Abstract
Background
There are limited data on the risk of hepatocellular cancer (HCC) in patients with non-alcoholic fatty liver disease (NAFLD). We aimed to estimate the risk of incident HCC among patients with NAFLD.
Methods
We conducted a retrospective cohort study from a total of 130 facilities in the Veterans Health Administration. Patients with NAFLD diagnosed between 1/1/2004 and 12/31/2008 were included and followed until HCC diagnosis, death or 12/31/2015. We also identified a gender and age-matched control cohort without NAFLD. We ascertained all new HCC cases from the Central Cancer Registry and manual chart reviews. We calculated incidence rates for HCC by NAFLD status as well as in subgroups of NAFLD patients. We used competing risk models to compare the risk of HCC in patients with vs. those without NAFLD. We reviewed electronic medical records of all HCC cases that developed in NAFLD patients without cirrhosis.
Results
We compared 296,707 NAFLD patients with 296,707 matched controls. During 2,382,289 person-years [PY] of follow-up, 490 NAFLD patients developed HCC (0.21/1000 PY). HCC incidence was significantly higher among NAFLD patients vs. controls (0.02/1000 PY; hazard ratio, 7.62, 95% confidence interval=5.76–10.09). Among patients with NAFLD, those with cirrhosis had the highest annual incidence of HCC (10.6 /1000 PY). Among patients with NAFLD cirrhosis, HCC risk ranged from 1.6 to 23.7 per 1000 PY based on other demographic characteristics; the risk of HCC was the highest in older Hispanics with cirrhosis. In medical record reviews, 20% of NAFLD patients with HCC had no evidence of cirrhosis.
Conclusions
Risk of HCC was higher in NAFLD patients than that observed in general clinical population. Most HCC cases in NAFLD developed in patients with cirrhosis. The absolute risk of HCC was higher than the accepted thresholds for HCC surveillance for most patients with NAFLD cirrhosis.
Keywords: Hepatitis, liver cancer, outcome, cohort
BACKGROUND
Hepatocellular cancer (HCC) is a rapidly increasing, highly fatal cancer.1–3 Major risk factors for HCC in the U.S. include hepatitis C virus (HCV), heavy alcohol drinking, and hepatitis B virus (HBV) infection. Recent studies reported the absence of any of these known major risk factors in a large proportion (20–40%) of patients with HCC.4 Some, if not all, of these HCC cases are speculated to have non-alcoholic fatty liver disease (NAFLD) as the underlying etiological risk factor.
NAFLD has become the leading cause of chronic liver disease in the U.S.5 It is posited as the hepatic manifestation of the metabolic syndrome, and is closely associated with diabetes and obesity. Coinciding with large increases in metabolic syndrome, the prevalence of NAFLD in the general population has doubled in the past 2 decades with estimates as high as 30%.6,7 NAFLD is often a non-progressive hepatic steatosis associated with few, if any, hepatic complications. However, at least 20–30% of patients with NAFLD develop progressive liver disease with necroinflammation and fibrosis that can result in cirrhosis in 10–20% of cases.8 NAFLD is the fastest growing cause of cirrhosis in the U.S.9–a concerning trend given the possible association between NAFLD related cirrhosis and HCC.10 HCC has also been reported to arise in patients with NAFLD in the absence of cirrhosis.11
Despite the increasing recognition of NAFLD and its potential association with HCC, there are currently mixed data regarding the exact magnitude of HCC risk in patients with NAFLD. Our previous systematic review of epidemiological studies (published through 2011) examining the NAFLD-HCC link found HCC risk ranging from 0% to 38% over 5 to 10 years of follow up.10 Most of the published studies included small or modest sized NAFLD cohorts and thus had few (or no) incident HCC cases, resulting in highly imprecise HCC risk estimates. Several of the largest studies relied on diagnostic codes to define NAFLD, and none reported efforts to validate codes against clinical data. Furthermore, most studies evaluated patients in the tertiary care settings. Thus, the generalizability of these findings to community-based clinical populations with NAFLD is unclear.
In summary, although the current evidence provides some support of an association between NAFLD and HCC, it is not possible to draw firm conclusions regarding the exact magnitude of HCC in NAFLD cases overall, let alone in subgroups defined by age, gender or race/ethnicity. Well-designed longitudinal cohort studies including large numbers of NAFLD cases with both sufficient follow up time and relevant number of HCC outcomes are needed to quantify this risk. We conducted a large retrospective cohort study to examine the risk of HCC among patients with NAFLD seen in the U.S. national Veterans Health Administration (VHA) system.
METHODS
Data Source
We used data from the national VHA Corporate Data Warehouse (CDW) and Central Cancer Registry (CCR). CDW includes all laboratory test results, inpatient and outpatient utilization, and diagnosis (ICD) codes. CDW also contains information from annual Alcohol Use Disorders Identification Test (AUDIT-C) screen and Vital Status files.12,13 AUDIT-C has been used to screen over 90% of VA outpatients nationwide since 2004.13 CCR is a centralized repository for over 750,000 VHA patients with cancer and includes information on date of diagnosis, primary site, and histology.
Study Cohorts
We evaluated all patients 18 years or older who had at least one visit to any of the VHA hospitals in the nation between January 1, 2003 and December 31, 2011 for this study.
