Abstract
AIM:
This study aimed to assess the association between body mass index and the development of severe hypoglycemia in patients with type 2 diabetes using the nationwide data of the entire Korean population.
METHODS:
The association between body mass index and severe hypoglycemia was retrospectively examined from the claims and national health examination data from 2002 to 2015. A total of 1,366,692 subjects with assigned clinic codes on type 2 diabetes and who were prescribed with anti-hypoglycemic agents were included. The primary outcome was a new development of severe hypoglycemia after the baseline health examination.
RESULTS:
A total of 37,682 subjects (2.7%) developed a severe hypoglycemic event during the follow-up period (mean of 8.6 years). An inverse J-shaped association was observed between body mass index and severe hypoglycemic events. The association between low body mass index and high-risk of severe hypoglycemia was similar among subjects who had never smoked, did not consume alcohol, did not use insulin, and had no major comorbidities, after adjusting multiple confounders. The association between low body mass index and severe hypoglycemia was found to be intensified in men, young people aged 30–49 years, people with major comorbidities, and insulin users.
CONCLUSIONS:
Body mass index and severe hypoglycemia were found to be inversely associated. Those individuals who fall into the category of having a low body mass index and a high-risk of severe hypoglycemia should be warned of the risk of a hypoglycemic event and properly educated on hypoglycemia in order to minimize the risk of hypoglycemia-related fatal outcomes.
Introduction
The American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD)’s Position Statement emphasizes the complexity of glycemic control and the importance of individualization of diabetes care [1]. In the recent trend toward individualization of diabetes care, hypoglycemia has been considered as a major barrier to optimal glucose control (which helps in preventing diabetes complications). One recent survey among global opinion leaders in diabetes showed that “the risk of hypoglycemia from treatment” and “the risk associated with hypoglycemia” were important factors that should be considered when setting patients’ glycemic targets [2]. Severe hypoglycemia is defined as an episode of hypoglycemia in which a person’s blood glucose levels become extremely low, requiring the assistance of another person for treatment [3]. Severe hypoglycemia reduces the quality of life, increases medical costs, and can lead to several fatal outcomes [4]. Thus, identifying the barriers to the proper treatment of hypoglycemia is an important and critical issue for the treatment of type 2 diabetes.
Obesity and low weight have been widely recognized as important risks to good health [5]. However, the obesity paradox, a medical hypothesis stating that obese or overweight patients with cardiovascular diseases seem to have a better survival prognosis, has been suggested previously [6]. Since then, many studies have been performed analyzing the association between body mass index (BMI) and increased risk for various diseases and mortality. The effects of being underweight or overweight on the risk of severe hypoglycemia are controversial. Some studies showed that lower BMI was associated with increased risk of severe hypoglycemia [7, 8]. However, since large-scale studies to elucidate the association between BMI and severe hypoglycemia have been conducted only in groups of people who were highly at risk of cardiovascular disease and in the Caucasian population, whether the findings of these studies can be applied to the general population remains uncertain. Furthermore, no studies have analyzed the risk of severe hypoglycemia based on BMI categories.
This study aimed to investigate the association between BMI and risk of severe hypoglycemia using insurance claims data on the entire Korean population. We also aimed to compare the associations in various subgroups to quantify the risk of severe hypoglycemia associated with an incremental increase in BMI.
Materials and Methods
Study population
The Catholic Medical Center Ethics Committee approved this study. The study was conducted in compliance with the Declaration of Helsinki. We conducted a nationwide retrospective study using the Korean National Health Insurance (NHI) Claims Database maintained by the Korean National Health Insurance Service (NHIS), a government-affiliated agency under the Korean Ministry of Health and Welfare from 2002 to 2015. This database consists of various sub-datasets including Qualification DB, Health Insurance Claim DB, Health-Check-up DB, and death information provided by the NHI program and the Medical Aid program [9]. The Korean NHI program is a compulsory social insurance scheme that covers approximately 97% of the population; the remaining 3% are covered under the Medical Aid program. NHI subscribers are recommended to perform standardized general medical checkups once in 2 years. The medical checkup usually involves checking for smoking status; alcohol consumption; physical activity; income level; measurement of height, weight, and blood pressure; and laboratory tests.
