Abstract
Calcium-calmodulin dependent protein kinase IIδ (CaMKIIδ) is an important regulator of cardiac electrophysiology, calcium (Ca) balance, contraction, transcription, arrhythmias and progression to heart failure. CaMKII is readily activated at mouths of dyadic cleft Ca channels, but because of its low Ca-calmodulin affinity and presumed immobility it is less clear how CaMKII gets activated near other known, extra-dyad targets. CaMKII is typically considered to be anchored in cardiomyocytes, but while untested, mobility of active CaMKII could provide a mechanism for broader target phosphorylation in cardiomyocytes. We therefore tested CaMKII mobility and how this is affected by kinase activation in adult rabbit cardiomyocytes. We measured translocation of both endogenous and fluorescence-tagged CaMKII using immunocytochemistry, fluorescence recovery after photobleach (FRAP) and photoactivation of fluorescence. In contrast to the prevailing view that CaMKII is anchored near its myocyte targets, we found CaMKII to be highly mobile in resting myocytes, which was slowed by Ca chelation and accelerated by pacing. At low [Ca], CaMKII was concentrated at Z-lines near the dyad but spread throughout the sarcomere upon pacing. Nuclear exchange of CaMKII was also enhanced upon pacing- and heart failure-induced chronic activation. This mobilization of active CaMKII and its intrinsic memory may allow CaMKII to be activated in high [Ca] regions and then move towards more distant myocyte target sites.
Keywords: calcium-calmodulin dependent protein kinase II, signal transduction, heart failure, calcium-dependent signaling
1. INTRODUCTION
Calcium/Calmodulin-dependent protein kinase II (CaMKII) is a key regulator of cardiomyocyte ion channels, calcium (Ca) balance, contraction and transcription [1, 2], and is known to be hyperactivated in pathological states such as heart failure (HF) and arrhythmias [3–6]. Acute CaMKII effects on ion channels and Ca handling proteins contribute to arrhythmogenesis by promoting early and delayed afterdepolarizations (EADs & DADs) and greater dispersion of repolarization and reentry. Chronic CaMKII activation, a hallmark of HF, can have major effects on cell survival and gene transcription by targeting calcineurin (CaN)[7] in the cytosol, and class II histone deaceytlases (HDACs) [5, 8]<sup>1,2</sup> in the nucleus. Several studies have also shown that acute or genetic inhibition of CaMKII limits arrhythmias and progression of HF [9–12], so CaMKII is now a therapeutic target in heart disease. However, little is known about how CaMKII localization or activation at its many known targets is regulated.
Of four CaMKII gene products (α, β, γ,δ), CaMKIIδ accounts for 85–90% of cardiomyocyte CaMKII activity and the rest is mostly CaMKIIγ [13, 14]. CaMKIIδ is alternatively spliced to encode δB and δC variants that differ by only an eleven amino acid nuclear localization sequence (NLS)[15]. CaMKII self-assembles as a dodecameric (or tetradecameric) holoenzyme, arranged as stacked hexameric (or heptameric) rings [16]. Each subunit has 3 domains: association, regulatory and catalytic. The C-terminal association domains bind to form a central hub in the holoenzyme that is connected by a linker to the regulatory domain that binds either the catalytic domain (inactive state), or calmodulin (CaM, active state). The regulatory domain binds Ca/CaM with a KD of 10–50 nM, which “opens” CaMKII so it can phosphorylate targets [17] including Thr287 on a neighboring subunit. This autophosphorylation raises Ca/CaM affinity (“CaM trapping”), slowing deactivation/reassociation with the catalytic domain. Thus, CaMKII autophosphorylation allows the kinase to remain partially active independently of Ca/CaM (activation memory). Several other posttranslational modifications in this same small region (oxidation, nitrosylation, and O-GlycNAcylation), were also recently shown to trigger this same autonomous activity [18–20].
Basal Ca/CaM affinity differs among CaMKII isoforms (γ >β>δ>α), but this affinity is much lower than that of other Ca/CaM effectors (e.g. calcineurin, nitric oxide synthase)[17]. CaMKII is therefore poised to turn on and off rapidly in locations with very high [Ca] spikes (e.g. at the dyadic cleft and neuronal synapses), but to work very much less well elsewhere [21–23]. While some CaMKII targets reside in the dyadic cleft between the transverse tubules and sarcoplasmic reticulum (SR) membranes (e.g. ryanodine receptor, RyR and L-type Ca channel, LTCC), it remains unclear how the widespread functional impact of CaMKII signaling is transduced to other membrane channels, cytosolic, SR, and nuclear proteins (e.g. Na channels, phospholamban, myofilaments, PLB and HDAC4). Under pathological conditions, the local microenvironment may amplify CaMKII signaling. For instance CaMKII oxidation upon increased oxidative stress may reset the Ca/CaM sensitivity permitting CaMKII activation at low Ca [24]. In HF, higher diastolic Ca in the junctional cleft and altered expression/distribution of Ca/CaM effectors and phosphatases result in increased RyR phosphorylation by CaMKII [25]. Whether microdomains at other CaMKII targets in the myocyte likewise bolster CaMKII activation in physiological or pathological conditions, or whether CaMKII translocation is needed for broader target phosphorylation is an open question.
Unlike protein kinase A anchors (AKAPs), remarkably little is known about CaMKII spatial targeting but local binding partners include αAKAP[26, 27], Cav 1.2 [28], βIV-spectrin [29], RyR [6] and HDAC [30]. Holoenzyme CaMKII composition also influences kinase targeting. Although CaMKIIδB tends to drive δB-δC complexes more nuclear (due to its NLS sequence), even δB expressed in a CaMKIIδ-knockout background is mostly cytosolic and CaMKIIδ signaling is more compartment-specific than subtype-specific [15, 31]. While neuronal CaMKIIα mobility is understood to play a key role in neuronal plasticity and memory [32–36], the prevailing view of cardiac CaMKII is as spatially confined, because of its large size [37] and aforementioned tethering interactions. Here we challenge the dogma of cardiac CaMKII as a stably anchored kinase at key target loci.
Using fluorescence recovery after photobleaching (FRAP) and photoactivation of fluorescence, we determined that tagged CaMKIIδ isoforms are highly mobile in cardiomyocytes at rest, and even more so when activated. At low Ca, endogenous CaMKII is concentrated along the Z-lines (and in the nucleus), but does not appear strictly tethered. Pacing, autophosphorylation and HF all resulted in enhanced CaMKII mobility and compartmental redistribution (net nuclear export and away from the Z-lines). These findings are in line with the notion that active CaMKII with its intrinsic memory moves from regions where it is most readily activated to more distant target sites in myocytes. We therefore suggest that the dynamic spatiotemporal regulation of CaMKII is an integral aspect of achieving the widespread functional impact of CaMKII signaling in the heart.
2. METHODS
An expanded methods section can be found in the online supplemental materials.
2.1. Cardiomyocyte isolation and culture
All animal experiments were conducted in compliance with the NIH guidelines for animal research and with approval by the Institutional Animal Care and Use Committee at the University of California Davis. Rabbit cardiomyocytes were isolated from young, 2–3 months old New Zealand White rabbits as previously described [38, 39]. Myocytes were also isolated from older healthy age-matched and heart failure rabbits as previously reported [39]. Heart failure was induced by combined aortic insufficiency and abdominal aortic stenosis as described [38]. After isolation, myocytes were cultured (<26 hrs) to express fluorescent CaMKII constructs or treated for western blotting or immunostaining procedures. For acute studies rabbit myocytes plated on laminin coated coverslips were superfused with normal Tyrode’s (135 mM NaCl, 5.4 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4, 23°C).
