Abstract
Background Information
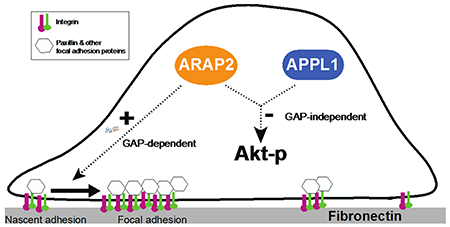
ARAP2, an Arf GTPase-activating protein (Arf GAP) that binds to adaptor protein with PH domain, PTB domain and leucine zipper motifs 1 (APPL1), regulates focal adhesions (FAs). APPL1 affects FA dynamics by regulating Akt. Here, we tested the hypothesis that ARAP2 affects FAs in part by regulating Akt through APPL1.
Results
We found that ARAP2 controlled FA dynamics dependent on its enzymatic Arf GAP activity. In some cells ARAP2 also regulated phosphoAkt (pAkt) levels. However, ARAP2 control of FAs did not require Akt and conversely, the effects on pAkt were independent of FAs. Reducing ARAP2 expression reduced the size and number of FAs in U118, HeLa and MDA-MB-231 cells. Decreasing ARAP2 expression increased pAkt in U118 cells and HeLa cells and overexpressing ARAP2 decreased pAkt in U118 cells; in contrast, ARAP2 had no effect on pAkt in MDA-MB-231 cells. An Akt inhibitor did not block the effect of reduced ARAP2 on FAs in U118. Furthermore, the effect of ARAP2 on Akt did not require Arf GAP activity, which is necessary for effects on FAs and integrin traffic. Altering FAs by other means did not induce the same changes in pAkt as those seen by reducing ARAP2 in U118 cells. In addition, we discovered that ARAP2 and APPL1 had coordinated effects on pAkt in U118 cells. Reduced APPL1 expression, as for ARAP2, increased pAkt in U118 and the effect of reduced APPL1 expression was reversed by overexpressing ARAP2. Conversely the effect of reduced ARAP2 expression was reversed by overexpressing APPL1.
Conclusions
We conclude that ARAP2 affects Akt signaling in some cells by a mechanism independent of FAs or membrane traffic.
Significance
Our results highlight an Arf GAP-independent function of ARAP2 in regulating Akt activity and distinguish the effect of ARAP2 on Akt from that on FAs and integrin trafficking, which requires regulation of Arf6.
ARAP2 is an Arf GAP that has previously been reported to affect focal adhesions (FAs) by regulating Arf6 and integrin trafficking and to bind to the adaptor proteins APPL1. Here, we report that ARAP2 suppresses pAkt levels in cells coordinately with APPL1 and independently of GAP activity and its effect on the dynamic behavior of FAs.
Introduction
ARAP2 is an Arf GAP composed of a SAM, 5 PH, Arf GAP, Ank repeat, Rho GAP and Ras Association domains (Kahn et al., 2008; Miura et al., 2002; Yoon et al., 2006b). It specifically uses Arf6 as a substrate (Chen et al., 2013a; Yoon et al., 2006b). ARAP2 localizes to and regulates focal adhesions (FAs) (Chen et al., 2014; Chen et al., 2013a; Yoon et al., 2006b). FAs are structures composed of clustered transmembrane proteins called integrins that bind to the extracellular matrix and link to the actin cytoskeleton and control cell migration, proliferation, survival and differentiation (Gardel et al., 2010; Geiger and Yamada, 2011; Parsons et al., 2010; Winograd-Katz et al., 2014). The effect of ARAP2 on FAs depends on the reduction of Arf6•GTP levels by the Arf6 GAP activity, which controls Rac1•GTP levels and endocytic traffic of integrins (Chen et al., 2014; Chen et al., 2013b). However, the reduction in Arf6•GTP levels alone is not sufficient to explain the effect of ARAP2 on FAs.
ARAP2 associates with adaptor protein with PH domain, PTB domain and leucine zipper motifs 1 (APPL1) in endosomes (Chen et al., 2014). APPL1 (Miaczynska et al., 2004; Mitsuuchi et al., 1999) has been found to regulate FA dynamics by control of Akt (Broussard et al., 2012). APPL1 is recruited by Rab5 to endosomes involved in traffic of transmembrane receptors including integrins (Miaczynska et al., 2004; Valdembri et al., 2009). APPL1 endosomes are platforms for signaling affecting both the MAP kinase and Akt pathways (Masters et al., 2017; Schenck et al., 2008; Zoncu et al., 2009). APPL1 also binds directly to Akt, which inhibits signaling through Akt1 (Broussard et al., 2012) and facilitates signaling through Akt2 (Cheng et al., 2009). APPL1 inhibition of Akt1 has been reported to stabilize FAs (Broussard et al., 2012). Thus, APPL1 may affect FAs by two mechanisms that are not mutually exclusive, regulation of integrin traffic and regulation of Akt.
The relationship of signaling through the Akt pathway to FAs is complex. Increased Akt signaling increases the turnover of FA (Broussard et al., 2012). However, FAs also function as signaling platforms, affecting several signaling pathways including Akt (Erez et al., 2005; Guo and Giancotti, 2004; Hehlgans et al., 2007; Hynes, 2002; Moreno-Layseca and Streuli, 2014; Webb et al., 2004b; Wehrle-Haller and Imhof, 2002). Proteins associated with FAs include the nonreceptor tyrosine kinases FAK and Src. FAK can directly bind to the p85 subunit of PI-3-kinase, which leads to the activation and production of the signaling phospholipid PIP3, resulting in the activation of Akt. FAK and Src phosphorylate p130CAS with the consequent recruitment of signaling adaptors, such as Crk, and exchange factors for Ras superfamily proteins, e.g. DOCK180, SOS, C3G, which activate Rac1, Ras and Rap1. Integrin linked kinase (ILK) has also been reported to directly phosphorylate and activate Akt (Persad and Dedhar, 2003). ARAP2, by affecting FAs, may influence these pathways and, conversely, the effects of ARAP2 on FAs may be mediated by changes in Akt signaling.
Here we test the hypothesis that ARAP2 affects FAs in part by controlling Akt. We considered this a plausible hypothesis given that ARAP2 binds to APPL1 and APPL1 has been found to affect FA dynamics by regulation of Akt. Our results refuted the idea that ARAP2 regulates FAs by controlling pAkt levels; however, in testing the hypothesis we discovered that ARAP2 and APPL1 coordinately regulate pAkt levels in some cells independently of effects on FAs.
Results
ARAP2 affects assembly/disassembly of FAs
In previous work, we found that loss of ARAP2 resulted in fewer and smaller FAs and reduced stress fibers, consistent with immature adhesive structures whereas increased ARAP2 activity increased FA size and number (Chen et al., 2013a; Yoon et al., 2006a). We found that regulation of Arf6 and subsequent changes in membrane traffic and Rac1 were necessary but not sufficient for the ARAP2-dependent changes in FAs. As a step towards identifying additional ARAP2-dependent mechanisms that may contribute to regulation of FAs, we characterized FA turnover in HeLa cells. Cells were treated with control or ARAP2 siRNA. GFP-paxillin was used as a FA marker and imaged over time using time-lapse TIRF microscopy. The kinetics of formation and disassembly of adhesions were quantified by measuring the change in fluorescence intensity of GFP-paxillin within single adhesions over time (Webb et al., 2004a). Adhesion kinetics can be characterized as having three phases, (i) assembly, (ii) stationary and (iii) disassembly. We found that reduced ARAP2 expression affected all three. The average lifetime of the adhesions in ARAP2 siRNA-transfected cells was significantly shorter than control cells (58 % of the controls) (Fig. 1A-D and G and Video 1), with accelerated assembly and disassembly rates and shorter stationary phase, consistent with those described for immature adhesions (Choi et al., 2008). Overexpression of FLAG tagged wild-type ARAP2 rescued this change in FA dynamics, with half-lives indistinguishable from cells transfected with control siRNA (Fig. 1E and G and Video 2). In contrast, an Arf GAP deficient mutant of ARAP2, FLAG-[R728K]ARAP2, failed to rescue the fast FA turnover in ARAP2 siRNA cells (Fig. 1F and G and Video 3). Based on these results, we conclude that ARAP2 contributes to the maturation of adhesions.