NAFLD Cohort:
We modified our previously published algorithm to define NAFLD in CDW.14 We examined the positive and negative predictive values of our operational definition in a sample of 300 patients (see below). Patients were classified as having NAFLD if they had two or more elevated ALT values (≥40 IU/ml for men and ≥31 IU/ml for women) in the ambulatory settings and more than 6 months apart, with no positive serologic testing for HBV (i.e., HBV surface antigen) or HCV (i.e., HCV RNA). We excluded patients if they had any alcohol related ICD-9 codes or positive AUDIT-C scores (≥ 4 in men and ≥3 in women) any time prior to or during study follow up. We also excluded patients with evidence of rare chronic hepatitides (hereditary hemochromatosis, primary biliary cirrhosis, primary sclersoing cholangitis, alpha-1 antitrypsin disease, or autoimmune hepatitis) defined based on ICD-9 codes.
We used the date of first elevated ALT as the index date of follow up for NAFLD cases. We included NAFLD patients with an index date from January 1, 2004 to December 31, 2008 in this analysis because AUDIT-C was implemented in the VA in 2004. We used 2008 as the cut-off to define study cohorts to allow sufficient follow up (minimum 5 years) for all patients.
Control Cohort:
We selected controls from individuals who had an ALT test performed from January 1, 2004 to December 31, 2008, but did not have NAFLD (as defined above) or any other documented liver related risk factor, as evidenced by persistently normal ALT, absence of positive tests for HBV and HCV, and no evidence of excess alcohol use (defined as absence of alcohol ICD-9 codes and all AUDIT-C scores <4.0 in men and <3.0 in women). We classified the date of first ALT test in the study timeframe as the index date of follow up for controls. We used random sampling without replacement to select a control cohort matched to NAFLD cases on gender, age at first ALT (index date), and duration from their first VA visit to the first ALT test date; the latter ensured that the control cohort was similar to cases in terms of duration of use of the VA healthcare system to allow equal ascertainment of NAFLD status and other baseline covariates.
Chart Validation of Cohort Definitions.
We estimated the positive and negative predictive value of our definitions of NAFLD cases and controls by comparing them with diagnoses derived from a structured review of the patients’ electronic medical records (EMR). We selected a random nationwide sample of 300 patients (150 NAFLD cases and 150 controls) from the matched cohorts for the EMR reviews. Two trained clinicians (YN, SM) abstracted the EMR using a standardized, detailed abstraction form to determine the presence ⁄ absence of NAFLD based on clinical, biochemical, radiological, or histological data from the EMR review, as previously described.14 The abstractors were blinded to the case and control status. Any disagreements were discussed and resolved after discussion with a third clinician (FK).
Variable Specification
Outcome:
We used a hierarchical approach to define the occurrence of HCC in the NAFLD and control cohorts. First, we examined the VA CCR for patients with possible HCC diagnosis based on primary site code C220 with histology codes 817XX through 818XX as well as text searches. We reviewed the EMR of selected cases identified as HCC per CCR to verify HCC diagnosis. We then identified patients with any ICD-9 code (155.0) for HCC from the inpatient and outpatient files of CDW. We examined the discordance of HCC diagnosis in the two groups of patients classified as HCC based on CCR and ICD-9 to identify patients who had an ICD-9 code but was not identified as HCC in the CCR data. We conducted a manual review of the EMR for each discordant patient to determine their true HCC status. This hierarchical approach ensured high validity of all the captured HCC cases.
Covariates:
These variables included age at index date of follow up, gender, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other). We defined body mass index (BMI) by using height and weight values within any time before to one year after and nearest to the index date during the study period. We defined diabetes and hypertension by ≥2 outpatient or ≥1 inpatient ICD-9 codes or ≥ 1 filled prescription of diabetes medications (oral hypoglycemic medications or insulin) or anti-hypertensives, respectively. We classified patients as having diabetes or hypertension at baseline if the first diagnostic code instance for the respective condition occurred any time before to 1 year after NAFLD index date. Similarly, we defined cirrhosis as ≥2 outpatient or ≥1 inpatient ICD-9 code for cirrhosis or its complications; we classified patients with cirrhosis at baseline if their first diagnosis date was recorded any time before to 1 year after NAFLD index date, or during follow up if the first diagnosis was recorded after 1 year of follow up and before the development of HCC or end of study).15 We also calculated FIB-4 to define liver fibrosis severity in our NAFLD cohort, starting within one year of the index date and for each subsequent year until the end of follow up. We calculated FIB-4 using laboratory results from AST, ALT and platelet tests performed in ambulatory settings and recorded within 6 months of each other, as previously described: FIB-4 = Age (years) × AST (U/L) / [PLT(109/L)×ALT1/2 (U/L)]. We excluded tests performed within 1 year of HCC diagnosis to avoid values that might be related to HCC. In the event of multiple tests in the same year, we calculated the median to define the annual value. We used the cut-off ≥2.67 because it is shown to be highly predictive of the presence of advanced fibrosis/cirrhosis in patients with NAFLD.16 It is plausible that FIB-4 values might fluctuate overtime. Given this, we classified patients as having persistently high FIB-4 if they had ≥ 2 values ≥2.67 during follow up or if they had a single FIB-4 test that was ≥2.67; more than 95% of patients met this definition based on the first criterion. When comparing our FIB-4 in NAFLD patients with cirrhosis codes, most of those with cirrhosis (77.3%) had a FIB-4 ≥2.67 and 68.1% had persistently high FIB-4.
Statistical Analyses
EMR Review Validation.
We calculated the positive predictive value (PPV), negative predictive value (NPV) for our modified definitions in correctly identifying NAFLD cases and controls in the EMR.