2.2. Immunocytochemistry
Cells either remained resting, were treated with BAPTA-AM, or were electrically field-stimulated with 5ms pulses of 20V for 10 min. Cells were fixed with 2% paraformaldehyde followed by standard immunocytochemistry staining or by expansion microscopy according to a previously published protocol [40]. The antibodies used were: goat anti-rabbit alexaFluor 488 (ThermoFisher, catalog number: A11034, lot number: 1751340) and goat anti-mouse alexaFluor 546 (ThermoFisher, Waltham, MA, catalog number: A11030, lot number: 51813A). CaMKIIδ (custom, 1:2000)[41], Alpha-Actinin (Sarcomeric) (Sigma-Aldrich, St. Louis, MO, catalog number: A7732, dilution 1:500), Alpha-Tubulin (Cell Signaling Technology, Danvers, MA, catalog number: 3873S, lot number: 11, 1:200), ATP Synthase Complex V (Thermo Fisher Scientific previously Novex, catalog number: 459240, lot number: K1948, 1:200), RyR (Thermo Fisher Scientific, clone: C3–33, catalog number: MA3–916, lot number: PC196808, 1:200), PLB (Badrilla, Leeds, UK, PLN, mAB A1, catalog number: A010–14, lot number: 642026,1:200). Phalloidin and DAPI stained F-actin and nuclei respectively.
2.3. Analysis
All fluorescence values were background subtracted. The CaMKII/actinin profile analysis was automated using Python version 3.5 (http://www.python.org) and the Spyder interactive development environment (http://www.pypi.python.org/pypi/spyder). Nuclear localization of CaMKII was measured using Image J and DAPI to define the nuclear region. Colocalization of CaMKII with other proteins was measured using the Image J plugin JaCOP (http://rsb.info.nih.gov/ij/plugins/track/jacop.html) [42]. FRAP and photoactivation analysis was with Image J. The colormap representing the time to peak of each pixel was made using Python.
All data are shown with error bars reflecting SEM.
3. Results
3.1. Dynamic CaMKII movement is altered by Ca and activation status
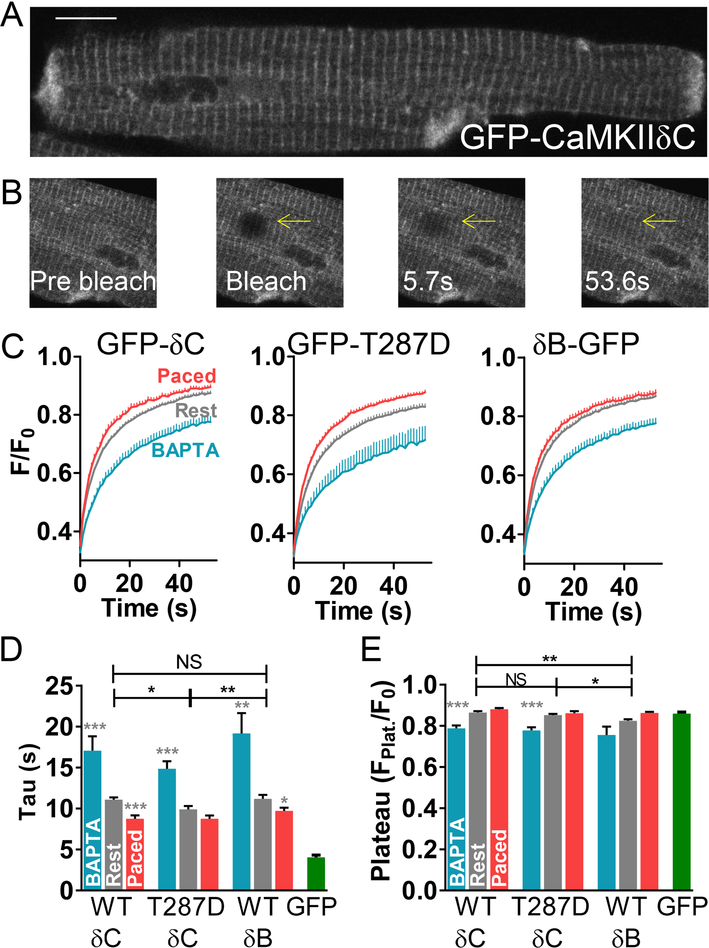
Confocal imaging of adenovirally expressed GFP-tagged CaMKIIδC (GFP-δC) in adult rabbit cardiomyocytes showed that at rest, GFP-δC is predominantly cytosolic (Fig 1A) and highly concentrated along Z-line/transverse striations (vs. cells expressing GFP alone, Fig S1 in SI Appendix). Expression of the δB splice variant (δB-GFP) also exhibited Z-line striations, but has significantly greater nuclear targeting, consistent with the presence of the NLS sequence (Fig S1). To measure the extent of CaMKII overexpression the ratio of GFP-δC to endogenous CaMKIIδ was assessed by western blotting (Fig S2). GFP tagged CaMKII expression was 63.2 ± 33% of endogenous CaMKII. Thus, GFP-CaMKIIδC is less than 1-fold overexpressed vs. endogenous CaMKII, localizes like endogenous CaMKIIδ and is likely to multimerize with endogenous CaMKII.
Figure 1.
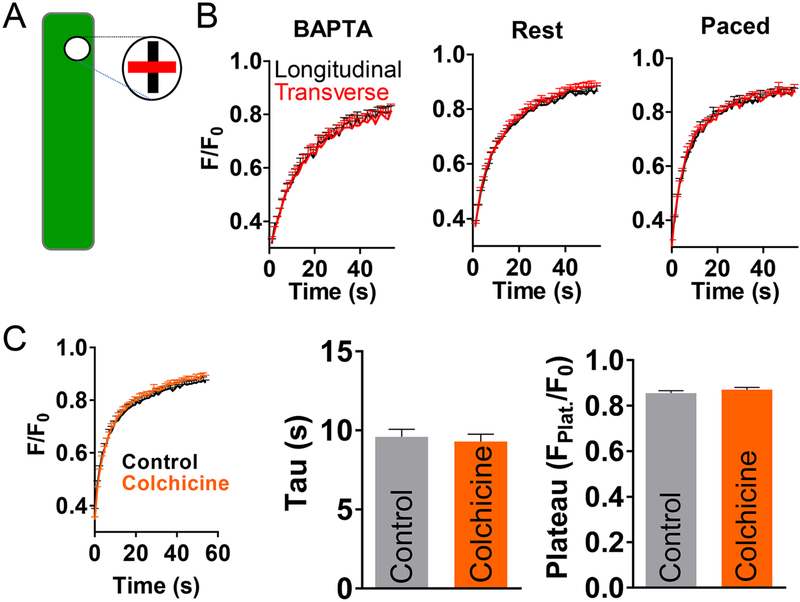
To measure CaMKII mobility, we bleached GFP-CaMKII in 10 μm regions of interest (ROIs) and monitored fluorescence recovery (FRAP). The time-constant of FRAP indicates the speed of mobile CaMKII, whereas incomplete recovery likely reflects CaMKII that is much less mobile. Fig 1B shows GFP-δC photobleach (to 30–40% of initial fluorescence) in a cytosolic region of interest (ROI, arrow) and subsequent recovery. In resting cells (Fig 1C-E, left), GFP-δC recovery time-constant was fast (τ ~11s) and nearly complete. Longer duration, control FRAP experiments (out to nearly 6 min) resulted in similar Fplat or tau (data not shown). The plateau of 0.86 ± 0.03 indicates a recovery of ~78% of the bleached GFP-δC. However, because GFP alone had a very similar plateau, this 78% may underestimate the fraction of mobile CaMKII. To promote CaMKII activation, myocyte calcium transients were induced by electrical pacing (0.5 Hz) for 10 min, prior to FRAP. GFP-δC moved slightly faster (τ ~9 s) after pacing. Next, to determine whether calcium was necessary for CaMKII mobility, FRAP was recorded in cells in which intracellular Ca was chelated by BAPTA-AM. In these myocytes, GFP-δC FRAP slowed (τ ~17 s) and recovery was also less complete while monitored. Control experiments confirmed that this incomplete recovery was not due to inadvertent photobleaching during our image acquisition (Fig S3). These results indicate very rapid mobility for nearly all of CaMKIIδ in adult cardiomyocytes, which is sensitive to intracellular Ca levels: Ca chelation (inactive CaMKII) increases CaMKII anchoring and slows the mobile fraction, whereas transient CaMKII activation by pacing results in a larger pool of more mobile CaMKII.
