Abstract
BACKGROUND:
Chronic rhinosinusitis (CRS) is associated with substantial productivity losses. Prior cross-sectional study has identified risk factors and symptom subdomains contributing to baseline productivity loss. This study evaluates correlations between post-treatment changes in symptom subdomain and productivity loss.
METHODS:
202 adult patients with refractory CRS were prospectively enrolled into an observational, multi-institutional cohort study between August, 2012 and June, 2015. Respondents provided pre-treatment and post-treatment SinoNasal Outcomes Test (SNOT-22) scores. Productivity losses were monetized using measures of absenteeism, presenteeism, lost leisure time, and United States government-estimated wage and labor rates.
RESULTS:
39 (19%) participants elected continued appropriate medical therapy (CAMT) and 163 (81%) elected endoscopic sinus surgery (ESS). CAMT patients experienced improvement in SNOT-22 total and rhinologic subdomain scores (both p≤0.039). ESS patients reported improvement in SNOT-22 total scores and all subdomains (all p<0.001). Mean monetized productivity losses were nearly unchanged following CAMT (−$200, p=0.887) but significantly reduced following ESS (−$5,015, p<0.001). Mean productivity losses were reported in CAMT patients reporting worse mean post-treatment extra-rhinologic, psychological, and sleep symptom severity scores, however no statistically significant linear correlations (r≤0.249; p≥0.126) were reported.
CONCLUSIONS:
Treatment modalities associate with different post-treatment productivity changes. Patients electing ESS experienced postoperative improvement in productivity distributed across all SNOT-22 symptom domains, suggesting productivity improvement correlates with multiple symptom domains. Patients electing CAMT had better baseline productivity compared to patients electing ESS, and this productivity level was maintained through treatment. Greater productivity loss occurred in patients with worse SNOT-22 scores in the extra-rhinologic, psychological, and sleep subdomains.
Keywords: Sinusitis, chronic disease, outcome assessment (health care), quality of life
INTRODUCTION
Chronic rhinosinusitis (CRS) is a common inflammatory disease associated with substantial health care costs.1 In the United States (U.S.), annual direct costs for managing CRS are estimated at $9 billion while various indirect costs are even greater at an estimated $13 billion.2, 3 Lost productivity, one component of indirect costs, has been shown to be associated with disease-specific quality of life,3 for which the 22-item SinoNasal Outcomes Test (SNOT-22) survey instrument is frequently employed to quantify.4
For patients with medically refractory disease, endoscopic sinus surgery (ESS) has been shown to improve productivity, while the results of continued appropriate medical therapy (CAMT) on productivity have been mixed.5–7 Previous cross-sectional analysis has demonstrated impacts on productivity loss in CRS correlates with worse sleep and psychological dysfunction, using the SNOT-22 5-subdomain model, as well as worse impairments in the emotional function when using the SNOT-22 4-subdomain model.8, 9 However, it remains unknown how specific symptom domain score changes correlate with productivity losses following independent treatment modalities for CRS. An improved understanding of these areas may facilitate more directed treatment paradigms with regards to both overall societal burden of CRS or to individual patient experience. Further, post-treatment differences following each treatment modality may help support informed patient decision-making.
MATERIALS & METHODS
Study Population and Inclusion Criteria
Prior to study recruitment, adult (≥18 years of age) patients were evaluated by fellowship trained rhinologists and diagnosed with CRS, as defined by current guidelines described by the American Academy of Otolaryngology-Head and Neck Surgery, per the standard of care.10, 11 CRS was considered medically refractory as patients had persistent symptoms despite previously completing appropriate medical therapy including, but not limited to: ~240ml. of saline irrigation Q.D., at least one course of either topical corticosteroid sprays/drops (≥21 days) or trial of oral corticosteroid therapy (≥5 days), and at least one trial (≥14 days) of broad spectrum or culture-directed antibiotics. All study participants remained on a regimen of undescribed, appropriate medical therapy per their individual standard of care for the study duration.
Study participants were prospectively enrolled into an observational, multi-center study cohort designed to evaluate CRS treatment outcomes in academic, tertiary referral centers. Investigational outcomes from this cohort study have been previously described.12–14 Enrollment sites included Departments/Division of Otolaryngology located at: Oregon Health & Science University (OHSU, Portland, OR.), Stanford University (Palo Alto, CA.), the Medical University of South Carolina (Charleston, SC.), and the University of Calgary (Calgary, Alberta, Canada). Enrollment meetings followed standard surgical counseling for all study participants who voluntarily elected either CAMT or ESS as subsequent treatment modalities. Study participants were assured of minimal study risk, voluntary informed consent, and that the standard of care was not modified due to study participation. The Institutional Review Board (IRB) at each enrollment location approved study protocols, as well as conducted annual review and data safety monitoring.
Comprehensive medical and social history was collected from each study participant during baseline enrollment meetings. Standard baseline measures of disease severity for patients with CRS, required for diagnostic purposes, was also collected for study analyses. Bilateral rigid endoscopy (0°-30°; Karl Storz; Tuttlingen, Germany) was conducted by each enrolling physician to visualize sinonasal pathology attributes including: nasal polyposis, discharge, edema, scarring, and crusting. Quantification of each endoscopy exam was completed in accordance with the Lund-Kennedy staging system (total score range: 0-20).15 Computed tomography (CT) imaging of the bilateral paranasal sinus regions (maxillary, anterior ethmoid, posterior ethmoid, sphenoid, frontal, and ostiomeatal complex) in both sagittal and coronal planes was also used to evaluate disease severity by each enrolling physician. Quantification of each CT scan was completed using the Lund-MacKay staging system (total score range: 0-24).16 Higher scores on either staging system reflect worse global disease severity or extent.