Using the claims data, we selected subjects who had received a health examination at least once between 2005 and 2008. Then, we selected subjects aged >30 years who underwent a national health examination and measured their body weight. Both International Classification of Disease (ICD)-10 codes and medication prescriptions were used to define diseases. Patients with type 2 diabetes were defined as patients who had been assigned with diagnostic clinic codes such as E11 or E14, both from inpatient or outpatient claims dataset, and who were prescribed oral hypoglycemic agents or insulin [10]. A total of 24,651 subjects without type 2 diabetes and who were aged <30 years were excluded. A total of 1,622 subjects with missing BMI data were also excluded. Previous history of severe hypoglycemia is one of the important risk factors for a subsequent severe hypoglycemic event. We excluded 2,971 subjects with severe hypoglycemic events in the 3 years before the health examination (3 years before the baseline point) in order to establish a wash-out period to avoid the effects of a previous severe hypoglycemic event. The final sample included 1,366,692 subjects (Supplementary Figure S1, Supplementary Figure S2).
Definition of variables
The primary end-point of this study was the development of severe hypoglycemia during the follow-up period. Patients with severe hypoglycemia were defined as those who had been assigned with diagnostic clinical codes for hypoglycemia (E1163, E1363, E1463, E160, E161, and E162) from the inpatient or emergency room claims dataset. The information about smoking status (never smoked; former smoker; current smoker), drinking habit (never drank alcohol; moderate drinker, ≤1 drink per day; heavy drinker, >1 drink per day), and physical activity (no exercise, 1–2 times per week, ≥3 times per week) were obtained using a standard questionnaire during the health examination. Socioeconomic status (SES) was categorized into three groups (lower 30%; mid 40%; upper 30%) based on income levels. Hypertension was defined using the ICD-10 code (I10-I13, I15) and prescription of anti-hypertensive drugs, or systolic and diastolic blood pressure of ≥140 mmHg and ≥90 mmHg, respectively. Anti-diabetic drugs included six classes (biguanide, sulfonylurea, a-glucosidase inhibitor, thiazolidinedione, meglitinide, and insulin) dispensed at the baseline period in Korea (2005–2008). Among the anti-diabetic agents, insulin and sulfonylurea are strongly associated with the development of hypoglycemia. Thus, insulin and sulfonylurea were used for the analysis. The subjects’ medical history, including the chronic obstructive pulmonary disease (COPD, J43–J44), all types of cancer (C00–C97), liver cirrhosis (K704, K746), end-stage renal disease (ESRD, N18, N19, Z49, Z905, Z94, and Z992 of ICD-10 code and R380, O7011–7020, O7017, and O7075 of the procedure code), and cardiovascular diseases (I21, I22, I63, and I64), was identified using the ICD-10 codes [11, 12]. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m²). BMI was categorized into five groups according to the BMI category of World Health Organization recommendations for Asians: underweight, <18.5 kg/m²: normal weight, 18.5–22.9 kg/m²; overweight, 23.0–24.9 kg/m²; obesity class I, 25.0–29.9 kg/m²; and obesity class II, ≥30 kg/m².
Statistical Analysis
Statistical analyses were performed using the SAS version 9.4 (SAS Institute, Cary, NC, USA). Restricted cubic spline curves were used to test and visualize the nonlinear relationship between BMI and risk of severe hypoglycemia using the “Hmisc” packages in R program. The results of continuous data are presented as the mean ± standard deviation, and categorical data are presented as the number and percentage (%) of the total data. Statistical analyses were performed using one-way analysis of variance (ANOVA) for continuous variables and chi-square test for categorical variables, to compare baseline values. We also evaluated the potential interaction between the baseline BMI, severe hypoglycemia, and major variables. The hazard ratio (HR) and 95% confidence intervals (CIs) were estimated using Cox proportional hazard models to analyze the association between severe hypoglycemia and other variables, such as age (as a categorical variable; 30–49 years, 50–64 years, 65– years) sex (male, female), smoking status (never smoked, former smoker, current smoker), alcohol consumption (never drank alcohol, moderate drinker, heavy drinker), physical activity (no exercise, 1–2 times per week, ≥3 times per week), SES (lower 30%, mid 40%, upper 30%), major comorbidities (the presence of major disease including COPD, cancer, liver cirrhosis, ESRD, cardiovascular disease), fasting plasma glucose (as continuous variables), use of insulin and sulfonylureas, and BMI. The datasets were complete for the 1,366,692 subjects included in the analysis, with the exception of missing data regarding smoking status (33,106 cases, 2.4% of total subjects) and alcohol consumption (32,556 cases, 2.4% of total subjects). These data were included in the baseline comparison analysis and the crude Cox regression analysis (except for the analysis of smoking status or alcohol consumption) and were not included in the multivariable Cox regression analysis. P-values of <0.05 were considered statistically significant.
