Abstract
Background & Aims:
The combined effects of healthy lifestyle factors on colorectal cancer (CRC) risk are unclear. We aimed to develop a healthy lifestyle score, to investigate the joint effects of modifiable lifestyle factors on reduction of CRC risk and determine whether associations differ with genetic risk.
Methods:
We collected data from a large population-based case–control study in Germany and used multiple logistic regression analyses to examine associations between the healthy lifestyle score (derived from 5 modifiable lifestyle factors: smoking, alcohol consumption, diet, physical activity, and body fatness) and CRC risk. We created a genetic risk score, based on 53 risk variants, to investigate the association of the healthy lifestyle score and risk of CRC, stratified by genetic risk.
Results:
We included 4092 patients with CRC and 3032 individuals without CRC (controls) in our analysis. In adjusted models, compared to participants with 0 or 1 healthy lifestyle factor, participants with 2 (OR, 0.85; 95% CI, 0.67–1.06), 3 (OR, 0.62; 95% CI, 0.50–0.77), 4 (OR, 0.53; 95% CI, 0.42–0.66), or 5 (OR, 0.33; 95% CI, 0.26–0.43) healthy lifestyle factors had increasingly lower risks of CRC (P trend <.0001). We found no differences among subgroups stratified by genetic risk score, history of colonoscopy, or family history of CRC. Overall, 45% of CRC cases (95% CI, 34%–53%) could be attributed to non-adherence to all 5 healthy lifestyle behaviors.
Conclusions:
In a large population-based case–control study, we identified a combination of lifestyle factors that appears to reduce risk of CRC, regardless of the patient’s genetic profile. These results reinforce the importance of primary prevention of CRC.
Keywords: DACHS, colon cancer, chemoprevention, food
Introduction
Despite significant progress in our understanding of prevention and early detection, colorectal cancer (CRC) remains the third most common cancer and the fourth most common cause of cancer related death worldwide 1. There is large geographical variation in CRC incidence rates with the highest incidence being reported in the “western” world (i.e. Australia and New Zealand followed by Europe and North America)1. A large body of evidence has established that many “western” lifestyle factors such as smoking2, alcohol consumption3, diet4–8, physical inactivity9, and body fatness10 are risk factors for CRC. However, since many of these lifestyle behaviors often coexist, investigating the combined impact of these lifestyle factors on CRC risk is highly relevant. Yet, little evidence currently exists.
To date, few studies have investigated the combined impact of healthy lifestyle behaviors on CRC risk11−16. Although the studies to date generally reported inverse associations between combined healthy lifestyle factors and CRC risk, the comparability of the studies is limited. The components included in the “lifestyle score” varied between studies and most used different methods for derivation of the score. Also, most of the previous studies did not consider prior use of colonoscopy, which strongly reduces CRC risk, and has been associated with a generally healthier lifestyle17. Furthermore, in the past few years, genomewide association analyses have identified more than 50 independent loci associated with the risk of CRC18. Although these genetic variants may only represent a small proportion of the heritable risk component of CRC, the combination of these single nucleotide polymorphisms (SNPs) to a genetic risk score could be relevant for risk stratification. A recent analysis found that a genetic risk score in the highest decile was associated with a 3 fold increase in CRC risk, compared to the lowest decile18. However, thus far it remains unclear whether healthy lifestyle still plays a role in subjects with increased genetic risk, and no study to date has investigated CRC risk incorporating both a healthy lifestyle score and a genetic risk score.
In this study, to further investigate CRC risk and lifestyle behaviors, we created a healthy lifestyle score based on recommendations of five potentially modifiable lifestyle factors – smoking, alcohol consumption, diet, physical activity, and body fatness. We examined associations between the healthy lifestyle score and risk of CRC including adjustment for other important lifestyle factors such as history of colonoscopy. Furthermore, we aimed to estimate the proportion of CRC cases that is attributable to the individual lifestyle factors as well as lack of adherence to all five healthy lifestyle behaviors. Finally, in a novel stratified analysis we investigated whether the association of the healthy lifestyle score and risk of CRC differed according to a genetic risk score.
Methods
Study design and study population
The DACHS study (Darmkrebs: Chancen der Verhütung durch Screening) is an ongoing populationbased case-control study conducted in southwest Germany since 2003. This analysis includes patients and controls recruited until 2014. Details of the DACHS study have been reported elsewhere19, 20. Briefly, patients with a histologically confirmed, first diagnosis of CRC (International Classification of Diseases, 10th Revision [ICD-10] codes C18-C20) are eligible to participate if they are at least 30 years of age (no upper age limit), can speak German, and are physically able to participate in an interview of about one hour. All 22 hospitals in the study area offering first line treatment to patients with CRC are involved in recruitment. Approximately 50% of all eligible patients in the study area are recruited. Incomplete recruitment of patients is largely due to lack of time among the clinicians in charge of notifying the study center in the routine setting. Community-based controls were randomly selected from population registries using frequency matching with respect to age, sex and county of residence (participation rate: 51%). The DACHS study was approved by the ethics committees of the University of Heidelberg and the state medical boards of Baden-Wuerttemberg and Rhineland-Palatinate. Written informed consent was obtained from each participant before taking part.
Data collection
Patients were informed about the study by their physicians, usually a few days after surgery. Patients participated in an interview with trained interviewers who collected information on patients’ sociodemographic, medical and lifestyle history using a standardized questionnaire. Patients who could not be recruited during their hospital stay were contacted by mail shortly after discharge by clinicians or clinical cancer registries. The median time between CRC diagnosis and interview was 24 days. Controls were contacted by the study center through mail and follow-up calls, and interviews were scheduled at their homes (participation rate: 51%). A minority of control participants not willing to participate in a personal interview provided some key information in a self-administered short questionnaire. However, as this questionnaire did not include a food frequency questionnaire (FFQ), these participants were excluded from this current analysis. In addition, we collected discharge letters and pathology reports for all cases.