Figure 1. Reduced ARAP2 expression promotes FA turnover.
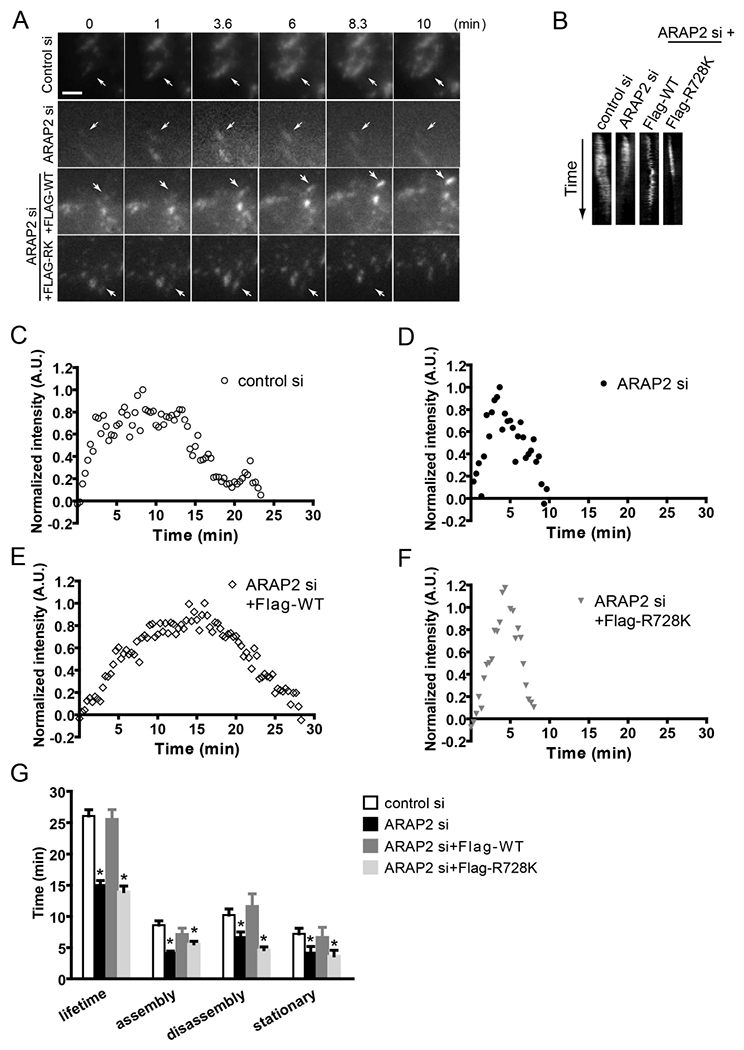
(A-G) HeLa cells treated with siRNA (control si, ARAP2 si) were transfected with a plasmid for expression of GFP-paxillin and either a vector control or different constructs for the expression of ARAP2 at a ratio of 1:7 (ARAP2 si+Flag-tagged WT, R728K), then plated onto fibronectin-coated (10 μg/ml) coverslips. The next day, images were obtained from a time-lapse TIRF series of GFP-paxillin during FA turnover. Data shown are (A) time-lapse images, (B) kymographs of GFP-paxillin adhesions, (C-F) examples of normalized fluorescent paxillin intensity change over time in the FAs, and (G) the average FA lifetime and length of assembly, disassembly and stationary phase from adhesions in each condition (n=14~19, ± s.e.m.). * p<0.05 indicates significantly different from control siRNA using Student’s t test. Scale bar, 2.5 μm.
ARAP2 affects the dynamic behavior of paxillin, a cytoplasmic element of FA plaques
The overall stability of FAs is determined by the exchange rate of the FA components in and out of the structure (Cohen et al., 2006; Edlund et al., 2001; Fraley et al., 2005; Gupton and Waterman-Storer, 2006; Lele et al., 2006; Lele et al., 2008; von Wichert et al., 2003; Wolfenson et al., 2011). Immature adhesions are reported to have faster exchange rates of the cytoplasmic plaque component, vinculin than do mature adhesions (Mohl et al., 2009). Therefore, if ARAP2 promoted maturation, cells with decreased ARAP2 expression may be expected to have adhesions with faster exchange of cytoplasmic components of the plaque than control cells and overexpression may be expected to have slower exchange rates. To determine if the exchange of FA components is affected by ARAP2 knockdown, we performed Fluorescence Recovery after Photobleaching (FRAP) analysis using GFP-paxillin as a model FA cytoplasmic component. In both control and ARAP2 siRNA-transfected cells, fluorescence recovery of GFP-paxillin in FAs fit a single exponential curve (Fig. 2A). Adhesions in cells with reduced ARAP2 had a shorter recovery time and a larger exchangeable fraction of GFP-paxillin (Fig. 2A and B). Conversely, fluorescence recovery of GFP-paxillin in cells overexpressing ARAP2 was slower but had no difference in the size of GFP-paxillin mobile fraction (Fig. 2C and D). These results are consistent with the idea that ARAP2 regulates the dynamics of FAs, which is controlled by several signaling pathways, including Rho-family GTP-binding proteins and Akt (Broussard et al., 2012). We have previously examined Rho family GTP-binding proteins (Chen et al., 2013b) but have not examined Akt.
Figure 2. ARAP2 modulates the exchange rate of paxillin in FAs.
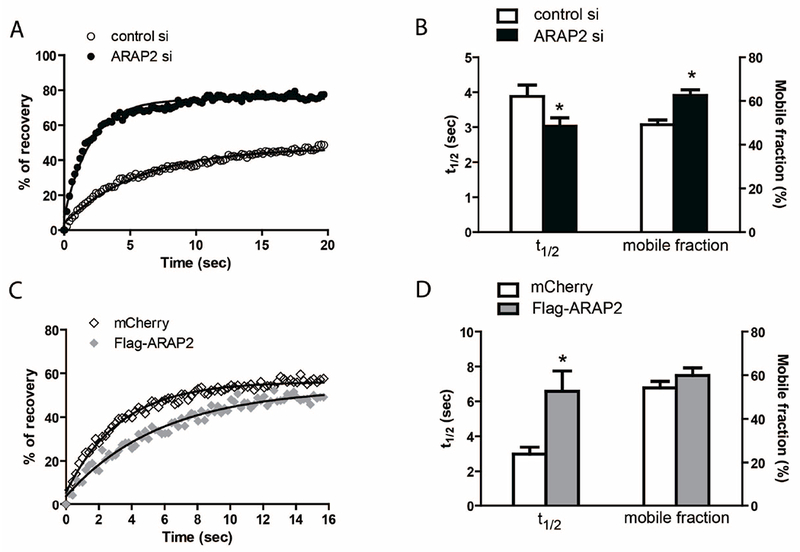
GFP-paxillin was co-transfected with siRNA (A, B), mCherry or Flag-ARAP2 at a ratio of 1:7 (C, D) into HeLa cells. Cells were plated onto fibronectin-coated glass bottom chambers and subsequently subjected to photo-bleaching and imaged on a spinning disk microscope. Examples of GFP-paxillin fluorescence recovery data in siRNA-transfected (A) and mCherry or Flag-ARAP2-transfected cells (C) fit a single exponential curve. (B, D) The average t1/2 and mobile fraction of GFP-paxillin fluorescence recovery in ten control siRNA (n=46), ten ARAP2 siRNA-transfected cells (n=48, ± s.e.m.), six mCherry (n=22) and six Flag-ARAP2-transfected cells (n=47, ± s.e.m.). * p<0.05 indicates different from control using Student’s t test.