Results
Characteristics of the study population
A total of 1,366,692 subjects were followed up from baseline to the end of the study period (mean follow-up duration, 8.6 ± 2.0 years). The mean age of subjects was 57.7 ± 11.7 years, 59.3% of whom were men. The mean BMI of subjects was 24.9 ± 3.2 kg/m². Table 1 presents the baseline characteristics stratified by BMI categories. Subjects with higher BMIs tended to be younger, with a higher ratio of women. Subjects with higher BMIs were less likely to be smokers, less likely to regularly exercise, and less likely to use insulin. Subjects with higher BMIs were more likely to have hypertension and cardiovascular diseases. However, the prevalence of COPD, cancer, liver cirrhosis, and ESRD was higher in subjects with lower BMIs.
Table 1.
Baseline characteristics according to body mass index category
| BMI (kg/m2) | P value | |||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–<23.0 | 23.0–<25.0 | 25.0–<30.0 | ≥30.0 | ||
| Total, n | 22724 | 355378 | 353679 | 549651 | 85260 | <0.001 |
| Age (year) | <0.001 | |||||
| 30–49 | 20 | 24 | 24 | 27 | 34 | |
| 50–64 | 31 | 43 | 47 | 47 | 43 | |
| 65– | 49 | 34 | 29 | 27 | 23 | |
| Sex (male) | 61 | 60 | 62 | 60 | 46 | <0.001 |
| Smoking status | <0.001 | |||||
| Never smoked | 59 | 63 | 65 | 67 | 72 | |
| Former smoker | 7.9 | 9.2 | 11 | 11 | 8.2 | |
| Current smoker | 34 | 28 | 25 | 23 | 20 | |
| Alcohol consumption | <0.001 | |||||
| Never drank alcohol | 65 | 61 | 59 | 59 | 64 | |
| Moderate consumption | 7.6 | 10 | 11 | 11 | 9.5 | |
| Heavy drinking | 28 | 29 | 31 | 31 | 27 | |
| Physical activity (%) | <0.001 | |||||
| None | 67 | 53 | 49 | 50 | 56 | |
| 1–2 times a week | 16 | 22 | 24 | 25 | 24 | |
| ≥3 times a week | 17 | 25 | 27 | 25 | 20 | |
| Socioeconomic status | <0.001 | |||||
| Lower 30% | 34 | 32 | 30 | 30 | 32 | |
| Mid 40% | 37 | 37 | 37 | 37 | 39 | |
| Upper 30% | 29 | 31 | 33 | 33 | 29 | |
| Glucose lowering medication | ||||||
| Insulin | 12 | 8.9 | 7.3 | 6.4 | 6.3 | <0.001 |
| Sulfonylurea | 40 | 49 | 52 | 51 | 51 | <0.001 |
| Metformin | 30 | 37 | 39 | 38 | 39 | <0.001 |
| Thiazolidinedione | 4.4 | 5.8 | 6.5 | 7.0 | 8.7 | <0.001 |
| Acarbose | 15 | 16 | 14 | 12 | 12 | <0.001 |
| Meglitinide | 3.6 | 3.3 | 2.9 | 2.5 | 2.4 | <0.001 |
| Hypertension | 41 | 48 | 56 | 64 | 74 | <0.001 |
| SBP (mmHg) | 125 ± 19 | 128 ± 18 | 131 ± 17 | 133 ± 17 | 136 ± 17 | <0.001 |
| DBP (mmHg) | 77 ± 11 | 78 ± 11 | 80 ± 11 | 82 ± 11 | 84 ± 11 | <0.001 |
| COPD | 15 | 10 | 9.2 | 9.2 | 9.6 | <0.001 |
| Cancer | 3.9 | 2.6 | 2.0 | 1.8 | 1.7 | <0.001 |
| Cardiovascular disease | 12 | 12 | 13 | 14 | 15 | <0.001 |
| Liver cirrhosis | 1.9 | 1.2 | 0.9 | 0.7 | 0.6 | <0.001 |
| ESRD | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | <0.001 |
| Fasting plasma glucose (mmol/L) | 8.7 ± 3.3 | 8.4 ± 2.9 | 8.2 ± 2.7 | 8.2 ± 2.6 | 8.2 ± 2.6 | <0.001 |
Values are presented as percentage or mean ± standard deviation.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease
Severe hypoglycemic events in all subjects with type 2 diabetes
The mean time to severe hypoglycemia development from enrollment was 5.1 ± 2.7 years. During the follow-up period, the incidence of severe hypoglycemia was 3.2 per 1000 patient years. Patients with severe hypoglycemia were older and had a lower proportion of current smokers (Table 2). The prevalence of hypertension, COPD, cancer, cardiovascular disease, liver cirrhosis, and ESRD was higher in patients who developed severe hypoglycemia during the follow-up period.