Primary Analyses
We calculated the annual and cumulative incidence rates for HCC in the NAFLD and control cohorts. We followed patients from index date to the development of HCC, death, or 12/31/2015, whichever was earlier. Because our objective was to examine the risk of incident HCC, we excluded patients with evidence of HCC prior to or within 12 months after NAFLD index date. We generated Kaplan– Meier curves to illustrate and compare the cumulative incidence functions of HCC by NAFLD status, and used the log-rank test to evaluate the difference between curves. We estimated hazard ratio (HR) and 95% confidence intervals (CIs) for the association between NAFLD and risk of HCC using the Fine and Gray proportional hazards models to adjust for the competing risk for death. Last, we conducted multivariable models adjusting for pre-specified variables of race/ethnicity, BMI, diabetes and hypertension to determine the independent effect of NAFLD on the risk of HCC.
Sensitivity Analyses.
We conducted several sensitivity analyses to examine the robustness of our results were sensitive to cohort specification. More than two-thirds (63.4%) of patients (70.1% in cases and 57.1% in controls) had received at least one HCV test during the study timeframe. Similarly, 83.2% of patients had at-least one documented AUDIT-C test [mean (SD) =5.5 (3.2) and 4.2 (3.4) AUDIT-C tests in cases and controls, respectively]. However, some degree of misclassification might have occurred where we included patients with undiagnosed HCV or alcohol use in our cohorts, especially if they did not have any HCV or AUDIT-C testing during the study period. To minimize any bias related to this potential misclassification, we conducted 2 sensitivity analyses: one limited to cases and controls who had complete rule outs (i.e., had ≥ 1 HCV test and ≥ 1 AUDIT-C test documented in the database; all negative) and the second after using multiple imputation of missing HCV and AUDIT-C data for cases and controls (see Supplementary materials for details of multiple imputation). Although VA is a semi-closed system, some patients might not seek regular healthcare at the VA. It is plausible that differences in healthcare utilization in cases and controls might differentially impact HCC ascertainment. We, therefore, limited our NAFLD case and control cohorts to patients with at least 2 visits in each of the 2 sequential years following NAFLD index to examine any potential bias related to differential follow up. For all sensitivity analyses, we recreated the matched case-control set, as done for the main analysis. It is also possible that some patients in the control cohort had NAFLD with persistently normal biochemistries and were misclassified. Because of the strong association between metabolic syndrome (especially diabetes) and NAFLD, we calculated the annual incidence rate for HCC in a subgroup of control subjects with diabetes.
Subgroup and Secondary Analyses.
We calculated the annual incidence rates for HCC in several pre-specified subgroups of patients with NAFLD including subgroups defined by gender, age, race/ethnicity, cirrhosis status, and FIB-4 categories. For the annual HCC incidence in patients with cirrhosis or high FIB-4, we calculated HCC risk from the date of cirrhosis diagnosis or first high FIB-4, whichever occurred first, to the development of HCC, death, or 12/31/2015. We also identified the subset of NAFLD patients that had documented evidence of hepatic steatosis on liver imaging (ultrasound, computerized tomography or magnetic resonance imaging tests) using our previously developed and validated natural language processing algorithm, and we calculated the annual incidence rate for HCC in this subgroup of NAFLD patients.17
HCC can occur in the absence of cirrhosis in NAFLD. We reviewed EMR of all HCC cases that were recorded in NAFLD patients without evidence of cirrhosis. A single trained clinician (SM) reviewed histopathology, imaging studies, clinical presentation, endoscopic findings and laboratory tests to ascertain cirrhosis status at the time of HCC diagnosis.11,18 We used pre-specified explicit criteria and classified a patient with cirrhosis if he/she had evidence of cirrhosis based on histopathology (i.e., stage 4 hepatic fibrosis before HCC diagnosis), radiology (i.e., morphological features or changes consistent with cirrhosis or portal hypertension on abdominal imaging before HCC diagnosis), or clinical complications (i.e., ascites, hepatic encephalopathy, variceal bleeding before HCC diagnosis). We defined patients as having “possible” cirrhosis if they did not have radiological, endoscopic and clinical criteria of cirrhosis but the FIB-4 values were ≥2.67.17 We classified patients as subjects without cirrhosis if they had a liver biopsy that documented non-cirrhosis histopathology within one year before HCC diagnosis; or in the absence of a liver biopsy, absence of cirrhosis related morphologic changes and signs of portal hypertension on liver imaging; absence of esophageal varices, gastric varices, and portal hypertensive gastropathy on upper endoscopy; and all FIB-4 values <2.67 within one year before HCC diagnosis.
All tests for statistical significance were two-sided at α = 0.05.
RESULTS
Patient characteristics
We identified 296,707 patients with NAFLD and 296,707 matched controls. The mean age at the time of first ALT elevation in NAFLD patients was 55.4 year (standard deviation, SD 13.2 years), 94.4% were men, 68.7% were white, 11.4% were African American (AA), and 5.3% were Hispanic. The median BMI was 30.7 (interquartile range, 27.5 to 34.6), 29.9% had diabetes, and 70.8% had hypertension. Only 0.4% of NAFLD patients had a diagnosis of cirrhosis at baseline; 1.4% had a diagnosis of cirrhosis anytime during the study duration. In total, 12.5% of NAFLD patients had evidence of persistently high FIB-4.
