Abstract
Background & Aims:
Burns remain the fifth cause of non-fatal pediatric injuries globally, with muscle cachexia being a hallmark of the stress response to burns. Burn-induced muscle wasting is associated with morbidity, yet the determinants of muscle protein catabolism in response to burn trauma remains unclear. Our objective was to determine the effect of patient and injury characteristics on muscle protein kinetics in burn patients.
Methods:
This retrospective, observational study was performed using protein kinetic data from pediatric patients who had severe burns (> 30% of the total body surface area burned) and underwent cross-limb stable isotope infusions between 1999 and 2008 as part of prospective clinical trials. Mixed multiple regression models were used to assess associations between patient/injury characteristics and muscle protein fractional synthesis rate (FSR), net balance (NB), and rates of phenylalanine appearance (Ra; index of protein breakdown) and disappearance (Rd; index of protein synthesis) across the leg.
Results:
A total of 268 patients who underwent 499 studies were analyzed. Increasing time post injury was associated with greater FSR (p < 0.001) and NB (p = 0.01). Males were more catabolic than females (as indicated by lower NB, p < 0.05 and greater Ra, p = 0.008), a consequence of higher protein breakdown rather than lower synthesis. Increasing burn size was associated with higher protein synthesis rate (as indicated by higher FSR, p < 0.05) and higher protein breakdown rates (as indicated by greater Ra, p = 0.001). FSR was negatively associated with age (p < 0.001).
Conclusions:
Data from this large patient cohort show that injury severity, sex, and time post injury influence skeletal muscle wasting in burned children. These findings suggest that individual patient characteristics should be considered when devising therapies to improve the acute care and rehabilitation of burn survivors.
Introduction
Burns are the fourth leading cause of severe injury, prompting an estimated 11 million people around the world to seek burn care annually (1). Improved understanding of the pathophysiological stress response to massive burns has resulted in a number of significant advances in patient care, which have considerably reduced morbidity and mortality (2). Yet, burns remain the fifth cause of non-fatal pediatric injuries globally (1), and in the U.S. alone, hospitalization costs for pediatric burn care were estimated at more than $211 million (3). While almost all patients now survive the initial burn trauma, they go on to develop several metabolic abnormalities (4) that may last more than 2 years after injury (5,6). Arguably the most detrimental metabolic derangement in response to severe burn trauma is excessive muscle wasting, given its involvement in growth retardation and long-term musculoskeletal disorders (7-9). Both minor and severe burn injuries are associated with long-term musculoskeletal morbidity (10).
Restriction of physical activity during acute burn treatment may partly mediate muscle loss (11), but it cannot fully explain the extent of muscle wasting. After severe burn injury, there is increased amino acid (AA) demand for wound healing and production of acute phase proteins (5,12). Muscle tissue, the main AA depot in the body, is thought to be catabolized to provide precursors for these functions (12-14). However, even with increased external provision of AA, skeletal muscle wasting cannot be readily reversed in burn survivors (15,16). An incomplete understanding of the physiologic mechanisms underlying muscle loss may account for current therapeutic strategies failing to halt and reverse this persistent catabolic response.
The mediators of burn-related muscle wasting remain largely unknown. The present study was conducted to investigate the effect of patient and injury characteristics on muscle protein kinetics in severely burned children during acute hospitalization. To this end, we analyzed data obtained from cross-limb stable isotope infusion studies conducted at Shriners Hospitals for Children - Galveston (SHC-G) over a 10-year period as part of prospective clinical trials. This is the first study to identify predictors of muscle protein kinetics in a large representative sample of pediatric patients with severe burn trauma, where protein turnover rates have been comprehensively determined using stable isotopic methods. The large sample size allows for the detection of associations, thus providing unique insights into the factors that influence burn-induced muscle wasting.
Materials and methods
Patients
The study population comprised children (< 18 years) admitted to SHC-G between August 1999 and January 2008 who had burns encompassing > 30% of their total body surface area (TBSA), who were prospectively studied as part of clinical trials conducted at SHC-G, and who had undergone ≥ 1 stable isotope infusion study during the acute hospitalization period (prior to the first hospital discharge) (Table 1). A total of 268 children who collectively underwent 499 stable isotope infusion studies were included in the final analysis. Part of the protein kinetics data for 97 of these patients (175 infusion studies) have been previously published (17-26) as secondary outcomes not related to the aims of this study. Brief description of those studies is presented in Supplemental Table 1. Patients were randomly assigned upon admission to receive standard of care (SOC) only or an additional pharmacologic agent depending on the clinical trial they were enrolled in (Table 1). The Institutional Review Board at the University of Texas Medical Branch in Galveston approved this study. Informed consent was obtained from patients’ guardians prior to enrollment.