Assessment of lifestyle factors
Extensive information on smoking history was collected during interviews. Participants provided information on their current as well as prior smoking behavior and if applicable the year in which they stopped smoking. Participants were classified as nonsmokers, if they had never smoked regularly or were former smokers and smoked < 30 pack years; and as smokers if they were smoking at the time of diagnosis or recruitment to the study or were former smokers and smoked ≥30 pack years (classification of former smokers based on findings from Tsoi et al 21). Further details have been provided previously22.
Information on alcohol consumption was assessed, where participants were asked how many drinks (beer [0.33L], wine [0.25L] or liquor [0.02L]) they had consumed on average per week at ages 20, 30, 40, 50, 60, 70, and 80, and in the last 12 months. Ethanol content of the beverage types (assuming 4, 8.6, and 33g of pure ethanol in 100ml of beer, wine or liquor, respectively) was derived from food composition tables and the average lifetime alcohol consumption was calculated based on self-recalled alcohol consumption at ages 20, 30, 40, 50, 60, 70, and 80 years. The mean daily lifetime amount of ethanol was calculated by dividing the total weekly ethanol amount by seven days. Further details have been provided previously23.
Participants were asked about the hours per week they spent with different physical activities over the past decades (i.e. hard exhausting work, light work spent walking or standing, walking, cycling, or doing sports). Based on task-specific metabolic equivalent of task (MET) values (3.3 MET-h/week for each hour walking, 6 MET-h/week for each hour cycling and 8 MET-h/week for each hour of sports), average recent non-occupational physical activity (walking, cycling or doing sports only) was calculated for each participant. Occupational activity (hard exhausting work, light work spent walking or standing) was not included in our physical activity variable given that most study participants were no longer engaged in occupational activity. Reported information from the most recent decade preceding the participants current age was used to derive the activity specific recent average MET-h/week (e.g. for patients aged 60–69, information from age 60 was used). Further details on the assessment of physical activity in the DACHS study have been reported previously24.
Dietary information was obtained by a 23-item FFQ, and consumption was assessed in 6 categories of predefined responses ranging from “never” to “multiple times per day”. Participants were asked to report their average frequency of consumption over the previous 12 months (controls) or before CRC diagnosis (cases). A diet quality score was created based on the availability of data from the FFQ and the updated evidence from the 2017 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) diet recommendations for prevention of CRC 25. Six main food groups (red and processed meat [as a negative component], fish, wholegrains, dairy foods, fruits, and vegetables [positive components]) were included in the diet quality score. Points were assigned depending on the frequency of consumption of the food groups and then summed up. The diet quality score ranged from 0 (lowest) to 50 (highest). Further details on the derivation of the diet quality score are provided in Supplementary Table 1. If information on any of the dietary items used to build the diet quality score were missing, the participants were excluded. Participants with a diet quality score in the highest 40% were considered to have a healthy diet. Further details on the assessment of diet in the DACHS study have been published previously26, 27.
Participants reported their current weight and height and their past weight at each decade from age 20 to 80 years. Body mass index (BMI; kg/m2) was calculated from recent weight and height (5–14 years earlier). Participants with a BMI <18.5kg/m2 were excluded. Further details on the assessment of BMI in the DACHS study have been published previously28.
Derivation of the healthy lifestyle score
A healthy lifestyle score was created by dichotomizing each lifestyle factor based on a priori knowledge of the risk factors for CRC 2–10, 21, 29, 30 (Supplementary Table 2). Participants were assigned one point for the following low risk lifestyle behaviors: nonsmoking (never smoker & former smoker (<30 pack years)), moderate alcohol consumption (adherent to WCRF/AICR recommendations: ≤24g/day men, ≤12g/day women29), a healthy diet (diet quality score ≥34: highest 40%), being physically active (meeting the WHO Global Recommendations on Physical Activity for Health: at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate and vigorous intensity physical activity [at least ~500 MET minutes] 31) and having a healthy weight (BMI >18.5 – <25kg/m2). The points for the five lifestyle factors were summed to obtain the healthy lifestyle score which ranged from 0 (least healthy) to 5 (most healthy).
Derivation of the genetic risk score
To investigate whether the association of the healthy lifestyle score and risk of CRC differed according to genetic risk, a genetic risk score was built based on 53 risk variants identified in previous genome-wide association studies. The score was calculated as the sum of risk alleles of the respective variants (0, 1 or 2 copies per risk allele). Full details on the derivation of the genetic risk score have been published recently18.
Multiple imputation of missing data
Missing data for school education, family history of CRC, history of colonoscopy, participation in a health check-up and use of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin (all less than 2% missing) was accounted for by multiple imputation using the Markov-Chain Monte Carlo method (N=10 imputed datasets, SAS procedure PROC MI) 32. Imputed values of categorical variables were rounded to the closest integer.
Statistical analysis
The distribution of the demographic and lifestyle characteristics of the study population according to case/control status was evaluated in descriptive analyses using the Pearson chi-square test or t-test. Unconditional multiple logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association of the individual as well as the combined lifestyle factors with risk of CRC. We calculated odds ratios as proxy indicators of the relative risk in this study. The basic model included adjustment for age and sex. The multivariable models included adjustment for age, sex, school education, family history of CRC, history of colonoscopy, participation in a health check-up and ever regular use of NSAIDs including aspirin. In analyses of the individual lifestyle factors, the multivariable models were additionally mutually adjusted for the other lifestyle factors and participants with 0 points (least healthy) were used as the reference group. In combined analyses, the healthy lifestyle score was modelled as a categorical variable (0 to 5 points) and as an ordinal variable (per 1 point increase in score; linear trend), and participants with no or only one healthy lifestyle factor were used as the reference group since only a small percentage of participants had a score of 0 points. Combined analyses were performed separately for sex and for cancer site (colon/rectum).