ARAP2 affects pAkt independently of FAs
Akt, regulated by the adaptor protein APPL1, has been found to affect FA dynamics (Broussard et al., 2012). ARAP2 binds to APPL1. Since the Arf6 GAP activity of ARAP2 is necessary but not sufficient for the ARAP2 effects on FAs, we considered the hypothesis that part of the effect of ARAP2 on FAs is mediated by Akt. As a first test of the hypothesis, we examined the effect of ARAP2 knockdown on FAs and pAkt levels in three cell lines: HeLa; U118 (a glioblastoma cell line) and; MDA-MB-231 (a breast cancer cell line). Cells were plated on fibronectin and serum starved for 5 hrs. ARAP2 knockdown by siRNA resulted in a reduction in the number of FAs in all three cell lines (Fig. 3A and B). Phosphorylation of T308 and S473 is required for maximum activation of Akt kinase. Thus, we determined the levels of Akt phosphorylation on these two key residues as a readout of Akt activity. The effect of ARAP2 knockdown on phosphoAkt (pAkt) was variable. In U118 cells, pT308Akt and pS473Akt were 2-4-fold greater in the knockdown cells than in controls. In HeLa cells, we did not detect a change in pT308Akt while pS473Akt was increased by approximately 50%. In MDA-MB-231 cells, ARAP2 knockdown had no effect on either pT308Akt or pS473Akt. To test the selectivity of ARAP2 for Akt, we examined the activation status of Erk, another kinase known to be activated by the formation of FAs and regulates FA dynamics (Webb et al., 2004b). No change in pERK was detected in all three cell lines. We conclude from these results that ARAP2 affects pAkt levels in some cells but an effect on pAkt is not necessary for the effects of ARAP2 on FAs in all cell types.
Figure 3. Lack of correlation between effects of ARAP2 on FAs and pAkt. (A, B) Effect of reduced ARAP2 expression on FAs.
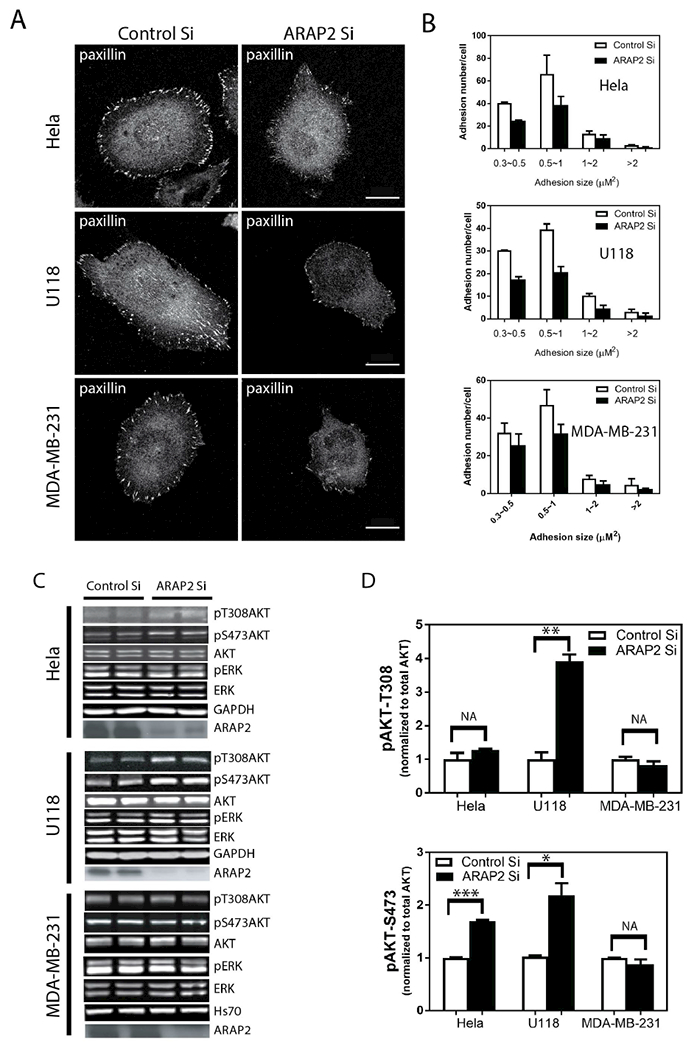
Hela, U118 and MDA-MB-231 cell were treated with either a control siRNA or ARAP2 siRNA for 72 hours, then plated onto fibronectin (10 μg/ml) coated coverslips for 5 hours. The cells were immunostained for paxillin to detect FAs. FAs in the indicated size ranges were counted as described in “Materials and Methods.” Representative images (A) and quantification (B) expressed as the mean±S.E. from three experiment are shown. In each experiment, approximately 20 cells were imaged, yielding a total of 60-65 cells from each condition for the quantification. Scale bars, 20 μm.
(C, D) Effect of reduced ARAP2 expression on pAkt and pErk. Cells treated with siRNA as described in (A) were lysed and total cell lysates were analyzed by immunoblotting with antibodies to the indicated proteins. Representative blots (C) and quantification of the signals using the Odyssey infra-red system (LI-COR) are shown.
To exclude the possibility that the increased pAkt in U118 cells is caused by off-targets effects of ARAP2 siRNA, we determined if expression of Flag-ARAP2 from a plasmid reversed the effect of the siRNA. In these experiments, pS473Akt levels were determined in single cells using quantitative immunofluorescence microscopy. The results correlated with immunoblotting results for pS473Akt from cells treated with siRNA (Fig. 4). In cells transfected with control siRNA and expressing Flag-ARAP2, detected with an antibody to the Flag epitope, pS473Akt levels were similar to or lower than cells transfected with control siRNA. Expression of Flag-ARAP2 in cells transfected with siRNA targeting ARAP2 reversed the effect of ARAP2 knockdown on pS473Akt.
Figure 4. The Arf GAP activity of ARAP2 is dispensable for its effects on Akt.