Exclusion Criteria
Due to potential disparity in global health and differential treatment approaches study participants were excluded from final analyses if they reported comorbid ciliary dyskinesia / cystic fibrosis. Participants were also excluded if they did not complete all patient reported outcome measures (PROMs) as instructed.
Patient Reported Outcome Measure (PROMs)
As the primary predictor of interest to this study, participants were asked to provide responses to the 22-item SinoNasal Outcome Test (SNOT-22), a validated survey instrument developed to enumerate sinonasal symptom severity (©2006, Washington University, St. Louis, MO).17 Symptom severity items are measured using Likert scale responses (score range: 0–5) where higher scores reflect worse symptom severity. Higher aggregate scores reflect worse daily functioning and/or symptom severity (total score range: 0-110). The 22-items of the SNOT-22 survey have been categorized into 5 distinct subdomains as well as 4 distinct subdomains based on different factor analyses.18, 19 In this analysis, the SNOT-22 items were summarized into both 5 and 4 distinct domains with minimal score cross-loading. The SNOT-22 5-subdomain model was described as previously published: rhinologic symptom domain (“need to blow nose”; “sneezing”; “runny nose”; “thick nasal discharge”; “sense of smell/taste”; “blockage/congestion of nose”, score range: 0-30), extra-nasal rhinologic symptom domain (“cough”; “post nasal discharge”; “thick nasal discharge”, score range: 0-15), ear / facial symptom domain (“sneezing”; “ear fullness”; “dizziness”; “ear pain”; “facial pain/pressure”, score range: 0-25), psychological dysfunction domain (“waking up tired”; “fatigue”; “reduced productivity”; “reduced concentration”; “frustrated/sad/restless”; “sad”; “embarrassed” score range: 0-35), and a sleep dysfunction domain (“difficulty falling asleep”; “waking up at night”; “lack of a good night’s sleep”; “waking up tired”; “fatigue”, score range: 0-25).18 The SNOT-22 4-subdomain model was described as previously published: a nasal symptom domain (“need to blow nose”; “sneezing”; “runny nose”; “cough”; “post nasal discharge”; “thick nasal discharge”; “sense of smell/taste”; “blockage/congestion of nose”, score range: 0-40), a sleep dysfunction domain (“difficulty falling asleep”; “waking up at night”; “lack of a good night’s sleep”; “waking up tired”; “fatigue”; “reduced productivity”; “reduced concentration”; “frustrated/sad/restless”, score range: 0-40), an otologic/facial pain domain (“ear fullness”; “dizziness”; “ear pain”; “facial pain/pressure”, score range: 0-20), and an emotional domain (“sad”; “embarrassed”, score range: 0-10).19
Study participants were asked to complete the SNOT-22 at both baseline enrollment meetings and during post-treatment evaluations up to 18-months. A minimal clinically important difference (MCID), as discernible by a patient, in SNOT-22 total scores has been historically defined as a post-treatment difference of 8.9 points.17
Monetization of Productivity Losses
As the primary outcome of interest to this study, participants were asked to provide responses to general economic impact and disease-related costing questionnaires during both baseline enrollment meetings and during post-treatment evaluations up to 18-months. Based on current recommendations regarding the minimization of recall bias, all economic impact questions were duration-based to measure the impact of CRS within the preceding 90 days.20–22
In order to operationalize costs associated with patient-specific productivity loss, the degree of lost productive time (LPT) was first measured, defined as the per-person work days lost due to refractory CRS. For this study we assumed the following mean paid work time per patient: 8 hours per day, 5 days per week with 4 weeks of vacation per year (i.e. 48 paid work weeks and a total of 240 paid work days per year). A total cumulative loss of 8 hours of work was assumed equal to 1 full work day missed. Additionally, absenteeism was quantified by asking both the number of full days of work missed and the number of work hours missed due to the impact of CRS. Presenteeism was measured based on the Quantity and Quality Questionnaire, substituting patient reported work performance for visual analogue scales, as has been previously performed.5, 23, 24 Patients were asked on mean the percentage (%) of reduced daily work performance due to CRS in the last 90 days. Healthy baseline performance was assumed to be 100% productivity. It was also assumed that the mean productivity level within the last 90 days was consistent over the course of the previous year. The annual number of work days missed due to CRS-related presenteeism was calculated using the following formula: P = (E – A)*p; where P is the number of missed work days due to presenteeism, E is the expected number of annual work days (i.e. 240), A is the work days missed due to absenteeism, and p is the % reduced performance at work.22 Annual lost productive time (LPT) was calculated by summing the work days lost from both presenteeism and absenteeism due to CRS.
Household productivity loss was calculated by asking patients how much time per day was consumed at home to care for symptoms associated with CRS. It was assumed the potential household productivity/leisure time available for weekdays is 7 hours per day (5:00pm to 12:00am) and on weekend days it is 15 hours per day (9:00am to 12:00am) for 52 weekends per year, consistent with prior studies evaluating productivity loss in CRS.3, 5 Therefore, 7 household hours lost per weekday, or 15 household hours lost per weekend, would approximate one household work day lost. Household productivity is reported separately from paid work days missed since it has a different monetary valuation.