Table 2.
Comparison of baseline parameters between the participants with and without severe hypoglycemia
| Severe hypoglycemia (−) | Severe hypoglycemia (+) | P value | |
|---|---|---|---|
| Number, n | 1329010 | 37682 | |
| BMI (kg/m2) | 24.9 ± 3.2 | 24.3 ± 3.4 | <0.001 |
| Age (year) | <0.001 | ||
| 30–49 | 26 | 6.1 | |
| 50–64 | 46 | 32 | |
| 65– | 28 | 62 | |
| Sex (male) | 60 | 45 | <0.001 |
| Smoking status (%) | <0.001 | ||
| Never smoked | 65 | 75 | |
| Former smoker | 10 | 7.3 | |
| Current smoker | 25 | 18 | |
| Alcohol consumption (%) | <0.001 | ||
| Never drank alcohol | 59 | 75 | |
| Moderate consumption | 10 | 6.1 | |
| Heavy drinking | 31 | 19 | |
| Physical activity (%) | <0.001 | ||
| None | 51 | 63 | |
| 1–2 times a week | 24 | 15 | |
| ≥3 times a week | 25 | 22 | |
| Socioeconomic status (%) | 0.02 | ||
| Lower 30% | 31 | 31 | |
| Mid 40% | 37 | 36 | |
| Upper 30% | 32 | 33 | |
| Glucose lowering medication | |||
| Insulin | 7.3 | 9.6 | <0.001 |
| Sulfonylurea | 52 | 14 | <0.001 |
| Metformin | 39 | 12 | <0.001 |
| Thiazolidinedione | 6.8 | 2.1 | <0.001 |
| Acarbose | 14 | 7.3 | <0.001 |
| Meglitinide | 2.8 | 2.6 | 0.01 |
| Hypertension (%) | 57 | 73 | <0.001 |
| SBP (mmHg) | 131 ± 17 | 134 ± 19 | <0.001 |
| DBP (mmHg) | 80 ± 11 | 80 ± 11 | <0.001 |
| COPD (%) | 9.3 | 16 | <0.001 |
| Cancer (%) | 2.1 | 2.4 | <0.001 |
| Cardiovascular disease (%) | 15 | 28 | <0.001 |
| Liver cirrhosis (%) | 0.9 | 1.5 | <0.001 |
| ESRD (%) | 0.1 | 0.5 | <0.001 |
| Fasting plasma glucose (mmol/L) | 8.3 ± 2.7 | 8.4 ± 3.4 | <0.001 |
Values are presented as percentage or mean ± standard deviation.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease
Association between BMI and severe hypoglycemia in the total and subgroup population
Figure 1 shows the relationship between BMI and risk of severe hypoglycemia. The lowest point was located between 25.0 kg/m2 and 29.9 kg/m2. After adjusting the potential confounders, the inverse relationship was associated between BMI and incidence of severe hypoglycemic event, with the lowest risk of severe hypoglycemia being in the BMI 25.0–29.9 kg/m² category (Supplementary table 1.). The highest risk of severe hypoglycemia was found in patients in the lowest BMI category.
Figure 1.