Comparison of FRAP in myocytes expressing GFP-δC vs. GFP alone proffered information as to whether CaMKII was moving at a speed consistent with simple diffusion (Fig 1D). The reported radius for the stable CaMKII multimer (in its more compact inactive state) is 7.25 nm [43] to 10 nm,[37, 44] with a height of 6 nm, whereas the hydrodynamic radius of GFP is 2.3 nm.[45] Assuming a spherical shape for both molecules (without the GFP on GFP-δC), we calculated that CaMKII should be roughly 4.4 times slower than GFP if moving simply by diffusion. Experimentally GFP-δC was 4.2, 2.7 and 2.1 times slower than GFP under BAPTA, resting and paced conditions respectively, indicating relatively free diffusion of the basal CaMKII complex, and conceivably facilitated diffusion for the less compact activated form of CaMKII.
Though CaMKII activity increases with pacing, [46] to assess whether a more fully activated state further enhances CaMKIIδ mobility, we performed FRAP with the autophos-phorylation-mimetic δC variant: GFP-T287D (Fig 1C-E, middle). Autonomous GFP-T287D moved faster than wildtype GFP-δC(WT) without stimulation (τ 9.9s, roughly half the change induced by pacing in WT). Note that the GFP-T287D is expected to multimerize with endogenous WT CaMKIIδ, which could slow its net diffusion, and explain its incomplete mimicry of active CaMKIIδ. Pacing had only a non-significant acceleration of GFP-T287D diffusion, but Ca chelation with BAPTA again slowed and reduced recovery (τ 15s and plateau 0.78 down from 0.85 at rest).
To test whether CaMKIIδ splice variants differ in their mobility or anchoring we also performed FRAP experiments with δB-GFP (Fig 1C-E, right). In resting myocytes, FRAP was equally fast (τ 11.2±0.5s vs. 11.1±0.3 for δC), but δB-GFP had a 5% lower recovery indicating δB is slightly more anchored. Together this data indicates that CaMKIIδ isoforms are predominantly and highly mobile and their mobility increases with activation.
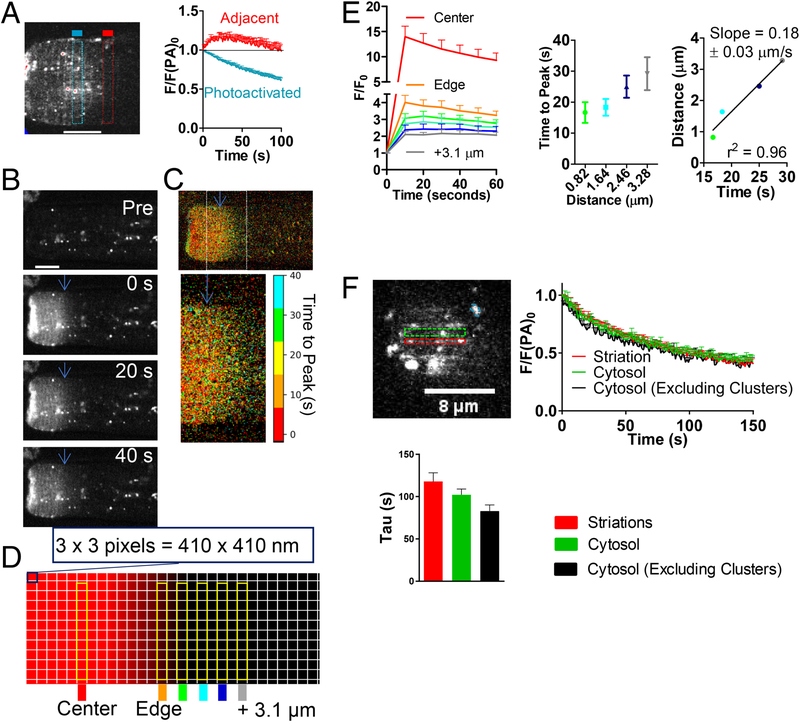
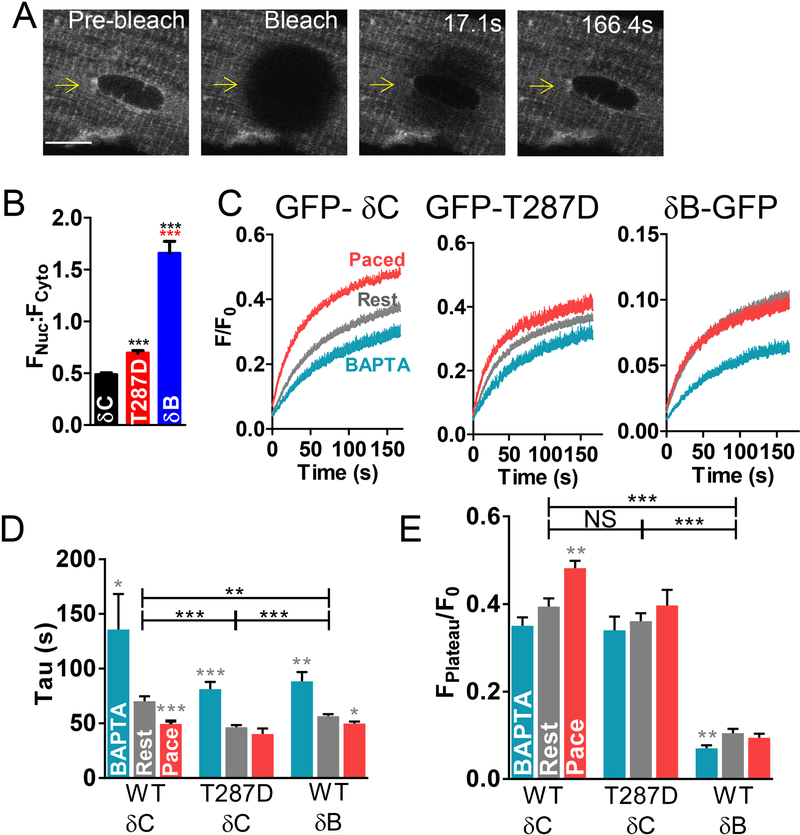
To more directly visualize dynamic GFP-CaMKIIδ translocation in real time in adult cardiomyocytes, photoactivatable-tagRFP-CaMKIIδ (PA- δC) was used, which is non-fluorescent until activated by 405 nm excitation. Upon photoactivation (PA) at the left end of a myocyte (Fig 2A and Figure S4), an expression pattern similar to GFP-δC emerged with a few “hotspots” initially in the activated region only. PA-δC then progressively declined (blue square and curve) as it diffused away from the activation site, but transiently rose and fell in the adjacent region as it diffused into and also out of (downstream of) that region (red square and curve).
Figure 2.
To estimate how fast CaMKII moves, we tracked the spread of the photoactivated CaMKII wave moving from the PA region. Figure 2B shows another cell before and at different times post-activation. Figure 2C shows a heat map of the myocyte in Fig 2B visualizing the fluorescence time to peak (TtP) in each pixel (for the initial image after PA time = 0s). The enlarged image focuses on the PA Edge (blue arrow). Pixels within the PA region reached peak fluorescence earlier (red-orange) than those further from the PA edge (more green). The RFP gradient dissipation was also analyzed by tracking the fluorescence in consecutive thin transverse ROIs (0.41 μm in width) over time (Fig 2D). Fluorescence normalized to pre-activation values was plotted for each region (Fig 2E, left). The center of the PA region showed robust photoactivation, and the Edge, by definition, also reached peak intensity immediately after PA. Beyond the edge, the ROIs showed a mild increase in fluorescence post PA, peaking only after 10–40s. That signal rises because of RFP-CaMKIIδ diffusing into this ROI, but also reflects some initial inadvertent PA in this ROI that would be declining after t=0. For each cell, the time to peak for individual ROIs was determined, with the first image after PA corresponding to 0 s. Figure 2E shows that TtP increased as ROI distance from Edge increased (Fig 2E, middle). A linear regression for the distance from Edge vs. time to peak yielded a slope of 0.18 μm/s (Fig 2E, right), indicating PA-δC moves across a half-sarcomere distance in approximately 5s.