Monetize productivity losses were then determined using the human capital approach. To accurately reflect the productivity cost to society, the median annual U.S. societal wage rate of $30,853 in the United States (U.S.) was used, based on the 2012 U.S. National Census. Assuming 48 work weeks per year, at 40 hours per week and 8 hours per day, this produces a mean daily income of $128.55 per patient.25 This value was used to monetize the paid work LPT. Household productivity loss was valued by assuming it was equal to the hourly wage of a housekeeper. Using the 2013 U.S. Department of Labor statistics, the mean hourly wage rate for a housekeeper was $10.49 which at 8 hours per day produced a daily household monetized LPT of $90.91.26
Database Management and Statistical Analysis
Study data was transferred to OHSU from each enrollment location for central data compilation and final analysis. Study data was manually entered into a HIPAA compliant relational database (Access, Microsoft Corp., Redmond, WA.) and secondary statistical analyses were conducted using SPSS software (IBM Corp., Armonk, NY.). Data normality was first evaluated for ordinal and continuous measures to guide subsequent comparison test selection. The primary predictive variable was defined as the post-treatment difference (post - pre) between baseline and last available SNOT-22 scores. The last available post-treatment assessment was used due to known historic stability in SNOT-22 measures between 6 and 18 months in this cohort, including up to 18 months after initial enrollment for the CAMT treatment group and 18 months postoperatively for participants electing ESS.27 Post-treatment differences (post - pre) in monetized productivity loss was defined as the primary outcome of interest. Descriptive analytics were completed for all study measures. Within-subject differences in both mean SNOT-22 scores and monetized productivity losses were evaluated using matched pair t-test statistics or Wilcoxon signed-rank tests, based on distributions of scaled data. Correlations between post-treatment differences in SNOT-22 responses and productivity losses for both the total cohort and each treatment modality were evaluated using Pearson’s two-tailed correlation coefficients (r). Mean productivity loss differences were further stratified across SNOT-22 symptom domain score differences for both the total cohort and each treatment modality. All statistical comparisons assumed a conventional 0.050 type-I error probability.
RESULTS
Final Study Cohorts
The preliminary cohort consisted of 299 study patients who met inclusion criteria and were enrolled between August, 2012 and June, 2015. A total of 202 study patients were selected for final analyses after exclusions for ciliary dyskinesia / cystic fibrosis (n=13; 4%) and non-compliance with completion of PROM surveys (n=84; 28%) during the study duration. Study participants electing ESS experienced an average wait time of 2.4 [standard deviation±4.2] weeks before their procedure. The final total cohort was followed from the time of enrollment for a mean of 14.9 [standard deviation ± 4.8] months. Throughout the Results and Discussion, findings from the SNOT-22 5-subdomain model are presented for concision. Results from the SNOT-22 4-subdomain model were parallel after statistical analysis and are delineated at times. Final study cohort characteristics, comorbid conditions, baseline measures of disease severity, mean PROM scores, and monetized productivity losses at baseline are described in Table 1 for both the total cohort and independent CAMT and ESS treatment modalities. Supplementary Table 1 describes these measures for the 4-subdomain model. None of the study participants electing ESS required a second ESS procedure within the study follow-up period.
Table 1:
Description of final study cohort characteristics (n=202)
| Baseline characteristics: | Continued Appropriate Medical Therapy (n=39) | Endoscopic Sinus Surgery (n=163) | Total Study Cohort (n=202) | |||
|---|---|---|---|---|---|---|
| Mean [±SD] | N (%) | Mean [±SD] | N (%) | Mean [±SD] | N (%) | |
| Age (years at enrollment) | 57.9 [±12.7] | ---- | 50.6 [±16.6] | ---- | 52.1 [±16.1] | ---- |
| Follow-up (months) | 14.3 [±4.5] | ---- | 15.0 [±4.9] | ---- | 14.9 [±4.8] | ---- |
| Females | ---- | 21 (54%) | ---- | 93 (57%) | ---- | 114 (56%) |
| White/Caucasian | ---- | 32 (82%) | ---- | 142 (87%) | ---- | 174 (86%) |
| African American | ---- | 5 (13%) | ---- | 12 (7%) | ---- | 17 (8%) |
| Asian | ---- | 0 (0%) | ---- | 6 (4%) | ---- | 6 (3%) |
| Hispanic/Latino | ---- | 1 (3%) | ---- | 8 (5%) | ---- | 9 (5%) |
| Previous sinus surgery | ---- | 26 (67%) | ---- | 96 (59%) | ---- | 122 (60%) |
| Nasal polyposis | ---- | 14 (36%) | ---- | 53 (33%) | ---- | 67 (33%) |