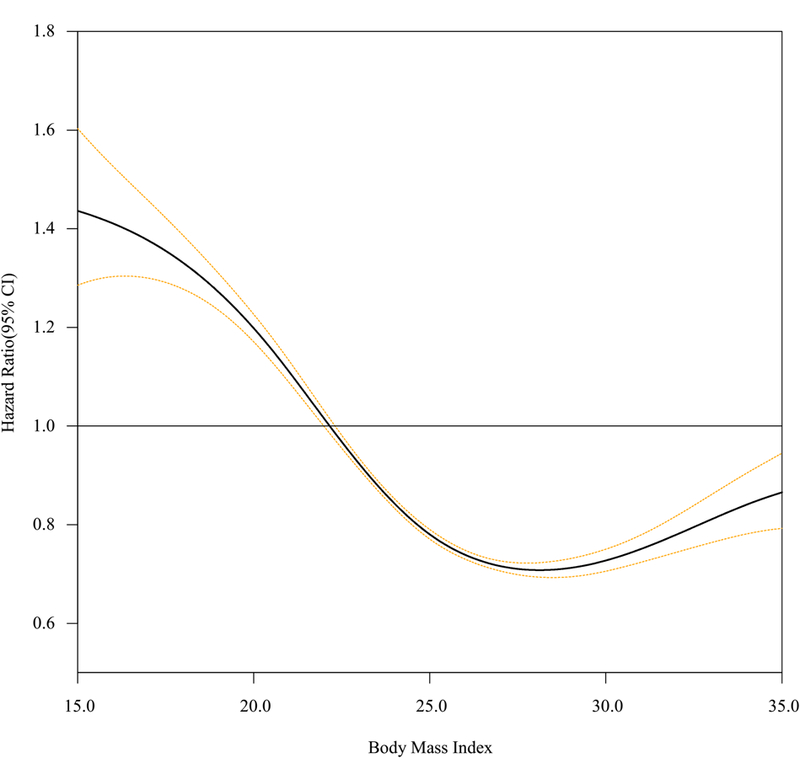
Restricted cubic spline curve of body mass index (BMI) and the risk of severe hypoglycemia in total population.
The association between BMI and severe hypoglycemia was found to be retained on further analysis stratified by subgroups. The association between low BMI and severe hypoglycemia weakened among the subjects >65 years and in women, and showed a steeper curve among the subjects aged 30–49 years and in men (Figure 1, Supplementary table 1). The relationships were similar among subjects who had never smoked, did not consume alcohol, and did not use of insulin (Figure 2, Supplementary table 1). Interactions of sex, age, smoking, alcohol consumption, and use of insulin were significant (P for interaction <0.001), whereas interactions of SES (P for interaction = 0.232), physical activity (P for interaction = 0.186) and sulfonylurea (P for interaction = 0.411) were insignificant on the association between BMI and severe hypoglycemia.
Figure 2.
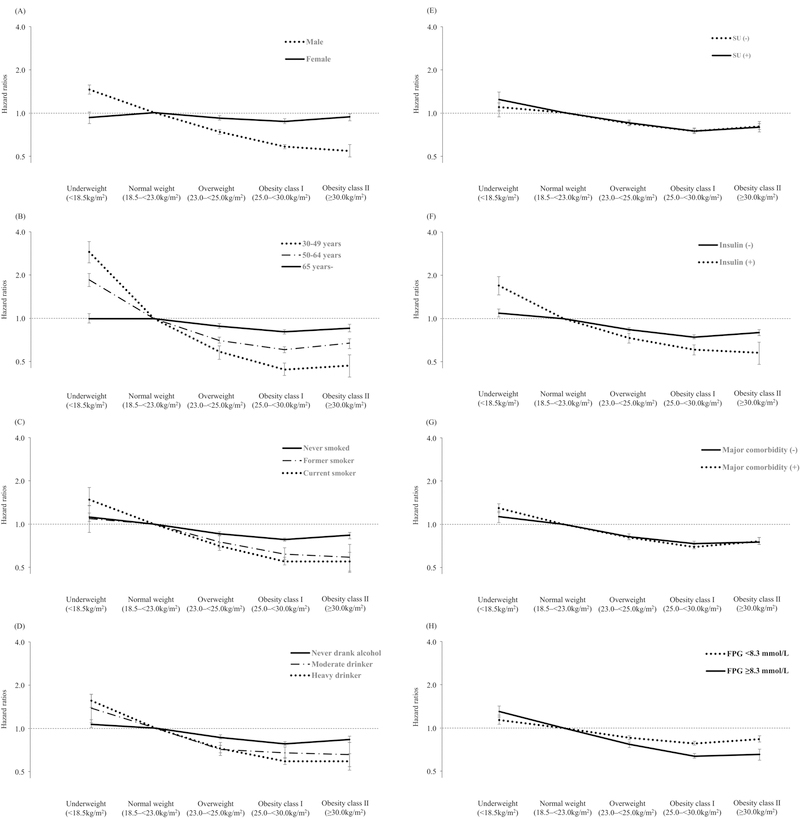
Subgroup analysis for severe hypoglycemia risks; (A) Sex (B) Age (C) Smoking status (D) Drinking habits (E) Use of sulfonylurea (F) Use of insulin, (G) Presence of major comorbidity, (H), Fasting plasma glucose. SU, sulfonylurea; FPG, fasting plasma glucose
We analyzed the association between BMI and risk of severe hypoglycemia within subgroups stratified by major chronic medical comorbidity status (Supplementary table 1). The highest severe hypoglycemia rate was found in subjects in the lowest BMI class, and the adjusted HR was lowest in the group of obese class I in all disease strata. The association between low BMI and severe hypoglycemia increased in subjects with at least one or more major comorbidities (P for interaction <0.001), in subjects with poor glycemic control (fasting plasma glucose ≥8.3 mmol/L, P for interaction <0.001) and in subjects with liver cirrhosis (P for interaction = 0.011). A similar association curve was observed between BMI and risks of severe hypoglycemia within the subgroup that included the participants without major chronic medical diseases.