Compared with controls, NAFLD patients were significantly more likely to be overweight or obese, have diabetes, hypertension, and cirrhosis diagnosis (all p-values <0.0001) (Table 1).
Table 1.
Demographic and clinical characteristics of patients with and without nonalcoholic fatty liver disease (NAFLD) at baseline. All p-values <0.0001
| Characteristic, N (%) | NAFLD (n=296,707) |
Non-NAFLD (n=296,707) |
|---|---|---|
| Age, mean (SD) | 55.4 (13.2) | 55.4 (13.1) |
| Gender | ||
| Men | 280,177 (94.4) | 280,177 (94.4) |
| Women | 16,530 (5.6) | 16,530 (5.6) |
| Race/ethnicity | ||
| White | 203,814 (68.7) | 175,487 (59.1) |
| African American | 33,755 (11.4) | 46,408 (15.6) |
| Hispanic | 15,839 (5.3) | 11,446 (3.9) |
| Other races | 7,803 (2.6) | 6,243 (2.1) |
| Missing | 35,496 (12.0) | 57,123 (19.3) |
| BMI median, IQR | 30.7 (7.0) | 28.7 (6.9) |
| Hypertension | 210,194 (70.8) | 178,483 (60.2) |
| Diabetes | 88,593 (29.9) | 69,072 (23.3) |
| Cirrhosis | 1,084 (0.4) | 534 (0.2) |
SD: standard deviation; IQR: interquartile range
The mean durations of follow-up for the NAFLD and control cohorts were 9.0 (SD 2.2) and 8.67 (SD 2.60) years, respectively. On average, patients had 2.71 (SD 0.52) visits to the VA in the first 2 years following index date.
Results of Chart Validation
We found high accuracy for our case and control definitions compared with chart reviews. In the entire sample, the PPV for the modified NAFLD algorithm was 89% (95% CI=84–94%) and NPV was 98% (95% CI=93 – 99%). Of the cases classified as NAFLD based on our EMR review, 40% had ≥ 1 abdominal imaging test performed before or after index date and 85% had a mention of findings suggestive of NAFLD (steatosis or increased echogenicity).
HCC case ascertainment
A total of 367 patients in the case and control cohorts were diagnosed with HCC during follow up in the VA CCR. We reviewed the EMR of 30 randomly selected cases from this group; all (100%) had confirmed HCC in EMR. An additional 17 patients were classified as possible HCC based on text searches of histology field in the CCR. We reviewed EMR of these patients and confirmed HCC in 5 patients. We identified an additional 406 patients in our cohorts who had ICD codes for HCC but not diagnosed as such in VA CCR. We reviewed EMR of all of these discordant cases and 173 (42.6%) had HCC confirmed in the EMR; these cases would have been missed based on CCR alone. This yielded a total of 545 patients with confirmed incident HCC in the combined cohorts.
Risk of HCC in NAFLD
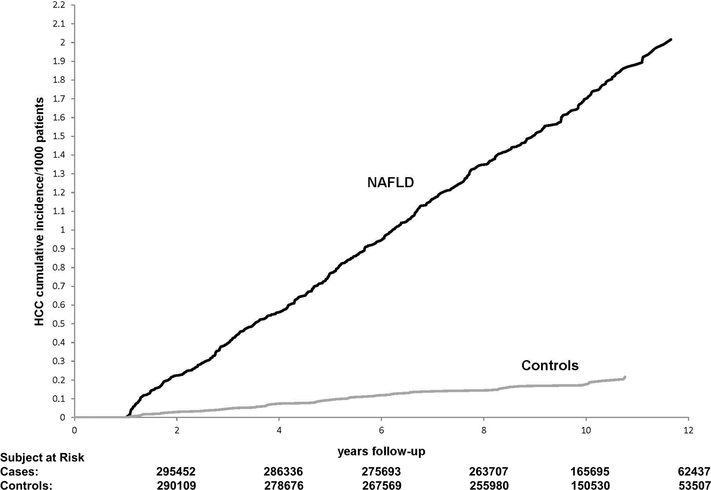
HCC developed in 490 patients with NAFLD during 2,382,289 PY follow up at an annual incidence rate of 0.21 per 1000 PY (95% CI, 0.19 to 0.22 per 1000 PY). This rate was significantly higher than the 0.02 (95% CI, 0.02 to 0.03) per 1000 PY incidence rate in the control group without NAFLD (55 HCC cases during 2,280,685 PY of follow-up). The 5 and 10-year cumulative incidence rates of HCC were 0.8 and 1.7 per 1000 patients in NAFLD vs. 0.09 and 0.18 per 1000 patients in controls.
NAFLD status was associated with higher HCC incidence throughout follow-up starting 1 year after index date and increasing over time (p value <0.0001) (Figure 1). In the unadjusted analysis, patients with NAFLD had a 8.6- fold higher risk of HCC than controls (unadjusted HR=8.61, 95% CI=6.54–11.37). In multivariable analyses, NAFLD remained associated with more than 7.6-fold higher risk of HCC after adjusting for race and features of metabolic syndrome (adjusted HR=7.62, 95% CI=5.76–10.09) (Table 2).
Figure 1:
Cumulative Incidence of hepatocellular cancer in patients with and without nonalcoholic fatty liver disease (NAFLD).
Table 2.