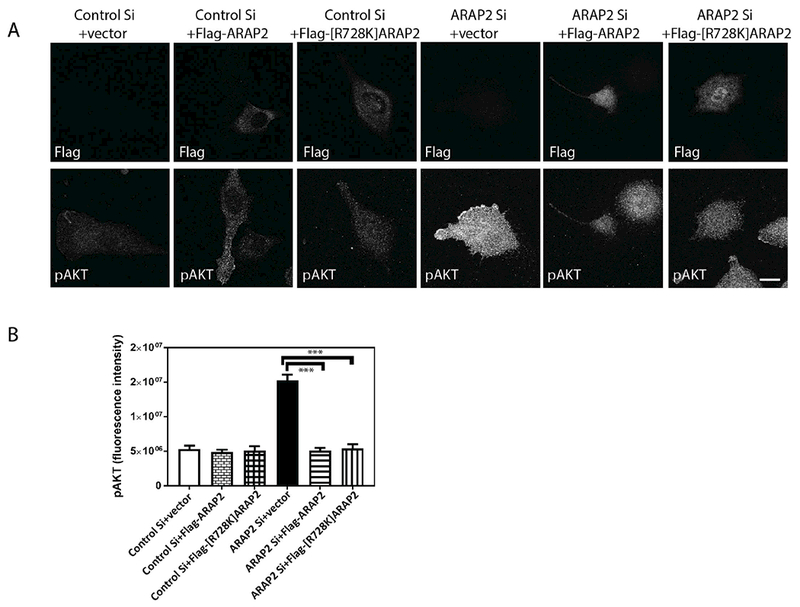
U118 cells treated with control or ARAP2 siRNA were transfected with plasmids for expressing either Flag-ARAP2 or Flag-[R728]ARAP2. The plasmid without cDNA for ARAP2 (vector) were used as a control. Cells were plated on fibronectin coated glass slides for 6 hrs, fixed and stained for pAkt and the Flag epitope, followed by immunofluorescence microscopy. (A) Representative images with the top panel showing staining for the Flag epitope to identify transfected cells and the lower panel staining for pS473Akt. (B) The summary data is shown as the mean±S.E. from quantifying 20-22 cells under each condition from 2 experiments. Scale bar, 20 μm. **, p<0.01, ***, p<0.001.
It is plausible that in U118 cells, the changes in FAs are consequent to changes in Akt activity. Given that ARAP2 affected pAkt levels in U118 cells, we next determined if Akt activity contributed to the effects of ARAP2 knockdown on FAs in these cells. U118 cells with ARAP2 knocked down were plated on fibronectin and treated with an Akt inhibitor, MK2206. The number and size of FAs and pAkt levels were compared in control or ARAP2 knockdown cells, with or without MK2206. MK2206 reduced pT308Akt and pS473Akt to undetectable levels (Fig 5A) but did not affect pERK, indicating a specific inhibition of Akt activity. However, MK2206 neither affected the number of FAs nor reversed the effect of ARAP2 knockdown on FAs (Fig 5B). Based on these results we conclude that Akt does not mediate ARAP2 effect on FAs.
Figure 5. ARAP2-dependent changes in FAs are not dependent on Akt.
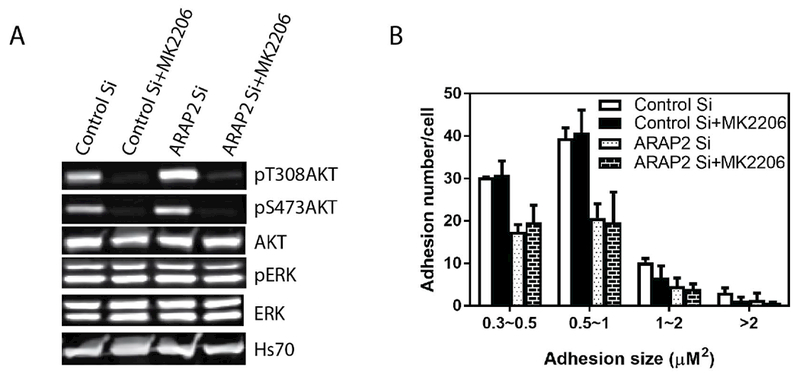
U118 cells transfected with control or ARAP2 siRNA were plated on fibronectin coated plates or glass coverslips for 5 hrs and treated with 5μM Akt inhibitor MK2206 for 1 hour prior to lysis or fixation. (A) Akt inhibition by MK2206. Cell lysates were fractionated by SDS-PAGE and transferred to nitrocellulose for immunoblotting for the indicated proteins. (B) Effects of reduced ARAP2 expression and Akt inhibition on FAs. Fixed cells were immunostained for paxillin, imaged with immunofluorescence microscopy and FAs quantified. Results shown are the summary of three experiments, with quantification of FAs from 20-22 cells for each condition for each experiment.
ARAP2 affected both FA dynamics and pAkt levels in U118. While Akt is not required for ARAP2 control of FAs, we considered an alternate hypothesis that Akt signaling was affected by FAs in U118 cells. To examine this possibility, we perturbed FAs by treatments that did not involve ARAP2. First, we reduced the size of the FAs in U118 cells by reducing expression of another Arf GAP, ASAP1, that also regulates FAs (Chen et al., 2016), As for ARAP2 knockdown, there was a reduction in the size of FAs (Fig. 6A and B); however, pT308Akt or pS473 levels did not increase; instead, there was a 20% decrease in pT308Akt (Fig. 6C). As a second test for an effect of FAs on Akt signaling, U118 cells plated on poly-L-lysine were compared to cells on fibronectin. FAs are assembled when integrins bind and are activated by their ligands, extracellular matrix proteins such as fibronectin. Cells on poly-L-lysine, which is not a ligand for integrins, had fewer and smaller FAs than those on fibronectin, which is a ligand for integrins (Fig. 6D and E). In contrast, there was no difference in pT308Akt or pS473Akt in cells plated on the two surfaces (Fig. 6F). With the lack of correlation of pAkt levels with FA size, we conclude that the ARAP2 regulation of pAkt levels is not a result of changes in FAs.
Figure 6. Changes in pAkt are independent of changes in FAs. (A, B and C) Reduced expression of ASAP1 reduces FA size but does not affect pAkt.
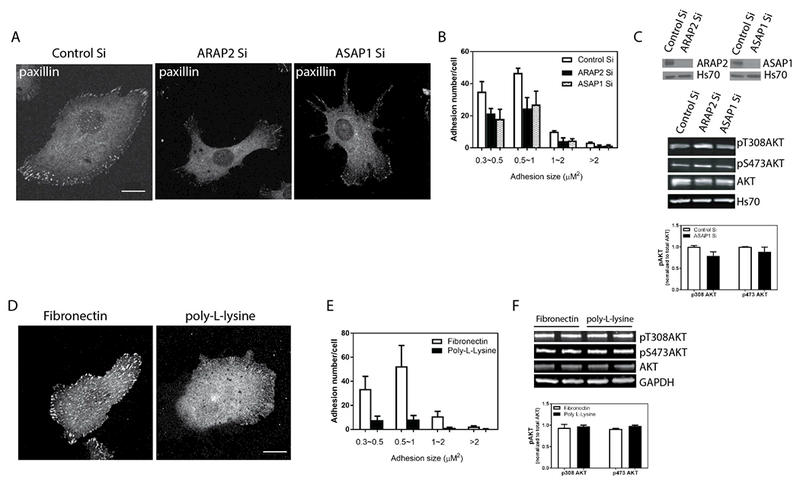
U118 cells were treated with siRNA for ARAP2 or ASAP1 and plated on fibronectin. The effects on FAs and on pAkt levels were determined. Representative images (A) and quantification of FAs (B), 20-22 cells in each of 2 experiments, are shown. (C) The upper panel is an immunoblot for ARAP2 and ASAP1, as indicted, of lysates of cells treated with siRNA targeting ARAP2 or ASAP1; the middle panel is a representative experiment showing the immunoblot of pT308Akt, , pS473Akt, total Akt and ASAP1; the lower panel is the summary of 3 experiments quantified by densitometry. The error bars are SEM. (D, E and F) Effect of integrin ligand on FAs and pAkt in U118 cells. U118 cells were plated on fibronectin or poly-L-lysine for 5 hours in serum free Opti-MEM prior to fixation or lysis. FAs and pAkt levels were analyzed as in Figure 5. Representative images (D) and quantification of FAs (E), 20-22 cells in each of 2 experiments are shown. (F) Upper panel is a representative immunoblot for pT308Akt, pS473Akt and total Akt and the lower panel is a summary of three experiments in which the immunoblots were quantified by densitometry. Error bars are SEM. Scale bars, 20 μm.