Conclusions
Our long-term retrospective study demonstrated that BMI and the incidence of severe hypoglycemic events were significantly associated. In general, there was an inverse J-shaped association between BMI and incidence of severe hypoglycemic events in Korean patients with type 2 diabetes. An increased risk of severe hypoglycemia in subjects with BMI of <18.5 kg/m², compared with the reference range (BMI 18.5–<23.0 kg/m²) was also found, with the lowest risk in the group of obese class I (BMI 25.0–<30.0 kg/m²). Further subgroup analyses stratified by smoking, alcohol consumption, and chronic comorbidity status did not change the general association trend between low BMI and severe hypoglycemia, and the intensity of the risk varied across age, sex, smoking, alcohol consumption, medication status, and comorbidity subgroups.
Asians generally tend to have lower BMIs and higher ratio of body fat contents with the same BMI compared to the Western population [13]. Obese Asians are vulnerable to insulin resistance and have an elevated risk of diabetes, hypertension, dyslipidemia, and cardiovascular disease [13]. Based on this evidence, the World Health Organization (WHO) has proposed lower optimal BMI cutoff points for obesity in the Asian population compared to the Caucasian population [14]. The findings of our study, applying WHO criteria for obesity in Asian people, are consistent with the results of previous analyses in Western countries [7, 8]. In this study, the risk of severe hypoglycemia was higher in the underweight category than in the group of obese class I categories.
One of the explanations of our findings on the association between BMI and severe hypoglycemia is “reverse causation” bias, in which weight loss resulting from chronic diseases can distort the independent relationship between underweight and risk of hypoglycemia. To evaluate and control the confounding or reverse causation bias, we stratified subjects with major chronic diseases at baseline. As a result, in patients without major comorbidities, the same relationship between BMI and development of severe hypoglycemia was found. We also found that the association between BMI and severe hypoglycemia varied according to the type of chronic diseases. In patients with liver cirrhosis, low BMI was significantly associated with an increased risk of severe hypoglycemia. The subgroup analysis of ESRD was limited by the presence of a small number of subjects in each BMI category; hence, the precise risk trend could not be determined.
Previous studies have identified the association between BMI and severe hypoglycemia in type 2 diabetes. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, a higher BMI (BMI ≥30.0 kg/m2 vs. <25.0 kg/m2) was associated with decreased risk of severe hypoglycemia (HR 0.65, 95% CI 0.50 to 0.85, P < 0.001) [7]. The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, another large-scale study conducted in patients with type 2 diabetes with high cardiovascular disease risk also showed that a 1.0 kg/m2 increase in BMI resulted in a 5% decrease in the risk of severe hypoglycemia [8]. Among the studies conducted in the Asian population, a recent large-scale study conducted by the Hong Kong Diabetes Registry investigated the association between BMI and severe hypoglycemia. In this study, Kong et al. suggested that a 1.0 kg/m² increase in BMI was associated with a 5% reduced risk of severe hypoglycemia [15]. However, the ACCORD study and ADVANCE trial were designed to include patients with high cardiovascular disease risk, and the study from the Hong Kong Diabetes Registry did not exclude patients with chronic illness, such as cancer and chronic liver and kidney diseases. Therefore, a notable limitation in these studies is reverse causation bias.