To investigate potential influences on the association between the combined lifestyle factors and risk of CRC, we performed analyses stratified by genetic risk score (<median/≥median), history of colonoscopy (yes/no), regular use of NSAIDs (yes/no), family history of CRC (yes/no), age (<70 /≥70 years) and according to cancer stage (I to IV). Interaction was tested by including a cross-product term along with the main effect terms in the models. In addition, adjusted population attributable fractions (PAFs) and 95% CIs were calculated to estimate the proportion of CRC cases that is attributable to the individual lifestyle factors as well as lack of adherence to the five healthy lifestyle factors. Estimation of PAFs was based on the method by Bruzzi et al33 using a formula proposed by Miettinen34. Bootstrapping (n=1000) was used to estimate 95% confidence intervals35.
The five healthy lifestyle factors were combined in all possible combinations (32 combinations in total); and for those combinations that were prevalent in more than 5% of the controls, we examined the association with risk of CRC. Odds ratios and 95% CI’s were calculated using participants who had no or only one healthy lifestyle factor as the reference group. We performed sensitivity analyses using a different cut-off (≤ / > median) for physical activity since almost 90% of participants met the WHO recommendations on physical activity. In addition, in a sensitivity analysis, patients with MSI-high tumors were excluded, to rule out the possibility that the results were biased due to the inclusion of Lynch syndrome patients.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Statistical tests were two-sided, with an alpha level of 0.05.
Results
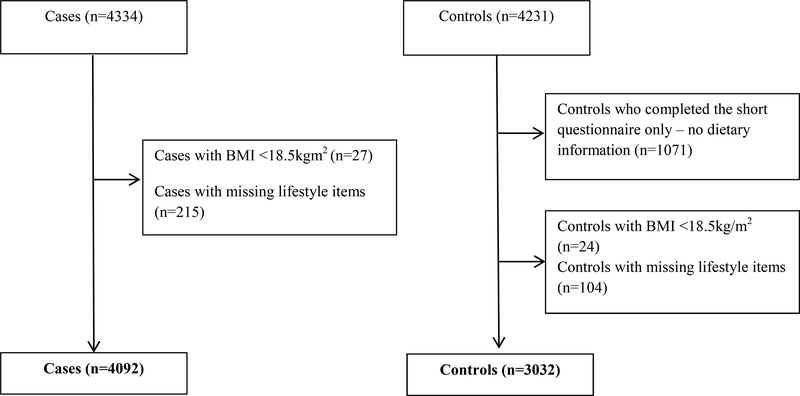
Overall, 4092 patients with CRC and 3032 control participants recruited in 2003–2014 were included in this analysis (Figure 1). The mean age of the cases and controls was 68.2 years and 60.8% of the participants were men (Table 1). The study population for the analyses on the genetic risk score was smaller than the overall study population because not all of the cases and controls have been genotyped as yet (N=4349, cases n=2235; controls, n=2114).
Figure 1.
Study participants
Table 1.
Baseline characteristics of participants by case and control status.
| Characteristics | Total | Controls | Cases | P value |
|---|---|---|---|---|
| N=7124 | N=3032 | N=4092 | ||
| Sex, n (%) | - | |||
| Female | 2792 (39.2) | 1186 (39.1) | 1606 (39.3) | |
| Male | 4332 (60.8) | 1846 (60.9) | 2486 (60.8) | |
| Age | - | |||
| Range | 32–99 | 33–99 | 32–96 | |
| Mean, (SD) | 68.2 (10.6) | 68.2 (10.4) | 68.2 (10.8) | |
| Education1, % | <0.0001 | |||
| <9 years | 4456 (62.7) | 1724 (56.9) | 2732 (66.9) | |
| 9–10 years | 1337 (18.8) | 643 (21.2) | 694 (17.0) | |
| >10 years | 1317 (18.5) | 660 (21.8) | 657 (16.1) | |
| Smoking status, % | ||||
| Current or former (≥30 pack years) | 1493 (21.0) | 546 (18.0) | 947 (23.1) | <0.0001 |
| Alcohol consumption, g/day, mean | ||||
| Women | 6.1 | 6.6 | 5.9 | 0.06 |
| Men | 23.7 | 21.9 | 25.1 | <0.0001 |
| Dietary quality score*, mean | 31.3 | 32.2 | 30.6 | <0.0001 |
| Leisure time physical activity, MET-h/week, mean | 42.5 | 46.1 | 39.9 | <0.0001 |
| BMI, kg/m2, mean | 26.8 | 26.3 | 27.2 | <0.0001 |
| 1st degree family history of CRC2, n (%) | ||||
| Yes | 927 (13.0) | 331 (10.9) | 596 (14.6) | <0.0001 |
| History of colonoscopy, n (%) | <0.0001 | |||
| Yes | 2675 (37.6) | 1693 (55.8) | 982 (24.0) | |
| History of colonoscopy in the preceding 10 years3, n (%) | <0.0001 | |||
| Yes | 2212 (31.1) | 1478 (48.8) | 734 (18.0) | |
| Participation in a health check up4, n (%) | <0.0001 | |||
| Yes | 6205 (87.6) | 2749 (91.2) | 3456 (84.9) | |
| NSAIDs5 n (%) | <0.0001 | |||
| Yes | 1967 (28.0) | 950 (31.7) | 1017 (25.3) | |
| Healthy lifestyle score | <0.0001 | |||
| 0 | 58 (0.81) | 12 (0.4) | 46 (1.1) | |
| 1 | 469 (6.6) | 149 (4.9) | 320 (7.8) | |
| 2 | 1502 (21.1) | 519 (17.1) | 983 (24.0) | |
| 3 | 2464 (34.6) | 1047 (34.5) | 1417 (34.6) | |
| 4 | 1922 (26.9) | 891 (29.4) | 1031 (25.2) | |
| 5 | 709 (9.9) | 414 (13.7) | 295 (7.2) | |
| Tumour location | ||||
| Colon | - | - | 2459 (60.1) | |
| Rectum | - | - | 1633 (39.9) | |
| Tumour stage6 | ||||
| I | - | - | 947 (23.2) | |
| II | - | - | 1257 (30.8) | |
| III | - | - | 1295 (31.8) | |
| IV | - | - | 577 (14.2) |
Data missing for 14 participants
Data missing for 6 participants
Data missing for 18 participants
Data missing for 38 participants
Data missing for 103 participants
Data missing for 16 cases
Diet quality score max 50 points
Abbreviations: MET, metabolic equivalent of task; BMI, body mass index; CRC, colorectal cancer; NSAIDs, non-steroidal antiinflammatory drug
Among the DACHS study population, 77% of cases and 82% of controls were non-smokers, 70% of cases and 73% of controls met the WCRF/AICR alcohol recommendations, 35% of cases and 46% of controls had a healthy diet quality, 84% of cases and 88% of controls met the physical activity recommendations, and 31% of cases and 39% of controls had a healthy BMI (Supplementary Table 2). Generally, among both cases and controls, females were more likely than males to meet the recommendations for all lifestyle factors except physical activity.