Table 1.
Patient characteristics.
| Characteristic1 | Patients (N = 268) |
|---|---|
| Total Metabolic Studies (obs) | 499 |
| Age at Burn (years) | 8 (5) |
| Male (%) | 71 |
| TBSA Burned (%) | 59 (17) |
| 3rd Degree TBSA burned (%) | 46 (24) |
| Admission Weight (kg) | 32 (21) |
| Study Weight (kg) | 27 (17) |
| Admission Height (cm) | 121 (31) |
| Admission z-scores | |
| Weight | −0.41 (1.3) |
| Height | −0.47 (1.4) |
| B.M.I. | −0.22 (1.5) |
| B2A (days) | 5 (16) |
| B2S (days) | 20 (11) |
| Length of Stay (days) | 33 (22) |
| Type of Burn (n) | |
| Chemical | 1 |
| Electrical/Flame | 18 |
| Electrical | 4 |
| Explosion/Flame | 1 |
| Flame | 200 |
| Scald | 44 |
| Treatment (n) | |
| SOC | 51 |
| β-blockers + SOC | 112 |
| Anabolic Agents + SOC | 37 |
| Antidiabetic + SOC | 37 |
| Other + SOC | 31 |
Reported as mean (SD) unless otherwise noted. B2A, time from burn to admission; B2S, time from burn to study; SOC, standard of care; TBSA, total body surface area
Patients received SOC, including fluid resuscitation, wound excision, and grafting within 48 hours of admission, and they were confined to bed rest for 5 days after each surgical procedure. Enteral nutrition was initiated upon admission and maintained until patients could consume sufficient nutrition ad libitum. The enteral formula used, Vivonex TEN (Sandoz Nutritional Corp., Minneapolis, MN), has a macronutrient composition of 82% carbohydrates, 15% protein, and 3% fat, and it was delivered at a rate of 1,500 kcalm−2 TBSA burned plus 1,500 kcalm−2 TBSA.
Patient and injury characteristics
Patient and injury characteristics were extracted from patient hospital charts. Age was defined as time from birth to burn, B2A as time from burn injury to admission, and B2S as time from burn injury to the stable isotope study. Weight and height were recorded on admission and study day. Z-score for weight, height and B.M.I on admission and study day were calculated using WHO (27) and CDC (28) for children aged 0 to 2 years old and 2 to 18 years old respectively. TBSA burned served as an index of burn size, and TBSA 3rd denoted size of full thickness (3rd degree) burns. Burn type was categorized into chemical, electrical/flame, electrical, explosion/flame, flame, or scald. Pharmacological agents that were administered in addition to SOC as part of randomized clinical trials during the data collection period (19992008) included β-blockers, anabolic agents, and anti-diabetes drugs (Table 1).
Metabolic studies
The metabolic study design is presented in Figure 1. Background Phe enrichment was determined from baseline blood samples. A primed (2 μmol·kg−1) constant (0.07 μmol·kg−1·h−1) infusion of D-5-Phe was initiated and continued for 5 hours. Phe enrichment and concentrations were determined in blood samples drawn simultaneously from the femoral artery and vein at 240, 260, 280, and 300 minutes, and they were analyzed as previously described (29). Indocyanine green (ICG) infusion between hours 3 and 4 was used to determine leg blood flow. For the determination of intracellular and protein-bound Phe enrichment (30,31), 2 muscle biopsies were taken from m. vastus lateralis at 120 and 300 minutes using a 5-mm Bergstrom needle (Stille, Stockholm, Sweden). Muscle samples were washed with cold saline to remove visible non-muscle tissue, immediately frozen with liquid nitrogen, and stored to −80oC for future analysis (32).
Figure 1.
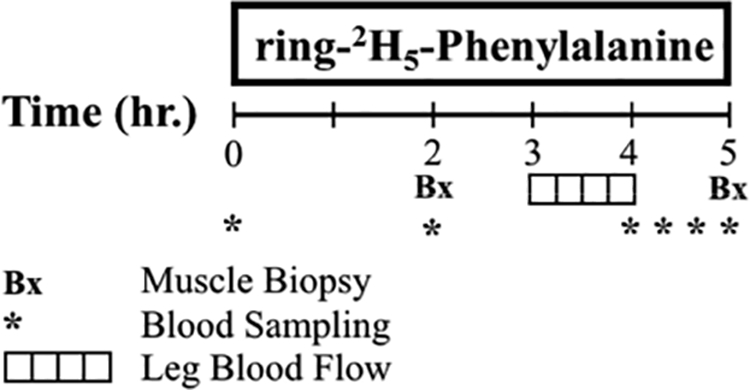
Metabolic study design
Calculations - outcome measures
The modified Fick’s equation was used to determine leg blood flow (31).