We previously reported that ARAP2 binds to APPL1. We investigated the possibility that ARAP2 functions with APPL1 to regulate Akt. Like ARAP2 knockdown, APPL1 knockdown in U118 cells resulted in increased pS473Akt levels and increased APPL1 expression reduced pS473Akt (Fig. 7A, B and C). To assess whether ARAP2 and APPL1 may function in the same pathway, we performed complementation experiments, determining if over-expression of APPL1 or ARAP2 could suppress the effect of knockdown of the other on pAkt. We found that the effect of APPL1 knockdown on pS473Akt levels was partly reversed by overexpressing ARAP2 (Fig. 7B and C). The effect of ARAP2 knockdown on pS473Akt was similarly reversed by overexpression of APPL1 (Fig. 7D and E). The results suggest that ARAP2 and APPL1 may coordinately regulate Akt.
Figure 7. APPL1 and ARAP2 depend on each other for regulation of Akt. (A, B and C) ARAP2 rescues the effect of APPL1 knockdown on pAkt.
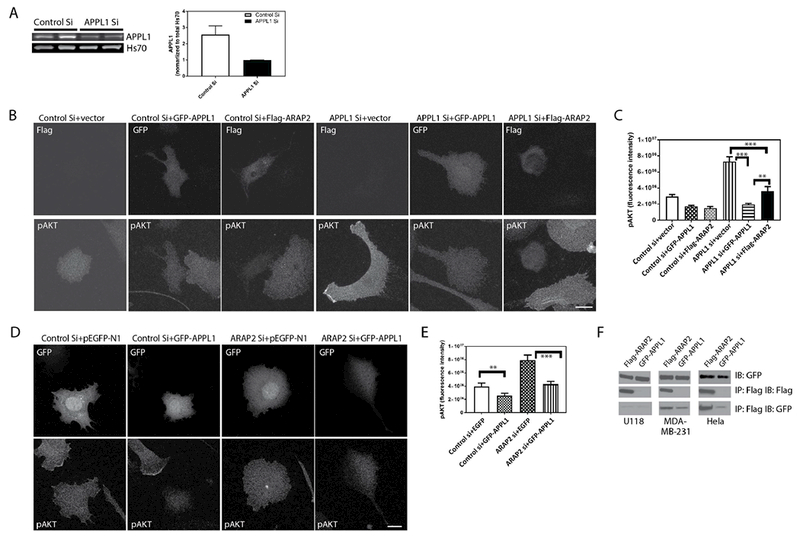
U118 cells treated with control or APPL1 siRNA and with expression vectors for either GFP-APPL1 or Flag-ARAP2 were plated on fibronectin for 5 hours in serum free medium prior to lysis and analysis of pS473Akt levels by quantitative IF. A. Left panel: Representative blot showing reduced APPL1 levels with siRNA treatment. Right panel: Quantification of immunoblot signals using using the Odyssey infra-red system (LI-COR). B. Representative images of cells. The upper panels are images indicating the transfected cells by GFP signal or by staining for Flag epitope. The lower panels are the cells stained for pS473Akt. C. Summary of three experiments. pAkt was quantified for 20-22 cells in each condition in three experiments. Error bars are SEM. (D and E) APPL1 rescues the effect of ARAP2 knockdown on pAkt. U118 cells were transfected with siRNA targeting ARAP2 and expression vectors for GFP-APPL1 or GFP (pEGFP-N1). D, representative images of cells in which pS473Akt was quantified are shown. The upper panels show cells with GFP signal to identify transfected cells. The lower panels are the cells stained for pS473Akt. E. shows the summarized quantitation of 20-22 cells for each condition from three experiments. Scale bars, 20 μm. F. Association of APPL1 and ARAP2 in three cell lines. U118, MDA-MB-231 and Hela cells were co-transfected with Flag-ARAP2 and GFP-APPL1. The cells were lysed after 24 h. Proteins from the lysates were immunoprecipitated (IP) with an antibody against the FLAG epitope, and the precipitates were immunoblotted (IB) with an antibody against FLAG or GFP. Error bars are SEM. **, p<0.01, ***, p<0.001.
We next examined whether the interaction of ARAP2 and APPL1 mediated the effect on Akt. Interaction of the proteins was assessed by co-immunoprecipitation in three cell lines expressing Flag-ARAP2 and GFP-APPL1 (Fig. 7F). APPL1 was recovered when precipitating ARAP2 from MDA-MB-231 or HeLa lysates, but was not detected in U118 cell lysates (Fig. 7F). Based on the results that ARAP2 knockdown did not affect Akt activity in MDA-MB-231 but ARAP2 co-immunoprecipitated with APPL1 (Fig. 3 and Fig. 7F), we conclude that binding of ARAP2 to APPL1 is not sufficient to regulate Akt.
We next considered whether the effect of ARAP2 on Akt was related to its effect on integrin trafficking or to its effect on Rac1. The GAP deficient mutant of ARAP2, Flag-[R728K]ARAP2, does not replace endogenous ARAP2 for control of integrin trafficking, signaling through Rac1 or FAs (Chen et al., 2014; Chen et al., 2013a). Here, we determined whether Arf GAP activity is necessary for effects on pAkt (Fig. 4). Expression of endogenous ARAP2 was reduced using siRNA and either Flag-ARAP2 or Flag-[R728K]ARAP2 was expressed from an siRNA resistant plasmid. pS473Akt levels were determined by quantitative immunofluorescence microscopy. Cells with reduced endogenous ARAP2 had increased levels of pS473Akt. Both wild type and Flag-[R728K]ARAP2 reversed the effect to the same extent, leading us to conclude that GAP activity is not required. The results support the idea that ARAP2 inhibits Akt independently of its control of integrin trafficking, Rac1 activation or FAs.
We considered that ARAP2 may be regulating another protein through direct binding to affect Akt. We screened for binding partners that might mediate the effect of ARAP2 on Akt. GST fusions of [1-813]ARAP2 and [875-1704]ARAP2 were expressed in U118 cells and in MDA-MB-231 cells (cells in which ARAP2 does not affect pAkt levels). Cells were lysed and the GST fusion proteins were precipitated with glutathione conjugated to agarose beads. Proteins that coprecipitated were identified by mass spectrometry. In this screen, proteins that precipitated with GST[1-813]ARAP2 or GST[875-1704]ARAP2 from U118 cells but not from MDA-MB-231 might mediate the effects on Akt. Non-muscle myosin IIA, tubulin, moesin and α-actinin were identified as potential binding partners in the screen, which will be examined in subsequent work.
Discussion
We set out to test the hypothesis that ARAP2 affects FAs in part by regulating the Akt pathway. We discovered that ARAP2 suppresses pAkt independent of GAP activity and effects on FAs. The effect of ARAP2 on Akt was similar to the effect of APPL1 and could replace APPL1 in suppressing pAkt. Thus, ARAP2 functions with APPL1 to regulate the Akt pathway independent of Arf GAP-mediated effects on FAs.