When comparing the baseline characteristics of the study participants, patients with CRC were more likely to have a lower level of education, to smoke, to have a higher BMI, were less likely to have had a previous colonoscopy in the preceding 10 years, were less likely to have participated in a health checkup and overall, had a lower healthy lifestyle score compared to control participants (Table 1).
Multivariable analyses revealed that each individual lifestyle factor was associated with a reduced risk of CRC: non-smoking (OR 0.82, 95% CI 0.72–0.94), recommended alcohol intake (OR 0.83, 95% CI 0.74–0.94), a healthy diet score (OR 0.70, 95% CI 0.63–0.78), recommended level of physical activity (OR 0.88, 95% CI 0.76–1.03) and a healthy BMI (OR 0.71, 95% CI 0.63–0.79) (Table 2). Combining lifestyle factors revealed that compared to participants with zero or one healthy lifestyle factor, participants with two, three, four or five healthy lifestyle factors showed increasingly lower risk of colorectal (p trend <0.0001), colon (p trend <0.0001) and rectal cancer (p trend <0.0001). Each additional healthy lifestyle factor (per 1 point increase in score) was associated with a 23% lower risk of CRC (OR 0.77, 95% CI 0.73–0.81), 23% lower risk of colon cancer (OR 0.77, 95% CI 0.73–0.81), and 23% lower risk of rectal cancer (OR 0.77, 95% CI 0.72–0.82) (Table 3). Overall, the estimated PAFs attributable to non-adherence to the healthy lifestyle factors were 4% for non-smoking, 5% for moderate alcohol intake, 19% for a healthy diet, 2% for physical activity, and 21% for a healthy weight. Together, 45% (35–53%) of CRC cases were attributable to non-adherence to all five of the healthy lifestyle behaviors (Table 4).
Table 2.
Association between the individual lifestyle factors and colorectal cancer in the DACHS study
| Lifestyle factor | Points | Description | Colorectal cancer |
||
|---|---|---|---|---|---|
| ncases(%)/ncontrols(%) | OR (95% CI)1 | OR (95% CI)2 | |||
| Smoking | 0 | Smoking: current smoker or former smoker (≥30 pack years) | 947 (23)/546 (18) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 | Non-smoking: never smoker or former smoker (<30 pack years) | 3145(77)/2486(82) | 0.72 (0.63–0.81) | 0.82 (0.72–0.94) | |
| Alcohol | 0 | Did not meet recommendations on alcoholic drinks 3 | 1227 (30)/810 (27) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 | Met recommendation on alcoholic drinks3 | 2865 (70)/2222(73) | 0.84 (0.76–0.94) | 0.83 (0.74–0.94) | |
| Diet | 0 | Unhealthy diet quality: diet score <344 | 2664(65)/1623(54) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 | Healthy diet quality: diet score ≥344 | 1428(35)/1409(47) | 0.61 (0.55–0.67) | 0.70 (0.63–0.78) | |
| Physical activity | 0 | Did not meet physical activity guidelines5 | 645 (16)/354(12) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 | Met physical activity guidelines5 | 3447 (84)/2678(88) | 0.70 (0.61–0.81) | 0.88 (0.76–1.03) | |
| BMI | 0 | Overweight or obese (BMI ≥ 25kg/m2) | 2841 (69)/1865 (62) | 1.00 (Ref.) | 1.00 (Ref.) |
| 1 | Healthy weight (18.5 < BMI < 25kg/m2) | 1251 (31)/1167 (38) | 0.69 (0.63–0.77) | 0.71 (0.63–0.79) | |
Model 1 adjusted for matching factors age and sex
Model 2 adjusted for matching factors age and sex; school education, family history of CRC, history of colonoscopy, participation in a health check-up, ever regular use of NSAIDs, and mutual adjustment for the other lifestyle factors
World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) (2007) Recommendation on alcoholic drinks: ≤24g/day men, 12g/day women
Diet score in the highest 40%
The WHO Global Recommendations on Physical Activity for Health (2010) recommend adults to engage in at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic physical activity throughout the week or an equivalent combination of moderate and vigorous intensity physical activity (At least ~500 MET minutes)
Abbreviations: CI: confidence intervals; OR: odds ratio; Ref.: Reference.
Table 3.