1) Blood Flow (BF):
CF and CC are the femoral and central venous ICG concentration, respectively. ICG was infused at a constant rate of 0.5 mg·min−1. Blood flow was normalized for leg volume of each patient. The essential AA Phe is not synthesized in the body and is only catabolized in the liver. Thus, the difference in the concentration of Phe between the femoral artery and vein represents the net balance (NB) of protein synthesis and breakdown across the leg. The 2-pool model was used to quantify Phe kinetics (31). For the determination of leg muscle protein fractional synthetic rate (FSR), the precursor-product method was used (30). The muscle intracellular enrichment was used as precursor and the bound muscle protein enrichment as product.
2) Leg protein net balance (NB): NB = (Ca – Cv) ∙ BF
3) Phe rate of disappearance (Rd):
4) Phe rate of appearance (Ra):
5) Fractional synthetic rate (FSR):
NB, Rd, and Ra are expressed as nmol·min−1·100 ml−1· leg. BF is leg blow flow in ml·min−1·100 ml−1 leg, Ca and Cv are the average femoral arterial and venous Phe concentrations over hours 4 and 5, expressed in nmol ml−1. Ea and Ev are the average Phe arterial and venous enrichments over the same period. EM and EP are the intracellular and protein-bound Phe enrichment, respectively. EP and EM are shown for t1 = 2 h and t2 = 5 h, when the two muscle biopsies were performed. Phe enrichments were expressed as tracer-to-tracee ratio (%).
Statistical analysis
Patient demographics were summarized as means, standard deviations, counts, or percentages, as appropriate. Because metabolic studies among different patients were performed at various time points after burn injury, mixed multiple regression models (33) were explored for each outcome (FSR, NB, Ra, Rd) with relation to age, sex, admission and study weight, z-scores for weight, height and B.M.I., burn type, total burned and 3rd-degree TBSA, B2A, and B2S, while blocking on subject to control for repeated measures. FSR was log-transformed to improve approximation of normality. B2S and B2A were log-transformed to reduce skewness. The models were compared by Bayesian Information Criteria (BIC), wherein a smaller BIC is indicative of a superior model. Likelihood ratio tests were used for pairwise model comparison. Sensitivity analyses were conducted to compare drug and SOC effects on each kinetic outcome using mixed linear regression models to adjust for confounding and repeated measures. Statistical analyses were performed using R statistical software (34)
Results
A total of 268 patients who collectively underwent 499 stable isotope infusions were included in the final analysis. Patients were 8 ± 5 years old at the time of injury, with 59 ± 17% TBSA burns and 46 ± 24% full-thickness TBSA burns. Infusion studies were performed at 5 ± 16 days after admission and 20 ± 11 days after burn. Patient characteristics are summarized in Table 1. FSR was measured in 235 patients, NB in 105 patients, and 2-pool model Phe kinetics in 100 patients.
Model selection process for independent variables
Patient and injury characteristics
Highly correlated variables, such as age with body weight or TBSA with 3rd degree burn, cannot be included in the same model. In FSR models, age was a better predictor than weight, while in pool models of Phe kinetics, weight was a stronger predictor than age. Z-scores for weight, height and B.M.I. on admission and z-scores for weight and B.M.I. on study day were not associated with any of the outcomes. For all outcomes, TBSA burned was a better predictor than 3rd degree TBSA burned, so TBSA burned was used in the final analysis. Study weight was, in every case, a better predictor than admission weight and was used in the final analysis. The addition of B2A to the models did not reveal any significant associations with any of the outcomes and did not affect the coefficients of the other predictors. Moreover, BIC was lower when B2A was not included. For these reasons, B2A was excluded from the final models. Type of burn injury did not have an effect on any outcome and was not included in the final analysis. No non-linear associations of the predictors with the outcomes were found.
Pharmacological treatment
To increase sample size and the probability of detection of true association of patients’ characteristics with kinetics parameters, the analysis was performed on the whole cohort of patients regardless of experimental treatment received. However, type of treatment was included as categorical variable in all models in order to obtain unbiased estimates of the size effects of patient characteristics. This study is not properly designed to assess the effects of the various pharmacological treatments; therefore, size effects for the levels of this variable are not presented here.
We performed sensitivity analysis by running the final models obtained from the total cohort using data obtained from patients who received SOC only (Supplemental Table 2). While some findings lost statistical significance, possibly due to marked decrease in sample size, the direction and magnitude of size effects of predictors where reasonably close.
