Abstract
The increasing incidence of colorectal cancer (CRC) in younger adults (age <50 years) has been widely reported. Using data from the Surveillance, Epidemiology, and End Results Program, young-onset CRC incidence rates decreased from 1975 through about 1990. Decreases were more prominent in the colon, a contrast with more recent increases in rectal cancer. Incidence rates subsequently increased, differing by time period and 5-year age group. This inflection point is consistent with a birth cohort effect and points to early life exposures – accumulated throughout the life course – that may increase cancer risk. Studying early life exposures among persons born after 1960 may advance our understanding of CRC in younger adults.
Keywords: Colorectal neoplasia, incidence, young adult, birth cohort
In contrast to dramatic declines in older populations, incidence of colorectal cancer (CRC) in the U.S. has increased among adults younger than age 50 years.1, 2 To clarify these observations and better understand the distribution of young-onset CRC in the population, we characterized incidence patterns over a 40-year period.
There were 70,400 incident cases of CRC diagnosed among young adults (age 20-49 years) during the period 1975 – 2014. The overall incidence rate was 9.5 per 100,000, increasing from 9.9 per 100,000 in 1975-80 to 11.7 per 100,000 in 2010-14 (Supplementary Table 1).
In contrast to increases over the entire time period, incidence (all younger ages combined) decreased between 1975-79 and 1990-94, from 9.9 to 8.3 per 100,000 (Supplementary Figure 1). There were particularly large decreases in the 45-49 year age group (Figure 1), among whom incidence decreased from 30.1 per 100,000 in 1975-79 to 24.3 per 100,000 in 1990-94. Younger age groups experienced a similar relative decrease during this period, although of smaller absolute magnitude.
Figure 1.
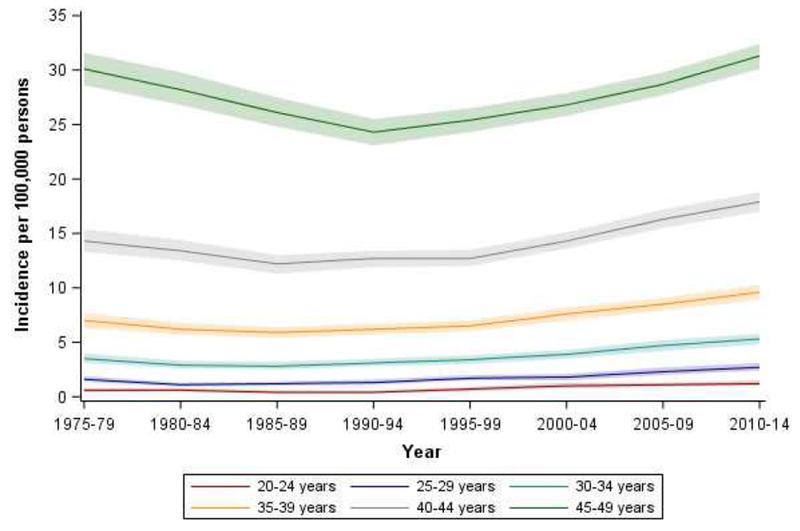
Age-specific incidence of young-onset colorectal cancer by 5-year age group and time period, SEER 9, 1975 – 2014
However, starting in about 1990, incidence increased from 8.3 per 100,000 to 11.8 per 100,000 in 2010-14 (Supplementary Figure 1). Increases in incidence differed by 5-year age group and time period. Age-specific incidence rates increased across successive birth cohorts after 1960, consistent with a birth cohort effect (Figure 2). Supplementary Table 1 shows detailed incidence rates by age and time period, where shaded cells correspond to time periods with the lowest incidence rate. For example, the incidence rate in the 30-34 age group was lowest in 1985-89, but the lowest incidence rate occurred later (1990-94) among 45-49 year olds. This pattern was prominent in whites (Supplementary Table 2) but not blacks (Supplementary Table 3).
Figure 2.
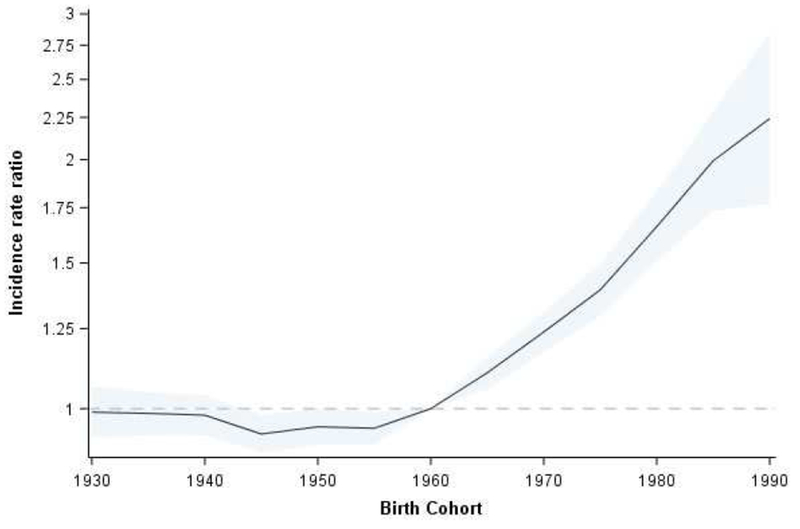
Incidence rate ratios by birth cohort
We observed similar trends, although of differing magnitude, by anatomic subsite (Supplementary Figure 2). Incidence of proximal and distal colon cancer decreased over a 15- year period starting in 1975, with notable decreases among 40-year olds. Rectal cancer rates differed little during this period. In contrast, from 1990-94 to 2010-14, incidence of rectal and distal colon cancer increased in all age groups. Increases in proximal cancer were smaller.