Association between non-alcoholic fatty liver disease (NAFLD) and hepatocellular cancer. Results of multivariable analysis
| Characteristic | Adjusted HR (95% CI) | P-value |
|---|---|---|
| NAFLD, ref no | -- | |
| Yes | 7.62 (5.76–10.09) | <0.0001 |
| Age, ref 46–64 years | -- | |
| ≤45 year | 0.05 (0.02–0.13) | <0.0001 |
| ≥65 years | 1.83 (1.53–2.18) | <0.0001 |
| Race/ethnicity, ref White | -- | |
| Hispanic | 1.59 (1.14–2.20) | 0.005 |
| African American | 0.74 (0.53–1.03) | 0.07 |
| Other race | 1.03 (0.59–1.78) | 0.92 |
| Missing | 1.16 (0.90–1.49) | 0.25 |
| Body mass index, ref <30 | -- | |
| ≥30 | 1.18 (0.99–1.42) | 0.06 |
| Diabetes, ref no | -- | |
| Yes | 3.03 (2.52–3.64) | <0.0001 |
| Hypertension, ref no | -- | |
| Yes | 1.10 (0.84–1.43) | 0.50 |
Sensitivity and Subgroup Analyses
Sensitivity analyses limited to patients with complete rule outs (i.e., those who had all negative HCV and AUDIT-C tests), after multiple imputation of missing HCV and AUDIT-C data, and after limting to patients with ≥ 2 visits following index date did not change the overall results (Table 3). In total, 32 of the 55 HCCs in the controls developed in patients with diabetes at baseline. In the subgroup of controls with diabetes, the annual incidence of HCC was 0.07 per 1000 PY (95% CI, 0.04–0.09), which was statistically significantly lower than the incidence in NAFLD cases with diabetes (0.45 per 1000 PY; 95% CI=0.40 – 0.51, p-value <0.0001).
Table 3.
Association between non-alcoholic fatty liver disease (NAFLD) and hepatocellular cancer. Results of sensitivity analyses.
| Group | HCC Incidence | |||
|---|---|---|---|---|
| HCC Cases | Total personyear (PY) of follow-up | IR (95% CI) (per 1000 PY) | Adjusted HR (95% CI) | |
| Limiting to patients with complete rule out for HCV and alcohol | ||||
| NAFLD (N=164,259) | 309 | 1,364,656 | 0.23 (0.20–0.25) | 5.86 (4.29–7.99) |
| Non-NAFLD (N=164,259) | 46 | 1,342,817 | 0.03 (0.03–0.05) | Ref |
| Using all patients with imputation of missing data on HCV and alcohol | ||||
| NAFLD (N=232,056) | 395 | 1,894,084 | 0.21 (0.19–0.23) | 6.5 (4.8–8.6) |
| Non-NAFLD (N=232,056) | xx | 1,865,435 | 0.03 (0.02–0.04) | Ref |
| Limiting to patients with at least 1 visit in each of the 2 consecutive follow up years following index date | ||||
| NAFLD (N=254,100) | 455 | 2,033,812 | 0.22 (0.20–0.25) | 6.81 (5.21–8.91) |
| Non-NAFLD (N=254,105) | 61 | 1,993,340 | 0.03 (0.02–0.04) | Ref |
Table 4 displays the annual incidence rates of HCC in key subgroups of NAFLD patients. HCC incidence was higher in men than in women (0.22 vs. 0.04 per 1000 PY, respectively) and in patients older than 65 years at NAFLD index (0.41 per 1000 PY) than in the younger age groups (0.01 and 0.21 per 1000 PY in patients aged <45 and 45–64 years at the time of NAFLD index, respectively). Hispanics with NAFLD had the highest HCC incidence (0.29 per 1000 PY) followed by Whites (0.21 per 1000 PY) and AAs (0.12 per 1000 PY). The high HCC rates among NAFLD patients of Hispanic ethnicity was evident across all ages but was the most prominent in the oldest group; HCC developed at a rate of 0.93 per 1000 PY in Hispanics who were older than 65 year at the time of NAFLD index. Of the 296,707 NAFLD patients, 53,441 patients had abdominal imaging with documented steatosis. HCC developed in 165 patients with hepatic steatosis during 433,378 PY follow up at an annual incidence rate of 0.45 per 1000 PY (95% CI, 0.39 to 0.54 per 1000 PY).
Table 4.
Incidence of hepatocellular cancer (HCC) in subgroup of patients with nonalcoholic fatty liver disease.