We found that the effects of ARAP2 on pAkt were independent of FAs. In previous work, we found that endocytic trafficking of integrins and changes in Rac1 contribute to the ARAP2-regulated changes in FAs. Although Arf6 GAP activity of ARAP2 was necessary for the changes observed in FAs, in Rac1 and in endocytic traffic, it was not sufficient to explain the full effect on FAs. We hypothesized that ARAP2 binds to other proteins that contribute to the effects on FAs. A plausible mechanism involved the ARAP2 binding partner APPL1. APPL1 had previously been reported to control FA dynamics by regulating Akt (Broussard et al., 2012). The effect of APPL1 on FA dynamics recapitulates that of ARAP2 reported in this study. We found that ARAP2 does contribute to the regulation of Akt in some cell lines, but our results exclude that this is the mechanism that contributes to ARAP2 control of FAs. In addition to Akt not mediating effects of ARAP2 on FAs, ARAP2-dependent changes in FAs did not affect pAkt. We tested this idea because ARAP2 controls FAs and endocytic trafficking, which are both implicated in the regulation of Akt (Han et al., 2011; Niit et al., 2015; Schenck et al., 2008; Zoncu et al., 2009). The Arf GAP deficient mutant of ARAP2 was as effective as wild type protein in regulating Akt but failed to rescue ARAP2 knockdown defects in either FAs or in endocytic trafficking of integrins. In unpublished studies, we found that ARAP2 knockdown reduces phosphoFAK levels in HeLa cells (Chen and Randazzo, unpublished), which would be predicted to reduce pAkt levels if the effect of ARAP2 on Akt were mediated by changes in FAs (Del Re et al., 2008). Therefore, we concluded that ARAP2 regulation of Akt was not secondary to effects on FAs.
An alternate hypothesis is that the effect on Akt is dependent on APPL1, independent of membrane traffic or FAs. We considered it plausible that ARAP2 binding to APPL1 could affect APPL1 regulation of Akt. ARAP2 and APPL1 knockdown both resulted in elevated pAkt levels. The effect of ARAP2 was rescued by overexpressing APPL1 and knockdown of APPL1 could be rescued by overexpressing ARAP2, indicating that ARAP2 and APPL1 function may be coordinated. We are examining possible mechanisms by which ARAP2 and APPL1 function together. Association of ARAP2 and APPL1 was more easily detected in cells in which ARAP2 knockdown did not affect pAkt than in cells in which it did. We can conclude that ARAP2 binding to APPL1 is not sufficient to down regulate Akt. In addition to direct binding, other mechanisms to be considered include parallel pathways, which would be indirect.
We may obtain insight into mechanism through screens for binding partners of ARAP2. In our initial proteomic screens, elements of the cytoskeleton were identified. Two hybrid screens did not identify candidates (unpublished data). Another possible mechanism is that ARAP2 binds and recruits a phosphatase that acts on Akt or upstream regulator of Akt. Protein phosphatase 1E and SH2-containing inositol 5’-phosphatase2 (SHIP2) have been found in two-hybrid screens for proteins that bind to another ARAP family member, ARAP1 (unpublished data). The differences we observed between cell types may provide clues about mechanism. MDA-MB-231 have an activating mutation in K-Ras whereas U118 cells have an inactivating mutation in PTEN (Bleeker et al., 2014). The effect of ARAP2 may be related to the metabolism of PIP3. PIP3 is critical for Akt activation (Manning and Toker, 2017). All ARAP members, including ARAP2, binds PIP3. The primary way to terminate Akt signaling is by PTEN, which converts PIP3 to PI(4,5)P2. It is possible that in cells such as U118 where PIP3 levels cannot be lowered by the defective PTEN, the recruitment of other inositol phosphatases by a PIP3 effector ARAP2 becomes pivotal to terminate Akt signaling.
The role of Arf GAPs in cellular signaling is relatively unexplored. Most work has focused on cross talk with Rho family proteins (Krugmann et al., 2002; Krugmann et al., 2004; Miura et al., 2002; Nishiya et al., 2005; Randazzo et al., 2007; Yoon et al., 2006b). Several papers have implicated Arf1 and Arf6 in the control of MAP kinase pathway (Boulay et al., 2008; Schlienger et al., 2014; Tushir and D’Souza-Schorey, 2007). Arf1 has been reported to regulate signaling through the Akt pathway (Boulay et al., 2008). In breast cancer cell lines, Arf1 controlled PI 3-kinase recruitment to the plasma membrane, leading to activation of PKD and Akt. Here we have reported that ARAP2 affects Akt signaling. GAP activity was not required, but we have not excluded a role for Arf6, which may affect activity by binding to the Arf GAP domain, possibly to recruit ARAP2 to a specific site. Other Arf GAPs bind to signaling proteins, e.g. ASAP1 binds to Src, Crk and FAK (Brown et al., 1998; Oda et al., 2003). ACAPs have been reported to be downstream of Akt signaling to control integrin recycling (Li et al., 2005).
In summary, we set out to test the idea that ARAP2 affects FAs in part by control of Akt. We refuted the hypothesis but discovered that ARAP2 affects pAkt levels in a subset of cells by a mechanism that is independent of membrane traffic, FAs and binding to APPL1. The complete mechanism by which ARAP2 affects FAs and the mechanism by which it regulates Akt in some cells is still being discovered.
Materials and Methods
Plasmids
Mammalian expression vectors for N-terminal FLAG-tagged ARAP2, [R728K]ARAP2, FLAG-tagged ARAP1, FLAG-tagged ACAP1 and GFP-APPL1 have been described (Chen et al., 2014). For, Flag-[1-813]ARAP2 and Flag-[875-1704]ARAP2, the coding region [1-813] and [875-1704] for ARAP2 was amplified by polymerase chain reactions and subcloned into the SalI and NotI sites of pCI (Promega) with a Flag tag (DYKDDDDK) for mammalian expression. For GST fusions of ARAP2, Coding region of [1-1704]ARAP1, [1-813]ARAP2 and [875-1704]ARAP2 was amplified by polymerase chain reactions and subcloned into the BamHI and KpnI sites of PSF-CMV-PURO-NH2-GST(sigma) with a GST tag for mammalian expression.
Antibodies and Reagents
Affinity purified rabbit anti-ARAP2 antibody (1186) and anti-ASAP1 polyclonal antibody were described before (Chen et al., 2014; Chen et al., 2013a; Randazzo et al., 2000). Anti-FLAG polyclonal Ab, monoclonal anti-flag M2 beads, and fibronectin, poly-L-lysine and MK2206 were purchased from Sigma-Aldrich. Anti-Paxillin monoclonal Ab (clone 349) and anti-APPL1 were from BD Biosciences (San Jose CA). Anti-phospho Akt308, Anti-phospho Akt473, anti-phospho ERK, anti-Akt, anti-ERK and anti-GAPDH polyclonal were from Cellular Signaling (Danvers, MA). Anti-Hs70 monoclonal antibody was from Santa Cruz. Anti-GFP polyclonal antibody and Alexa Fluor-labeled secondary antibodies were from Invitrogen (Carlsbad, CA). Horseradish-peroxidase-conjugated anti-mouse and anti-rabbit IgG Abs were from Bio-Rad. IRDye® 800CW Donkey anti-Rabbit IgG (H + L) and IRDye® 680RD Goat anti-Mouse IgG (H + L) were from LI-COR biosciences (Lincoln, NE).
Cell Culture and Transfection
Hela, U118 and MDA-MB-231 cell were maintained at 37 °C in Dulbecco’s modified Eagle’s medium supplemented with 100 units/ml penicillin, 100ug/ml streptomycin, and 10% fetal bovine serum (FBS). siRNA against ARAP2 (GUAAGAAGACAUUGGGUUA), APPL1 (GACAAGGTCTTTACTAGGTGTATTT), ASAP1 (smart pool Cat#MU-031961-01-0002) and control siRNA (siCONTROL Non-Targeting siRNA#4) were purchased from Thermo Scientific (Lafayette, CO). Cells were transfected with 40 nM siRNA using Dharmafect (Thermo Scientific, Lafayette, CO) according to the manufacturer’s instructions. For rescue experiments, 48 h after siRNA transfection, cells were transfected again with plasmids expressing Flag-tagged ARAP2 and GFP tagged APPL1 using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA).