The final regression models included 5 independent variables: sex, age or weight, TBSA burned, log-transformed B2S, and pharmacological treatment.
Amino acid metabolism— mixed regression model results
FSR, Phe enrichments and concentrations, and pool-model kinetics are summarized in Table 2. Mean values for Phe NB across the leg were negative. Blood flow and Phe kinetic values were significantly (2- to 3-fold) higher than those published for healthy individuals (35), indicating that burn patients were hyperdynamic with increased leg protein turnover.
Table 2.
Phenylalanine measures, kinetics, and FSR.
| Parameter | Mean (SD) |
|---|---|
| Phe Concentration/Enrichment | |
| Arterial Concentration (nmol·ml−1) | 111 (34) |
| Venous Concentration (nmol·ml−1) | 112 (35) |
| Arterial Enrichment (%) | 4.44 (1.5) |
| Venous Enrichment (%) | 4.02 (1.7) |
| Phe Kinetics | |
| NB (nmol·min−1·100 ml−1·leg) | −8 (73) |
| Rd (nmol·min−1·100 ml−1·leg) | 127 (87) |
| Ra (nmol·min−1·100 ml−1·leg) | 134 (76) |
| FSR (%·h−1) | 0.16 (0.13) |
| Leg Blood Flow (ml·min−1·100 ml−1·leg) | 13 (7) |
For NB, n = 107 (obs = 160). For Rd, and Ra, n = 100 (obs = 152). For FSR, n = 235 (obs = 431). FSR, fractional synthetic rate; NB, net balance; Ra, rate of disappearance; Rd, rate of disappearance
The best fit model for FSR is presented in Table 3. Increasing age was associated with a decrease in FSR (p < 0.001). Burn size was positively associated with muscle FSR (p = 0.019). Each 1% increase in time post-burn resulted in a 0.51% increase in skeletal muscle FSR (p < 0.001) (Figure 2).
Table 3.
Final mixed multiple regression models for FSR, NB, Ra, and Rd adjusted for type of experimental treatment.
| Model A: Log(FSR) | Model B: NB | ||||||
|---|---|---|---|---|---|---|---|
| n (obs) = 235 (431) | n (obs) = 105 (160) | ||||||
| Coeff. | SE | p value | Coeff. | SE | p value | ||
| Sex (Male) | −0.03 | 0.234 | 0.8 | Sex (Male) | −28 | 13.4 | 0.04 |
| Age | −0.04 | 0.008 | <0.001 | Weight | 0.52 | 0.39 | 0.19 |
| TBSA | 0.01 | 0.002 | 0.019 | TBSA | −0.19 | 0.37 | 0.6 |
| Log(B2S) | 0.51 | 0.068 | <0.001 | Log(B2S) | 28.3 | 10.7 | 0.01 |
| Model C: Ra | Model D: Rd | ||||||
| n (obs) = 100 (152) | n (obs) = 100 (152) | ||||||
| Coeff. | SE | p value | Coeff. | SE | p value | ||
| Sex (Male) | 38.3 | 14 | 0.008 | Sex (Male) | 4.14 | 18.3 | 0.9 |
| Weight | −1.09 | 0.37 | 0.006 | Weight | −0.74 | 0.46 | 0.11 |
| TBSA | 1.22 | 0.34 | 0.001 | TBSA | 0.8 | 0.46 | 0.09 |
| Log(B2S) | −0.36 | 11.2 | 0.9 | Log(B2S) | 24.2 | 13.2 | 0.072 |
Best mixed regression models. Type of experimental treatment was included as categorical variable in all models to obtain estimates of size effects of clinical characteristic independent from experimental treatment. The coefficients for each level of this variable are intentionally left out. FSR and B2S were log- transformed. n refers to the numbers of patients, and obs is the number of metabolic studies included in the analysis. B2S, time from burn to metabolism study; FSR, fractional synthesis rate; NB, net balance; Ra, rate of appearance; Rd, rate of disappearance; TBSA: total body surface area burned.
Figure 2. Time course of fractional synthesis rate (FSR) during the acute period after injury.