| Asthma | ---- | 14 (36%) | ---- | 67 (41%) | ---- | 8 1(40%) |
| Allergy (mRAST/skin prick) | ---- | 21 (54%) | ---- | 103 (63%) | ---- | 124 (61%) |
| Septal deviation | ---- | 3 (8%) | ---- | 42 (26%) | ---- | 45 (22%) |
| Turbinate hypertrophy | ---- | 0 (0%) | ---- | 19 (12%) | ---- | 19 (9%) |
| Depression (self-reported) | ---- | 3 (8%) | ---- | 15 (9%) | ---- | 18 (9%) |
| OSA | ---- | 4 (10%) | ---- | 23 (14%) | ---- | 27 (13%) |
| Tobacco use | ---- | 1 (3%) | ---- | 5 (3%) | ---- | 6 (3%) |
| Alcohol use | ---- | 19 (49%) | ---- | 56 (34%) | ---- | 75 (38%) |
| Corticosteroid dependency | ---- | 4 (10%) | ---- | 20 (12%) | ---- | 24 (12%) |
| Diabetes mellitus (Type I/II) | ---- | 5 (13%) | ---- | 15 (9%) | ---- | 20 (10%) |
| GERD | ---- | 8 (21%) | ---- | 42 (26%) | ---- | 50 (25%) |
| Lund-Kennedy endoscopy score | 5.2 [±3.5] | ---- | 5.1 [±3.6] | ---- | 5.2 [±3.6] | ---- |
| Lund-Mackay CT score | 11.5 [±5.3] | ---- | 10.7 [±6.5] | ---- | 10.8 [±6.4] | ---- |
| SNOT-22 total score | 45.6 [±18.1] | ---- | 52.8 [±21.6] | ---- | 51.4 [±21.1] | ---- |
| Rhinologic symptom domain | 15.0 [±6.3] | ---- | 16.1 [±7.0] | ---- | 15.9 [±6.8] | ---- |
| Extra-nasal rhinologic symptom domain | 7.6 [±3.3] | ---- | 8.4 [±3.9] | ---- | 8.3 [±3.8] | ---- |
| Ear / facial symptom domain | 8.5 [±4.9] | ---- | 9.0 [±5.3] | ---- | 8.9 [±5.2] | ---- |
| Psychological dysfunction domain | 12.4 [±7.1] | ---- | 16.1 [±8.4] | ---- | 15.4 [±8.3] | ---- |
| Sleep dysfunction domain | 11.5 [±6.3] | ---- | 13.8 [±6.8] | ---- | 13.4 [±6.7] | ---- |
| Monetized productivity losses ($) | $4,510 [±$6,817] | ---- | $8,516 [±$11,949] | ---- | $7,742 [±$11,242] | ---- |
SD, standard deviation; N, sample size; mRAST, modified radioallergosorbent testing; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disease; CT, computed tomography; SNOT-22, 22-item SinoNasal Outcome Test
Post-treatment Improvements per Treatment Modality
Mean post-treatment changes are described in Table 2 for both the total cohort and CAMT and ESS treatment modalities. Similar post-treatment changes were identified with the SNOT-22 4-subdomain model and are listed in Supplementary Table 2. For those participants electing ESS, the reported mean percentage (%) of daily work performance improved from 84% to 92% postoperatively (p<0.001). For participants electing CAMT, the reported mean daily work performance changed only marginally from 89% to 88% (p=0.856). Highly significant mean within-subjects improvements (p<0.001) were reported for the entire cohort for all mean SNOT-22 scores and monetized productivity losses, as indicated by negative mean change measures. Similarly, unadjusted significant mean improvement over time (p<0.001) was similarly reported by the subgroup of study participants electing ESS alone. For study participants electing CAMT however, significant mean improvements were only reported for the SNOT-22 total (p=0.039) and the rhinologic symptom domain (p=0.011) between baseline and post-treatment scores. Mean monetized productivity losses were significantly reduced following ESS intervention (−$5,015; p<0.050) and nearly unchanged for patients electing CAMT (−$200; p=0.887) using the Wilcoxon signed rank test for non-normally distributed data.
Table 2:
Mean post-treatment improvements in patient reported outcome measures and monetized productivity losses (n=202)
| Continued Appropriate Medical Therapy (n=39) | Endoscopic Sinus Surgery (n=163) | Total Study Cohort (n=202) | ||||
|---|---|---|---|---|---|---|
| Post-treatment Score | Change (Δ) | Post-treatment Score | Change (Δ) | Post-treatment Score | Change (Δ) | |
| Mean [±SD] | Mean [±SD] | Mean [±SD] | ||||
| SNOT-22 total score | 38.8 [±24.8] | −6.8 [±19.9]* | 28.3 [±21.3] | −24.5 [±23.0]* | 30.4 [±22.3] | −21.1 [±23.5]* |
| Rhinologic symptom domain | 12.0 [±7.4] | −3.0 [±7.0]* | 8.7 [±6.1] | −7.4 [±7.8]* | 9.3 [±6.5] | −6.6 [±7.8]* |
| Extra-nasal rhinologic symptom domains | 6.5 [±3.8] | −1.2 [±3.8] | 4.9 [±3.6] | −3.6 [±4.2]* | 5.2 [±3.7] | −3.1 [±4.2]* |
| Ear / facial symptom domain | 6.8 [±5.5] | −1.7 [±5.4] | 4.6 [±4.6] | −4.5 [±5.0]* | 5.0 [±4.8] | −3.9 [±5.2]* |
| Psychological dysfunction domain | 11.1 [±8.9] | −1.3 [±6.8] | 8.4 [±8.5] | −7.7 [±8.6]* | 8.9 [±8.7] | −6.5 [±8.7]* |
| Sleep dysfunction domain | 10.9 [±8.0] | −0.6 [±6.2] | 7.8 [±6.8] | −6.0 [±7.0]* | 8.4 [±7.1] | −4.9 [±7.2]* |
| Monetized productivity losses ($) | $4,310 [±$6,265] | −$200 [±$8,742] | $3,501 [±$5,021] | −$5,015 [±$12,545]* | $3,657 [±$5,277] | −$4,085 [±$12,039]* |
SD, standard deviation; SNOT-22, 22-item SinoNasal Outcome Test
indicates significant within-subject improvements over time using matched pair t-test statistics (p<0.050)