Immunofluorescence Staining, Confocal Microscopy and image analysis
To quantify pAKT level, siRNA treated U118 cells were transfected, using Lipofectamine 3000, with plasmids directing expression of relevant proteins. 24 h later, the cells were replated on fibronectin-coated coverslips and maintained in serum-free Opti-MEM (Invitrogen) for 5 h prior to fixation and immunostaining. Images from fixed cells were collected with a Leica SP8 laser scanning confocal microscope, using a 63×, 1.4 NA objective (Leica Microsystems Inc, Buffalo Grove IL). 2-3 μm Z stacks with a spacing of 0.3 μm were taken of the area contacting the coverslip. For pAKT, series of confocal images were exported, the total intensity of pAKT were measured for transfected cell by Imaris 8 (Bitplane, Zurich, Switzerland). Leica AF software was used to produce images. Maximum intensity projection were exported and then Adobe PhotoShop and Illustrator (Adobe Systems Inc, San Jose CA) were used to prepare composite figures. Scale bars were removed from the original images and were replaced with a more visible version in the final composite image. Focal adhesions were analyzed as described previously (Chen et al., 2014; Chen et al., 2013a).
Time-lapse TIRF microscopy
To analyze the effect of ARAP2 on the dynamics of FAs, ARAP2-silencing HeLa cells transiently co-transfected with pEGFP-paxillin and various ARAP2 constructs (ratio=1:7) were mounted on slides with double-stick tape in phenol red-free culture medium with 25 mM Hepes (pH= 7.4) and 10units/ml Oxyrase and imaged by TIRF using a ×100 1.49 NA Plan objective (Nikon) with an ~100 nm evanescent field depth on a Cascade II:1024 EMCCD (Photometrics) (Shin, 2010). Temperature was maintained at 37oC with an airstream incubator (Nevtek) and focus was maintained using PerfectFocus(™) (Nikon). TIRF images of EGFP-paxillin were captured at 20s intervals using a 512B EMCCD (Photometrics). Analysis of the duration of FA assembly, disassembly and total lifetime, the intensity of pEGFP-paxillin in FAs in TIRF images was performed. We hand-outlined a single FA that was able to see their total lifetime (assembly, maturation, and disassembly) in the paxillin channel, and recorded the integrated intensity of pEGFP-paxillin in the region for each image over time. The mean of the background was considered as the mean of the intensity in this region before the assembly of the FA. The integrated intensity of a region without FA for each image over time was considered as a factor to correct photobleach. Therefore, the intensity value of a single FA in each image was corrected by mean background subtraction, photobleach correction (division), and normalization to the maximal intensity in the series. This normalized integrated intensity values were then plotted as a function of time. A significant rise in intensity, indicating the initiation of recruitment of the pEGFP-paxillin in the region, was considered as the first image of FA assembly in the series. The first image of slowing the assembly rate was considered as the last image of FA assembly. Additionally, a significant fall in intensity was considered as the first image of FA disassembly in the series. The first image showing the normalized intensity lower than background was considered as the last image of FA disassembly and total lifetime.
FRAP
3×104 siRNA or plasmid-transfected cells were plated on Lab-TekII 4-chambered coverglass (Nalge Nunc International, Naperville, IL) coated with 10 μg/ml fibronectin. The movements of GFP-tagged paxillin in live cells were monitored with a Zeiss Axiovert 200 microscope equipped with a PerkinElmer UltraView spinning disc confocal system (PerkinElmer, Boston, MA) using a 63× 1.4 NA plan Neofluar oil immersion objective. Cells were imaged in real time on stage preheated to 37°C with CO2 chamber using an Orca-ERII camera (Hamamatsu, Bridgewater, NJ). Cells were co-transfected with Flag-ARAP2 and GFP-Paxillin at a ratio of 7:1 to ensure ARAP2 coexpression in GFP-Paxillin positive cells. GFP-Paxillin labeled adhesions were bleached using the 488 nm laser at full power. During fluorescence recovery, images were captured at 200~500 ms intervals for less than 3 min at a reduced laser intensity (40~60 %) to minimize undesired photobleaching. The integrated fluorescence intensity was recorded in pre-bleach and recovery image series and normalized so that the pre-bleach value was 100 %. The recovery halftime (t½) and mobile fraction were calculated using the normalized recovery values with a one phase exponential association in GraphPad Prism (GraphPad Software, La Jolla, CA).
Supplementary Material
Acknowledgments
The work was supported by the intramural program of the National Cancer Institute, National Institutes of Health (Project BC 007365). We thank Dr. Marielle E. Yohe for discussions and William Shin for help with TIRF microscopy.
References
- Bleeker FE, Lamba S, Zanon C, Molenaar RJ, Hulsebos TJ, Troost D, van Tilborg AA, Vandertop WP, Leenstra S, van Noorden CJ, et al. (2014). Mutational profiling of kinases in glioblastoma. BMC Cancer 14, 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay PL, Cotton M, Melancon P, and Claing A (2008). ADP-ribosylation factor 1 controls the activation of the phosphatidylinositol 3-kinase pathway to regulate epidermal growth factor-dependent growth and migration of breast cancer cells. The Journal of biological chemistry 283, 36425–36434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Lin WH, Majumdar D, Anderson B, Eason B, Brown CM, and Webb DJ (2012). The endosomal adaptor protein APPL1 impairs the turnover of leading edge adhesions to regulate cell migration. Molecular Biology of the Cell 23, 1486–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, and Randazzo PA (1998). ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Molecular and Cellular Biology 18, 7038–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-W, Luo R, Jian X, and Randazzo PA (2014). The Arf6 GTPase-activating Proteins ARAP2 and ACAP1 Define Distinct Endosomal Compartments That Regulate Integrin α5β1 Traffic. Journal of Biological Chemistry 289, 30237–30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PW, Jian X, Heissler SM, Le K, Luo R, Jenkins LM, Nagy A, Moss J, Sellers JR, and Randazzo PA (2016). The Arf GTPase-activating Protein, ASAP1, Binds Nonmuscle Myosin 2A to Control Remodeling of the Actomyosin Network. The Journal of biological chemistry 291, 7517–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PW, Jian X, Yoon HY, and Randazzo PA (2013a). ARAP2 signals through Arf6 and Rac1 to control focal adhesion morphology. J Biol Chem [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PW, Jian XY, Yoon HY, and Randazzo PA (2013b). ARAP2 Signals through Arf6 and Rac1 to Control Focal Adhesion Morphology. Journal of Biological Chemistry 288, 5849–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KKY, Iglesias MA, Lam KSL, Wang Y, Sweeney G, Zhu WD, Vanhoutte PM, Kraegen EW, and Xu AM (2009). APPL1 Potentiates Insulin-Mediated Inhibition of Hepatic Glucose Production and Alleviates Diabetes via Akt Activation in Mice. Cell Metabolism 9, 417–427. [DOI] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, and Horwitz AR (2008). Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 10, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DM, Kutscher B, Chen H, Murphy DB, and Craig SW (2006). A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J Biol Chem 281, 16006–16015. [DOI] [PubMed] [Google Scholar]
- Del Re DP, Miyamoto S, and Brown JH (2008). Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection. The Journal of biological chemistry 283, 35622–35629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M, Lotano MA, and Otey CA (2001). Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil Cytoskeleton 48, 190–200. [DOI] [PubMed] [Google Scholar]
- Erez N, Bershadsky A, and Geiger B (2005). Signaling from adherens-type junctions. Eur J Cell Biol 84, 235–244. [DOI] [PubMed] [Google Scholar]