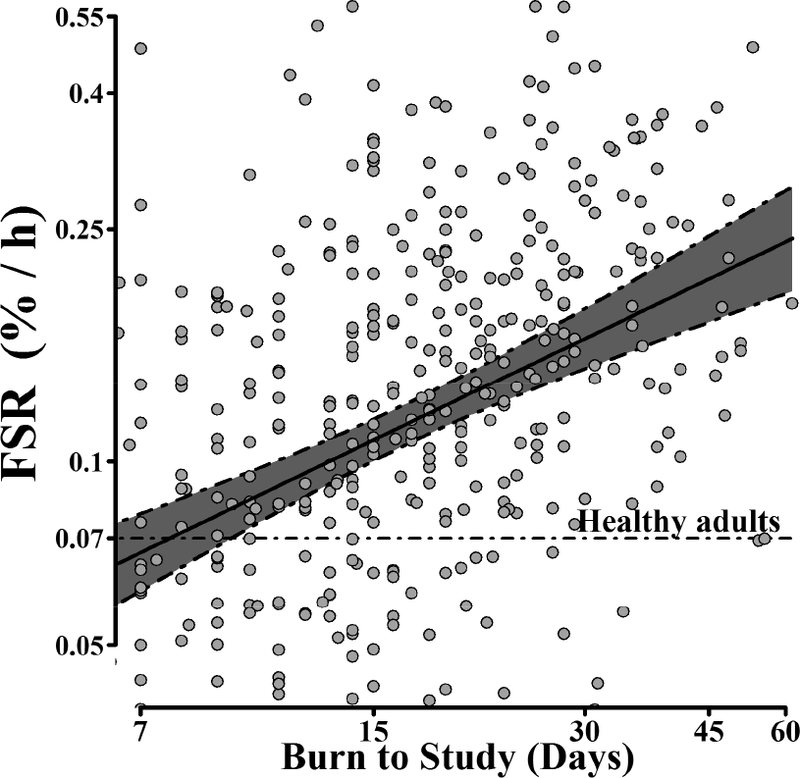
Predicted mean of FSR conditional on time from burn injury to study, with 95% CI adjusted for sex, age, total body surface area burned and experimental treatment (n = 235, obs = 431); Data points correspond to actual FSR measurements (not adjusted for covariates) Both FSR and burn to study axes are in logarithmic scale.
The best fit models for NB, Ra, and Rd are presented in Table 3. NB was higher in females than in males (p < 0.04) (Figure 3), and increasing time post-burn (B2S) was also associated with a higher NB (p = 0.01) (Figure 4). Ra was positively associated with TBSA burned (p = 0.001), indicating higher protein turnover with increasing burn severity. Ra was higher in males (p < 0.008) (Figure 3).
Figure 3. Effect of sex on protein net balance (NB) and rate of appearance (Ra) during the acute period after injury.
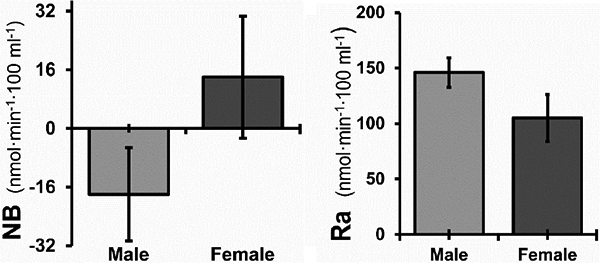
Means are shown with the 95% CI adjusted for weight, total burned surface area, time from burn injury to metabolic study, and experimental treatment. For NB, n = 105 and obs = 160 (male, n = 73; p = 0.04). For Ra, n = 100 and obs = 152 (male, n = 71; p = 0.008).
Figure 4. Time course of protein net balance (NB) during the acute period after injury.
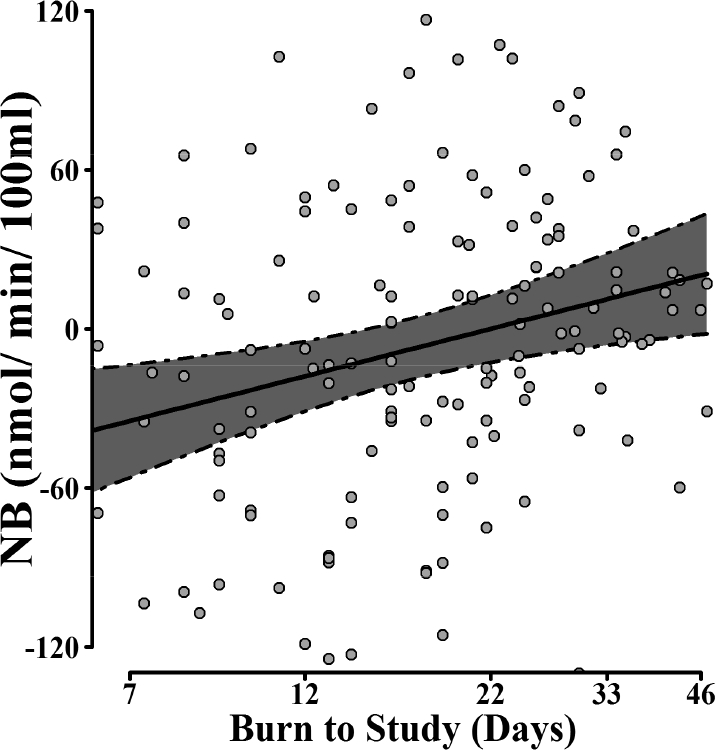
Predicted NB mean conditional on time from burn injury to metabolic study, with 95% CI adjusted for sex, weight, total body surface area burned, and experimental treatment. n = 105 and obs = 160. Data points correspond to actual NB measurements (not adjusted for covariates). Burn to study axis is in logarithmic scale.