Like in GFP- δC, PA-δC exhibited sarcomeric striations, but also sporadic clusters. To assess differences in CaMKII translocation to and/or anchoring at particular sites, higher magnification and smaller PA regions were used (allowing more complete PA in a shorter activation time, Fig 2F). Dissipation of the RFP fluorescence in the cytosol (excluding the clusters found in that region) was faster than that in clusters (τ 82±9s vs. 130±12s), moreover there was a tendency for slower diffusion from striations vs. cytosolic regions devoid of clusters.
3.2. Endogenous CaMKIIδ moves away from the Z-line/dyadic cleft during pacing
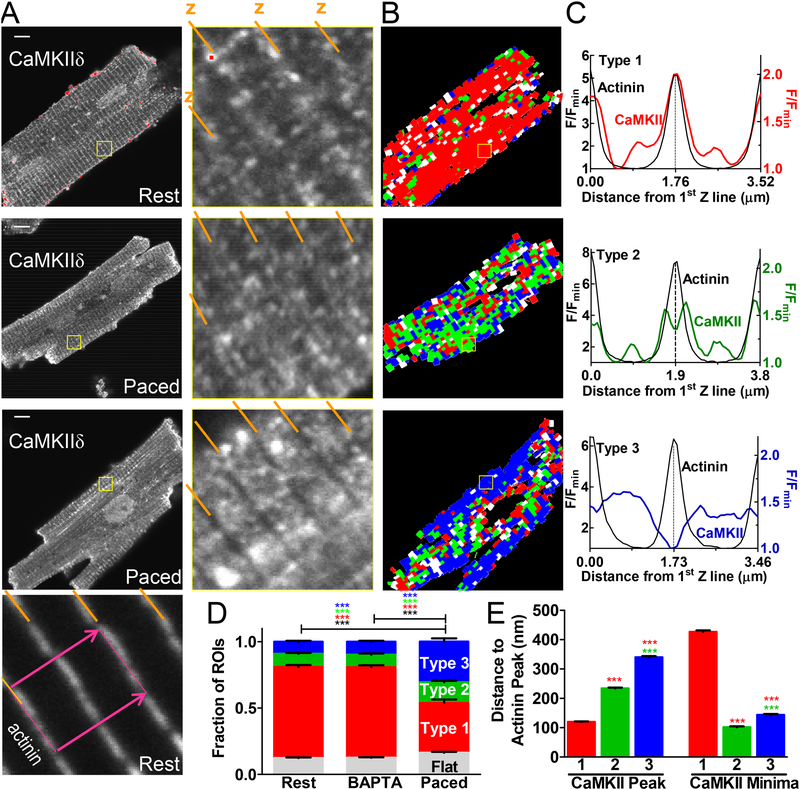
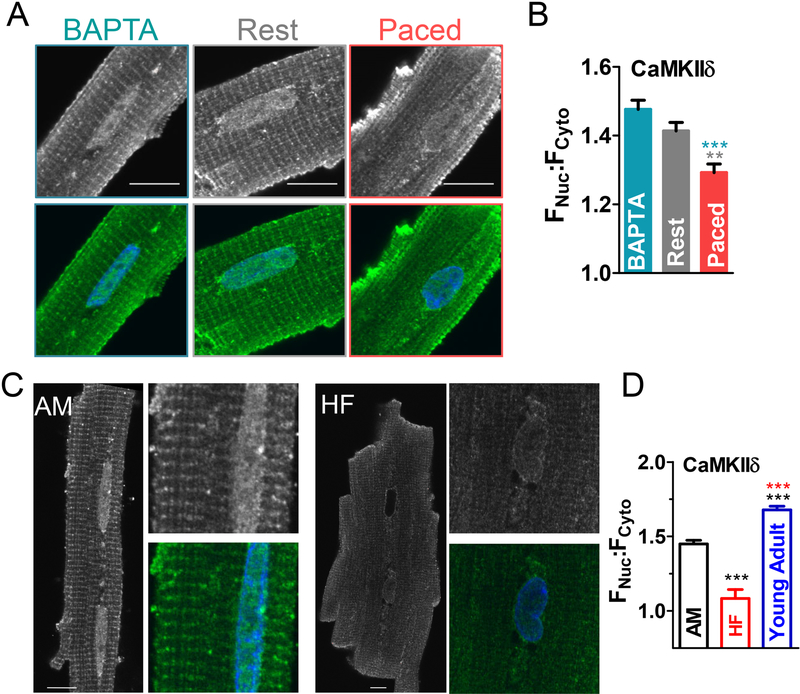
Having established that GFP-tagged CaMKIIδ is mobile and responsive to Ca signals in myocytes, we next tested endogenous CaMKIIδ movement using immunofluorescence. Myocytes were stained with a custom antibody specific for CaMKIIδ (verified in CaMKIIδ knockout mouse cardiomyocytes), and for the Z-line protein α-actinin (Fig 3A and Fig S5A). At the sarcomeric level, CaMKIIδ is highly concentrated at the Z-line (α-actinin indicated by the diagonal lines in enlargements) with additional mid-sarcomere and nuclear signal. Indeed, α-actinin was sharply-defined as Z-lines, while CaMKIIδ had broader distribution. This is consistent with the known association of CaMKII with RyR2 that surround the 200 nm diameter T-tubules that are centered at the Z-line. Pacing at 0.5 Hz for 10 min shifted the distribution of CaMKIIδ toward the mid-sarcomere (Fig 3A middle and bottom rows, Fig S6).
Figure 3.
Novel image-analysis was developed to assess the emerging distribution patterns. Guided by well-defined α-actinin Z-lines, confocal images were divided into ~500 rectangles (depending on cell size) 1.2 μm wide x 2.4 μm long (see bottom left panel of Figure 3A and S5A). This yields longitudinal sarcomeric fluorescence profiles (Fig S5B and 3B-C). Each profile (~500 per myocyte) was analyzed in 59 resting, 60 BAPTA treated and 60 paced myocytes (93,109 profiles total). These sarcomeric profiles were sorted based on position of the CaMKIIδ maxima and minima relative to the α-actinin peak. Exemplars across 2 full sarcomeres (Fig 3A, bottom) are shown in Fig 3C for each pattern (or type).
Type 1 profiles (red) have a large CaMKIIδ peak very near the Z-line, often having a smaller M-line peak at mid-sarcomere (Fig 3C and Fig S10). Type 2 profiles (green in Fig 3C) exhibit a relative CaMKIIδ nadir at the Z-line, with two nearby peaks on either side of the Z-line. Type 3 profiles (blue in Fig 3C) have an absolute CaMKIIδ nadir at the Z-line (i.e. opposite to type 1). A minority of profiles did not fit these 3 exemplar types (~10%, white) categorized as “flat” (i.e. no detectable CaMKIIδ peak greater than 110% of the minima). Every analyzed myocyte contained a mixture of these distribution patterns (Fig 3B, 3D and S5A), but in resting and BAPTA-treated cells Type 1 sarcomeres dominate (68.5 ± 1.6% and 68.2 ± 1.5%, respectively). In paced cells Type 1 drops to 37.4 ± 0.2, while the sum of type 2+3 increased dramatically from ~19% to 46% (with 15.7 ± 1% Type 2 and 30.6 ± 2.4% Type 3). Put another way, the ratio of Type 1/(Type 2 +3) drops more than 4-fold from 3.6 to 0.8 upon pacing. Thus, upon activation CaMKIIδ spreads from the Z-line through the sarcomere, consistent with the mobility assessed by FRAP in Figure 1.