| Group | HCC Incidence | ||
|---|---|---|---|
| HCC cases | Total person-year (PY) of follow-up | IR (95% CI) (per 1000 PY) | |
| Age (year) | |||
| ≤45 | 4 | 563,905 | 0.01 (0.00–0.02) |
| 46–64 | 281 | 1,312,276 | 0.21 (0.19–0.24) |
| ≥65 | 205 | 506,108 | 0.41 (0.35–0.46) |
| Gender | |||
| Men | 484 | 2,242,258 | 0.22 (0.20–0.24) |
| Women | 6 | 140,030 | 0.04 (0.02–0.09) |
| Race/ethnicity | |||
| White | 345 | 1,638,140 | 0.21 (0.19–0.23) |
| African American (AA) | 32 | 273,494 | 0.12 (0.08–0.17) |
| Hispanic | 38 | 129,745 | 0.29 (0.21–0.40) |
| Age and race/ethnicity | |||
| <65 year | |||
| White | 199 | 1,259,727 | 0.16 (0.14–0.18) |
| AA | 24 | 242,465 | 0.10 (0.06–0.15) |
| Hispanic | 20 | 110,350 | 0.18 (0.11–0.28) |
| ≥65 year | |||
| White | 146 | 378,413 | 0.39 (0.33–0.45) |
| AA | 8 | 31,029 | 0.26 (0.11–0.51) |
| Hispanic | 18 | 19,395 | 0.93 (0.55–1.47) |
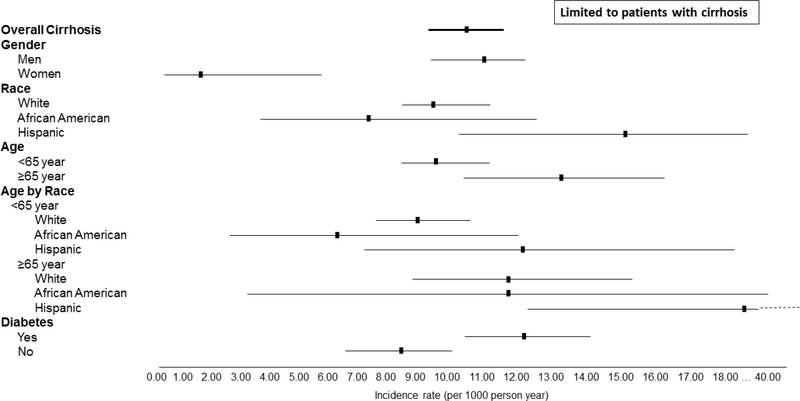
We also examined the incidence of HCC in NAFLD patients with vs. those without established cirrhosis diagnosis. HCC developed at an annual incidence rate of 0.08 per 1000 PY in NAFLD patients without cirrhosis (compared with 0.02 per 1000 PY incidence rate in the control group without NAFLD). In contrast, the annual incidence of HCC was 10.6 per 1000 PY in patients with cirrhosis diagnosis. Among patients with NAFLD cirrhosis, HCC risk ranged from 1.6 to 23.7 per 1000 PY based on other demographic characteristics (Figure 2, Supplementary Table 1); the risk of HCC was the highest in older Hispanics with cirrhosis (Figure 2, Supplementary Table 1).
Figure 2:
Incidence of hepatocellular cancer in different subgroups of non-alcoholic fatty liver disease patients with cirrhosis
We further examined the effect of FIB-4 score with or without cirrhosis diagnosis on HCC risk. High FIB-4 in the presence of cirrhosis diagnosis was associated with the highest HCC risk (13.5 per 1000 PY). The HCC risk was 0.39 per 1000 PY in patients who had high FIB-4 in the absence of cirrhosis diagnosis compared with 0.04 per 1000 PY in patients with no cirrhosis and persistently low FIB-4. Supplementary Table 1 displays the risk of HCC in the latter 2 subgroups based on other demographic and clinical characteristics.
A total of 193 HCCs developed in NAFLD patients without evidence of documented cirrhosis diagnosis during study follow up. Based on our manual EMR review, 98 (50.7% of 193; 20.0% of 490) patients with HCC had evidence of absence of cirrhosis. A total of 62 (32.1% of 193) patients had EMR evidence of cirrhosis; an additional 20 (10.3% of 193) were classified as having possible cirrhosis during follow up; of the patients with cirrhosis or possible cirrhosis, 62% had evidence of high FIB-4 during follow up. Cirrhosis status could not be determined in 13 (6.7% of 193) patients. With the exception of 5 patients, all patients had NAFLD as underlying etiology; yet NAFLD was mentioned as possible etiology in fewer than 15% of charts.
DISCUSSION
Our study has 4 key findings.
First, the risk of HCC in patients with NAFLD was significantly higher than that in age and gender matched controls without NAFLD or other major liver disease risk factors. HCC incidence rates became more elevated with longer follow up and the high relative risk persisted after adjusting for race and metabolic syndrome features.
Second, despite the relative increase in HCC risk, the absolute risk of HCC was low in NAFLD. HCC developed in 490 patients during average 9 years of follow up at an annual incidence of 0.21 per 1000 PY. This translated into a 5 and 10 year cumulative HCC risk of 0.8 and 1.7 per 1000 patients, respectively. The absolute risk varied in key subgroups. The risk of HCC was higher in men and older patients while it was significantly lower in women and patients who were younger than 45 years at the time of NAFLD index. There was a significant race effect in our analyses, where Hispanics had a higher and AA had a lower risk of developing HCC than whites. The absolute risk of HCC was the highest among older Hispanics, with annual incidence of 0.93 per 1000 PY in this subgroup of patients. This racial disparity persisted in NAFLD patients with or without cirrhosis. This variability may be explained by differences in genetic predisposition, health behaviors, and prevalence of other etiological risk factors such as metabolic syndrome in racial/ethnic groups. Notwithstanding the underlying mechanisms, our results highlight the importance of HCC risk reduction in older men and Hispanics with NAFLD.
Third, cirrhosis was the most common as well the strongest risk factor for HCC in NAFLD. HCC risk estimates reached or exceeded the cut-offs (0.8–2.3% per year) beyond which HCC surveillance may become cost-effective19 for all subgroups with cirrhosis, with few exceptions; the risk of HCC was low in AA and women with cirrhosis. If confirmed in future studies, these findings will have important implications for HCC surveillance guidelines in subgroups with NAFLD cirrhosis.