Discussion
To our knowledge, this is the first time that predictors of muscle protein kinetics have been determined in a large representative sample of severely burned pediatric patients who underwent cross-limb stable isotope studies. This study encompasses a 10-year collection of prospective data from 499 stable isotope infusion studies on 268 patients. Smaller studies have implicated certain patient and injury characteristics in burn-induced catabolism. However, none have explored these relationships using comprehensively defined protein turnover rates. We used data from 2-pool A-V balance models, which provide a unique insight into leg protein turnover after massive burn trauma. We found that increasing injury severity, increasing time post-burn, and male sex were associated with changes in several muscle protein metabolism parameters, indicating greater muscle protein turnover during acute hospitalization. In addition, age was a significant predictor of muscle protein synthesis in these severely burned children.
Analysis of skeletal muscle FSR revealed a significant negative association with age and a significant positive association with burn size, indicating that muscle protein turnover is greater in younger children and with more severe burns. In contrast to our previous work showing that FSR was higher in females than males, this study found no effect of sex on FSR (26). This may be attributable to the fact that patients were studied up to 2 years post-injury in our previous study, but were only studied during their acute hospitalization on the current study. Nevertheless, in both studies, age and time post-burn were predictors of muscle FSR (26), supporting the conclusion that burns induce dynamic changes in muscle protein turnover.
We have previously shown that muscle FSR markedly increases in children 7 to 28 days after burn trauma (26). However, here we found that FSR continued to increase over the duration of acute hospitalization period, which ranged from 3 to 60 days post-burn. This association remained significant after adjusting for burn severity and time to admission. In healthy individuals, daily FSR (a mean of postprandial and post absorptive values) is approximately 0.075%·h−1 (≈ 1.8%·day−1), which corresponds to several hundred grams of newly synthesized muscle protein per day (36). A significant amount of ATP is required per mole of AAs incorporated into protein, making muscle protein synthesis is an energetically expensive process (37). Indeed, protein turnover has been estimated to account for around 25% of basal metabolic rate in healthy individuals (38). Interestingly, we found that, after burn, FSR is 2 to 3 times greater than values reported for healthy individuals, where muscle protein FSR equals approximately 4–6%·day−1. Clearly then, increased muscle protein synthesis places a significant energetic demand on severely burned patients. Moreover, we have recently shown that the efficiency of mitochondrial ATP production in skeletal muscle declines post-burn (6,13). Because muscle mitochondria produce the ATP required for muscle protein synthesis, our current data suggest that muscle protein turnover might make a greater contribution to burn-induced hypermetabolism than previously thought (37).
A major finding of the current study was that muscle protein NB was higher in females (by ~28 nmol·min−1100 ml−1) than males. Moreover, the Ra of AA from the leg was higher in males, while the Rd did not differ between sexes. These findings suggest that severely burned males are more catabolic than girls, owing to greater muscle breakdown. There is currently no evidence to suggest that severely burned females cope better than males in terms of their metabolic response. Whether greater muscle protein catabolism and AA release in males (as indicated by a higher AA Ra) is deleterious or a beneficial aspect of the acute stress response to burns in males is beyond the scope of this hypothesis-generating observational study. However, this interesting observation deserves further investigation, especially given that tailoring treatments based on patient characteristic such as sex and age remains an important goal.
Muscle protein NB was also positively associated with time post-burn, suggesting that burn-induced protein catabolism gradually moderates as patient’s progress from the acute to the rehabilitative phase. No statistically significant association of AA Ra or Rd with time post-burn (B2S) was found. However, even though not statistically significant, the coefficient of B2S in the Rd was positive and high (≈ 24 nmol·min−1·100 ml−1 leg increase in protein synthesis per log-day post-injury, p = 0.07). The finding that FSR and likely Rd—both indices of muscle protein synthesis albeit derived from different stable isotope approaches—increased with time in our study while the index of protein breakdown, Ra, remained unaffected, suggests that the improvement in NB observed with increasing time post-burn is attributable to increased capacity for muscle synthesis rather than reduced muscle breakdown. We have previously shown that a long-term improvement in NB occurs from 6 to 12 months post-burn owing to a decrease in protein breakdown (8). The current study extends this observation by showing that this gradual improvement in NB following burn trauma likely begins around 1 month post-injury and is mediated by increased protein synthesis.