Bivariate Correlations with Productivity Loss
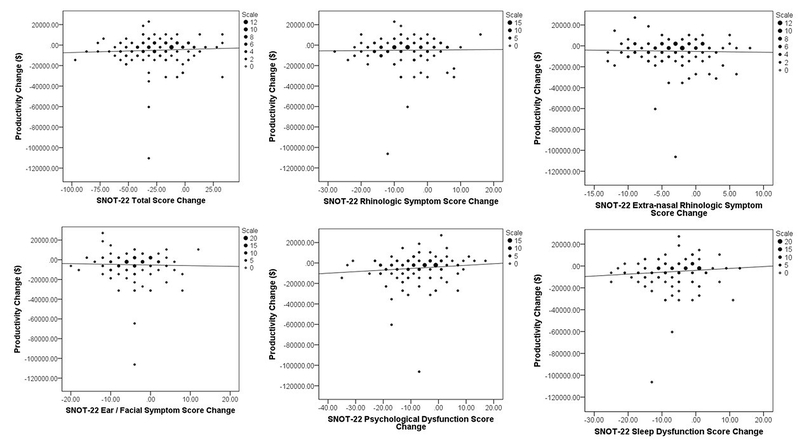
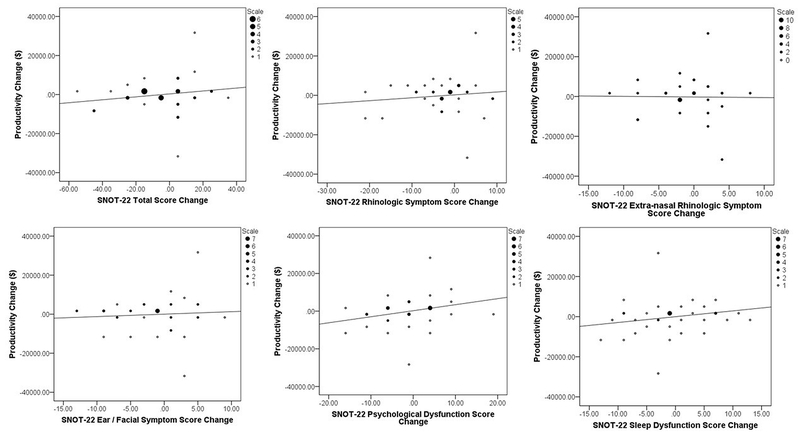
The total ranges of monetized productivity loss changes were also graphed against changes in SNOT-22 total and subdomain scores for both treatment modalities (Figures 1–2) to evaluate linear trends in scaled response scores. Bivariate correlations coefficients (r) were evaluated between post-treatment improvements in SNOT-22 responses and changes in productivity losses for both the total cohort and each treatment modality (Table 3). No significant correlations were found within either the ESS subgroup (all p≥0.175) or CAMT treatment subgroup (all p≥0.126). Similar findings were identified when the SNOT-22 4-subdomain model was applied are listed in Supplementary Table 3. Without regard for treatment modality, significant but weak correlations were found between worse productivity loss and both worse SNOT-22 psychological dysfunction and sleep dysfunction scores, without adjustment for potential covariate associations.
FIGURE 1:
Binning plots of monetized productivity loss changes versus changes in SNOT-22 total and subdomain scores for both treatment modalities for study participants electing endoscopic sinus surgery.
FIGURE 2:
Binning plots of monetized productivity loss changes versus changes in SNOT-22 total and subdomain scores for both treatment modalities for study participants electing continued appropriate medical therapy.
Table 3:
Bivariate correlation coefficients between post-treatment changes in monetized productivity loss and improvements in patient-reported outcome measures
| Continued Appropriate Medical Therapy (n=39) | Endoscopic Sinus Surgery (n=163) | Total Study Cohort (n=202) | |
|---|---|---|---|
| r (p-value) | r (p-value) | r (p-value) | |
| SNOT-22 total score | 0.171 (p=0.299) | 0.055 (p=0.487) | 0.112 (p=0.112) |
| Rhinologic symptom domain | 0.119 (p=0.472) | 0.015 (p=0.846) | 0.063 (p=0.375) |
| Extra-nasal rhinologic symptom domain | −0.013 (p=0.937) | −0.026 (p=0.738) | 0.012 (p=0.863) |
| Ear / facial symptom domain | 0.078 (p=0.635) | −0.027 (p=0.730) | 0.023 (p=0.746) |
| Psychological dysfunction domain | 0.249 (p=0.126) | 0.107 (p=0.175) | 0.162 (p=0.021) |
| Sleep dysfunction domain | 0.205 (p=0.212) | 0.097 (p=0.220) | 0.151 (p=0.032) |
SNOT-22, 22-item SinoNasal Outcome Test. r, Pearson’s two-tailed correlation coefficient for normally distributed scale data.
Mean Productivity Loss Changes across SNOT-22 Score Improvements
Individual post-treatment changes for the total study cohort in SNOT-22 total scores ranged between −97 and 32 points. The majority of participants (80%) reported at least a 1-point improvement following their elected treatment modality while 143 patients (71%) reported at least one MCID value (≥8.9 points). Differences in both SNOT-22 total scores (Table 4) and symptom domain scores (Tables 5–6) were stratified to evaluate mean changes in productivity loss categories for the total cohort and both treatment modalities. The greatest productivity gains were seen in patients who underwent ESS and experienced the largest improvement in SNOT-22 score. Larger productivity losses were found in CAMT patients reporting worse post-treatment extra-rhinologic, psychological, and sleep symptom severity scores; no such differences in productivity losses were seen in patients electing ESS.
Table 4:
Mean changes in productivity loss stratified across ordinal post-treatment differences in SNOT-22 total scores
| Improvement → | Post-treatment SNOT-22 total score differences: | Overall N (%) | Changes in mean monetized productivity loss ($) | ||
| Continued Appropriate Medical Therapy (n=39) | Endoscopic Sinus Surgery (n=163) | Total Study Cohort (n=202) | |||
| Mean ± SD | Mean ± SD | Mean ± SD | |||
| −80 to −97 | 2 (1%) | N/A | −$11,679 [±$3,227] | −$11,679 [±$3,227] | |
| −60 to −79 | 6 (3%) | N/A | −$2,952 [±$5,023] | −$2,952 [±$5,023] | |
| −40 to −59 | 34 (17%) | −$4,872 [±$6,526] | −$5,878 [±$5,837] | −$5,760 [±$5,824] | |
| −20 to −39 | 63 (31%) | $243 [±$2,987] | −$5,404 [±$18,458] | −$4,687 [±$17,360] | |
| −9 to −19 | 38 (19%) | $1,451 [±$2,851] | −$3,323 [±$7,147] | −$2,569 [±$6,856] | |
| 8 to −8 | 39 (19%) | −$3,394 [±$10,374] | −$6,494 [±$10,828] | −$5,461[±$10,645] | |
| 9 to 20 | 14 (7%) | $7,605 [±$12,479] | −$866 [±$4,404] | $2,764 [±$9,448] | |
| 21 to 32 | 6 (3%) | −$241 [±$80] | −$5,168 [±$17,245] | −$3,526 [±$13,598] | |
SD, standard deviation; SNOT-22, 22-item SinoNasal Outcome Test. Negative mean values reflect a post-treatment reduction monetized productivity losses. Positive mean values reflect a post-treatment increase in monetized productivity loss. N/A, no data available within the possible range of scale responses.