- Fraley TS, Pereira CB, Tran TC, Singleton C, and Greenwood JA (2005). Phosphoinositide binding regulates alpha-actinin dynamics: mechanism for modulating cytoskeletal remodeling. J Biol Chem 280, 15479–15482. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, and Waterman CM (2010). Mechanical Integration of Actin and Adhesion Dynamics in Cell Migration In Annual Review of Cell and Developmental Biology, Vol 26, Schekman R, Goldstein L, and Lehmann R, eds., pp. 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, and Yamada KM (2011). Molecular Architecture and Function of Matrix Adhesions. Cold Spring Harbor Perspectives in Biology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WJ, and Giancotti FG (2004). Integrin signalling during tumour progression. Nature Reviews Molecular Cell Biology 5, 816–826. [DOI] [PubMed] [Google Scholar]
- Gupton SL, and Waterman-Storer CM (2006). Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell 125, 1361–1374. [DOI] [PubMed] [Google Scholar]
- Han JW, Lee HJ, Bae GU, and Kang JS (2011). Promyogenic function of Integrin/FAK signaling is mediated by Cdo, Cdc42 and MyoD. Cell Signal 23, 1162–1169. [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, and Cordes N (2007). Signalling via integrins: Implications for cell survival and anticancer strategies. Biochimica Et Biophysica Acta-Reviews on Cancer 1775, 163–180. [DOI] [PubMed] [Google Scholar]
- Hynes RO (2002). Integrins: Bidirectional, allosteric signaling machines. Cell 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Kahn RA, Bruford E, Inoue H, Logsdon JM, Nie ZZ, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, et al. (2008). Consensus nomenclature for the human ArfGAP domain-containing proteins. Journal of Cell Biology 182, 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, et al. (2002). Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Molecular Cell 9, 95–108. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Williams R, Stephens L, and Hawkins PT (2004). ARAP3 is a PI3K- and rap-regulated GAP for RhoA. Current Biology 14, 1380–1384. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, and Ingber DE (2006). Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol 207, 187–194. [DOI] [PubMed] [Google Scholar]
- Lele TP, Thodeti CK, Pendse J, and Ingber DE (2008). Investigating complexity of protein-protein interactions in focal adhesions. Biochem Biophys Res Commun 369, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, and Hsu VW (2005). Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta 1 to control cell migration. Developmental Cell 9, 663–673. [DOI] [PubMed] [Google Scholar]
- Manning BD, and Toker A (2017). AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters TA, Tumbarello DA, Chibalina MV, and Buss F (2017). MYO6 Regulates Spatial Organization of Signaling Endosomes Driving AKT Activation and Actin Dynamics. Cell Rep 19, 2088–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, and Zerial M (2004). APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456. [DOI] [PubMed] [Google Scholar]
- Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, and Testa JR (1999). Identification of a chromosome 3p14.3-21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18, 4891–4898. [DOI] [PubMed] [Google Scholar]
- Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu KJ, Hirsch DS, Resau J, Zheng Y, and Randazzo PA (2002). ARAP1: A point of convergence for Arf and Rho signaling. Molecular Cell 9, 109–119. [DOI] [PubMed] [Google Scholar]
- Mohl C, Kirchgessner N, Schafer C, Kupper K, Born S, Diez G, Goldmann WH, Merkel R, and Hoffmann B (2009). Becoming stable and strong: the interplay between vinculin exchange dynamics and adhesion strength during adhesion site maturation. Cell Motil Cytoskeleton 66, 350–364. [DOI] [PubMed] [Google Scholar]
- Moreno-Layseca P, and Streuli CH (2014). Signalling pathways linking integrins with cell cycle progression. Matrix Biology 34, 144–153. [DOI] [PubMed] [Google Scholar]
- Niit M, Hoskin V, Carefoot E, Geletu M, Arulanandam R, Elliott B, and Raptis L (2015). Cell-cell and cell-matrix adhesion in survival and metastasis: Stat3 versus Akt. Biomolecular concepts 6, 383–399. [DOI] [PubMed] [Google Scholar]
- Nishiya N, Kiosses WB, Han JW, and Ginsberg MH (2005). An alpha(4) integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nature Cell Biology 7, 343–U347. [DOI] [PubMed] [Google Scholar]
- Oda A, Wada I, Miura K, Okawa K, Kadoya T, Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, et al. (2003). CrkL directs ASAP1 to peripheral focal adhesions. Journal of Biological Chemistry 278, 6456–6460. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, and Schwartz MA (2010). Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature Reviews Molecular Cell Biology 11, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S, and Dedhar S (2003). The role of integrin-linked kinase (ILK) in cancer progression. Cancer metastasis reviews 22, 375–384. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, and Cooper JA (2000). The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America 97, 4011–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo PA, Inoue H, and Bharti S (2007). Arf GAPs as regulators of the actin cytoskeleton. Biology of the Cell 99, 583–600. [DOI] [PubMed] [Google Scholar]
- Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, and Zerial M (2008). The endosomal protein Appl1 mediates akt substrate specificity and cell survival in vertebrate development. Cell 133, 486–497. [DOI] [PubMed] [Google Scholar]
- Schlienger S, Campbell S, and Claing A (2014). ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Molecular Biology of the Cell 25, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin WD, Fischer RS, Kanchanawong P, Kim Y, Lim J, Myers KA, Nishimura Y, Plotnikov SV, Thievessen I, Yarar D, Sabass B, and Waterman CM. (2010). A Versatile, Multicolor Total Internal Reflection Fluorescence and Spinning-Disk Confocal Microscope System for High-Resolution Live Cell Imaging in Live Cell Imaging. A Laboratory Manual , Second edn (Cold Spring Harbor, NY: : Cold Spring Harbor Laboratory Press; ). [Google Scholar]
- Tushir JS, and D’Souza-Schorey C (2007). ARF6-dependent activation of ERK and Rac1 modulates epithelial tubule development. The EMBO journal 26, 1806–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, et al. (2009). Neuropilin-1/GIPC1 Signaling Regulates alpha 5 beta 1 Integrin Traffic and Function in Endothelial Cells. Plos Biology 7, 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, and Sheetz MP (2003). Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J 22, 5023–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, and Horwitz AF (2004a). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 6, 154–161. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, and Horwitz AF (2004b). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biology 6, 154–+. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B, and Imhof BA (2002). The inner lives of focal adhesions. Trends in Cell Biology 12, 382–389. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz SE, Fassler R, Geiger B, and Legate KR (2014). The integrin adhesome: from genes and proteins to human disease. Nature Reviews Molecular Cell Biology 15, 273–288. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Bershadsky A, Henis YI, and Geiger B (2011). Actomyosin-generated tension controls the molecular kinetics of focal adhesions. J Cell Sci 124, 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, and Randazzo PA (2006a). ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. J Cell Sci 119, 4650–4666. [DOI] [PubMed] [Google Scholar]
- Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, and Randazzo PA (2006b). ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. Journal of Cell Science 119, 4650–4666. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, and De Camilli P (2009). A Phosphoinositide Switch Controls the Maturation and Signaling Properties of APPL Endosomes. Cell 136, 1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