Fourth, ~20% of the overall NAFLD-related HCC cases occurred in the absence of cirrhosis in our cohort. Other studies have reported even a higher proportion of NAFLD-related HCC cases (10–75%) that developed in the absence of cirrhosis.20–22 Therefore, it is plausible that carcinogenesis can occur in NAFLD in the absence of advanced fibrosis/cirrhosis. However, our results show that cirrhosis is the main precursor lesion for most HCCs in NAFLD – a group that will need to be targeted in HCC screening programs. The overall risk in patients without cirrhosis is too low to recommend HCC screening at this time until future studies identify biomarkers that can reliably identify at risk subgroups among the general masses with NAFLD.
Our case definition for NAFLD captured those with biochemically apparent NAFLD and therefore did not include individuals that might have had evidence of hepatic steatosis yet who never developed abnormal liver biochemistries during the study period. Our control group may have included some misclassified cases with NAFLD and therefore the calculated risk estimates for HCC in NAFLD are likely to be conservative. To further evaluate this, we examined the risk of HCC in a subgroup of controls with diabetes (as a surrogate of possibly misclassified NAFLD). Indeed, more than half of the HCC cases in our control group developed in patients with diabetes; however, the HCC risk was still substantially lower in this subgroup (0.07 per 1000 PY) than the risk in patients classified as NAFLD (especially with diabetes) per our explicit definition. Our study shows that biochemically apparent NAFLD may be the most relevant condition in terms of HCC risk. Our previous data show that most of these patients with apparent NAFLD remain unrecognized in routine practice. 23 Only 40% of patients in the NAFLD cohort had any abdominal imaging. Of these, the annual incidence rate of HCC was 0.45 per 1000 PY in the subgroup with evidence of hepatic steatosis on imaging reports. We believe this estimate may represent risk of HCC in most patients with NAFLD diagnosed as part of routine care (i.e., patients who receive abdominal imaging). However, our overall estimate may be more reflective of the HCC risk in the larger population of patients with NAFLD, including many individuals who may never receive (or need) abdominal imaging. Collectively, these findings also suggest that a population-based study that relies on hepatic steatosis (per imaging or biopsy) may significantly limit the generalizability of HCC risk estimates to general patients with NAFLD. Overall, we believe that our comprehensive evaluation and quantification of HCC risk for different patient subgroups is very useful to public health authorities and health plans that are trying to use similar data to manage patients with NAFLD.
Our study has several strengths. We combined the advantages of the comprehensive fully automated nationwide VA clinical, laboratory, and administrative databases with those of direct complementary manual EMR abstractions. We examined the validity of our algorithm for identifying patients with NAFLD using these automated data, including those who have not received a formal diagnosis of NAFLD. We verified all HCC diagnoses and adjudicated cirrhosis status for all HCC cases without documented cirrhosis diagnosis. We were thus able to examine a large cohort of patients with NAFLD for an extended follow-up period – a feat not otherwise achievable using other extant data sources without sacrificing the accuracy and completeness of data on the main confounders and outcomes.
Our study has limitations. Although, we relied on both ICD code and the longitudinal history of serial AUDIT-C results to determine alcohol use, we might still have underestimated alcohol use in our cohorts. However, we believe that the any misclassification bias related to alcoholic liver disease is quite minimal as demonstrated by the high PPV and NPV of our cohort definitions. Similarly, we might have missed some cases with undiagnosed HCV and HBV and these missed etiological factors might underlie the few cases of HCC observed in our control cohort. To address this, we conducted a sensitivity analysis limited to matched cases and controls all of whom had at least one documented HCV and one AUDIT-C test, without substantial changes in our findings. In total, 37% of patients received a test for HBV. However, we did not limit the cohorts to patients who had HBV testing because the overall proportion of patients with HBV infection is too low in the U.S. (and the VA) to have a meaningful effect on the risk estimates. It is possible that a small proportion of patients presenting to the VA with established NAFLD cirrhosis, including some with normal ALT, might not have been captured using our definition and therefore were included in the control cohort. However, this misclassification would have biased our results towards the null and thus enhance the confidence in the HCC risk difference. We used previously validated ICD codes to define cirrhosis. Although, the cirrhosis algorithm was highly predictive of presence of cirrhosis diagnosis in EMR in our previous study, we might have missed patients with cirrhosis especially if cirrhosis was not recognized (and coded as such) as part of routine care.24 However, our results suggest that ascertainment bias in cirrhosis definition is likely small. For example, in the subgroup of NAFLD patients with HCC, most patients with cirrhosis could be identified based on ICD codes or high FIB-4. Similarly, the risk of HCC was minimal in the subgroup with no cirrhosis code combined with low FIB-4, suggesting that few cirrhosis patients were missed. Our findings show that incorporating information from non-invasive markers for fibrosis may improve identification of the at risk groups at the population level. For example, we found that a FIB-4 > 2.67 was associated with a high HCC risk especially in those with documented cirrhosis (close to 1.35% per year), whereas absence of cirrhosis combined with low FIB-4 was associated with the lowest risk of HCC in this cohort (0.004% per year); these two factors can be easily applied in clinical practice to identify the at-risk groups for targeted evaluation and risk modification among the masses of individuals with NAFLD, most of who do not develop HCC. Some risk factors like smoking status were missing for cases or controls. We also did not have data on NASH in the study database. However, we calculated FIB-4 to define liver fibrosis severity and found a stepwise increase in the risk of HCC with increasing FIB-4. Lastly, our study is limited to Veterans and thus the results may not be generalizable to individuals with NAFLD seen in other healthcare systems. However, the biological processes of disease progression are likely similar in veterans and non-veterans with NAFLD.