Table 5:
Mean changes in productivity loss stratified across ordinal post-treatment differences in SNOT-22 symptom domain scores for participants electing CAMT (n=39)
| ← Worsening Improvements → | SNOT-22 symptom domain score differences: | Rhinologic Symptom Domain | Extra-nasal rhinologic symptom domain | Ear / facial symptom domain | Psychological dysfunction domain | Sleep dysfunction domain |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| −31 to −35 | ---- | ---- | ---- | N/A | ---- | |
| −26 to −30 | N/A | ---- | ---- | N/A | ---- | |
| −21 to −25 | N/A | ---- | N/A | N/A | N/A | |
| −16 to −20 | −$4,577 [±$8,036] | ---- | N/A | N/A | N/A | |
| −11 to −15 | $2,185 [±$291] | N/A | $401 [±$997] | −$3,418 [±$6,026] | −$5,680 [±$6,476] | |
| −6 to −10 | $136 [±$3,013] | −$3,698 [±$8,045] | −$2,101 [±$5,480] | −$695 [±$1,851] | −$1,296 [±5,953] | |
| −5 to 0 | $754 [±$5,603] | $1,067 [±$4,303] | $203 [±$4,307] | −$1,943 [±$9,106] | $682 [±$11,854] | |
| 1 to 5 | −$3,373 [±$12,246] | $1,291 [±$14,279] | $667 [±$14,702] | $2,812 [±$10,223] | $1,162 [±$6,545] | |
| 6 to 10 | $4,584 [±$18,936] | N/A | −$277 [±$130] | $4,315 [±$5,890] | $2,873 [±$4,219] | |
| 11 to 15 | N/A | N/A | N/A | N/A | $581 [±$1,243] | |
| 16 to 20 | N/A | ---- | N/A | N/A | N/A | |
| 21 to 25 | N/A | ---- | N/A | N/A | N/A | |
| 26 to 30 | N/A | ---- | ---- | N/A | ---- | |
| 31 to 35 | ---- | ---- | --- | N/A | ---- |
SD, standard deviation; SNOT-22, 22-item SinoNasal Outcome Test. Negative mean values reflect a post-treatment reduction monetized productivity losses. Positive mean values reflect a post-treatment increase in monetized productivity loss. N/A, no data available within the possible range of scale responses. CAMT, continued appropriate medical therapy.
Table 6:
Mean changes in productivity loss stratified across ordinal post-treatment differences in SNOT-22 symptom domain scores for participants electing ESS (n=163)
| ← Worsening Improvements → | SNOT-22 symptom domain score differences: | Rhinologic Symptom Domain | Extra-nasal rhinologic symptom domain | Ear / facial symptom domain | Psychological dysfunction domain | Sleep dysfunction domain |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| −31 to −35 | ---- | ---- | ---- | −$8,625 [±$7,507] | ---- | |
| −26 to −30 | −$9,691 [±$415] | ---- | ---- | N/A | ---- | |
| −21 to −25 | −$6,291 [±$6,767] | ---- | N/A | −$2,994 [±$4,135] | −$4,507 [±$6,206] | |
| −16 to −20 | −$3,354 [±$6,867] | ---- | −$4,360 [±$8,712] | −$9,517 [±$15,033] | −$5,958 [±$7,369] | |
| −11 to −15 | −$6,428 [±$18,861] | −$5,702 [±$9,605] | −$1,271 [±$10,633] | −$5,703 [±$8,716] | −$8,935 [±$21,981] | |
| −6 to −10 | −$2,789 [±$9,357] | −$4,777 [±$11,751] | −$4,647 [±$6,612] | −$6,261 [±$19,036] | −$7,129 [±$13,097] | |
| −5 to 0 | −$7,024 [±$12,818] | −$5,089 [±$13,894] | −$6,285 [±$15,630] | −$3,561 [$8,070] | −$1,710 [±$8,167] | |
| 1 to 5 | −$2,715 [±$8,196] | −$4,272 [±$9,442] | −$2,216 [±6,789] | $669 [±$10,351] | −$4,524 [±$8,374] | |
| 6 to 10 | −$8,287 [±$12,499] | −$10,254 [±$16,936] | −$12,544 [±$13,100] | −$7,196 [±$10,252] | −$1,913 [±$9,445] | |
| 11 to 15 | N/A | N/A | N/A | $4,452 [±$5,672] | −$10,394 [±$16,799] | |
| 16 to 20 | N/A | ---- | N/A | N/A | N/A | |
| 21 to 25 | N/A | ---- | N/A | N/A | N/A | |
| 26 to 30 | N/A | ---- | ---- | N/A | ---- | |
| 31 to 35 | ---- | ---- | --- | N/A | ---- |
SD, standard deviation; SNOT-22, 22-item SinoNasal Outcome Test. Negative mean values reflect a post-treatment reduction monetized productivity losses. Positive mean values reflect a post-treatment increase in monetized productivity loss. N/A, no data available within the possible range of scale responses. ESS, endoscopic sinus surgery.