We also measured the longitudinal distances between the α-actinin peak and the different CaMKIIδ peaks and minima to get an idea of which targets CaMKII might be near in the Type 2 distribution. Type 1 CaMKIIδ profiles, by definition had a peak closest to the Z-line (120 ± 1.9 nm away), while the nearest Type 2 and 3 peaks were 234 ± 2.4 and 340 ±4 nm from the Z-line, respectively (Fig 3E). CaMKII minima for Type 2 and Type 3 were both near the Z-line, while the Type 1 minima was approximately a quarter sarcomere length away (426 nm). Myocytes co-stained for the ryanodine receptor (RyR), a known CaMKII target at the junctional SR-membrane were subjected to the same analysis, and CaMKII localization vs. RyR and Z-line were quite similar at confocal microscope resolution (Fig S7). Skeletonization of the RyR signal was a less regular lattice vs. α-actinin (with some longitudinal projections), but likewise CaMKII showed increases in Type 2+3 sarcomeres upon pacing. Thus at rest CaMKIIδ is focused at Z-lines (& M-lines), but with activation it moves longitudinally, pausing at type 2 peak mid-sarcomeric targets (possibly titin and myosin binding protein C, MyBP-C).
3.3. Expansion microscopy and mid-subsarcomeric CaMKIIδ
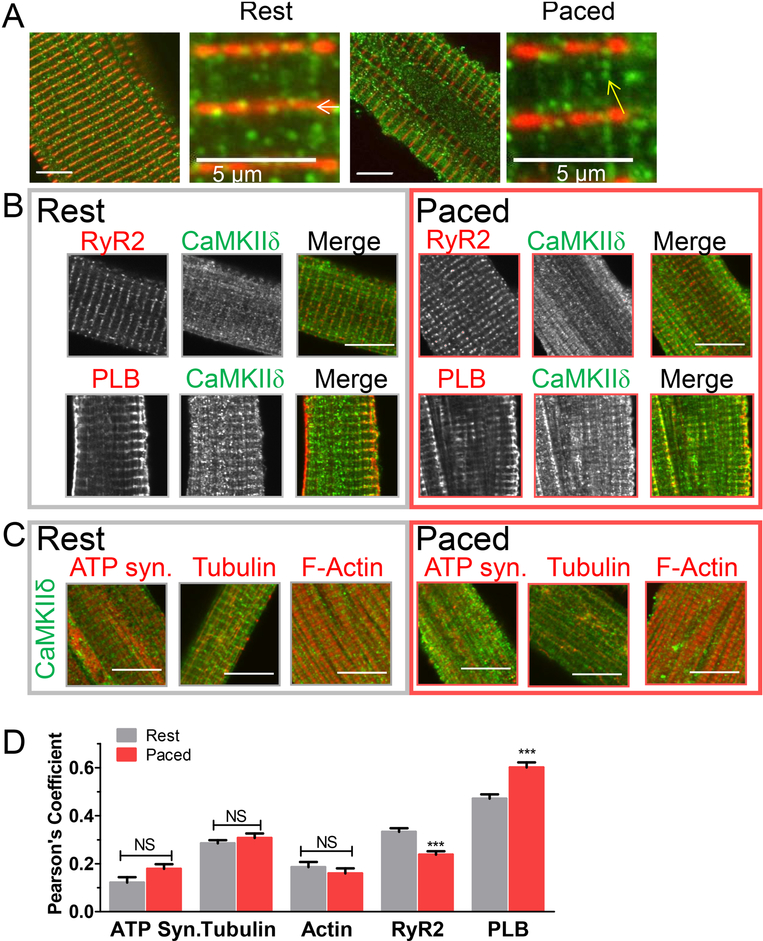
To better visualize CaMKIIδ subcellular targeting, expansion microscopy (ProExM) was used to enhance spatial resolution of CaMKIIδ/actinin-stained cardiomyocytes ~1.4-fold (Fig 4A).[40]. This approach highlighted the clustered nature of CaMKIIδ spatial distribution, with clusters aligned in both transverse (white arrow) and longitudinal directions (yellow arrow) in resting and paced cells. Because local control is key to CaMKIIδ activation and signaling we sought to identify ultrastructural CaMKIIδ microdomain or interacting proteins at rest and upon activation by pacing. Visualization of RyR clusters (Fig 4B & S8, left), confirmed clearer transverse striation of RyR than CaMKIIδ. Moreover, while CaMKIIδ and RyR clusters overlapped, lone CaMKIIδ and RyR clusters were also present. As in our standard confocal analysis (Fig S7), RyR-CaMKIIδ co-localization decreased with pacing (Fig 4B, D). In contrast, phospholamban (PLB, another CaMKII SR target protein) showed increased co-localization with CaMKIIδ upon pacing (Fig 4B, D). PLB showed transverse striations with some longitudinal signal (potentially corresponding to junctional and longitudinal SR respectively; Fig S8). Notably, CaMKIIδ was more highly co-localized with PLB than RyR, especially after pacing (Fig 4D).
Figure 4.
MyBP-C, another CaMKII substrate, is also a potential destination for CaMKII. Indeed, MyBP-C exhibits double striations in the sarcomere (Fig S9) as do Type 2 profiles. However, MyBP-C-actinin sarcomeric analysis looks more like Type 3 distribution, with the absolute minima of MyBP-C overlapping with the α-actinin peak, and the MyBP-C peak 465 nm from the Z-line (Fig S9B-C), far from the Type 2 CaMKIIδ peaks (at 234 nm) (and close to the Type 1 CaMKII minima at 426 nm Fig 3E). This suggests that the Type 2 peaks are not created by CaMKII diffusion stopping at MyBP-C. Titin is another sarcomeric CaMKII target as a Type 2 peak candidate. However, the phosphorylation sites are expected to be 100–150 nm from the Z-line at the sarcomere lengths in Fig 3,[47] and we find titin much closer to the Z-line than the Type 2 CaMKII peak (Fig S9D). Many Type 1 CaMKII distributions exhibited a secondary mid-sarcomeric peak (M-line; Fig 3C) on average 893 ± 13 nm from the Z-line. Analysis showed this hump to be detectable in 30.1 ± 2.7% of Type 1 profiles, but only 7.9 ± 1.5% of Type 2 profiles (Fig S10B).
CaMKII co-localization with mitochondria (which are in longitudinal chains) was assessed using CaMKIIδ and F1/F0-ATP synthase antibodies. However, CaMKII-mitochondrial colocalization was low at baseline and did not change significantly upon pacing (Fig 4C-D). Tubulin is another longitudinal target candidate. While baseline CaMKIIδ-tubulin co-localization was apparent, not all longitudinal CaMKIIδ clusters coincided with tubulin nor did pacing affect co-localization. CaMKIIδ targeting to F-actin in the thin filaments, was likewise low under both resting and paced conditions.
3.4. Microtubules do not influence CaMKIIδ mobility in myocytes
CaMKIIδ recovery in Fig 1D, even at rest was faster than predicted based on size-adjusted comparison to freely mobile GFP. Here we tested whether CaMKII diffusion is facilitated via microtubules, or occurs preferentially in the longitudinal vs. transverse direction. Figure 5A-B compares FRAP of GFP-δC in the longitudinal vs. transverse direction. If CaMKII moved along microtubules, faster recovery in the longitudinal direction would be expected. However FRAP recovery was symmetrical and not different between transverse and longitudinal ROIs in BAPTA-treated, resting, and paced cells (Fig 5A-B). As lateral projections of the microtubule network could account for similar longitudinal and transverse recovery, the effect of microtubule disruption was also assessed. Pre-treatment with 250 μM colchicine (30 min) had no effect on either GFP-δCFRAP tau or plateau (Fig 5C). Thus microtubules do not appear to facilitate CaMKIIδ translocation in cardiomyocytes.
Figure 5.