To increase the sample size, we analyzed data from all available studies, regardless of pharmacological treatment. However, type of treatment was included as categorical variable in all models in order to obtain unbiased estimates of the size effects of patient characteristics. However, type of treatment was included as categorical variable in all models in order to obtain unbiased estimates of the size effects of patient characteristics on muscle protein kinetics. Notably, this study was not designed to determine the effects of these agents on muscle metabolism and for this reason, we do not present here the size effects of each treatment type as obtained in the final models. Futhermore, to assess the validity of the results obtained by the total cohort of patients, we performed sensitivity analysis by running the final models using data obtained from patients who received SOC only (Supplemental Table 2). While some findings lost statistical significance, possibly due to marked decrease in sample size, the direction and magnitude of size effects of predictors where reasonably close.
The current study has several strengths. It is the first to investigate determinants of muscle protein kinetics in response severe burns. Given the invasiveness, cost, and translational value of data derived from these cross-limb stable isotope infusion studies, the sample size of our current study is considerable. Our statistical analysis approach allowed us to include almost all the available data, while controlling for dependency due to multiple studies being conducted in many of our subjects.
This study also has limitations. The fact that the data analyzed spanned 10 years likely introduced some variation. That is, small changes in SOC occurred during this time as a consequence of staff turnover, technological advances, and changes in laboratory personnel, equipment, and reagents. However, we did not detect any effect of year on outcomes, suggesting that these changes had no significant influence on the data. Given the high heterogeneity in our sample, the size effect of the predictors may have been higher with a more controlled experimental environment.
Conclusions
Patient characteristics such as age, sex, body weight, and burn size are associated with changes in several parameters of muscle protein metabolism. Protein turnover was positively associated with time post-burn throughout acute treatment, suggesting that contributions of muscle protein turnover to burn-induced hypermetabolism are more substantial than previously thought. Further, females were less catabolic than males, mainly because of lower rates of muscle breakdown. These data suggest that the stress response to massive trauma is not uniform and underscore the need for a more individualized approach to patient care.
Supplementary Material
Acknowledgments
We sincerely thank the patients who took part in this study; without their cooperation, this research could not have been performed. This work was supported by grants from NIH (P50 GM060338, R01 GM056687, T32 GM008256, R01 HD049471, K01 HL070451), NIDILRR (90DP00430100), Shriners of North America (SHC 84080, SHC 84090), and the Department of Surgery at UTMB.
Abbreviations
- AA
amino acids
- B2A
time from injury to admission
- B2S
time from injury to metabolic study
- BF
blood flow
- BIC
Bayesian Information Criteria
- Ca
arterial phenylalanine concentration
- Cc
central vein indocyanine green concentration
- Cf
femoral vein indocyanine green concentration
- Cv
venous phenylalanine concentration
- Ea
arterial enrichment
- EM
free intracellular enrichment
- EP
protein bound enrichment
- Ev
venous enrichment
- ICG
indocyanine green
- NB
net balance
- Phe
phenylalanine
- Ra
rate of appearance
- Rd
rate of disappearance
- FSR
fractional synthesis rate
- SHC
Shriners Hospitals for Children
- SOC
standard of care
- TBSA
total body surface area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.WHO. WHO, The global burden of disease: 2004 update. WHO; 2014; [Google Scholar]
- 2.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–902. [DOI] [PubMed] [Google Scholar]
- 3.Shields BJ, Comstock RD, Fernandez SA, Xiang H, Smith GA. Healthcare Resource Utilization and Epidemiology of Pediatric Burn-Associated Hospitalizations, United States, 2000. J Burn Care Res 2007;28(6):811–26. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RR. Review: acute versus chronic response to burn injury. Circ Shock. 1981;8(1):105–15. [PubMed] [Google Scholar]
- 5.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011. Jan;6(7):e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter C, Herndon DN, Borsheim E, Bhattarai N, Chao T, Reidy PT, et al. Long-Term Skeletal Muscle Mitochondrial Dysfunction is Associated with Hypermetabolism in Severely Burned Children. J Burn Care Res. 2016;37(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000. August;128(2):312–9. [DOI] [PubMed] [Google Scholar]
