potential cures. The work by Turner and colleagues provides greater insight and a clear conclusion that we must also focus upon patient nutritional status, metabolism, and cancer cachexia in order to maximize the benefit of immunotherapy.
Acknowledgments
Supported by the following funding: K12-CA133250 (C.C. Coss), P30 CA016058 (C.C. Coss, M. A. Phelps)
Footnotes
Disclosure of Potential Conflicts of Interest
CC Coss – No potential conflicts of interest to disclose
SK Clinton – No potential conflicts of interest to disclose
MA Phelps – No potential conflicts of interest to disclose
REFERENCES
- 1.Turner D, Kondic AG, Anderson KM, Robinson A, Garon EB, Riess JW, et al. Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clinical cancer research : an official journal of the American Association for Cancer Research 2018. doi 10.1158/1078-0432.CCR-18-0415. [DOI] [PubMed] [Google Scholar]
- 2.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nature reviews Disease primers 2018;4:17105 doi 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 3.Ryman JT, Meibohm B. Pharmacokinetics of Monoclonal Antibodies. CPT: pharmacometrics & systems pharmacology 2017;6(9):576–88 doi 10.1002/psp4.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flint TR, Fearon DT, Janowitz T. Connecting the Metabolic and Immune Responses to Cancer. Trends in molecular medicine 2017;23(5):451–64 doi 10.1016/j.molmed.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67(2):320–32 doi 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]