3.5. Activation increases CaMKIIδ nuclear translocation
Because Ca/CaM signals altered cytosolic CaMKIIδ mobility, we also tested whether they influence nuclear translocation of CaMKIIδB and δC. Figure 6 shows nuclear FRAP experiments analogous to Fig 1. At baseline, splice variants target differentially, with δB-GFP being ~3.4-fold more nuclear than δC-GFP (Fig 6B). Autophosphorylation-mimetic T287D- δC-GFP was likewise more nuclear (~1.43-fold), suggesting that CaMKII activation stimulates nuclear targeting. Upon photobleach of the nucleus and nuclear envelope GFP-δCFRAP was significantly slower than cytosolic FRAP (Fig 6A). And as in the cytosol, BAPTA slowed nuclear FRAP whereas activation with pacing accelerated FRAP (Fig 6C-D). Likewise, the constitutively active T287D recovered faster in the nucleus than WT and there was no additional effect of pacing. The CaMKIIδB splice variant containing an NLS (δB-GFP) had faster nuclear FRAP (as expected), despite similar FRAP kinetics for cytosolic GFP-δC and δB-GFP (Fig 1D). The plateau for δB-GFP was much smaller, which might be due to bleached δB-GFP remaining bound within the nucleus. These results indicate CaMKII activation enhances nuclear import.
Figure 6.
The effect of CaMKII activation on nuclear targeting was also assessed on endogenous CaMKIIδ using immunocytochemistry. Here, activation with pacing decreased nuclear localization (Fnuc/Fcy to = 1.48±0.03 in BAPTA, 1.41±0.02 at rest and 1.29±0.02 with pacing, Fig 7B). Considering the nuclear FRAP results, this suggests that both nuclear import and export of CaMKIIδ are enhanced with pacing, but the effect on nuclear export dominates at steady state.
Figure 7.
To examine whether chronic CaMKII activation (as seen in HF6) mimics the acute effects of activation, nuclear localization of endogenous CaMKIIδ was examined in myocytes isolated from HF vs. age-matched (AM) vs. young adult rabbits (the latter group was included to assess age-effects, Fig 7C). While AM myocytes already exhibited lower nuclear CaMKIIδ, this was more pronounced in HF cardiomyocytes (Fnuc/Fcy to was 1.08±0.06 in HF vs. 1.45±0.03 in AM and 1.68±0.02 in young adult, Fig 7D). This suggests that chronic activation of CaMKII during HF results in reduced nuclear CaMKII, to a greater extent than acute activation or aging.
4. DISCUSSION
CaMKII has emerged as a nodal signal in the development of heart failure and arrhythmias, with many known phosphorylation targets throughout the myocyte that directly contribute to cardiac pathology.[1, 2] So understanding the precise cellular and molecular steps controlling CaMKII activity in cardiomyocytes is crucial. Over the past decade attention has focused on identifying these phosphorylation targets and posttranslational modifications (PTMs) that promote chronic CaMKII activation (phosphorylation, oxidation, O-GlcNacylation, nitrosylation) and pathological consequences. This has made the present study especially timely in demonstrating where myocyte CaMKIIδ is positioned and how it moves toward different target loci.
This study redefines our prior view of cardiac CaMKII as a spatially confined, targeted signaling molecule in adult cardiomyocytes. We show that CaMKIIδ moves rapidly, and that that mobility is promoted by activation. At rest, CaMKIIδ is concentrated near Z-lines and the RyR, but during pacing or activation it translocates longitudinally within the sarcomere. This translocation, coupled with the intrinsic activation memory of CaMKII provides a plausible mechanism to explain how CaMKII can phosphorylate known myocyte targets at large distances from the Ca channels that produce the high local [Ca2+]i that is required to activate CaMKII. This introduces an important new dimension to cardiac CaMKII signaling which may be essential to its major functional impact in cardiac myocyte function and pathological remodeling.
4.1. CaMKIIδ is highly mobile in cardiac myocytes
Despite clear baseline Z-line concentration, the vast majority of cytosolic CaMKIIδ (~80%) is highly mobile, while the remainder is more firmly anchored (Fig 1). This suggests that while CaMKIIδ binds to specific known targets at the Z-line (e.g. RyR and L-type Ca channels),[6, 28] that most of these sites have sufficiently low affinity that CaMKII can dissociate within 3–10 s in the myocyte environment (and diffuse away or potentially rebind). Notably, the immobile fraction is much higher for nuclear CaMKIIδ, and especially for CaMKIIδB (>80%; Fig 6D), suggesting that a relatively high fraction of nuclear CaMKIIδB is more tightly bound to nuclear sites.
CaMKII mobility is further enhanced during physiological pacing, under conditions where CaMKII is known to be partially activated [46] and that mobility is mimicked by autonomously active CaMKIIδC-T287D (Fig 1). Thus, physiological CaMKII activation promotes mobility. We were surprised that BAPTA (which makes [Ca2+]i very low) was able to reduce CaMKII mobility even below that seen in resting myocytes (Fig 1C-D). That is, we expected little difference in CaMKII activation in these conditions. So, we cannot rule out that a Ca-dependent process other than CaMKII activation state might modulate CaMKII mobility or binding. On the other hand, this rest vs. BAPTA difference was not present for endogenous CaMKIIδ (Fig 3D), so the exogenous GFP-CaMKIIδ (Fig 1) might also be slightly activated in resting myocytes and account for the lower mobility with BAPTA. In that case Fig 1 may underestimate the influence of activation on CaMKIIδ mobility (vs. endogenous CaMKIIδ in Figure 3). This limitation would also hamper detection of more detailed heart-rate dependent changes in CaMKII mobility and localization. Here we tested how Ca- and autophosphorylation influence CaMKIIδ mobility, but it seems likely that mobility would also be promoted by other PTMs that promote CaMKIIδ autonomous activity (oxidation at M281/M282, O-GlcNAcylation at S280 and S-nitrosylation at C290). [1,19]
The overexpression of GFP-CaMKIIδ that is required for direct mobility measurements might be more mobile than endogenous CaMKIIδ that may already be bound to target sites that impede mobility. We think this is a minor factor here for several reasons. First, the level of GFP-CaMKII expressed is less than the endogenous CaMKII (Fig S2). Second, the exogenous GFP-CaMKII is expected to multimerize with endogenous myocyte CaMKII into stable dodecamers, such that endogenous and GFP-tagged CaMKII move together. Third, the addition of the GFP tag would most likely slow mobility due to its added size. Fourth, our parallel studies of endogenous CaMKIIδ showed striking CaMKII movement, albeit as snapshots of CaMKIIδ shifting from mostly Type 1 to Type 2–3 patterns upon pacing (Fig 3–4). We conclude that the kinetics revealed by our FRAP and photo-activation studies are good approximations of mobility of endogenous CaMKIIδ, however any overexpression is likely to perturb the bound to unbound ratio to some extent. Moreover, the PA studies show direct movement of CaMKIIδ in myocytes from Z-line to M-line in ~5 sec. Indeed, our 3 complementary approaches all indicate high CaMKIIδ mobility in myocytes, that is accelerated by activation.
The illumination used to bleach GFP may also result in local oxidative stress, which could potentially activate the kinase. Because an open conformation is necessary for oxidation of CaMKII, any oxidative effect would be minimal for the BAPTA-treated myocytes, but may be more present in rest and especially paced conditions. If oxidation enhances kinase mobility similarly to pacing, oxidation could obscure differences between resting and paced conditions, and augment differences between BAPTA-treated and resting cells.
CaMKII primarily exists as a stable dodecamer in cells. While monomers can exchange between the holoenzyme it requires activation and occurs over a much slower time scale than the translocation reported here.[48, 49] Our FRAP studies suggest that CaMKIIδ at rest may diffuse, as expected based on its dodecameric size (vs. GFP) without being greatly delayed by high-affinity binding. The high mobility of CaMKII is likely due to interactions with its targets which are transient and low affinity, as opposed to more stable interactions which would restrict its ability to move. However, CaMKIIδ moved faster when activated, raising the possibility of facilitated diffusion (e.g. along microtubules as for CaMKIIα in neuronal dendrites).[32, 33] However, our FRAP studies show that CaMKIIδ diffusion was symmetrical (not preferentially longitudinal) and not influenced by microtubule disruption (Fig 5). We conclude that CaMKIIδ movement does not occur via microtubules, but diffuses relatively freely in ventricular myocytes, and that many of its potential interactions with partners are of relatively low affinity (high off-rate), such that they minimally hinder diffusion. Of course, there is also a CaMKIIδ fraction that appears immobile (20% of cytosolic CaMKIIδ) which may bind especially strongly to key targets, with limited movement over this time frame.