Association between the healthy lifestyle score and colorectal cancer in the DACHS study
| Lifestyle score | Colorectal cancer | Colon cancer | Rectal cancer | ||||
|---|---|---|---|---|---|---|---|
| ncases(%)/ncontrols(%) | OR (95% CI)1 | OR (95% CI)2 | ncases(%)/ncontrols(%) | OR (95% CI)2 | ncases(%)/ncontrols(%) | OR (95% CI)2 | |
| All participants | |||||||
| 0 or 1 | 366(9)/161(5) | 1.00 (Ref.) | 1.00 (Ref.) | 213(9)/161(5) | 1.00 (Ref.) | 153 (9)/161(5) | 1.00 (Ref.) |
| 2 | 983(24)/519(17) | 0.81(0.65–1.00) | 0.85 (0.67–1.06) | 560(23)/519(17) | 0.80 (0.62–1.03) | 423(26)/519(17) | 0.93 (0.70–1.24) |
| 3 | 1417(35)/1047(35) | 0.56 (0.46–0.69) | 0.62 (0.50–0.77) | 865(35)/1047(35) | 0.60 (0.47–0.76) | 552(34)/1047(35) | 0.64 (0.49–0.84) |
| 4 | 1031(25)/891(29) | 0.47(0.38–0.58) | 0.53 (0.42–0.66) | 643(26)/891(29) | 0.52 (0.40–0.66) | 388(24)/891(29) | 0.53 (0.40–0.70) |
| 5 | 295(7)/414(14) | 0.28 (0.22–0.36) | 0.33 (0.26–0.43) | 178(7)/414(14) | 0.31 (0.23–0.41) | 117(7)/414(14) | 0.37 (0.26–0.51) |
| Per 1 point increase in score | 0.74 (0.71–0.78) | 0.77 (0.73–0.81) | 0.77 (0.73–0.81) | 0.77 (0.72–0.82) | |||
| P trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Men | |||||||
| 0 or 1 | 330(13)/140(8) | 1.00 (Ref.) | 1.00 (Ref.) | 187(13)/140(8) | 1.00 (Ref.) | 143(13)/140(8) | 1.00 (Ref.) |
| 2 | 717(29)/398(21) | 0.77 (0.61–0.96) | 0.82 (0.64–1.06) | 390(28)/398(21) | 0.78(0.59–1.02) | 327(30)/398(21) | 0.91 (0.67–1.23) |
| 3 | 846(34)/686(37) | 0.52 (0.42–0.65) | 0.59 (047–0.75) | 489(35)/686(37) | 0.58 (0.45–0.76) | 357(33)/686(37) | 0.61 (0.46–0.82) |
| 4 | 492(20)/460(25) | 0.46 (0.36–0.58) | 0.54 (0.42–0.70) | 285(20)/460(25) | 0.52 (0.39–0.69) | 207(19)/460(25) | 0.56 (0.41–0.77) |
| 5 | 101(4)/162(9) | 0.27 (0.19–0.36) | 0.33 (0.23–0.47) | 59(4)/162(9) | 0.32 (0.22–0.48) | 42(4)/162(9) | 0.33 (0.21–0.51) |
| Per 1 point increase in score | 0.74 (0.70–0.79) | 0.78 (0.73–0.83) | 0.78 (0.73–0.84) | 0.78 (0.72–0.84) | |||
| P trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Women | |||||||
| 0 or 1 | 36 (2)/21(2) | 1.00 (Ref.) | 1.00 (Ref.) | 26(2)/21(2) | 1.00 (Ref.) | 10 (2)/21(2) | 1.00 (Ref.) |
| 2 | 266(17)/121(10) | 1.27 (0.71–2.27) | 1.04 (0.56–1.91) | 170(16)/121(10) | 0.93 (0.49–1.77) | 96 (17)/121(10) | 1.26 (0.52–3.05) |
| 3 | 571(35)/361(31) | 0.91 (0.52–1.59) | 0.77 (0.43–1.37) | 376(36)/361(31) | 0.67 (0.36–1.25) | 195(35)/361(31) | 0.88 (0.37–2.07) |
| 4 | 539(34)/431(36) | 0.72 (0.42–1.26) | 0.61 (0.34–1.10) | 358(34)/431(36) | 0.56 (0.30–1.03) | 181(32)/431(36) | 0.64 (0.27–1.50) |
| 5 | 194(12)/252(21) | 0.44 (0.25–0.79) | 0.40 (0.22–0.73) | 119(11)/252(21) | 0.33 (0.18–0.63) | 75(13)/252(21) | 0.49 (0.20–1.18) |
| Per 1 point increase in score | 0.74 (0.69–0.80) | 0.75 (0.69–0.82) | 0.74 (0.68–0.82) | 0.75 (0.67–0.84) | |||
| P trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
Model 1 adjusted for matching factors age and sex
Model 2 adjusted for matching factors age and sex; and the factors school education, family history of CRC, history of colonoscopy, participation in a health check-up, ever regular use of NSAIDs
Abbreviations: CI: confidence intervals; OR: odds ratio; Ref.: Reference
Table 4.
Population attributable fractions (PAFs)1 according to individual lifestyle factors and the combined healthy lifestyle score
| Lifestyle factor | Colorectal cancer |
|
|---|---|---|
| Proportion of cases with risk factor | PAF2 (95% CI) | |
| Smoking | 947 (23%) | 4% (0%−7%) |
| Alcohol | 1227 (30%) | 5% (2%−8%) |
| Diet | 2664 (65%) | 19% (14%−24%) |
| Physical activity | 645 (16%) | 2% (0%−4%) |
| BMI ≥25kg/m2 | 2841 (69%) | 21% (15%−26%) |
| Healthy lifestyle score <5 points | 3797 (93%) | 45% (34%−53%) |
Estimation of adjusted PAFs was based on the method by Bruzzi et al by using the formula of Miettinen, PAF= Pdis(E) (RR-1)/(RR), where PAF represents the adjusted PAF, Pdis(E) is the proportion of the exposed among the diseased (i.e. among those with colorectal cancer), and RR is the relative risk estimated from the odds ratio.
PAF adjusted for matching factors age and sex; and for the factors school education, family history of CRC, history of colonoscopy, participation in a health check-up, ever regular use of NSAIDs, and mutual adjustment for the other lifestyle factors Abbreviations: PAF: Population attributable fraction.
Among men, a higher lifestyle score was significantly associated with reduced risk of colorectal, colon and rectal cancer (Table 3). Among women, a higher lifestyle score was associated with reduced risk of colorectal (OR 0.40, 95% CI 0.22–0.73), colon (OR 0.33, 95% CI 0.18–0.63) and rectal cancer (OR 0.49, 95% CI 0.20–1.18). However, the differences between men and women regarding risk of colorectal (pheterogeneity=0.88), colon (pheterogeneity=0.93) and rectal cancer (pheterogeneity=0.77) did not seem to be meaningful.