The individual survey item #16 of the SNOT-22 instrument determines the severity of reduced productivity in patients with CRS and serves as a type of internal control for measuring productivity losses in this patient population. For the overall cohort, we found significant (p<0.001) improvement between the baseline average score (2.4 [SD±1.5]) and the post-treatment mean score (1.4 [SD±1.5]) with only a weak, nonparametric two-tailed correlation between post-treatment changes in item #16 and changes in productivity loss (r=0.176; p=0.012). For study participants electing ESS, we found significant (p<0.001) improvement between the baseline average score of SNOT-22 item #16 (2.6 [SD±1.5]) and post-operative mean score (1.3 [SD±1.5]) with a non-significant correlation (r=0.121; p=0.125) between post-operative changes in item #16 and changes in productivity loss. For study participants electing CAMT we found non-significant (p=0.243) improvement between baseline average scores of the SNOT-22 item #16 (2.0 [SD±1.4]) and post-treatment mean scores (1.7 [SD±1.5]) again with a non-significant correlation (r=0.198; p=0.226) between post-treatment changes in item #16 and changes in productivity loss.
DISCUSSION
This study analyzed productivity changes among SNOT-22 subdomains associated with both CAMT and ESS treatment for medically refractory CRS. Recent work has shown that both measures of increased sleep and psychological dysfunction significantly correlate with pre-treatment productivity loss among CRS patients.8 This current analysis was completed to determine if changes in specific SNOT-22 subdomain scores were correlated with improvements in productivity following each treatment modality. While the CAMT and ESS groups were both presented in this analysis, since post-treatment productivity differences for each treatment group have not been previously fully described, our intent was not to directly compare between-subject differences in those values. The ESS and CAMT groups are distinct, with the ESS cohort reporting worse pre-treatment productivity relative to the CAMT group. It is likely that differential pre-treatment productivity loss was a driver of treatment decision making, as has been previously found in this cohort using SNOT-22 total scores.28
Results from this investigation demonstrate that patients who elect ESS report significant improvement in productivity post-operatively. Within the cohort electing ESS, there were no significant correlations between improvements in monetized productivity gain and postoperative changes in any SNOT-22 subdomain scores. These data suggest that significant predictors of productivity improvements following ESS are likely multi-factorial and not correlated with symptom severity as measured by specific subdomain scores of the SNOT-22 instrument. While prior work has shown that mean productivity losses are correlated with impairments in the sleep and psychological dysfunction in a cross-sectional fashion,8 results from the current study demonstrate that the majority of post-treatment improvements in SNOT-22 subdomain scores are not significantly correlated with reported changes in productivity status.
Our findings demonstrate very limited correlations between enhanced productivity and overall improvement in symptom severity ranges (Table 4). While a small number of patients who underwent ESS and experienced very large SNOT-22 improvements (>80 points) had the greatest productivity change, there was no such correlation for the remainder of the SNOT-22 improvement categories for either ESS or CAMT groups. This may be due to the fact that post-treatment productivity improvements are due to a reduced frequency or intensity of acute exacerbations, which might not be captured by the SNOT-22 questionnaire when it was administered. As most ESS patients experienced productivity improvements regardless of the magnitude of change in SNOT-22 score, it is also possible that changes relating to productivity may be impacted by different or additional factors than those captured by the SNOT-22 score. Alternative explanations might also include other factors such as unmeasured confounding, recall bias, or a response shift in the form of recalibration, reconceptualization, or reprioritization.18
This analysis demonstrates that CAMT was not significantly correlated with productivity improvements over time; this may be anticipated as CAMT patients had better baseline productivity compared to ESS patients and therefore had less potential for productivity improvements. While CAMT patients experienced a statistically significant improvement in mean SNOT-22 total score of 6.8 [±19.9] points, this change failed to reach the MCID threshold of 8.9 points,17 suggesting the mean symptomatic improvement may not be clinically meaningful or particularly discernible to this treatment group.
Prior outcomes studies have shown similar findings for CAMT with respect to baseline productivity and productivity loss. Smith et al. reported on a prospective cohort of refractory CRS patients awaiting ESS and determined that patients undergoing medical therapy while awaiting surgery experienced a mean worsening in SNOT-22 total scores of 8.5 points, as well as an increase in missed days of work.7, 17 Rudmik et al. analyzed a prospective group of CRS patients with mildly reduced baseline productivity that elected medical treatment.6 Their findings demonstrated a non-significant reduction in annual productivity costs with CAMT, concluding that CAMT may maintain baseline productivity status for selected patients. The results of the current study show minimal effect of CAMT on mean monetized productivity loss and align with prior research in this area.
Findings from this study must be interpreted in light of the fact that ESS and CAMT patients are intrinsically different patient populations. For example, patients with worse disease-specific quality of life have been shown to be more likely to select ESS.28 For the current CAMT treatment group, there was an mean trend between worse productivity loss and post-treatment changes in SNOT-22 total scores as well as in the sleep, psychological dysfunction, and extra-rhinologic subdomain scores. These findings expand on prior work from this cohort which has shown that worse impairment in both sleep and psychological dysfunction subdomains contributed to baseline monetized productivity loss8 and that the decision to pursue ESS is significantly correlated with worse impairment in the sleep and psychological dysfunction domains.13
Interpretations of this study are subject to several potential limitations. Patients had refractory CRS and were enrolled from academic, tertiary care rhinology practices and the results may not be externally valid beyond those populations or beyond the observed ~15 month period of follow-up time. There is also a possibility for differential recall bias since patients in both treatment cohorts were asked to provide economic and productivity data from the preceding 90 day period, potentially resulting in either over-reporting or under-reporting their productivity losses. Additionally, the cohort of patients electing CAMT is also of limited sample size and likely underpowered to detect significance between some of the mean differences reported here, especially among stratified subgroups of mean productivity loss or mean symptom severity improvement. Finally, patient-reported productivity metrics were approximated and monetized using general findings from the U.S. National Census and Department of Labor.23,24 These estimated productivity loss values can provide general guidelines for interpreting productivity losses in CRS at the population level, however should not be specifically applied to patient-level data.