In summary, our study is the first to quantify the absolute risk of HCC in a large, geographically and ethnically diverse cohort of NAFLD patients compared with controls. We found that the risk of HCC was higher in patients with NAFLD than that observed in general clinical population. Risk of HCC in most NAFLD patients with cirrhosis was higher than the currently accepted thresholds for starting HCC surveillance. A total of 80% of NAFLD-related HCC developed in the setting of underlying cirrhosis. The absolute risk of HCC in patients who do not develop cirrhosis is too low to recommend HCC surveillance.
Supplementary Material
Table 5.
The annual risk of hepatocellular cancer (HCC) in groups of patients with non-alcoholic fatty liver disease stratified by the presence of cirrhosis diagnosis and /or FIB-4 during follow up.
| Group |
Subject | HCC cases | Total personyear (PY) of follow-up | IR (95% CI) (per 1000 PY) |
|---|---|---|---|---|
| Cirrhosis diagnosis and high FIB-4 | 2,871 | 252 | 18598 | 13.55 (11.93–15.33) |
| Cirrhosis without high FIB-4 | 1,364 | 45 | 9323 | 4.82 (3.52–6.46) |
| High FIB-4 without cirrhosis diagnosis | 34,392 | 101 | 259942 | 0.39 (0.31–0.47) |
| Neither cirrhosis diagnosis nor high FIB-4 | 258,074 | 92 | 2094427 | 0.04 (0.04–0.05) |
FIB-4 values available for 95% of the cohort. Patients with no cirrhosis diagnosis and missing FIB-4 are excluded from the table.
Acknowledgments
GRANT SUPPORT: This material is based upon work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The works is also supported in part by the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, Texas and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Footnotes
CONFLICTS OF INTEREST: None to report
DISCLAIMER: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs or the United States
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 5/1/2016 2016;122(9):1312–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan DE, Chapko MK, Mehta R, Dai F, Skanderson M, Aytaman A, Baytarian M, D’Addeo K, Fox R, Hunt K, Pocha C, Valderrama A, Taddei TH; VOCAL Study Group. Healthcare Costs Related to Treatment of Hepatocellular Carcinoma Among Veterans With Cirrhosis in the United States. Clin Gastroenterol Hepatol. 2017. July 26 pii: S1542–3565(17)30861–3. doi: 10.1016/j.cgh.2017.07.024. [Epub ahead of print]. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JD, Larson JJ, Watt KD, Allen AM, Wiesner RH, Gores GJ, Roberts LR, Heimbach JA, Leise MD. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin Gastroenterol Hepatol. 2017. May;15(5):767–775. e3. doi: 10.1016/j.cgh.2016.11.034. Epub 2016 Dec 21. PubMed PMID: ; PubMed Central PMCID: PMC5401787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver diseaserelated hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13(3):594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol 2007;17(11):863–869 [DOI] [PubMed] [Google Scholar]
- 6.Williams CD, Stengel J, Asike MI et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140(1):124–131. [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the Burden of Nonalcoholic Fatty Liver Disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol. 2016. February;14(2):301–8.e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34(3):274–285. [DOI] [PubMed] [Google Scholar]
- 9.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59(6):2188–2195. [DOI] [PubMed] [Google Scholar]
- 10.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10(12):1342–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016. January;14(1):124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lapham GT, Achtmeyer CE, Williams EC, Hawkins EJ, Kivlahan DR, Bradley KA. Increased documented brief alcohol interventions with a performance measure and electronic decision support. Med Care. 2012. February;50(2):179–87 [DOI] [PubMed] [Google Scholar]
- 14.Husain N, Blais P, Kramer J, Kowalkowski M, Richardson P, El-Serag HB, Kanwal F. Nonalcoholic fatty liver disease (NAFLD) in the Veterans Administration population: development and validation of an algorithm for NAFLD using automated data. Aliment Pharmacol Ther. 2014. October;40(8):949–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, Li J, El-Serag HB. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–7 [DOI] [PubMed] [Google Scholar]
- 16.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. October 2009;7(10):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redman JS, Natarajan Y, Hou JK, Wang J, Hanif M, Feng H, Kramer JR, Desiderio R, Xu H, El-Serag HB, Kanwal F. Accurate Identification of Fatty Liver Disease in Data Warehouse Utilizing Natural Language Processing. Dig Dis Sci. 2017;62(10):2713–2718 [DOI] [PubMed] [Google Scholar]
- 18.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, Kanwal F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66(2):355–362 [DOI] [PubMed] [Google Scholar]
- 19.Cucchetti A, Cescon M, Erroi V, Pinna AD. Cost-effectiveness of liver cancer screening. Best Pract Res Clin Gastroenterol. 2013. December;27(6):961–72 [DOI] [PubMed] [Google Scholar]
- 20.Paradis V, Zalinski S, Chelbi E et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49(3):851–859 [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto E, Yatsuji S, Tobari M et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol 2009;44 Suppl 19:89–95. [DOI] [PubMed] [Google Scholar]
- 22.Ertle J, Dechene A, Sowa JP et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128(10):2436–2443. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N, Younossi Z, Lavine JE et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142(7):1592–1609. [DOI] [PubMed] [Google Scholar]
- 24.Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10–4. [DOI] [PubMed] [Google Scholar]
- 25.Walker M, El-Serag HB, Sada Y, Mittal S, Ying J, Duan Z, Richardson P, Davila JA, Kanwal F. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther. 2016. March;43(5):621–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.