In stratified analyses we assessed whether the association of the healthy lifestyle score and CRC risk differed according to a recently published genetic risk score (Table 5). We found that participants with more healthy lifestyle factors had a lower risk of CRC irrespective of the genetic risk score (< median genetic risk score: OR 0.34, 95% CI 0.20–0.57; ≥ median genetic risk score: OR 0.37, 95% CI 0.24–0.58 respectively). Additionally, we did not observe any differences in associations between the healthy lifestyle score and CRC risk according to history of colonoscopy, regular use of NSAIDs, family history of CRC, age, or cancer stage (Supplementary Table 3).
Table 5.
Association between the healthy lifestyle score and colorectal cancer in the DACHS study by genetic risk score
| Lifestyle score | Colorectal cancer | Pinteraction | ||
|---|---|---|---|---|
| ncases(%)/ncontrols(%) | OR (95% CI)1 | |||
| Genetic risk score | ||||
| <median | 0 or 1 | 74 (9)/62(6) | 1.00 (Ref.) | |
| 2 | 190(23)/167(16) | 0.88 (0.57–1.37) | ||
| 3 | 298(36)/376 (36) | 0.66 (0.44–0.99) | ||
| 4 | 218(26)/311(29) | 0.60 (0.39–0.92) | ||
| 5 | 54(6)/141(13) | 0.34 (0.20–0.57) | ||
| Per 1 point increase in score | 0.79 (0.71–0.87) | |||
| P trend | <0.0001 | 0.79 | ||
| ≥median | 0 or 1 | 113(8)/59(6) | 1.00 (Ref.) | |
| 2 | 349(25)/190(18) | 0.98 (0.66–1.46) | ||
| 3 | 468(33)/348(33) | 0.74 (0.50–1.07) | ||
| 4 | 352(25)/295(28) | 0.64 (0.43–0.94) | ||
| 5 | 119(8)/165(16) | 0.37 (0.24–0.58) | ||
| Per 1 point increase in score | 0.78 (0.72–0.85) | |||
| P trend | <0.0001 | |||
Adjusted for matching factors age and sex; and for the factors school education, family history of CRC, history of colonoscopy, participation in a health check-up, ever regular use of NSAIDs Abbreviations: CI: confidence intervals; OR: odds ratio; Ref.: Reference
We conducted analyses according to different combinations of two, three and four healthy lifestyle factors prevalent in at least 5% of our control population compared to zero or one factors (Supplementary Table 4). Although none of the observed associations were as protective as the combination of five factors, the risk of CRC was lower for some combinations. The three factor combination of non-smoking and moderate alcohol consumption and physical activity was associated with a lower risk of CRC (OR 0.69, 95% CI 0.55–0.87), and the combination of non-smoking, a healthy diet and physical activity, was associated with an even lower risk (OR 0.51, 95% CI 0.37–0.69). Among the four factor combinations, the combination of non-smoking, moderate alcohol consumption, physical activity and a healthy weight, was associated with a lower risk of CRC (OR 0.53, 95% CI 0.41–0.69) and the combination of nonsmoking, moderate alcohol consumption, a healthy diet and physical activity was similarly protective (OR 0.52, 95% CI 0.41–0.66).
In sensitivity analyses, the results did not markedly change after including a different cut off for physical activity (≤ / > median) in the healthy lifestyle score (results not shown) or in sensitivity analyses excluding all patients with MSI-high tumors in a subset of patients with available data on microsatellite instability status (results not shown).
Discussion
Using data from a large epidemiological study, we found that overall, almost half (45%) of all CRC cases could potentially be prevented through healthy lifestyle modifications. We have shown that a healthy lifestyle score including five potentially modifiable lifestyle factors (non-smoking, moderate alcohol consumption, a healthy diet, physical activity, and a healthy weight) was inversely associated with CRC risk. Interestingly, this strong inverse association remained present for all subgroup analyses including stratification by genetic risk score, history of colonoscopy and by family history of CRC. These results provide evidence for the importance of a healthy lifestyle in preventing CRC and reinforce the enormous potential of primary prevention.
Our study, the largest to date, adds to previously reported studies on lifestyle factors and risk of CRC and is the first to incorporate information on a genetic risk score. To our knowledge, a limited number of studies have investigated associations between a combination of healthy lifestyle factors and risk of CRC11–16, 36–38. Although the studies generally reported inverse relations between healthy lifestyle factors and CRC risk, the effect sizes varied, and most of the studies did not have information available on history of colonoscopy. Moreover, the comparability of the studies is limited, as the studies used different lifestyle factors and different assessments, different definitions of adherence, as well as different methodological approaches. Two European prospective cohort studies using similar methodological approaches as ours, reported inverse associations between healthy lifestyle factors (healthy weight, physical activity, non-smoking, moderate alcohol intake and a healthy diet) and CRC risk11, 12. A Danish study found that 23% (9–37%) of CRC cases could have been prevented if all participants followed the five healthy lifestyle recommendations12 and similarly, results from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, found that 16% (7–24%) of new CRC cases were attributable to non-adherence to the same five healthy lifestyle factors11. Two Asian studies, one prospective cohort16 and one retrospective case-control study13, reported protective effects for a healthy lifestyle, but in addition to healthy lifestyle factors, both studies included sleep in their score. Two prospective cohort studies from the US also examined the combination of lifestyle factors in relation to CRC risk14, 15. However, there was again heterogeneity in the number and definitions of the healthy lifestyle factors addressed. Using data from the Health Professionals Follow up Study, one study reported that if all men followed an unhealthy lifestyle (obesity, physical inactivity, alcohol consumption, early adulthood cigarette smoking, red meat consumption, and low intake of folic acid from supplements) compared to men in the low risk score group, the PARs ranged from 39% (23%−58%) to 55% (27%80%)14. In the Nurse’s Health Study, women with an unhealthy lifestyle (smoking, high BMI, low physical activity, daily consumption of red or processed meat, not participating in CRC screening, and consumed low daily amounts of folate) had almost a 4-fold higher cumulative risk of colon cancer by age 70 years compared to women who had a healthy diet and lifestyle15. In recent years, a number of other studies have also investigated CRC risk according to adherence to the WCRF/AICR guidelines. A recent case-control study from Spain investigating adherence to the WCRF/AICR recommendations (body fatness, physical activity, foods and drinks that promote weight gain, plant foods, animal foods and alcoholic drinks) and CRC risk, found a 46% lower risk of CRC for individuals in the highest category compared to individuals with low adherence to the recommendations36. Similar findings were also reported in the EPIC study investigating associations between a WCRF/AICR score and CRC risk37. In contrast, the Framingham Offspring Cohort found no association between adherence to the WCRF/AICR guidelines and CRC risk (HR 0.87, 95% CI 0.68–1.12), but the number of included CRC patients was very small (n=63)38.