4.2. CaMKIIδ Target Neighborhoods and Phosphorylation in Cardiac Myocytes
CaMKIIδ binds to, co-immunoprecipitates with and phosphorylates numerous myocyte targets including RyR and L-type Ca channels (in the junctional cleft), HDAC4 (in nucleus and cytosol), InsP3 receptors (at the nuclear envelope), titin and MyBP-C (on the myofilaments) and PLB (throughout the SR).[1] The low affinity of CaMKII for Ca-CaM (vs. e.g. nitric oxide synthase and calcineurin) poises CaMKII for activation only where local [Ca]i is very high, such as near Ca channels and RyRs in the junctional cleft, and at nuclear InsP3 receptors.[21, 22, 50, 51] This allows responsive phosphorylation that regulates these local Ca channels, but not distant targets on the myofilaments or SR. The latter targets may require both CaMKIIδ translocation and sustained CaMKII active state (memory) that is induced by regulatory domain autophosphorylation, oxidation, O-GlcNAcylation and S-nitrosylation. Those post-translational modifications in the regulatory domain can greatly prolong CaMKII active state (autonomy) even after [Ca]i decline and CaM dissociation from the enzyme.
We hypothesize that CaMKIIδ is activated at junctional clefts at the Z-line (Type 1 peaks) and that activated CaMKIIδ diffuses away, potentially pausing at targets near the Type 2 twin peaks (234 nm away from Z-lines) on their way to more uniform sarcomeric distribution. However, neither MyBP-C nor titin’s phosphorylation peaks align with that locus (further from and closer to Z-line, respectively). It is possible that the concept is correct, but that the Type 2 hump reflects a composite of these targets, phospholamban (where co-localization increased upon pacing; Fig 4D). There may also be a steric diffusional restriction upon transition from Iband to A-band (where both thick and thin filaments overlap). The increase in Type 2, 3, and “Flat” profiles with pacing suggests that CaMKII is moving towards a large number of targets rather than one specific microdomain, reflecting its widespread functional impact.
Our results do not dispute CaMKII scaffolds that have previously been identified, nor do they exclude the possibility that interactions with other proteins may create local pools of inactive and active kinase [52, 53]. Rather they provide insight into how CaMKII reaches targets outside of areas where it is most readily activated. Our data provide an explanation as to how CaMKII manages to reach its targets in separate microdomains.
4.3. CaMKIIδB vs. CaMKIIδC and Nuclear translocation
CaMKIIδB is more concentrated in the nucleus, based on its NLS, but CaMKIIδB and δC coexist in holoenzyme dodecamers, such that CaMKIIδ signaling is more location-specific than subtypespecific.[31] Indeed, in the cytosol both splice variants behaved quite similarly, with only slightly more δB-GFP appearing immobile (smaller plateau in Fig 1). Nuclear FRAP was much slower than in the cytosol, consistent with nuclear pore translocation of CaMKIIδ being rate-limiting. Nuclear CaMKIIδB FRAP was faster reflecting the NLS that δC lacks. Likewise the recovery for CaMKIIδB is far less complete, indicating increased nuclear anchoring of this variant. The aforementioned hetero-multimerization with endogenous CaMKIIδ (B and C) could cause us to underestimate these differences, suggesting that the 11 amino acid NLS in CaMKIIδB may also contain interaction domains with other proteins. Pacing also speeds nuclear FRAP. Taken together with our finding that pacing and HF result in a net CaMKIIδ shift out of the nucleus, activation appears to increase exchange of CaMKIIδ between cytosolic and nuclear compartments (with the balance in favor of export). This agrees with evidence that kinase activation and autophosphorylation (at Serine 332) promotes cytosolic retention of CaMKII.[54] The nuclear import/export balance is likely affected by additional factors such as GPCR stimuli and duration of activation signals, since at rest T287D is more nuclear than the WT CaMKIIδ.
In conclusion, we used FRAP, photoactivation, and immunocytochemistry to disprove the hypothesis that cardiac CaMKII signaling is spatially confined. We found CaMKII to be remarkably mobile, with mobility increasing upon activation. Activated CaMKIIδ moved away from the Z-lines and out of the nucleus independently of microtubules and likely towards numerous different targets. Since CaMKIIδ is most readily activated by high Ca/CaM signals such as at the dyadic cleft, this translocation may be essential to CaMKII signal spread in myocytes. It likely also explains how mathematical CaMKII models, lacking this mobility, do not explain significant cytosolic CaMKII activation or target phosphorylation. Our work adds a new dimension to the spatiotemporal regulation of CaMKII signaling and its widespread functional intracellular impact.
Supplementary Material
Cardiac CaMKII is predominantly mobile
CaMKII mobility varies with calcium/kinase activation
Active CaMKII moves from the dyadic cleft towards cytosolic targets including PLB
Heart failure promotes movement of CaMKII out of the nucleus
Acknowledgments
We would like to thank Drs. Samantha Harris and Henk Granzier for helpful discussions and MyBPc and Titin antibodies.
This work was supported by NIH grants P01-HL0980101 and R01-HL030077 (DMB), R01HL103933 (JB) and R01-HL142282 (DMB/JB).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Anderson ME, Brown JH, Bers DM, CaMKII in myocardial hypertrophy and heart failure, J Mol Cell Cardiol 51(4) (2011) 468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mattiazzi A, Bassani RA, Escobar AL, Palomeque J, Valverde CA, Vila Petroff M, Bers DM, Chasing cardiac physiology and pathology down the CaMKII cascade, Am J Physiol Heart Circ Physiol 308(10) (2015) H1177–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P, Identification and expression of deltaisoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium, Circulation research 84(6) (1999) 713–21. [DOI] [PubMed] [Google Scholar]
- [4].Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittkopper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS, Inhibition of elevated Ca2+/calmodulindependent protein kinase II improves contractility in human failing myocardium, Circulation research 107(9) (2010) 1150–61. [DOI] [PubMed] [Google Scholar]
- [5].Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM, Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure, Circulation research 102(6) (2008) 695–702. [DOI] [PubMed] [Google Scholar]
- [6].Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM, Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure, Circulation research 97(12) (2005) 1314–22. [DOI] [PubMed] [Google Scholar]
- [7].MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR, CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes, Circulation research 105(4) (2009) 316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH, CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses, The Journal of biological chemistry 282(48) (2007) 35078–87. [DOI] [PubMed] [Google Scholar]
- [9].Hoeker GS, Hanafy MA, Oster RA, Bers DM, Pogwizd SM, Reduced Arrhythmia Inducibility With Calcium/Calmodulin-dependent Protein Kinase II Inhibition in Heart Failure Rabbits, J Cardiovasc Pharmacol 67(3) (2016) 260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kreusser MM, Lehmann LH, Wolf N, Keranov S, Jungmann A, Grone HJ, Muller OJ, Katus HA, Backs J, Inducible cardiomyocyte-specific deletion of CaM kinase II protects from pressure overloadinduced heart failure, Basic research in cardiology 111(6) (2016) 65. [DOI] [PubMed] [Google Scholar]
- [11].Neef S, Steffens A, Pellicena P, Mustroph J, Lebek S, Ort KR, Schulman H, Maier LS, Improvement of cardiomyocyte function by a novel pyrimidine-based CaMKII-inhibitor, J Mol Cell Cardiol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pellicena P, Schulman H, CaMKII inhibitors: from research tools to therapeutic agents, Frontiers in pharmacology 5 (2014) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grimm M, Ling H, Willeford A, Pereira L, Gray CB, Erickson JR, Sarma S, Respress JL, Wehrens XH, Bers DM, Brown JH, CaMKIIdelta mediates beta-adrenergic effects on RyR2 phosphorylation and SR Ca(2+) leak and the pathophysiological response to chronic beta-adrenergic stimulation, J Mol Cell Cardiol 85 (2015) 282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kreusser MM, Lehmann LH, Keranov S, Hoting MO, Oehl U, Kohlhaas M, Reil JC, Neumann K, Schneider MD, Hill JA, Dobrev D, Maack C, Maier LS, Grone HJ, Katus HA, Olson EN, Backs J, Cardiac CaM Kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy, Circulation 130(15) (2014) 1262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gray CB, Heller Brown J, CaMKIIdelta subtypes: localization and function, Frontiers in pharmacology 5 (2014) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hunter T, Schulman H, CaMKII structure--an elegant design, Cell 123(5) (2005) 765–7. [DOI] [PubMed] [Google Scholar]