CONCLUSIONS
Selection of surgical or medical therapy is correlated with different post-treatment productivity changes. CRS patients who undergo ESS experience a considerable improvement in productivity post-operatively that is distributed across all SNOT-22 subdomains, suggesting that productivity improvements occur as a result of improvements across multiple symptom areas. Patients with CRS who undergo CAMT had better baseline productivity compared to patients electing ESS, while this mean productivity level was maintained during treatment. Among CAMT patients, greater productivity loss occurred in patients with worse mean scores in the sleep, psychological dysfunction, and extra-rhinologic SNOT-22 subdomains, as well as mean SNOT-22 total scores. Due to potential sample size limitations in the CAMT group, a larger cohort may reveal additional trends between productivity changes following treatment and symptom subdomains.
Supplementary Material
Acknowledgments
Financial Disclosures: T.L.S., J.C.M., and Z.M.S. were supported for this investigation by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (R01 DC005805; Co-PI: T.L.S./Z.M.S.). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT02720653. This funding organization did not contribute to the design or conduct of this study; preparation, review, approval or decision to submit this manuscript for publication. There are no relevant financial disclosures for D.M.B. or L.R. A.S.D. is a consultant for Intersect ENT, Stryker Endoscopy and Olympus, none of which are affiliated with this investigation. Z.M.S. is a consultant for Olympus and 480 Biomedical, neither of which are affiliated with this investigation.
Footnotes
Potential Conflicts of Interest: None
The abstract for this manuscript was accepted for podium presentation to the American Rhinologic Society during the annual Combined Otolaryngology Spring Meetings in National Harbor, MD, April 18-22nd, 2018. Abstract #2236
REFERENCES
- 1.DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy. 2016; 30(2):134–139. [DOI] [PubMed] [Google Scholar]
- 2.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope. 2015;125(7):1547–1556. [DOI] [PubMed] [Google Scholar]
- 3.Rudmik L, Smith TL, Schlosser RJ, et al. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope. 2014; 124(9):2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50(1):1–12. [DOI] [PubMed] [Google Scholar]
- 5.Rudmik L, Smith TL, Mace JC, et al. Productivity costs decrease after endoscopic sinus surgery for refractory chronic rhinosinusitis. Laryngoscope. 2016; 126(3):570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudmik L, Soler ZM, Smith TL, et al. Effect of Continued Medical Therapy on Productivity Costs for Refractory Chronic Rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2015; 141(11):969–973. [DOI] [PubMed] [Google Scholar]
- 7.Smith KA, Rudmik L. Impact of continued medical therapy in patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014; 4(1):34–38. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury NI, Mace JC, Smith TL, et al. What drives productivity loss in chronic rhinosinusitis? A SNOT-22 subdomain analysis. Laryngoscope. 2018; 128(1): 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell AP, Phillips KM, Hoehle LP, et al. Depression symptoms and lost productivity in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017; 118(3):286–289. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007; 137(3 Suppl):S1–31. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015; 152(2 Suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 12.Alt JA, Smith TL, Schlosser RJ, et al. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014; 4(9):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeConde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014; 4(12):972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudmik L, Soler ZM, Mace JC, et al. Economic evaluation of endoscopic sinus surgery versus continued medical therapy for refractory chronic rhinosinusitis. Laryngoscope. 2015; 125(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–40. [DOI] [PubMed] [Google Scholar]
- 16.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 17.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009; 34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 18.DeConde AS, Bodner TE, Mace JC, et al. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014; 140(8):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng AL, Wesely NC, Hoehle LP, et al. A validated model for the 22-item Sino-Nasal Outcome Test subdomain structure in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017; 7(12):1140–1148. [DOI] [PubMed] [Google Scholar]
- 20.Revicki DA, Irwin D, Reblando J, et al. The accuracy of self-reported disability days. Med Care. 1994; 32(4):401–404. [DOI] [PubMed] [Google Scholar]
- 21.Severens JL, Mulder J, Laheij RJ, et al. Precision and accuracy in measuring absence from work as a basis for calculating productivity costs in The Netherlands. Soc Sci Med. 2000; 51(2):243–249. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Bansback N, Anis AH. Measuring and valuing productivity loss due to poor health: A critical review. Soc Sci Med. 2011;72(2):185–192. [DOI] [PubMed] [Google Scholar]
- 23.Brouwer WB, Koopmanschap MA, Rutten FF. Productivity losses without absence: measurement validation and empirical evidence. Health Policy. 1999;48(1):13–27. [DOI] [PubMed] [Google Scholar]
- 24.Koopmanschap MA. PRODISQ: a modular questionnaire on productivity and disease for economic evaluation studies. Expert Rev Pharmacoecon Outcomes Res. 2005;5(1):23–28. [DOI] [PubMed] [Google Scholar]
- 25.U.S. National Census: income, poverty, and health insurance coverage in the United States: 2012. Available: https://www.census.gov/prod/2013pubs/p60-245.pdf. Accessed June, 2018.
- 26.U.S. Department of Labor. Occupational employment statistics, May 2016. Available: http://www.bls.gov/oes/current/oes372012.htm. Accessed June, 2018.
- 27.DeConde AS, Mace JC, Alt JA, et al. Longitudinal improvement and stability of the SNOT-22 survey in the evaluation of surgical management for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(3):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soler ZM, Rudmik L, Hwang PH, et al. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.