- 9.Biolo G, Declan Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87(7):3378–84. [DOI] [PubMed] [Google Scholar]
- 10.Randall SM, Fear MW, Wood FM, Rea S, Boyd JH, Duke JM. Long-term musculoskeletal morbidity after adult burn injury: a population-based cohort study. BMJ Open. 2015. September 11 ;5(9):e009395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119(1):109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30(4):331–8. [DOI] [PubMed] [Google Scholar]
- 13.Porter C, Herndon DN, Sidossis LS, Børsheim E. The impact of severe burns on skeletal muscle mitochondrial function. Burns. 2013;39(6):1039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter C, Hurren NM, Herndon DN, Børsheim E. Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma. 2013;3(1) :9–17. [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46(5):573–8. [DOI] [PubMed] [Google Scholar]
- 16.Porter C, Cotter M, Diaz EC, Jennings K, Herndon DN, Børsheim E. Amino acid infusion fails to stimulate skeletal muscle protein synthesis up to 1 year after injury in children with severe burns. J Trauma Acute Care Surg. 2013;74(6):1480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233(4):556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of Catabolism by Beta-blocakde After Severe Burns. N Engl J Med. 2001. October 25;345(17):1223–9. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SE, Thomas SJ, Dasu MR, Ferrando A a, Chinkes DL, Wolfe RR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237(6):801–810-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003. April;54(4):755-61–4. [DOI] [PubMed] [Google Scholar]
- 21.Herndon DN, Dasu MRK, Wolfe RR, Barrow RE. Gene expression profiles and protein balance in skeletal muscle of burned children after beta-adrenergic blockade. Am J Physiol Endocrinol Metab. 2003;285(4):783–9. [DOI] [PubMed] [Google Scholar]
- 22.Hart DW, Wolf SE, Zhang XJ, Chinkes DL, Buffalo MC, Matin SI, et al. Efficacy of a highcarbohydrate diet in catabolic illness. Crit Care Med. 2001;29(7):1318–24. [DOI] [PubMed] [Google Scholar]
- 23.Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI, Herndon DN. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236(4):450-6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeschke MG, Williams FN, Finnerty CC, Rodriguez NA, Kulp GA, Ferrando A, et al. The effect of ketoconazole on post-burn inflammation, hypermetabolism and clinical outcomes. PLoS One. 2012;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branski LK, Herndon DN, Pereira C, Mlcak RP, Celis MM, Lee JO, et al. Longitudinal assessment of Integra in primary burn management: a randomized pediatric clinical trial. Crit Care Med. 2007;35(11):2615–23. [DOI] [PubMed] [Google Scholar]
- 26.Diaz EC, Herndon DN, Lee J, Porter C, Cotter M, Suman OE, et al. Predictors of muscle protein synthesis after severe pediatric burns. J Trauma Acute Care Surg. 2015;78(4):816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. WHO-Weight-for-Age [Internet]. World Health Organization; [cited 2018 Feb 15]. Available from: http://www.who.int/childgrowth/standards/weight_for_age/en/
- 28.Centers for Disease Prevention and Control. Growth Charts - Clinical Growth Charts [Internet].[cited 2018 Feb 15]. Available from: https://www.cdc.gov/growthcharts/clinical_charts.htm
- 29.Tuvdendorj D, Chinkes DL, Zhang X-J, Sheffield-Moore M, Herndon DN. Skeletal muscle is anabolically unresponsive to an amino acid infusion in pediatric burn patients 6 months postinjury. Ann Surg. 2011. ;253(3):592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe RR, Chinkes DL. Measurement of the Synthesis of Specific Proteins In: Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2005. p. 325–60. [Google Scholar]
- 31.Wolfe RR, Chinkes DL. Arterial-Venous Balance Technique to Measure Amino Acid Kinetics In: Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. John Wiley & Sons, Inc; 2005. p. 381–420. [Google Scholar]
- 32.Sheffield-Moore M, Wolfe RR, Gore DC, Wolf SE, Ferrer DM, Ferrando AA. Combined effects of hyperaminoacidemia and oxandrolone on skeletal muscle protein synthesis. Am J Physiol. 2000;278:E273-9. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro J, Bates D, DebRoy S SD and RCT. Linear and Nonlinear Mixed Effects Models. 2014. [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2017. [Google Scholar]
- 35.Bell JA, Volpi E, Fujita S, Cadenas JG, Sheffield-Moore M, Rasmussen BB. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. JNutr. 2006;136(5):1249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006. September;84(3):475–82. [DOI] [PubMed] [Google Scholar]
- 37.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23(3):160–8. [DOI] [PubMed] [Google Scholar]
- 38.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