Contrary to the well-known recent increases in young-onset CRC, we observed a clear pattern of decreasing incidence rates over the 15-year period from 1975 through about 1990. Interestingly, decreasing rates were most prominent in the colon, a contrast with the more recent increases in rectal cancer. The observed incidence trends are consistent with a birth cohort effect, suggesting exposures in early childhood or adolescence may contribute to young-onset CRC.
Although increases in young-onset CRC have been widely reported,1, 2 decreasing rates in the 1970s and 80s, particularly among the 40-year age group, have not been discussed. These early declines occurred before CRC incidence rates decreased in older adults, which started around 1985, most likely due to screening.3 Data from the Connecticut Tumor Registry show CRC incidence started to decrease as early as 1950 among young women, and mortality also decreased among both men and women during this period.4 Based on limited evidence, some have speculated that dietary patterns during the Great Depression and World War II5 contributed to lower risk of CRC among persons born during this time period. Demographic shifts (e.g., population age structure and size) may have also contributed to decreasing incidence rates among younger adults.
Starting with persons born around 1960, CRC incidence rates increased across successive birth cohorts. There were even larger increases among more recent birth cohorts. For example, incidence was higher among 40-year olds born in 1970 (17.9 per 100,000) than among 40-year olds born in 1950 (12.7 per 100,000). Birth cohort effects point to early life exposures – or exposures accumulated throughout the life course – that may increase risk of cancer.6 Higher incidence rates among “Generation X” may implicate risk factors, such as antibiotic use,7–9 increasingly prevalent during their childhood. Indeed, prescriptions for broad-spectrum antibiotics, associated with disruptions to gut microbiota, nearly tripled from 1980 to 1992.10 Ear and upper respiratory infections accounted for much of the rampant antibiotic prescribing in the 1980s, and physician visits associated with these diagnoses also increased during the same period. We have only recently begun to understand the complexity of gut microbiota, and how antibiotics shape its ecology. Growing evidence suggests antibiotics in infancy or early childhood influences microbial diversity and composition,11 which may then promote colorectal carcinogenesis in adulthood.7, 9
Birth cohort effects also illustrate the importance of reconsidering the timing and duration of well-established CRC risk factors. Many have hypothesized that obesity has driven recent increases in young-onset CRC. Measuring obesity during vulnerable windows of growth and development (e.g., birthweight, childhood obesity) may advance our understanding of its role in young-onset CRC and identify periods of exposure conferring the greatest risk. Exposures occurring later in life but with short latent periods, such as weight gain in young adulthood, may also contribute to risk.
Early, decreasing incidence rates and birth cohort patterns appeared more prominent in younger whites. We also observed a higher incidence of proximal colon, and lower incidence of rectal, cancer among blacks. Sparse data may have limited our ability to observe birth cohort patterns in blacks, particularly during the earliest years of the SEER registries. Alternatively, the discordant trends may point to racial differences in CRC biology or distribution of risk factors. Differential exposure to environmental- or lifestyle-related factors may explain why overall incidence rates are higher among young blacks, and the age-related acceleration in incidence starts at a younger age. For example, blacks have experienced a sustained influence of early life exposures, such as type II diabetes12 or childhood obesity,13 compared to more marked changes in prevalence that have occurred over time among whites. Others have noted racial differences in inflammatory response and immune surveillance14 may play a role in colorectal carcinogenesis.
The largest absolute decreases in incidence during the 1970s and 80s occurred in the proximal and distal colon. But since 1990, increases in incidence have been primarily driven by higher rates of rectal cancer, particularly among whites. Differences in incidence rates by anatomic subsite make a strong case for teasing apart risk factors for colon vs. rectal cancer. Many etiologic studies group colon and rectal cancers together as a single disease, but studies that have examined risks separately suggest diet, family history, obesity, and smoking may have different effects on risk.15 Although not fully understood, reasons for these observations may include differences in: sensitivities to insulin effects; exposure to fecal matter; and pH levels influencing susceptibility to environmental risk factors.15
In summary, the recent increase in young-onset CRC was preceded by decreasing rates from 1975 through 1990. Patterns of CRC in younger populations provide etiologic clues for understanding mechanisms of young-onset disease. Specifically, the sharp pivot in incidence curves, where incidence decreased and then increased, as well as trends across birth cohorts, suggest early life exposures may play a prominent role in disease development. Studying population shifts in the distribution of CRC risk factors, as well as their timing and duration, may advance our understanding of young-onset CRC and its causes.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Cancer Institute (P30CA142543) and National Center for Advancing Translational Sciences (KL2TR001103 to Dr. Murphy) at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest or financial disclosures.
References
- 1.Siegel RL, et al. J Natl Cancer Inst 2017;109. [Google Scholar]
- 2.Bailey CE, et al. JAMA Surg 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CC, et al. Clin Gastroenterol Hepatol 2017;15:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu KC, et al. J Natl Cancer Inst 1994;86:997–1006. [DOI] [PubMed] [Google Scholar]
- 5.Svensson E, et al. Cancer Causes Control 2005;16:215–23. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, et al. Chapman and Hall/CRC Press, 2013.
- 7.Boursi B, et al. Pharmacoepidemiol Drug Saf 2015;24:534–42. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, et al. Gut 2018;67:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dik VK, et al. Dig Dis Sci 2016;61:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCaig LF, et al. JAMA 1995;273:214–9. [PubMed] [Google Scholar]
- 11.Cho I, et al. Nature 2012;488:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koopman RJ, et al. Ann Fam Med 2005;3:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CL, et al. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace K, et al. Cancer Epidemiol Biomarkers Prev 2018; Epub 2018 May 16. [Google Scholar]
- 15.Wei EK, et al. Int J Cancer 2004;108:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