In line with our findings, in the most recent updated report on diet, nutrition, physical activity and CRC from the WCRF/AICR (2017)25, it was estimated that 1 of 2 cases of CRC in the US could be prevented through diet and lifestyle such as eating healthy, being physically active and maintaining a healthy weight. In our study, out of the five lifestyle factors, healthy diet and low body fatness were those components of the lifestyle score that showed substantially higher PAFs for CRC. However, despite this, when examining the different combinations of factors, none of the combinations were as protective as the combination of five factors. Nevertheless, the compelling results from our study together with previous findings provide strong support that a large proportion of CRC cases can be prevented though lifestyle modification. However, when interpreting the PAFs from our study, it should be taken into account that the PAF in reality could be even higher. It is possible that due to the simplicity of our healthy lifestyle score, the true effect of the observed associations is underestimated. Moreover, given that our score did not include all lifestyle behaviors that could influence CRC risk, such as NSAID use 39, hormone replacement therapy40 or specific dietary components (e.g. vitamin D, dietary fiber, calcium)4–6, the percentage of CRC cases that could be prevented through diet and lifestyle modification may be much higher. Also, interestingly, we found strong inverse associations between a healthy lifestyle score and CRC risk irrespective of prevalent genetic risk variants. Although it has been reported previously among participants from the DACHS study that a higher genetic risk score was associated with increasing risk of CRC18, our results provide evidence that lifestyle factors may reduce risk regardless of the patient’s genetic risk profile. Our results provide encouraging support of current diet and lifestyle recommendations for CRC prevention which emphasize a healthy diet and lifestyle for everyone. From a public health perspective, these results should be used to reinforce the importance of primary prevention to policymakers, as well as convince individuals of the importance of following lifestyle recommendations. Furthermore, these five lifestyle factors that are important for CRC prevention are also important for overall health and prevention of chronic diseases such as cardiovascular disease or diabetes, thereby highlighting the benefit of a healthy lifestyle in disease prevention. Clinical research should aim to further investigate other possibilities of how individuals could better implement diet and lifestyle recommendations.
There are several strengths of our study. Firstly, this is a large population based study with the largest number of CRC cases to date, to investigate the association between healthy lifestyle factors and CRC risk. Other strengths include the comprehensive collection of medical, dietary, and lifestyle information during personal interviews which enabled us to incorporate the most prevalent lifestyle factors convincingly linked to CRC. Previous studies investigating adherence to the WCRF/AICR guidelines did not include all relevant lifestyle factors in the score because they were not part of the WCRF/AICR recommendations, i.e. smoking. Also, we could incorporate information on history of colonoscopy, as a confounder as well as in stratified analyses, which was not available in the majority of the previous studies. Our study for the first time also incorporated information on a genetic risk score. There are also several potential limitations of our study which deserve careful consideration. First, we cannot rule out the possibility of selection bias, in particular in the recruitment of controls. Control participants reported overall healthier lifestyles compared to patients with CRC and therefore may have been more health conscious which would have resulted in overestimation of effects and PAFs. However, we were able to adjust for some “proxy” measures of health consciousness in our study such as education, history of colonoscopy and participation in a health check-up. Second, ascertainment of lifestyle factors in the DACHS study was based on self-reported information; therefore we cannot rule out possible recall bias or misclassification of exposures, potentially leading to an underestimation of the effects. Third, since there is currently no standard or validated dietary scoring system for CRC, we used a simplified diet quality score to rank subjects on their intake of healthy foods, which may not sufficiently account for the complexity of dietary intake. Nevertheless, in sensitivity analyses using alternative methods to construct the diet quality score, the results did not substantially change. Fourth, neither the FFQ nor the physical activity questionnaire used in this study was validated. However, since a validated questionnaire specific for measuring physical activity levels in a predominately elderly study population such as ours is lacking, we included comprehensive assessment of physical activity levels at various phases of life in the standardized questionnaire. Fifth, although physical activity levels were quite high in our study (mean: 42.5 MET hours/week), sensitivity analyses showed that even with a lower cutoff to fulfil the physical activity recommendations, our results would not change much. Finally, although many potential confounders were considered in multivariable analyses, residual confounding cannot be ruled out.
In summary, we found that diet and lifestyle have a major role in CRC. Our data show that at least 45% of CRC cases could be preventable by adopting healthy lifestyle behaviors regardless of the patient’s genetic profile. Although it is unrealistic that a population will fully adhere to all five healthy lifestyle recommendations, these findings suggest that primary prevention of CRC should remain a priority and future work should focus on new possibilities to encourage individuals to implement healthy lifestyle behaviors.
Supplementary Material
Acknowledgements
The authors thank Ute Handte-Daub, Ansgar Brandhorst and Petra Bächer for their excellent technical assistance. The authors thank the study participants and the interviewers who collected the data. The authors also thank the following hospitals and cooperating institutions that recruited patients for this study: Chirurgische Universitätsklinik Heidelberg, Klinik am Gesundbrunnen Heilbronn, St. Vincentiuskrankenhaus Speyer, St. Josefskrankenhaus Heidelberg, Chirurgische Universitätsklinik Mannheim, Diakonissenkrankenhaus Speyer, Krankenhaus Salem Heidelberg, Kreiskrankenhaus Schwetzingen, St. Marienkrankenhaus Ludwigshafen, Klinikum Ludwigshafen, Stadtklinik Frankenthal, Diakoniekrankenhaus Mannheim, Kreiskrankenhaus Sinsheim, Klinikum am Plattenwald Bad Friedrichshall, Kreiskrankenhaus Weinheim, Kreiskrankenhaus Eberbach, Kreiskrankenhaus Buchen, Kreiskrankenhaus Mosbach, Enddarmzentrum Mannheim, Kreiskrankenhaus Brackenheim, and Cancer Registry of Rhineland-Palatinate, Mainz.