- [17].Gaertner TR, Kolodziej SJ, Wang D, Kobayashi R, Koomen JM, Stoops JK, Waxham MN, Comparative analyses of the three-dimensional structures and enzymatic properties of alpha, beta, gamma and delta isoforms of Ca2+-calmodulin-dependent protein kinase II, The Journal of biological chemistry 279(13) (2004) 12484–94. [DOI] [PubMed] [Google Scholar]
- [18].Erickson JR, Joiner M.-l.A., Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME, A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation, Cell 133(3) (2008) 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM, S-Nitrosylation Induces Both Autonomous Activation and Inhibition of Calcium/Calmodulin-dependent Protein Kinase II delta, The Journal of biological chemistry 290(42) (2015) 25646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM, Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation, Nature 502(7471) (2013) 372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saucerman JJ, Bers DM, Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes, Biophys J 95(10) (2008) 4597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saucerman JJ, Bers DM, Calmodulin binding proteins provide domains of local Ca2+ signaling in cardiac myocytes, J Mol Cell Cardiol 52(2) (2012) 312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Song Q, Saucerman JJ, Bossuyt J, Bers DM, Differential integration of Ca2+-calmodulin signal in intact ventricular myocytes at low and high affinity Ca2+-calmodulin targets, The Journal of biological chemistry 283(46) (2008) 31531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A, Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species, Circulation research 105(12) (2009) 120412. [DOI] [PubMed] [Google Scholar]
- [25].Fischer TH, Eiringhaus J, Dybkova N, Forster A, Herting J, Kleinwachter A, Ljubojevic S, Schmitto JD, Streckfuss-Bomeke K, Renner A, Gummert J, Hasenfuss G, Maier LS, Sossalla S, Ca(2+) /calmodulin-dependent protein kinase II equally induces sarcoplasmic reticulum Ca(2+) leak in human ischaemic and dilated cardiomyopathy, European journal of heart failure 16(12) (2014) 1292–300. [DOI] [PubMed] [Google Scholar]
- [26].Singh P, Salih M, Tuana BS, Alpha-kinase anchoring protein alphaKAP interacts with SERCA2A to spatially position Ca2+/calmodulin-dependent protein kinase II and modulate phospholamban phosphorylation, The Journal of biological chemistry 284(41) (2009) 28212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bayer KU, Harbers K, Schulman H, alphaKAP is an anchoring protein for a novel CaM kinase II isoform in skeletal muscle, EMBO J 17(19) (1998) 5598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS, CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation, The Journal of cell biology 171(3) (2005) 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ, A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice, The Journal of clinical investigation 120(10) (2010) 3508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN, Histone deacetylase 5 acquires calcium/calmodulin-dependent kinase II responsiveness by oligomerization with histone deacetylase 4, Mol Cell Biol 28(10) (2008) 3437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH, Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes, Circulation research 109(12) (2011) 1354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lemieux M, Labrecque S, Tardif C, Labrie-Dion E, Lebel E, De Koninck P, Translocation of CaMKII to dendritic microtubules supports the plasticity of local synapses, The Journal of cell biology 198(6) (2012) 1055–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lu HE, MacGillavry HD, Frost NA, Blanpied TA, Multiple spatial and kinetic subpopulations of CaMKII in spines and dendrites as resolved by single-molecule tracking PALM, The Journal of neuroscience : the official journal of the Society for Neuroscience 34(22) (2014) 7600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mora RJ, Roberts RW, Arnold DB, Recombinant probes reveal dynamic localization of CaMKIIalpha within somata of cortical neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience 33(36) (2013) 14579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shen K, Meyer T, Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation, Science (New York, N.Y.) 284(5411) (1999) 162–6. [DOI] [PubMed] [Google Scholar]
- [36].Tsui J, Inagaki M, Schulman H, Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation, The Journal of biological chemistry 280(10) (2005) 9210–6. [DOI] [PubMed] [Google Scholar]
- [37].Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J, Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme, Cell 123(5) (2005) 849–60. [DOI] [PubMed] [Google Scholar]
- [38].Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM, Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure, Circulation research 85(11) (1999) 1009–19. [DOI] [PubMed] [Google Scholar]
- [39].Hegyi B, Bossuyt J, Ginsburg KS, Mendoza LM, Talken L, Ferrier WT, Pogwizd SM, Izu LT, Chen-Izu Y, Bers DM, Altered Repolarization Reserve in Failing Rabbit Ventricular Myocytes: Calcium and beta-Adrenergic Effects on Delayed- and Inward-Rectifier Potassium Currents, Circulation. Arrhythmia and electrophysiology 11(2) (2018) e005852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tillberg PW, Chen F, Piatkevich KD, Zhao Y, Yu CC, English BP, Gao L, Martorell A, Suk HJ, Yoshida F, DeGennaro EM, Roossien DH, Gong G, Seneviratne U, Tannenbaum SR, Desimone R, Cai D, Boyden ES, Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies, Nature biotechnology 34(9) (2016) 987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huke S, Bers DM, Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII, J Mol Cell Cardiol 42(3) (2007) 590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bolte S, Cordelieres FP, A guided tour into subcellular colocalization analysis in light microscopy, Journal of microscopy 224(Pt 3) (2006) 213–32. [DOI] [PubMed] [Google Scholar]
- [43].Hoelz A, Nairn AC, Kuriyan J, Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II, Mol Cell 11(5) (2003) 1241–51. [DOI] [PubMed] [Google Scholar]
- [44].Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK, Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains, The Journal of biological chemistry 275(19) (2000) 14354–9. [DOI] [PubMed] [Google Scholar]
- [45].Hink MA, Griep RA, Borst JW, van Hoek A, Eppink MH, Schots A, Visser AJ, Structural dynamics of green fluorescent protein alone and fused with a single chain Fv protein, The Journal of biological chemistry 275(23) (2000) 17556–60. [DOI] [PubMed] [Google Scholar]
- [46].Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM, Fluorescence resonance energy transferbased sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes, Circulation research 109(7) (2011) 729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H, Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity, Biophys J 79(6) (2000) 3226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bhattacharyya M, Stratton MM, Going CC, McSpadden ED, Huang Y, Susa AC, Elleman A, Cao YM, Pappireddi N, Burkhardt P, Gee CL, Barros T, Schulman H, Williams ER, Kuriyan J, Molecular mechanism of activation-triggered subunit exchange in Ca(2+)/calmodulin-dependent protein kinase II, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, Groves JT, Kuriyan J, Correction: Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity, Elife 3 (2014) e02490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA, Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II, The Journal of biological chemistry 280(16) (2005) 15912–20. [DOI] [PubMed] [Google Scholar]
- [51].Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM, Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling, The Journal of clinical investigation 116(3) (2006) 675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Griffith LC, Lu CS, Sun XX, CaMKII, an enzyme on the move: regulation of temporospatial localization, Molecular interventions 3(7) (2003) 386–403. [DOI] [PubMed] [Google Scholar]
- [53].Lu CS, Hodge JJ, Mehren J, Sun XX, Griffith LC, Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation, Neuron 40(6) (2003) 1185–97. [DOI] [PubMed] [Google Scholar]
- [54].Heist EK, Srinivasan M, Schulman H, Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting, The Journal of biological chemistry 273(31) (1998) 19763–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.