A previous study suggested the hypothesis that normal weight persons with diabetes have a different genetic susceptibility to diabetes and are associated with more severe and advanced diabetes [16, 17]. The group with metabolically obese normal weight phenotypes may be prone to exposure to other diseases and “genetically loaded” toward other comorbidities. They may also experience a higher risk of severe hypoglycemia compared to the metabolically healthy obese group [16]. A lower BMI may partially reflect the effects of malnutrition, which has been associated with a higher risk of hypoglycemia [18]. In patients who have low body weight, the capacity of insulin secretion may be decreased [19, 20]. Thus, the use of insulin treatments may be more common in patients with diabetes with low body weight and can cause more hypoglycemic events. In our study, groups with low to normal BMI used more insulin compared to those in the obese category at baseline, and insulin user showed an intensified effect modification about the relationship between low BMI and severe hypoglycemia.
In our study, diabetic patients with low or even normal weight and those of young age had greater risks of severe hypoglycemia compared to obese patients. The magnitude on the risk of developing severe hypoglycemia in younger subjects with low BMI was much higher than that in older subjects with low BMI. Older patients with diabetes may have greater risk factors for severe hypoglycemia, such as cognitive impairment, renal impairment, hepatic impairment, or polypharmacy [4, 21, 22]. In contrast, younger patients have lower numbers of other major severe hypoglycemia risk factors. Low BMI can have a much greater impact on the development of severe hypoglycemia in young patients. In addition, young patients are more likely to comply with the recommendations for intensive glycemic control [1, 23]. BMI was strongly associated with severe hypoglycemia in the male group compared with female groups in the relationship between BMI and severe hypoglycemia in this analysis. To the best of our knowledge, previous studies examining the relationship between BMI and severe hypoglycemia based on sex difference have not been conducted yet. We examined male and female BMI distribution with age, insulin treatment, and comorbidities; however, we failed to find any clear explanation of the sex difference in severe hypoglycemia development observed in this study. The association between low BMI and severe hypoglycemia increased in the group with smokers and heavy drinkers. Smoking and heavy drinking were related to multiple severe comorbidities, which closely related with a development of severe hypoglycemia. In contrast, SES and physical activity did not affect the relationship between BMI and severe hypoglycemia. Low BMI was also strongly associated with severe hypoglycemia in the group with poorly controlled glycemic levels (fasting plasma glucose > 8.3 mmol/L), and this may be due to the high intensity of the hypoglycemic agent or the possibility that the poor glycemic control group may include more patients at high risk for severe hypoglycemia, such as long diabetes duration, old age, polypharmacy, and diabetes complications.
In this study, the risk of severe hypoglycemia was slightly increased in the group with obesity class II (BMI, ≥30 kg/m2) compared to that with obesity class I (BMI, 25–29 kg/m2). Generally, more intensive glycemic control and higher medication dosage which can possibly influence hypoglycemia may be applied in severely obese patients because of insulin resistance [23, 24]. In addition, severe obesity associated with various diseases or states such as kidney dysfunction and autonomic neuropathy may attenuate the defensive mechanism of hypoglycemia [25–27]. Further research is required to confirm this association.
This study has several limitations. First, as this study was based on a medical claims database, misclassification of diagnoses may be possible. Second, this study includes a potential selection bias. A health examination, conducted at least once in 2 years, is recommended by the NHI for all subscribers to health insurance, although not compulsory. Hence, patients included in this study may have been otherwise healthier people or those who happened to be more concerned about their health than the others. Third, the claims database did not include detailed information on patients’ history of diabetes, such as the duration of disease, dosage of medications, and beta cell function. Nevertheless, the strength of this study lies in the fact all relevant medical data for the Korean population were included in the analyses. Furthermore, to the best of our knowledge, this is the largest population-based analysis on the association between BMI and severe hypoglycemia to date.
In conclusion, our results indicate an inverse J-shaped relationship between BMI and the risk of severe hypoglycemia. Especially, underweight patients with type 2 diabetes who are young, men, had chronic comorbidities, and insulin user showed greater risks of severe hypoglycemia. Thus, this category of patients should be warned about the risk of hypoglycemic event and properly educated on hypoglycemia and self-adjustment of medication doses when they receive intensive treatment for diabetes in order to minimize the risk of severe hypoglycemia and hypoglycemia-related fatal outcomes.