Grant support: This work was supported by the German Research Council (BR 1704/6–1, BR 1704/6–3, BR 1704/6–4, BR 1704/6–6, CH 117/1–1, BR 1704/17–1, HO 5117/2–1), the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815), the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Germany, and the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (NIH R01 CA195789; U01 CA137088; and R01 CA059045). ). The funders play no role in the design of the study, the collection, analysis and interpretation of data; and in the decision to approve publication of the finished manuscript. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- DACHS
Darmkrebs: Chancen der Verhütung durch Screening
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FFQ
food frequency questionnaire
- ICD-10
International Classification of Diseases, 10th Revision
- MET
metabolic equivalent of task
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OR
odds ratio
- PAF
population attributable fraction
- SNPs
single nucleotide polymorphisms
- WCRF/AICR
World Cancer Research Fund/American Institute for Cancer Research
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Registration: This observational study has been registered in the German Clinical Trials Register (ID DRKS00011793), which is a primary registry in the WHO Registry Network.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon, France: 2013. [Google Scholar]
- 2.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406–15. [DOI] [PubMed] [Google Scholar]
- 3.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Lau R, Chan DS, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011;141:106–18. [DOI] [PubMed] [Google Scholar]
- 5.Aune D, Lau R, Chan DS, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol 2012;23:37–45. [DOI] [PubMed] [Google Scholar]
- 6.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Bmj 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011;6:e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr PR, Walter V, Brenner H, et al. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int J Cancer 2016;138:293–302. [DOI] [PubMed] [Google Scholar]
- 9.Boyle T, Keegel T, Bull F, et al. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:1548–61. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8:e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. Bmj 2010;341:c5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hang J, Cai B, Xue P, et al. The Joint Effects of Lifestyle Factors and Comorbidities on the Risk of Colorectal Cancer: A Large Chinese Retrospective Case-Control Study. PLoS One 2015;10:e0143696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platz EA, Willett WC, Colditz GA, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000;11:579–88. [DOI] [PubMed] [Google Scholar]
- 15.Wei EK, Colditz GA, Giovannucci EL, et al. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. Am J Epidemiol 2009;170:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odegaard AO, Koh WP, Yuan JM. Combined lifestyle factors and risk of incident colorectal cancer in a Chinese population. Cancer Prev Res (Phila) 2013;6:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- 18.Weigl K, Chang-Claude J, Knebel P, et al. Strongly enhanced colorectal cancer risk stratification by combining family history and genetic risk score. Clin Epidemiol 2018;10:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Chang-Claude J, Rickert A, et al. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol 2012;30:2969–76. [DOI] [PubMed] [Google Scholar]
- 21.Tsoi KK, Pau CY, Wu WK, et al. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2009;7:682–688.e1–5. [DOI] [PubMed] [Google Scholar]
- 22.Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: populationbased study from Germany. Int J Cancer 2015;137:1433–45. [DOI] [PubMed] [Google Scholar]
- 23.Walter V, Jansen L, Ulrich A, et al. Alcohol consumption and survival of colorectal cancer patients: a population-based study from Germany. Am J Clin Nutr 2016;103:1497–506. [DOI] [PubMed] [Google Scholar]
- 24.Walter V, Jansen L, Knebel P, et al. Physical activity and survival of colorectal cancer patients: Populationbased study from Germany. Int J Cancer 2017;140:1985–1997. [DOI] [PubMed] [Google Scholar]
- 25.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physcial Activity and Colorectal Cancer., 2017.
- 26.Carr PR, Jansen L, Walter V, et al. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am J Clin Nutr 2016;103:192–200. [DOI] [PubMed] [Google Scholar]
- 27.Carr PR, Jansen L, Bienert S, et al. Associations of red and processed meat intake with major molecular pathological features of colorectal cancer. Eur J Epidemiol 2017;32:409–418. [DOI] [PubMed] [Google Scholar]
- 28.Walter V, Jansen L, Hoffmeister M, et al. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr 2016;104:1110–1120. [DOI] [PubMed] [Google Scholar]
- 29.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC, 2007. [Google Scholar]
- 30.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer, 2011.
- 31.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, 2010. [PubMed]
- 32.Yuan Y Multiple Imputation Using SAS Software. Journal of Statistical Software 2011;45:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzi P, Green SB, Byar DP, et al. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 1985;122:904–14. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol 1974;99:325–32. [DOI] [PubMed] [Google Scholar]
- 35.Miller D. Bootstap 101:obtain robust confidence intervals for any statistic. Proceedings of the 29th annual SAS users group international conference Vol 29. Montreal, Canada: SAS Institute Inc,. 2004. [Google Scholar]
- 36.Romaguera D, Gracia-Lavedan E, Molinuevo A, et al. Adherence to nutrition-based cancer prevention guidelines and breast, prostate and colorectal cancer risk in the MCC-Spain case-control study. Int J Cancer 2017;141:83–93. [DOI] [PubMed] [Google Scholar]
- 37.Romaguera D, Vergnaud AC, Peeters PH, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr 2012;96:150–63. [DOI] [PubMed] [Google Scholar]
- 38.Makarem N, Lin Y, Bandera EV, et al. Concordance with World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) guidelines for cancer prevention and obesity-related cancer risk in the Framingham Offspring cohort (1991–2008). Cancer Causes Control 2015;26:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol 2012;23:1403–15. [DOI] [PubMed] [Google Scholar]
- 40.Lin KJ, Cheung WY, Lai JY, et al. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer 2012;130:419–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.