Supplementary Material
Acknowledgments
This work was performed by the cooperation with National Health Insurance Service (NHIS), and the National Health Information Database made by NHIS was used (No. NHIS-2016 -1-137). The authors wish to acknowledge the financial support of the St.Vincent’s hospital, research institute of medical science (SVHR-2017-05) and Takeda Pharmaceuticals Korea.
Footnotes
Conflict of interest disclosure
Nothing to declare
References
- [1].Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35(6):1364–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cahn A, Raz I, Kleinman Y, Balicer R, Hoshen M, Lieberman N, et al. Clinical Assessment of Individualized Glycemic Goals in Patients With Type 2 Diabetes: Formulation of an Algorithm Based on a Survey Among Leading Worldwide Diabetologists. Diabetes Care 2015;38(12):2293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yun JS, Ko SH. Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes. Korean J Intern Med 2015;30(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yun JS, Ko SH. Risk Factors and Adverse Outcomes of Severe Hypoglycemia in Type 2 Diabetes Mellitus. Diabetes Metab J 2016;40(6):423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9(1):13–27. [DOI] [PubMed] [Google Scholar]
- [6].Goel K, Lopez-Jimenez F, De Schutter A, Coutinho T, Lavie CJ. Obesity paradox in different populations: evidence and controversies. Future Cardiol 2014;10(1):81–91. [DOI] [PubMed] [Google Scholar]
- [7].Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363(15):1410–8. [DOI] [PubMed] [Google Scholar]
- [9].Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J 2014;38(5):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data Analytic Process of a Nationwide Population-Based Study Using National Health Information Database Established by National Health Insurance Service. Diabetes Metab J 2016;40(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee H, Choi EK, Rhee TM, Lee SR, Lim WH, Kang SH, et al. Cirrhosis is a risk factor for atrial fibrillation: A nationwide, population-based study. Liver international : official journal of the International Association for the Study of the Liver 2017. [DOI] [PubMed]
- [12].Lee SR, Choi EK, Rhee TM, Lee HJ, Lim WH, Kang SH, et al. Evaluation of the association between diabetic retinopathy and the incidence of atrial fibrillation: A nationwide population-based study. Int J Cardiol 2016;223:953–7. [DOI] [PubMed] [Google Scholar]
- [13].Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363(9403):157–63. [DOI] [PubMed] [Google Scholar]
- [14].Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368(9548):1681–8. [DOI] [PubMed] [Google Scholar]
- [15].Kong AP, Yang X, Luk A, Ma RC, So WY, Ozaki R, et al. Severe hypoglycemia identifies vulnerable patients with type 2 diabetes at risk for premature death and all-site cancer: the Hong Kong diabetes registry. Diabetes Care 2014;37(4):1024–31. [DOI] [PubMed] [Google Scholar]
- [16].Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308(6):581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS genetics 2012;8(5):e1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27(1):5–15. [DOI] [PubMed] [Google Scholar]
- [19].Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52(1):102–10. [DOI] [PubMed] [Google Scholar]
- [20].Funakoshi S, Fujimoto S, Hamasaki A, Fujiwara H, Fujita Y, Ikeda K, et al. Analysis of factors influencing pancreatic beta-cell function in Japanese patients with type 2 diabetes: association with body mass index and duration of diabetic exposure. Diabetes Res Clin Pract 2008;82(3):353–8. [DOI] [PubMed] [Google Scholar]
- [21].Kim JT, Oh TJ, Lee YA, Bae JH, Kim HJ, Jung HS, et al. Increasing trend in the number of severe hypoglycemia patients in Korea. Diabetes Metab J 2011;35(2):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35(4):787–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med 2011;154(8):554–9. [DOI] [PubMed] [Google Scholar]
- [24].Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55(6):1577–96. [DOI] [PubMed] [Google Scholar]
- [25].Chalmers L, Kaskel FJ, Bamgbola O. The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv Chronic Kidney Dis 2006;13(4):352–64. [DOI] [PubMed] [Google Scholar]
- [26].Yun JS, Kim JH, Song KH, Ahn YB, Yoon KH, Yoo KD, et al. Cardiovascular autonomic dysfunction predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care 2014;37(1):235–41. [DOI] [PubMed] [Google Scholar]
- [27].Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115(3):387–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


